Abstract
The synthesis of vitamin D is altered by the granulomatous inflammation of sarcoidosis leading to increased production of 1, 25-dihydroxyvitamin D. Mounting evidence suggests that vitamin D is an immunomodulating hormone that inhibits both antigen presentation by cells of the innate immune system, and the cytokine release and proliferation of Th1 cells. These and other extraskeletal health benefits have led to an increase in vitamin D assessment and pharmacological supplementation in the general population. This review highlights the altered synthesis and general immunomodulating properties of vitamin D with a special emphasis on known interactions with sarcoidosis. In addition, the assessment of vitamin D nutritional status, its pharmacological supplementation, and the management of bone health in patients with sarcoidosis are reviewed.
Keywords: Sarcoidosis; vitamin D; 1, 25-dihydroxyvitamin D
Vitamin D metabolism has been the focus of intense investigation over the past 30 years providing substantial insight into handling of vitamin D by granulomas. In addition, its role as an immune-modulating hormone has been increasingly explored because of the known epidemiological association between hypovitaminosis D and autoimmune disease incidence. As a consequence, vitamin D status with serum 25-hydroxyvitamin D level is assessed with increasing frequency in general medical care, and depleted patients are supplemented. This review highlights the data that are propagating the increasing vitamin D assessment of the population, the general immunologic role of vitamin D with a special focus on antigen-presenting cells and T cells, the impact and management of bone health in sarcoidosis, and clinical utility of vitamin D nutritional assessment and correction as it relates specifically to patients with sarcoidosis.
VITAMIN D SYNTHESIS
Humans acquire vitamin D predominantly from ingestion of foods rich in vitamin D2 (plant-derived ergocalciferol), and vitamin D3 (cholecalciferol), or foods supplemented with vitamin D3 (dominant form). In addition to the ingested forms of vitamin D, after exposure to ultraviolet radiation, 7-dehydrocholesterol is converted to vitamin D3 in the skin. These sources of vitamin D are dependent upon latitude, daily sun exposure, food tolerance (lactose intolerance), and use of vitamin D–fortified foods and supplements. Regardless of the vitamin D source, two key hydroxylation steps are needed prior to the formation of the metabolically active 1, 25-dihydroxyvitamin D (Fig. 1). First, 25-hydroxylation occurs in hepatocytes, under the influence of rather poorly regulated 25-hydroxylase (CYP27A1) enzyme, resulting in the formation of 25-hydroxyvitamin D (D3 or D2). This is the circulating and storage form of vitamin D; it is the best available index of vitamin D nutrition and is measured in ng/mL of serum.1 A second hydroxylation step, via 1α-hydroxylase (CYP27B1), leads to the formation of the metabolically active form of vitamin D (D3 or D2), 1, 25-dihydroxyvitamin D (measured in pg/mL of serum). This CYP27B1 is a tightly regulated enzyme in contrast to CYP27A1 and is expressed in almost all tissues of the body, but the circulating 1,25-dihydroxyvitamin D predominantly comes from the renal 1α hydroxylation under normal physiological conditions.
Figure 1.
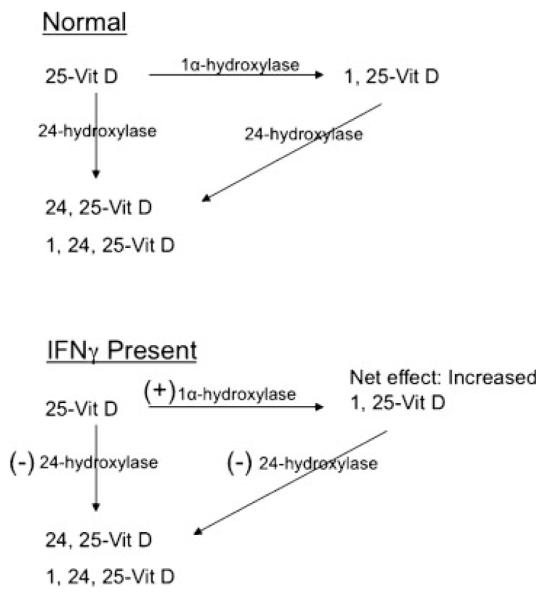
Interferon-γ effects on vitamin D3 synthesis.
VITAMIN D DEFICIENCY
Several studies suggest that vitamin D insufficiency is widespread in the population. Although the definitions of vitamin D deficiency and insufficiency are different in two separate analyses of the National Health and Nutrition Examination Survey III (NHANES III) dataset, both suggest inadequate vitamin D stores are common.2,3 Looker et al performed a stratified analysis of the NHANES III cohort (n = 18,875) accounting for seasonal differences in vitamin D sampling.2 Vitamin D deficiency was present in 1% of the population (<17.5 nmol/L or 7 ng/mL) and insufficiency reported in 21 to 58% (<62.5 nmol/L or 25 ng/mL) depending on age, gender, and sampling latitude. Zadshir et al, using higher cutoff points in the same cohort, found severe deficiency (<25 nmol/L or 10 ng/mL) in 1 to 3% and mild to moderate deficiency (<70 nmol/L or 28 ng/mL) to be 40 to 51% depending on gender.3 However, race is the largest independent determinant of hypovitaminosis D, with 5 to 11% of blacks having severe deficiency and 75% having mild to moderate deficiency.3
In a recent vitamin D assessment (n = 59) of our predominantly female (71%) and African American (86%) sarcoidosis patients, 49% had severe deficiency (25-hydroxyvitamin D <10 ng/mL), 48% had mild deficiency (10 to 28 ng/mL), and only one patient had a normal level above 28 ng/mL as defined by Zadshir et al.3 Despite near universal vitamin D deficiency, 71% of the patients (41/58) had serum 1, 25-dihydroxyvitamin D levels at or above the median clinical value (33.5 pg/mL) in the reference population. This suggests that, despite low 25-hydroxyvitamin D levels, the active form 1, 25-dihydroxyvitamin D is normal in the majority of patients with sarcoidosis.
Given the recent studies suggesting increased autoimmune disease risk with reduced vitamin D intake and high deficiency rates among the general population the supplementation guidelines are being challenged.4 The recommended vitamin D supplementation doses have been traditionally based on requirements needed to promote bone health, ranging from 5 to 15 μg/d (200 to 600 IU/day). However, in light of the association between vitamin D deficiency and diseases such as cancer and autoimmune disease, the 13th Workshop Consensus for Vitamin D Nutritional Guidelines recommended 25-hydroxyvitamin D levels of at least 20 ng/mL. It has been suggested that the upper level (UL) (2000 IU/day, 50 μg) be reevaluated and supplementation targeted to approach this level.4
PHYSIOLOGICAL EFFECTS OF VITAMIN D
Calcitriol (1, 25-dihydroxyvitamin D) is the most metabolically active form of vitamin D. Although the serum levels of 1, 25-hydroxyvitamin D (pg/mL) are three orders of magnitude lower than that of 25-hydroxyvitamin D (ng/mL), the binding affinity to the vitamin D receptor (VDR) is greater for 1, 25-dihyroxyvitamin D. The classical systemic effect of 1, 25-dihydroxyvitamin D is to help maintain serum calcium within a narrow range in concert with parathyroid hormone (PTH).5 With reductions in serum calcium, the parathyroid gland releases PTH, which increases the expression of 1α-hydroxylase by renal tubular cells. Hydroxylation of 25-hydroxyvitamin D by 1α-hydroxylase (CYP27B1), leads to 1, 25-dihydroxyvitamin D. Calcitriol (1, 25-dihydroxyvitamin D) attempts to increase serum calcium to normal levels by increasing intestinal absorption of calcium and increasing bone resorption. These effects at the cellular level are at least partially modulated through calcitriol binding to the VDR and subsequent transcription of genes expressed in vitamin D response elements (VDREs). A negative feedback loop, whereby calcitriol inhibits further PTH release and 1α-hydroxylase mRNA transcription, prevents the development of hypercalcemia. Granulomas can increase 1, 25-dihyroxyvitamin D production, leading to hypercalciuria or hypercalcemia, but only in a minority of sarcoidosis patients.6 Meyrier et al studied 39 untreated patients with sarcoidosis and found that 30% had hypercalciuria despite the institution of a low calcium diet. This suggests that a calcium source other than increased intestinal calcium absorption was leading to hypercalciuria.7 A second group, which had normal calcium excretion on a low calcium diet, developed hypercalcemia with an oral calcium load suggesting increased intestinal absorption. Elevated 1, 25-dihydroxyvitamin D levels and more extensive sarcoidosis involvement have been reported to be associated with abnormal calcium handling.7,8
VITAMIN D RECEPTOR DISTRIBUTION IN THE IMMUNE SYSTEM
Vitamin D modulates calcium and phosphorus homeostasis through its binding to the VDR, which has a high degree of affinity for 1, 25-dihydroxyvitamin D.9 Upon binding of calcitriol to the VDR, in the presence of the retinoid X receptor (RXR) cofactor, this ligand–receptor complex binds to various promoter regions (vitamin D responsive elements) of vitamin D responsive genes. These genes promote or suppress several products involved in bone health, including osteocalcin, PTH, calbindin, CYP24A (24-hydroxylase), or CYP27B1 (1α-hydroxylase).5 The distribution of VDR is diverse but includes the key target organs in bone and mineral homeostasis—bone, kidney, and intestine. In addition, VDR is found in immune cells depending upon their stage of activity. As a general rule, monocytes, macrophages, and lymphocytes have low-level VDR expression, but upon activation they acquire VDR in the presence of 1, 25-dihydroxyvitamin D.10–12
There are different polymorphisms within the VDR gene, but only the Bsm1 and Fok1 polymorphisms have been studied in patients with sarcoidosis. Bsm1 is likely a nonfunctional RFLP however; evidence suggests that its relationship to other RFLPs (Apa-Taq and polyA tails) may make it a marker of VDR protein numbers. In contrast, the Fok1*F allele introduces a truncated (three-amino acid) protein that appears to be more active at VDRE. The Bsm1*B allele frequency is increased in Japanese patients with sarcoidosis compared with normal controls; however, this genotype was not associated with differences in Scadding radiographic stage, the number of organs involved, calcitriol levels, or hypercalcemia.13,14 In African American families with sarcoidosis, the Fok1 VDR gene polymorphism (Fok1*f allele) was increased in those with more severe disease.15 The Fok1 and Taq1 polymorphisms are associated with the extent or severity of tuberculosis and leprosy, respectively, suggesting that VDR polymorphisms may modulate granulomatous inflammation.16–19
CYTOKINE EFFECTS ON 1α-HYDROXYLASE ACTIVITY
In renal tubular cells, 1α-hydroxylase (CYP27B1) expression is tightly regulated by PTH, phosphorus, and 1, 25-dihydroxyvitamin D itself. However, this is not the predominant control mechanism for 1α-hydroxylase activity in immune cells. Rather, immune cell 1α-hydroxylase expression is controlled predominantly by locally synthesized interferon-γ (IFN-γ), which is highly expressed in sites of granulomatous inflammation20 (Table 1). Calcitriol production is increased in monocytes cultured with cytokines IFN-γ, tumor necrosis factor-α (TNF-α), and interleukin 1 and 2 (IL-1/IL-2), and its production is reduced with dexamethasone.21,22
Table 1.
Interferon-γ Effects on Vitamin D Metabolism
An additional distinction between renal tubular and immune cell 1α-hydroxylase control is the effect of 1, 25-dihydroxyvitamin D itself. In renal tubular cells, calcitriol inhibits 1α-hydroxylase activity in a product-substrate-dependent manner, providing a negative feedback loop on its continued synthesis.23,24 However, in murine macrophages and human monocytes this negative feedback loop is less effective.22,24,25
In addition, calcitriol induces 24-hydroxylase (CYP24A1) enzyme activity, which diverts 25-hydroxyvitamin D to noncalcemic and less active polar metabolites (Table 1). This catabolic pathway is induced by 1, 25-dihydroxyvitamin D in monocytes but not in alveolar macrophages.25 By utilizing the human monocyte THP-1 cell line, Dusso et al found that IFN-γ blocked 1, 25-dihydroxyvitamin D–mediated inhibition of 1αhydroxylase enzyme activity and reduced the mRNA for 24-hydroxylase expression (promoted by 1, 25-dihydroxyvitamin D).25 These findings suggest that a local environment, rich in IFN-γ, can perpetuate continued production of 1, 25-dihydroxyvitamin D without a significant feedback loop to halt its synthesis or a breakdown pathway to reduce its levels. This unabated synthesis of 1, 25-dihydroxyvitamin D should lead to feedback inhibition of ongoing antigen-presenting cell (APC) and T cell activation.
It has also been hypothesized that 25-hydroxyvitamin D binding to alternative nuclear receptors may be an additional mechanism leading to persistent calcitriol levels.26 Pregnane X receptor (PXR) is a nuclear receptor similar to VDR and increases 25-hydroxyvitamin D through increased 25-hydroxylase and inhibition of 24-hydroxylase (CYP24A1) expression.27–29 It has been suggested that both 25-hydroxy- and 1, 25-dihyroxyvitamin D are antagonists of PXR which would lead to a scenario of low 25-hydroxyvitamin D and possibly elevated 1, 25-dihydroxyvitamin D, a pattern found clinically in sarcoidosis.26 This theory is supported by the observations of Blaney et al, in which patients with autoimmune disease (n = 30) had low 25-hydroxyvitamin D (30%) but nearly all had significantly elevated 1, 25-dihydroxyvitamin D levels (90%).30
VITAMIN D ASSOCIATIONS WITH AUTOIMMUNE DISEASE INCIDENCE
Recently, several population-based studies have associated vitamin D deficiency with the prevalence of various autoimmune diseases. These findings have led to an increased assessment and supplementation of vitamin D in the adult general medical population. There have been discrepant reports of association between vitamin D intake and rheumatoid arthritis (RA) incidence in prospective cohort studies. Merlino et al reported a lower incidence of RA with increasing baseline vitamin D intake in the Iowa Women’s Health Study.31 In contrast, no such association for either RA or systemic lupus erythematosis (SLE) was found in the larger Nurses’ Health Study I and II.32 In addition, high-dose vitamin D intake reduced the incidence of type 1 diabetes by 78% in a prospective birth cohort.33 Despite these large observational findings, no randomized, controlled trial of pharmacological or supplemental vitamin D has been performed to suggest causation.
EFFECTS OF VITAMIN D IN ANIMAL MODELS OF AUTOIMMUNE DISEASE
Prior to the population-based studies, in vivo and in vitro studies suggested vitamin D is an important immunomodulatory hormone. Lemire et al have reported significant reductions in proteinuria, alopecia, and antinuclear antibody titers in a murine SLE model when supplemented with 1, 25-dihydroxyvitamin D.34 Similarly, Cantorna et al, reported 1, 25-dihydroxyvitamin D supplementation reduced the onset of RA (90% vs 45%) and markers of joint inflammation in murine arthritis models.35 In the nonobese diabetic (NOD) model of type 1 diabetes, 1, 25-dihydroxyvitamin D supplementation reduced the onset of diabetes (56% vs 8%) and pancreatic islet infiltration by lymphocytes.36 A similar finding has been reported in an IL-10 knock-out murine model of inflammatory bowel disease with reductions in histopathologic inflammation.37 Considering the central role of Th1-mediated inflammation in autoimmune disease, these data suggest 1, 25-dihydroxyvitamin D may modulate T-lymphocyte activity with alterations in disease manifestations.
EFFECT OF VITAMIN D ON IMMUNE CELLS AND BRONCHIAL EPITHELIUM
Monocytes have low-level VDR expression. However, after exposure to 1, 25-dihydroxyvitamin D they mature and express markers of macrophage lineage such as β-acetylglucosaminidase, Fc receptor, OKM1 complement antigen (C3), and IFN-γ receptor.10,38–40 Investigation by Liu et al provides an important link between infection and local 1, 25-dihydroxyvitamin D–induced changes in innate immune cells.41 Upon Mycobacterium tuberculosis antigen binding to toll-like receptors (TLR 2/1), VDR and CYP27B1 are differentially expressed by monocytes and macrophages. In addition, cathelicidin and its antimicrobial product LL-37 are synthesized when exposed to 1, 25-dihydroxyvitamin D, reducing Mycobacterium burden. Calcitriol’s effect on monocyte cellular proliferation is inhibitory in most studies, suggesting a phenotype of antigen recognition but induction of tolerance.38,39,42
Dendritic cells (DCs) are resident phagocytic cells within tissues recognized for high levels of environmental antigen exposure (i.e., skin and lung). These cells are particularly adept at phagocytosis, antigen processing, and presentation to the cellular arm of the immune system. Human monocytes can be stimulated to become dendritic cells upon exposure to granulocyte-macrophage-colony stimulating factor (GM-CSF) and IL-4. Penna et al have eloquently shown that human monocytes will not progress to immature DCs, as marked by CD1a expression, upon culture with calcitriol.43 In addition, immature DCs will not acquire the antigen-presentation characteristics common to APCs, such as CD80/86, CD40, and major histocompatibility complex-class II (MHC-II) expression (Table 2). Similar findings were reported by Piemonti et al with the additional finding that immature dendritic cells have a heightened capacity for endocytosis as reflected by increased mannose receptor uptake of dextran.44 Despite an increase in antigen uptake, the dendritic cells induced T cell hyporesponsiveness. Similar findings have also been reported in allogeneic pancreatic transplantation in mice with calcitriol effects as substantial as mycophenolate.45 These altered dendritic cells also exhibit a reduced capacity to stimulate CD4+ cells, interrupting an essential interaction of innate and cellular immunity that is needed for antigen-specific inflammation.
Table 2.
Immunomodulatory Effects of 1, 25-dihydroxyvitamin D3
| 1, 25-dihydroxyvitamin D3 Effects | References | |
|---|---|---|
| ANTIGEN-PRESENTING CELLS | Antigen uptake, reduced presentation | |
| IL-12 production | ↓ | 43,45,49 |
| Co-stimulatory signal (CD40, CD80/86) expression | ↓ | 45,48 |
| Major histocompatibility complex (MHC expression) | ↓ | 48 |
| T CELLS | Hypoproliferative | |
| IFN-γ production | ↓ | 20,45,85 |
| IL-2 production | ↓ | 84,85 |
| Cellular proliferation | ↓ | 43,46 |
IFN, interferon; IL, interleukin.
CD4+ cell proliferation is inhibited in the presence of 1, 25-dihydroxyvitamin D43,46,47 (Table 2). Currently numerous mechanisms have been proposed to explain this anergy induction. First, as described previously, APCs do not express antigen well when exposed to calcitriol. Reduced MHC-II and co-stimulatory signal expression (CD80/86, CD40) prevent APC engagement and activation of CD4+ cells.43,48 In addition, 1, 25-dihydroxyvitamin D reduces macrophage release of IL-12, a potent stimulator of CD4+ proliferation.45,49 The third mechanism involves a reduction of IL-2, a positive autoregulatory Th1 cytokine.11,20,50 All of these 1, 25-dihydroxyvitamin D–mediated effects reduce T cell activation and proliferation.
The regulatory T cell (Treg), a subclass of CD4+ CD25+ cells expressing FoxP3 and low-level or absent CD127 (IL-7 receptor), is a potent inhibitor of general CD4+ proliferation. Tregs induce anergy in CD4+ cells through direct cell–cell contact and IL-2, and indirectly through a reduction of molecules important for antigen presentation and co-stimulatory signaling by APCs. Calcitriol has been found to qualitatively improve the suppressive function and number of tregs in an expanding CD4+ population.51,52
Bronchial epithelial cells are continually exposed to antigenic stimuli and must maintain a phenotype of tolerance to immunogenic but noninvasive material. Consistent with this observation, Hansdottir et al have found that human bronchial epithelial cells constitutively express 1α-hydroxylase mRNA and produce 1, 25-dihyroxyvitamin D without any stimulation.53 The consequence of this 1, 25-dihyroxyvitamin D synthesis and its subsequent binding to VDR are the expression of antimicrobial LL-37 and soluble CD14 from epithelial cells. CD14, a pathogen recognition receptor (PRR), detects pathogen-associated molecular patterns (PAMPs), particularly lipopolysaccharide (LPS), a constituent of the cell walls of gram-negative organisms. These highly conserved antigen detection and bacterial control mechanisms are increased with exposure to vitamin D.53,54 These findings suggest that 1, 25-dihyroxyvitamin D plays a significant role in immune surveillance and local bacterial control in the normal human airway.
THE IMPORTANCE OF DISORDERED CALCIUM METABOLISM IN SARCOIDOSIS
Calcium metabolism is abnormal in a minority of patients with sarcoidosis. The A Case-Control Etiologic Study of Sarcoidosis (ACCESS) cohort (n = 736) found hypercalcemia and/or hypercalciuria in 3.7% of newly diagnosed (<6 months) patients.6 A recent epidemiology survey of incident cases (n = 1027) in Japan reported hypercalcemia in 7.4% of patients.55 Although these large prospective cohort studies provide a comprehensive assessment of total body disease involvement in newly diagnosed patients, they likely underestimate the prevalence of hypercalciuria because urinary calcium excretion was not assessed in a systematic manner. In addition, the complications of disordered calcium metabolism, such as nephrolithiasis or nephrocalcinosis, are unlikely to develop within the observation time periods of these studies. Hypercalcemia is more likely to develop in Caucasians, males, age >40, and in patients with both the HLA-DRB1*1101 allele and exposure to insecticide, although this interaction explained only four of the 22 cases of hypercalcemia.6,56 Intrinsic renal dysfunction is an important determinant of hypercalcemia in patients with sarcoidosis. Mahévas et al have recently reported a case series of 47 patients with renal failure and sarcoidosis.57 Among this cohort of patients with biopsy-proven interstitial nephritis (46 of 47) and an estimated glomerular filtration rate (eGFR) <90 mL/min per 1.73 m2, hypercalcemia was present in 34% of subjects. Among this high-risk population, only three patients had nephrolithiasis and one had nephrocalcinosis. This suggests that baseline chronic renal insufficiency is an important determinant of hypercalcemia, independent of hypercalcemia-related intravascular volume depletion.
ALVEOLAR MACROPHAGE METABOLISM OF VITAMIN D IN SARCOIDOSIS
In an effort to determine the pathogenesis of disordered calcium metabolism in sarcoidosis, Adams et al studied the pulmonary alveolar macrophages (PAMs) of six patients with sarcoidosis.58 Lipid extract of unstimulated PAMs contained 1, 25-dihyroxyvitamin D. In addition, incubation of macrophages with 25-hydroxyvitamin D resulted in the production of 1, 25-dihydroxyvitamin D. However, 1, 25-dihydroxyvitamin D could only be identified in patients with Scadding stage II or III disease compared with patients with stage I radiographs and two patients with idiopathic pulmonary fibrosis.
Reichel et al cultured PAMs from six patients with sarcoidosis and nine normal subjects to determine what factors regulate 1, 25-dihydroxyvitamin D production in the lung.20 Confirming the results of Adams et al, sarcoidosis PAMs were spontaneously able to synthesize 1, 25-dihydroxyvitamin D. However, control PAMs required IFN-γ or LPS to be added to their culture to obtain the same effect. The most likely explanation for these discrepant findings is that the IFN-γ was present within the sarcoid lung prior to cell harvest, rather than differential control of 1α-hydroxylase activity in sarcoidosis. Reichel et al also reported that the 1, 25-dihydroxyvitamin D feedback loop is less effective in sarcoidosis PAMs compared with controls. In addition, 24-hydroxylase activity is reduced in PAMs. These findings, specifically studied in sarcoidosis, are consistent with the observations of Dusso et al, whereby IFN-γ significantly impairs the 1, 25-dihydroxyvitamin D feedback mechanism and that the 24-hydroxylase pathway is of marginal importance in the alveolar macrophage.25 Overall, IFN-γ release by the activated Th1 cell enhances macrophage production of 1, 25-dihydroxyvitamin D. Calcitriol then leads to feedback inhibition of Th1 proliferation and ongoing release of IFN-γ by binding to the VDR present in activated T cells. In sarcoidosis it seems, the local ongoing production of 1, 25-dihydroxyvitamin D, induced by IFN-γ, is inadequate to control persistent Th1 inflammation.
DIFFERENTIAL DIAGNOSIS OF HYPERCALCEMIA IN PATIENTS WITH SARCOIDOSIS
In patients with sarcoidosis and hypercalcemia, excessive levels of 1, 25-dihydroxyvitamin D lead to increased intestinal absorption of calcium and/or bone resorption. However, it is important to recognize additional causes of hypercalcemia, including primary hyperparathyroidism and malignancy, which account for 62 to 81% of hypercalcemic states in ambulatory and hospital settings.59 Intact PTH or PTH-related peptide (PTHrP) testing is helpful in distinguishing primary hyperparathyroidism from alternative etiologies such as malignancy (lymphoma, metastatic bone carcinoma) or granulomatous disease (sarcoidosis, fungal infections, mycobacterial infections).
ASSESSMENT AND MANAGEMENT OF HYPERCALCEMIA AND/OR HYPERCALCIURIA
Given the wide variation in reporting of hypercalcemia and/or hypercalciuria among various populations there is no currently accepted standard guideline for the assessment calcium metabolism in patients with sarcoidosis. It is our observation that hypercalcemia can be quite common in a significant minority of patients. In some individuals with sarcoidosis, hypercalcemia may be the only indication for steroid therapy. Adequate hydration and prednisone 40 mg/day for 1 week usually correct acute severe hypercalcemia. We recommend a reduction to 20 mg daily within the first 1 to 2 weeks and attempts at maintenance of 10 mg prednisone daily or every other day therapy with attempts at discontinuing prednisone if chronic renal dysfunction is not present. However, many patients with chronic kidney disease require more frequent monitoring of serum calcium levels and creatinine. We do not screen patients without nephrolithiasis for isolated hypercalciuria (>300 mg/24 h) based on the following observations: (1) The complications of hypercalciuria (nephrolithiasis or nephrocalcinosis) are uncommon even among high-risk sarcoidosis populations57; (2) isolated hypercalciuria alone is not an indication for prednisone therapy; and (3) the risk of side effects with corticosteroid and hydroxychloroquine therapy are far greater than the risk of nephrolithiasis.
MANAGEMENT OF THE 25-VITAMIN D–DEFICIENT SARCOIDOSIS PATIENT
The vitamin D nutritional status is increasingly assessed in the medical care of adults. The decision to provide supplementation for deficient patients as a primary prevention strategy should be based on the perceived needs and risks of supplementation. Despite observational associations of hypovitaminosis D with autoimmune disease, colon cancer, breast cancer, cardiovascular disease, or all-cause mortality, no randomized, controlled interventional trials of vitamin D supplementation have been performed to show a reduction in primary prevention of these diseases in adults.60–64 In our clinic we assess both 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D upon initial evaluation. We have found that 25-hydroxyvitamin D deficiency, either mild (<28 ng/mL) or severe (10 ng/mL), is nearly universal (58 of 59 patients); however, 1, 25-dihydroxyvitamin D is above the clinical median in 71% of patients. Given these data, we do not recommend pharmacological vitamin D (50,000 IU) doses for patients with low 25-hydroxyvitamin D(Table 3). This aggressive assessment and education of patients regarding vitamin D has been very helpful in preventing or removing patients from potentially harmful pharmacological doses (50,000 IU) of vitamin D. However, most patients with sarcoidosis appear to tolerate vitamin D 200 to 400 IU daily without manifesting hypercalciuria and/or hypercalcemia.
Table 3.
Henry Ford Hospital Multidisciplinary Recommendations for the Assessment and Management of Vitamin D in Patients with Sarcoidosis*
|
Level of evidence: expert opinion. No published observational or randomized clinical data are available to determine the safety of pharmacological vitamin D dosing (50,000 IU) in the sarcoidosis population.
BONE HEALTH AND SARCOIDOSIS
The bone health of patients with sarcoidosis has been extensively studied through the assessment of bone mineral density (BMD) as a surrogate for future fracture risk. It has been suggested that the granulomatous reaction of sarcoidosis, separate from the concomitant corticosteroid use, may lead to a reduced BMD. The evidence for this is an observational series by Montermurro et al, which assessed the vertebral cancellous bone mineral content (VCMC) of patients with untreated sarcoidosis.65 This analysis found the BMD in sarcoidosis patients was 1.1 ± 0.3 standard deviation (SD) units lower than age-sex-matched controls. The underlying mechanism is believed to be related to granuloma-derived osteoclast stimulating factor or the direct stimulation of osteoclasts by excessive levels of 1, 25-dihydroxyvitmamin D.
Therapeutic corticosteroids are the main factor in reducing BMD in patients with sarcoidosis requiring treatment. Van Staa et al, utilizing the United Kingdom General Practice Research Database (GPRD), reported a 20 to 30% increase of any osteoporotic fracture with corticosteroid use among the general population.66,67 Although fracture prevalence has not been reported in patients with sarcoidosis, the effects of corticosteroids on BMD are well described. Rizzato et al found the VCMC score of prednisone-treated sarcoidosis patients (n = 64) was 1.33 ± 0.9 SD units (Z-score) below age-sex-matched controls (n = 190).68 Montemurro et al have since reported that the decline of BMD occurs predominantly within the first year of corticosteroid use.69 In contrast to these observations, Heijckmann et al reported no difference in the femoral neck and trochanter BMD when comparing sarcoidosis patients (past and current corticosteroid treatment) with population-normalized values (Z-scores).70 However, patients who never used corticosteroids had a relative increase in trochanter Z-score (+0.45, 95% CI 0.15–0.76, p = 0.004). In contrast to the Rizzato and Montemurro studies, Heijckmann et al investigated the hip rather than the spine and utilized dual x-ray absorptiometry (DXA) rather than quantitative computed tomography (QCT). It has been suggested that the spine trabecular bone is more sensitive to glucocorticoid effects and that QCT is more sensitive than DXA in assessing BMD deficits.71
In patients undergoing corticosteroid treatment for sarcoidosis, two observational studies have reported a preservation of BMD with calcitonin. Rizzato et al, in a matched (age, sex, and total steroid dose) cohort reported less decline of VCMC in patients taking calcitonin compared with those not taking calcitonin (−2.15% vs −14.11%, respectively, p < 0.01).72 A subsequent analysis of 64 patients by Montemurro et al reported similar findings.73 In the general adult population, bisphosphonate therapy is effective in the treatment of osteoporosis with reductions in vertebral or hip fracture by 20 to 30%.74,75 Gonnelli et al, in a controlled clinical trial, randomized 30 patients with sarcoidosis undergoing corticosteroid therapy to receive either alendronate (5 mg/day) or placebo for the prevention of corticosteroid-induced bone mineral loss.76 After 1 year of therapy, there was less decline in bone density in the alendronate group compared with placebo (+0.8% vs −4.5%, p < 0.01). Current guidelines are discrepant on when to initiate bisphosphonate therapy with long-term glucocorticoid therapy. The American College of Physicians recommends bisphosphonate therapy for individuals with a DXA T-score lower then −1.5.77 In contrast, the American College of Rheumatology recommends bisphosphonate therapy in patients requiring prednisone >5 mg/day for greater than 3 months, independent of bone densitometry testing results.78
Vitamin D has traditionally been recommended with calcium supplementation as a primary prevention strategy to reduce the risk of osteoporotic fractures. A meta-analysis of eight randomized treatment trials found that >700 to 800 IU/d of vitamin D reduced hip fractures (pooled relative risk, 0.74, 95% CI 0.61 to 0.88) and nonvertebral fractures (pooled relative risk, 0.77, 95% CI 0.68 to 0.87) with a number needed to treat (NNT) of 45 and 27, respectively.79 Given that 1, 25-dihydroxyvitamin D levels are above the median reference value in 71% of patients, despite near universal insufficient 25-hydroxyvitamin D (58 of 59 patients) levels (<28 ng/mL) in our sampled patients, we do not recommend high-dose vitamin D supplementation. Rather, consistent with the American College of Rheumatology recommendations, we institute bisphosphonate therapy at the start of prednisone therapy, independent of the DXA T-score testing or results.78
CAN 1, 25-DIHYDROXYVITAMIN D PROVIDE A CLUE TO SARCOIDOSIS PHENOTYPES?
Interferon-γ is the likely stimulus for the continued production of 1, 25-dihydroyxvitamin D in sarcoid granulomas. Measurement of calcitriol, a common clinically available test, may be a marker of ongoing inflammation driving the granulomatous reaction. Infante et al investigated calcitriol levels in patients who underwent gallium-67 scans. No correlation existed between serum calcitriol levels and pulmonary Ga-67 intake nor was there a difference in calcitriol levels between those with active (n = 26) compared with those with inactive disease (n = 5) (44.6 ± 3.7 vs 35.8 ± 6.4, NS).80 The largest limitation to this study is the lack of objective criteria to define active disease or describing activity as acute versus chronic phenotype.
In addition to investigating the vitamin D levels in a cross-sectional cohort of patients with sarcoidosis, we associated 1, 25-dihydroxyvitamin D levels with disease phenotypes.81 Utilizing the Sarcoidosis Clinical Activity Classification (SCAC) proposed by Prasse et al,82 we determined that 1, 25-dihydroxyvitamin D levels are increased in patients with activity class 6 (subacute onset, with treatment needs >1 year or more than one treatment course) compared with other activity classes (47.2 ± 14.7 pg/mL vs 38.8 ± 11.0 pg/mL, p = 0.02). Increasing quartiles of 1, 25-dihydroyxvitamin D were associated with increased risk of disease chronicity (OR 2.7, 95% CI 1.18, 6.19, p = 0.02). This association was not affected by current immunosuppression and was independent of radiographic stage and race (OR 2.8, 95% CI 1.2, 6.6, p = 0.02). In fact, 71% of patients with 1, 25-dihydroxyvitamin D levels in the highest quartile (>52 pg/mL) needed to receive multiple courses of immunosuppression or more than 1 year of therapy (SCAC 6). This finding highlights a paradox whereby the ongoing IFN-γ-mediated production of 1, 25-dihydroxyvitamin D does not lead to feedback inhibition of ongoing Th1 inflammation in patients with sarcoidosis.
SUMMARY
Vitamin D is an important immunomodulatory hormone with many intersections in the management of patients with sarcoidosis. The increased synthesis of 1, 25-dihydroxyvitamin D, in the face of reduced 25-hydroxyvitamin D levels, creates both a diagnostic and a therapeutic dilemma in the management of patients with sarcoidosis.
REFERENCES
- 1.Rao DS. Perspective on assessment of vitamin D nutrition. J Clin Densitom. 1999;2:457–464. doi: 10.1016/s1094-6950(06)60411-3. [DOI] [PubMed] [Google Scholar]
- 2.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 3.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15(4 Suppl 5):S5–97–101. [PubMed] [Google Scholar]
- 4.Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103:204–205. doi: 10.1016/j.jsbmb.2006.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 6.Baughman RP, Teirstein AS, Judson MA, et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 7.Meyrier A, Valeyre D, Bouillon R, Paillard F, Battesti JP, Georges R. Different mechanisms of hypercalciuria in sarcoidosis: correlations with disease extension and activity. Ann N Y Acad Sci. 1986;465:575–586. doi: 10.1111/j.1749-6632.1986.tb18534.x. [DOI] [PubMed] [Google Scholar]
- 8.Adams JS, Gacad MA, Anders A, Endres DB, Sharma OP. Biochemical indicators of disordered vitamin D and calcium homeostasis in sarcoidosis. Sarcoidosis. 1986;3:1–6. [PubMed] [Google Scholar]
- 9.DeLuca HF, Schnoes HK. Metabolism and mechanism of action of vitamin D. Annu Rev Biochem. 1976;45:631–666. doi: 10.1146/annurev.bi.45.070176.003215. [DOI] [PubMed] [Google Scholar]
- 10.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 11.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 12.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 13.Niimi T, Tomita H, Sato S, et al. Vitamin D receptor gene polymorphism and calcium metabolism in sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:266–269. [PubMed] [Google Scholar]
- 14.Niimi T, Tomita H, Sato S, et al. Vitamin D receptor gene polymorphism in patients with sarcoidosis. Am J Respir Crit Care Med. 1999;160:1107–1109. doi: 10.1164/ajrccm.160.4.9811096. [DOI] [PubMed] [Google Scholar]
- 15.Rybicki BA, Maliarik MJ, Poisson LM, Iannuzzi MC. Sarcoidosis and granuloma genes: a family-based study in African-Americans. Eur Respir J. 2004;24:251–257. doi: 10.1183/09031936.04.00005904. [DOI] [PubMed] [Google Scholar]
- 16.Bellamy R, Ruwende C, Corrah T, et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:721–724. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 17.Roy S, Frodsham A, Saha B, Hazra SK, Mascie-Taylor CG, Hill AV. Association of vitamin D receptor genotype with leprosy type. J Infect Dis. 1999;179:187–191. doi: 10.1086/314536. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355:618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 19.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HAP, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1987;84:3385–3389. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyetko MR, Hsu CH, Wilkinson CC, Patel S, Young E. Monocyte 1 alpha-hydroxylase regulation: induction by inflammatory cytokines and suppression by dexamethasone and uremia toxin. J Leukoc Biol. 1993;54:17–22. doi: 10.1002/jlb.54.1.17. [DOI] [PubMed] [Google Scholar]
- 22.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal AM, Jones G, Kooh SW, Fraser D. 25-hydroxyvitamin D3 metabolism by isolated perfused rat kidney. Am J Physiol. 1980;239:E12–E20. doi: 10.1152/ajpendo.1980.239.1.E12. [DOI] [PubMed] [Google Scholar]
- 24.Overbergh L, Decallonne B, Valckx D, et al. Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin Exp Immunol. 2000;120:139–146. doi: 10.1046/j.1365-2249.2000.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dusso AS, Kamimura S, Gallieni M, et al. Gamma-interferon-induced resistance to 1,25-(OH)2 D3 in human monocytes and macrophages: a mechanism for the hypercalcemia of various granulomatoses. J Clin Endocrinol Metab. 1997;82:2222–2232. doi: 10.1210/jcem.82.7.4074. [DOI] [PubMed] [Google Scholar]
- 26.Marshall TG. Vitamin D discovery outpaces FDA decision making. Bioessays. 2008;30:173–182. doi: 10.1002/bies.20708. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Hashizume T, Shuhart MC, et al. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1alpha,25-dihydroxyvitamin D(3): implications for drug-induced osteomalacia. Mol Pharmacol. 2006;69:56–65. doi: 10.1124/mol.105.017392. [DOI] [PubMed] [Google Scholar]
- 28.Li T, Chen W, Chiang JY. PXR induces CYP27A1 and regulates cholesterol metabolism in the intestine. J Lipid Res. 2007;48:373–384. doi: 10.1194/jlr.M600282-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Zhou C, Assem M, Tay JC, et al. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J Clin Invest. 2006;116:1703–1712. doi: 10.1172/JCI27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaney GP, Albert PJ, Proal AD. Vitamin D metabolites as clinical markers in autoimmune and chronic disease. Ann N Y Acad Sci. 2009;1173:384–390. doi: 10.1111/j.1749-6632.2009.04875.x. [DOI] [PubMed] [Google Scholar]
- 31.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG, Iowa Women’s Health Study Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50:72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 32.Costenbader KH, Feskanich D, Holmes M, Karlson EW, Benito-Garcia E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis. 2008;67:530–535. doi: 10.1136/ard.2007.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 34.Lemire JM, Ince A, Takashima M. 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity. 1992;12:143–148. doi: 10.3109/08916939209150321. [DOI] [PubMed] [Google Scholar]
- 35.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998;128:68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- 36.Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia. 1994;37:552–558. doi: 10.1007/BF00403372. [DOI] [PubMed] [Google Scholar]
- 37.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 38.Clohisy DR, Bar-Shavit Z, Chappel JC, Teitelbaum SL. 1,25-Dihydroxyvitamin D3 modulates bone marrow macrophage precursor proliferation and differentiation: up-regulation of the mannose receptor. J Biol Chem. 1987;262:15922–15929. [PubMed] [Google Scholar]
- 39.Amento EP, Bhalla AK, Kurnick JT, et al. 1 alpha,25-dihydroxyvitamin D3 induces maturation of the human monocyte cell line U937, and, in association with a factor from human T lymphocytes, augments production of the monokine, mononuclear cell factor. J Clin Invest. 1984;73:731–739. doi: 10.1172/JCI111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuckerman SH, Schreiber RD. Up-regulation of gamma interferon receptors on the human monocytic cell line U937 by 1,25-dihydroxyvitamin D3 and granulocyte-macrophage colony stimulating factor. J Leukoc Biol. 1988;44:187–191. doi: 10.1002/jlb.44.3.187. [DOI] [PubMed] [Google Scholar]
- 41.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 42.Ohta M, Okabe T, Ozawa K, Urabe A, Takaku F. In vitro formation of macrophage-epithelioid cells and multinucleated giant cells by 1 alpha,25-dihydroxyvitamin D3 from human circulating monocytes. Ann N Y Acad Sci. 1986;465:211–220. doi: 10.1111/j.1749-6632.1986.tb18497.x. [DOI] [PubMed] [Google Scholar]
- 43.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 44.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 45.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 46.Lemire JM, Adams JS, Kermani-Arab V, Bakke AC, Sakai R, Jordan SC. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol. 1985;134:3032–3035. [PubMed] [Google Scholar]
- 47.Provvedini DM, Manolagas SC. 1 Alpha,25-dihydroxyvitamin D3 receptor distribution and effects in subpopulations of normal human T lymphocytes. J Clin Endocrinol Metab. 1989;68:774–779. doi: 10.1210/jcem-68-4-774. [DOI] [PubMed] [Google Scholar]
- 48.Griffin MD, Lutz WH, Phan VA, Bachman LA, McKean DJ, Kumar R. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun. 2000;270:701–708. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]
- 49.D’Ambrosio D, Cippitelli M, Cocciolo MG, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3: involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsui T, Takahashi R, Nakao Y, et al. 1,25-Dihydroxyvitamin D3-regulated expression of genes involved in human T-lymphocyte proliferation and differentiation. Cancer Res. 1986;46:5827–5831. [PubMed] [Google Scholar]
- 51.Gorman S, Kuritzky LA, Judge MA, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+ CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 52.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3) J Cyst Fibros. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008;31:372–379. doi: 10.1183/09031936.00075307. [DOI] [PubMed] [Google Scholar]
- 56.Rossman MD, Thompson B, Frederick M, et al. ACCESS Group HLA and environmental interactions in sarcoidosis Sarcoidosis Vasc Diffuse Lung Dis. 2008;25:125–132. [PubMed] [Google Scholar]
- 57.Mahévas M, Lescure FX, Boffa JJ, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009;88:98–106. doi: 10.1097/MD.0b013e31819de50f. [DOI] [PubMed] [Google Scholar]
- 58.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lafferty FW. Differential diagnosis of hypercalcemia. J Bone Miner Res. 1991;6(Suppl 2):S51–59. doi: 10.1002/jbmr.5650061413. discussion S61. [DOI] [PubMed] [Google Scholar]
- 60.Ding EL, Mehta S, Fawzi WW, Giovannucci EL. Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: reanalysis of Women’s Health Initiative randomized trial. Int J Cancer. 2008;122:1690–1694. doi: 10.1002/ijc.23311. [DOI] [PubMed] [Google Scholar]
- 61.Chlebowski RT, Johnson KC, Kooperberg C, et al. Women’s Health Initiative Investigators Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100:1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaCroix AZ, Kotchen J, Anderson G, et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64:559–567. doi: 10.1093/gerona/glp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 64.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Women’s Health Initiative Investigators Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 65.Montemurro L, Fraioli P, Rizzato G. Bone loss in untreated longstanding sarcoidosis. Sarcoidosis. 1991;8:29–34. [PubMed] [Google Scholar]
- 66.van Staa TP, Leufkens HG, Cooper C, Leufkens HGM, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 67.van Staa TP, Geusens P, Pols HAP, de Laet C, Leufkens HGM, Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM. 2005;98:191–198. doi: 10.1093/qjmed/hci029. [DOI] [PubMed] [Google Scholar]
- 68.Rizzato G, Tosi G, Mella C, Montemurro L, Zanni D, Sisti S. Prednisone-induced bone loss in sarcoidosis: a risk especially frequent in postmenopausal women. Sarcoidosis. 1988;5:93–98. [PubMed] [Google Scholar]
- 69.Montemurro L, Fraioli P, Riboldi A, Delpiano S, Zanni D, Rizzato G. Bone loss in prednisone treated sarcoidosis: a two-year follow-up. Ann Ital Med Int. 1990;5(3 Pt 1):164–168. [PubMed] [Google Scholar]
- 70.Heijckmann AC, Huijberts MS, De Vries J, et al. Bone turnover and hip bone mineral density in patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24:51–58. [PubMed] [Google Scholar]
- 71.Rizzato G, Fraioli P. Natural and corticosteroid-induced osteoporosis in sarcoidosis: prevention, treatment, follow up and reversibility. Sarcoidosis. 1990;7:89–92. [PubMed] [Google Scholar]
- 72.Rizzato G, Tosi G, Schiraldi G, Montemurro L, Zanni D, Sisti S. Bone protection with salmon calcitonin (sCT) in the long-term steroid therapy of chronic sarcoidosis. Sarcoidosis. 1988;5:99–103. [PubMed] [Google Scholar]
- 73.Montemurro L, Schiraldi G, Fraioli P, Tosi G, Riboldi A, Rizzato G. Prevention of corticosteroid-induced osteoporosis with salmon calcitonin in sarcoid patients. Calcif Tissue Int. 1991;49:71–76. doi: 10.1007/BF02565124. [DOI] [PubMed] [Google Scholar]
- 74.Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281–289. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 75.Karpf DB, Shapiro DR, Seeman E, et al. Alendronate Osteoporosis Treatment Study Groups Prevention of nonvertebral fractures by alendronate: a meta-analysis. JAMA. 1997;277:1159–1164. [PubMed] [Google Scholar]
- 76.Gonnelli S, Rottoli P, Cepollaro C, et al. Prevention of corticosteroid-induced osteoporosis with alendronate in sarcoid patients. Calcif Tissue Int. 1997;61:382–385. doi: 10.1007/s002239900352. [DOI] [PubMed] [Google Scholar]
- 77.Qaseem A, Snow V, Shekelle P, Hopkins RJ, Jr, Forciea MA, Owens DK, Clinical Efficacy Assessment Subcommittee of the American College of Physicians Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:404–415. [PubMed] [Google Scholar]
- 78.American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. Arthritis Rheum. 2001;44:1496–1503. doi: 10.1002/1529-0131(200107)44:7<1496::AID-ART271>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 79.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 80.Infante JR, Pacheco C, Torres-Avisbal M, Vallejo JA, González FM, Latre JM. Pulmonary activity in sarcoidosis: 67Ga uptake quantification and plasma determination of 1,25-dihydroxyvitamin D [in Spanish] Rev Esp Med Nucl. 2002;21:275–280. doi: 10.1016/s0212-6982(02)72088-0. [DOI] [PubMed] [Google Scholar]
- 81.Kavathia D, Buckley JD, Rao D, Rybicki B, Burke R. Elevated 1, 25-dihydroxyvitamin D levels are associated with protracted treatment in sarcoidosis. Respir Med. 2010;104:564–570. doi: 10.1016/j.rmed.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prasse A, Katic C, Germann M, Buchwald A, Zissel G, Müller-Quernheim J. Phenotyping sarcoidosis from a pulmonary perspective. Am J Respir Crit Care Med. 2008;177:330–336. doi: 10.1164/rccm.200705-742OC. [DOI] [PubMed] [Google Scholar]
- 83.Adams JS, Gacad MA, Diz MM, Nadler JL. A role for endogenous arachidonate metabolites in the regulated expression of the 25-hydroxyvitamin D-1-hydroxylation reaction in cultured alveolar macrophages from patients with sarcoidosis. J Clin Endocrinol Metab. 1990;70:595–600. doi: 10.1210/jcem-70-3-595. [DOI] [PubMed] [Google Scholar]
- 84.Reichel H, Koeffler HP, Barbers R, Norman AW. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab. 1987;65:1201–1209. doi: 10.1210/jcem-65-6-1201. [DOI] [PubMed] [Google Scholar]
- 85.Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest. 1987;79:1659–1664. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]


