Abstract
AIM: To examine the association between interferon (IFN) therapy and loss of hepatitis B surface antigen (HBsAg) in inactive HBsAg carriers.
METHODS: This was a retrospective cohort study in inactive HBsAg carriers, who were treatment-naive, with a serum HBsAg level < 100 IU/mL and an undetectable hepatitis B virus (HBV) DNA level (< 100 IU/mL). All the 20 treated patients received subcutaneous PEG-IFN alfa-2a 180 μg/wk for 72 wk and were then followed for 24 wk. There were 40 untreated controls matched with 96 wk of observation. Serum HBsAg, HBV DNA, and alanine aminotransferases were monitored every 3 mo in the treatment group and every 3-6 mo in the control group.
RESULTS: Thirteen (65.0%) of 20 treated patients achieved HBsAg loss, 12 of whom achieved HBsAg seroconversion. Mean HBsAg level in treated patients decreased to 6.69 ± 13.04 IU/mL after 24 wk of treatment from a baseline level of 26.22 ± 33.00 IU/mL. Serum HBV DNA level remained undetectable (< 100 IU/mL) in all treated patients during the study. HBsAg level of the control group decreased from 25.72 ± 25.58 IU/mL at baseline to 17.11 ± 21.62 IU/mL at week 96 (P = 0.108). In the control group, no patient experienced HBsAg loss/seroconversion, and two (5.0%) developed HBV reactivation.
CONCLUSION: IFN treatment results in HBsAg loss and seroconversion in a considerable proportion of inactive HBsAg carriers with low HBsAg concentrations.
Keywords: Chronic hepatitis B surface antigen carriers, Inactive hepatitis B surface antigen carriers, Interferon, Peginterferon alfa-2a, Hepatitis B surface antigen loss/seroconversion
Core tip: This study examined the association between interferon (IFN) therapy and loss of hepatitis B surface antigen (HBsAg) in inactive HBsAg carriers. This was a retrospective cohort study in inactive HBsAg carriers with a serum HBsAg level < 100 IU/mL and a persistently undetectable hepatitis B virus (HBV) DNA level (< 100 IU/mL). All the 20 treated patients received subcutaneous PEG-IFN alfa-2a 180 μg/wk for 72 wk and were then followed for 24 wk. IFN treatment resulted in HBsAg loss (65.0%) and seroconversion in a considerable proportion of inactive HBsAg carriers with low HBsAg concentrations. In the control group, no patient experienced HBsAg loss/seroconversion, and 2 (5.0%) developed HBV reactivation.
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is the leading cause of end-stage liver disease or hepatocellular carcinoma (HCC) throughout the world. In the nature history of chronic HBV infection, inactive hepatitis B surface antigen (HBsAg) carriers, defined as HBsAg positive, hepatitis B envelope antigen (HBeAg)-negative/antiHBe-positive, undetectable HBV DNA level and normal alanine aminotransferases (ALT) levels, frequently have good long-term clinical outcomes and thus are not recommended for antiviral treatment[1-3]. However, this inactive carrier status was not always sustained. Fourteen percent to 24% of inactive carriers have reactivation after years of quiescent disease, and 4.2% to 20% of them reverse back to HBeAg positivity[4-7], with increased cumulative probabilities of reactivation of hepatitis B after years of follow-up[8]. Compared to a control subcohort (negative for HBsAg), inactive HBsAg carriers have higher risks of hepatocellular carcinoma and liver-related death[9], especially in countries with a high prevalence of HBV infection[8,9]. In contrast, 100% and 90% of patients had improvement and stable liver inflammation and liver fibrosis[10], respectively, and no HCC occurred in patients with HBsAg clearance after interferon (IFN) treatment[11]. Nevertheless, spontaneous HBsAg loss occurred in inactive carriers only at rates from 1% to 1.5% per year observed in Caucasians[12] and Asians[13]. HBsAg clearance usually indicates recovery from HBV infection, and has been an aim of antiviral therapy[2]. In the real life, HBsAg positive people were restricted in many aspects such as work, diet, and cosmetic surgery, in China, and many of them hope to obtain HBsAg loss through effective methods.
IFN treatment exerts direct antiviral as well as immunoregulatory effects[14], and can induce specific and nonhepatotoxic degradation of nuclear HBV covalently closed circular DNA (cccDNA)[15], and increased HBV-specific T-cell responses in chronic HBV infected patients with undetectable levels of serum HBV DNA[16]. This retrospective cohort study was conducted to evaluate the efficacy of PEG-IFN alfa-2a treatment in chronic inactive carriers with a low HBsAg level.
MATERIALS AND METHODS
Selection of patients
A retrospective cohort study including inactive HBsAg carriers attending the department of hepatology, Beijing Ditan Hospital, Capital Medical University between May 2008 and August 2012 was conducted. We diagnosed inactive HBsAg carriers based on their history of HBV infection, HBV DNA level, serological markers, and liver function. Patients with cirrhosis, which was diagnosed as liver stiffness > 9 kPa or presence of portal hypertension (spleen enlargement with a reduction in platelet count) by FibroScan and ultrasonic examinations, were excluded. Patients who were treatment-naive, HBsAg positive, anti-HBs-negative and HBeAg negative for more than 6 mo, had a persistently undetectable HBV DNA level (< 100 IU/mL) and normal ALT levels (< 19 IU/mL for females and < 30 IU/mL for males, measured every 3-6 mo) during the preceding 2 years, and serum HBsAg < 100 IU/mL on two occasions during the month prior to enrollment were included in the study. Patients with other liver diseases or co-infection of hepatitis C virus, hepatitis D virus, and human immunodeficiency virus, as well as those who had a history of immunosuppressive or antiviral drug usage were excluded. HBV genotyping cannot be performed due to an undetectable HBV DNA level in the subjects; however, epidemiological studies showed the HBV genotypes in China were mainly genotypes B and C[17,18].
Participants in the treatment group contained all the patients who were willing to receive IFN treatment for achieve HBsAg clearance and had completed 72 wk of treatment with PEG-IFN alfa-2a and 24 wk of follow-up after completing the treatment. There were 40 controls matched for age, sex, and HBsAg level, and undetectable HBV DNA with persistently normal ALT levels, and they were selected from 284 untreated patients who attended the clinic and completed 96 wk of observation during the same period as treated patients.
Ethics approval
The study adhered to the Declaration of Helsinki and ethics approval was obtained from the Beijing Ditan Hospital of Capital Medical University Institutional Review Board. Written informed consent was obtained from all subjects before enrolment.
Treatment and follow-up
The treated cohort comprised 20 patients who had received subcutaneous PEG-IFN alfa-2a at a dose of 180 μg/wk for 72 wk and had been followed for 24 wk after completing the treatment, while the control cohort comprised 40 matched patients who had finished 96 wk of observation.
None of the participants received immunosuppressive or oral antiviral drugs during the study period. In the treated patients, serum HBsAg, anti-HBs and HBV DNA levels were measured once every 3 mo, and peripheral blood neutrophil and platelet counts, and liver and kidney function tests were performed once every 1-3 mo. These biomarkers were measured once every 3-6 mo in controls.
Safety and efficacy assessments
Kidney and liver function biomarkers, including serum creatinine, blood urea nitrogen, ALT, aspartate aminotransferase, albumin and total bilirubin (Tbil), were measured with an automated biochemical analyzer. Peripheral blood neutrophil and platelet counts were measured with an automatic blood cell analyzer.
HBV DNA was measured with a commercially available real-time fluorescence PCR kit with a detection limit of 100 IU/mL (Piji Company, Shenzhen City, China). HBsAg concentrations were quantified by an automated chemoluminescent microparticle immunoassay (Architect i2000 HBsAg quantitative assay, Abbott Laboratories, Abbott Park, IL, United States, sensitivity < 0.05 IU/mL; dynamic range 0.05-250 IU/mL). HBsAg loss was defined as HBsAg concentration < 0.05 IU/mL. Anti-HBs was measured with an Architect i2000 kit (Abbott Laboratories, dynamic range of 0.00-1000 mIU/mL), with concentrations ≥ 10 mIU/L being considered positive. The primary efficacy endpoints were HBsAg loss and seroconversion.
Statistical analysis
Unless otherwise stated, clinical and biological outcomes before and after treatment are expressed as mean ± SD or median (range), and were compared using paired Student’s t-tests, with a P-value less than 0.05 being considered statistically significant. Qualitative variables are presented as counts and percentages and were compared using Fisher’s exact tests. All statistical analyses were performed using SPSS statistical software version 13.0 (Chicago, IL, United States).
RESULTS
Patients and clinical characteristics
A total of 60 inactive chronic HBsAg carriers were included in the study, 20 of whom were in the treated group and 40 in the control group. There were no significant differences in the baseline characteristics between the treated and control groups (Table 1). However, in the treatment group, the patients who achieved HBsAg loss had a lower baseline HBsAg level of 8.09 (3.81-22.50) IU/mL and were younger (age of 31.46 ± 12.16 years) than patients without HBsAg loss after treatment [baseline HBsAg level of 18.95 (2.85-83.00) IU/mL and age of 38.24 ± 9.25 years], but there was no significant difference.
Table 1.
Baseline characteristics and outcomes at the end of treatment and follow-up
| Characteristic | Treatment group | Control group | P-value |
| No. | 20 | 40 | |
| Mean age at entry in year ± SD | 33.80 ± 11.45 | 33.85 ± 8.37 | 0.985 |
| Age > 40 yr, n (%) | 4 (20.0) | 11 (27.5) | 0.527 |
| Men:women, n | 15:5 | 30:10 | 1.000 |
| Mean baseline ALT (U/L) ± SD | 23.46 ± 8.78 | 21.24 ± 10.26 | 0.874 |
| HBsAg level (IU/mL) | |||
| Mean ± SD | 26.22 ± 33.00 | 25.72 ± 5.58 | 0.949 |
| Median (Q1, Q3) | 11.36 (3.52-37.40) | 15.81 (4.59-40.15) | 0.714 |
| 95%CI of patients with 10-100 IU/mL, n (%) | (10.77, 41.75), 10 (50.0) | (17.54, 33.90), 22 (55.0) | |
| Patients with < 10 IU/mL, n (%) | 10 (50.0) | 18 (45.0) | |
| Mean decline in HBsAg level at EOT (IU/mL) ± SD | 22.33 ± 29.45 | 5.76 ± 17.67 | 0.009 |
| Median HBsAg level at EOT (IU/mL) | 0.04 (0.02, 0.55) | 13.21 (2.97, 30.31) | 0.003 |
| (Q1, Q3) | 95%CI: (-0.68, 8.53) | 95%CI: (12.8, 27.12) | |
| Mean decline in HBsAg level at EOF (IU/mL) ± SD | 23.36 ± 29.47 | 8.61 ± 19.32 | 0.023 |
| Median HBsAg level at EOF (IU/mL) | 0.045 (0.02, 2.44) | 5.69 (1.50, 20.88) | 0.007 |
| (Q1, Q3) | 95%CI: (-1.63, 7.43) | 95%CI: (10.20, 24.03) | |
| HBsAg loss, n (%) | 13 (65.0) | 0 (0) | 0.000 |
| HBsAg seroconversion, n (%) | 12 (60.0) | 0 (0) | 0.000 |
| HBV DNA reactivation, n (%) | 0 (0) | 2 (5.0) | 0.309 |
ALT: Alanine aminotransferase; EOF: End of follow-up; EOT: End of treatment; HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus.
HBsAg kinetics and clinical outcomes
HBsAg levels decreased with increasing treatment period in the treated group (Figure 1). Among patients treated with PEG-IFN alfa-2a, the mean HBsAg level decreased by 55.98% from baseline to week 12 (from 26.22 ± 33.00 to 11.59 ± 20.83 IU/mL, P = 0.108), by 74.59% from baseline to week 24 (to 6.69 ± 13.04 IU/mL, P = 0.024 vs baseline), and was 0.045 IU/mL (range, 0.02-2.44 IU/mL) at the end of follow-up (week 96). Of the 20 treated patients, 13 achieved HBsAg loss, of whom 12 occurred during treatment and 1 at follow-up time, with a mean of 40.62 ± 22.74 mo after the initiation of treatment, in which 12 achieved HBsAg seroconversion (Table 1). Eighty percent (8/10) of patients with an HBsAg level < 10 IU/mL achieved HBsAg loss after treatment. In the remaining seven treated patients, the mean HBsAg level decreased by 66.93% from baseline to the end of follow-up (from 37.43 ± 38.69 to 8.20 ± 15.69 IU/mL, P = 0.049). Serum HBV DNA remained undetectable (< 100 IU/mL) in all treated patients during the treatment and follow-up periods, and no return to HBsAg positivity occurred in all patients during the study course. In contrast, the mean HBsAg level of the control group remained relatively stable over 96 wk and was 25.72 ± 25.58 IU/mL at baseline and 17.11 ± 21.62 IU/mL at week 96 (P = 0.108; Figure 1). No patients in the control group experienced HBsAg loss/seroconversion, and two (5.0%) experienced HBV reactivation, defined as return of serum HBV DNA to positivity from undetectable level (< 100 IU/mL) (Table 1).
Figure 1.
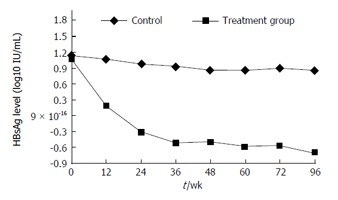
Mean hepatitis B surface antigen level decreased in a time-dependent manner in treated patients and was significantly lower at week 24 than at baseline. HBsAg: Hepatitis B surface antigen.
Safety
Among all patients in the treated group, peripheral blood neutrophil count decreased, which was lower than 0.85 × 109/L in 13 (65.0%) individuals. The platelet count also decreased, which was lower than 6.0 × 1012/L in eight (40.0%) patients, and dose reductions were not required. Serum creatinine and blood urea nitrogen remained stable during treatment with PEG-IFN alfa-2a. Five patients had a loss of body weight and six patients had mild hair loss during treatment. There were no thyroid dysfunction and neuropsychiatric adverse effects, including depression, delirium, irritability and agitation. All adverse reactions disappeared 3-6 mo after the therapy was discontinued.
ALT levels increased during treatment in 18 of 20 (90.0%) treated patients, and 9 (45.0%) individuals experienced an ALT level > 80 IU/L. However, bilirubin levels remained within normal limits throughout the treatment and follow-up periods in all treated patients. Normalization of ALT levels coincided with HBsAg loss and/or the end of treatment, and was maintained during follow-up.
DISCUSSION
HBsAg level reflects the transcriptional activity of the cccDNA and is used as a proxy measure of HBV infection and for treatment guidance[19-21]. HBsAg declines during treatment and its level at the end of treatment can predict HBeAg seroconversion in HBeAg-positive patients[22-24] and sustained viral response in HBeAg-negative patients[25-27]. Thus, inactive HBsAg carriers were not recommended for antiviral therapy[1-3]. However, this inactive state was not always sustained. A long-term follow-up study showed cumulative probabilities of hepatitis relapse in inactive HBsAg carriers of 10.2%, 17.4%, 19.3%, 20.2% and 20.2% after 5, 10, 15, 20 and 25 years of follow-up, respectively, with an annual rate of 1.55%[28]. Another long-term longitudinal study (up to 23 years) showed that 1%-17% of inactive carriers reverted back to HBeAg-positive chronic hepatitis[4]. Cirrhosis and HCC may still develop in some inactive HBsAg carriers[28-30]. In contrast, no cirrhosis or HCC occurred in patients with HBsAg loss after IFN treatment, indicating that HBsAg clearance is currently the only parameter associated with an excellent long-term prognosis[10], and the strongest factor predicting excellent long-term outcome in HBV infected individuals is HBsAg loss, spontaneously or after treatment[10]. Therefore, it could be speculated that inactive HBsAg carriers can get further improvement in outcomes if HBsAg loss could be achieved after IFN treatment.
This study contained all participants who were inactive carriers with HBsAg < 100 IU/mL and wished to achieve HBsAg clearance by PEG-IFN alfa-2a treatment during the study period. Despite the lack of liver pathology for diagnosis, the patients could be considered as inactive for having undetectable HBV DNA and persistent normal ALT for 2 years, serum HBV DNA < 100 IU/mL and HBsAg < 100 IU/mL at enrollment. It has been reported that HBsAg < 1000 IU/mL with HBV DNA < 2000 IU/mL can distinguish inactive from active carriers with a diagnostic accuracy of 94.3%, sensitivity of 91.1%, specificity of 95.4%, positive predictive value of 87.9%, and negative predictive value of 96.7%[31]. Although the present study was not a randomized controlled study, all treated inactive carriers with HBsAg < 100 IU/mL and matched controls according to age, sex, and HBsAg and ALT levels were included for eliminating the bias.
Effects, including the probability of HBsAg clearance, can be enhanced by extended therapy with PEG-IFN alfa-2a[32]. In our study the patients were given 72 wk of treatment. After 12 wk of treatment with PEG-IFN alfa-2a, HBsAg levels decreased significantly compared with baseline levels. Furthermore, at the end of study, HBsAg loss occurred in most of treated patients, and HBsAg levels in the remaining seven treated carriers who did not achieve HBsAg loss decreased significantly. In contrast, mean HBsAg level of the control group remained constant during 96 wk of observation and no patients experienced HBsAg loss. These results suggest that inactive HBsAg carriers could benefit from PEG-IFN alfa-2a treatment.
In the present study, all participants had HBsAg < 100 IU/mL and they may have a good long-term clinical outcome, even HBsAg loss, after long-term follow-up. However, it was reported that spontaneous HBsAg loss in patients with HBsAg < 100 IU/mL occurred in a mean period of 86.6 ± 29 mo (range, 26-115) after the baseline visit with an annual rate of 1.6%[33], and in the present study after 72 wk treatment of PEG-IFN alfa-2a, HBsAg clearance occurred in 65% of treated objects. In a study by Tseng et al[34], HBsAg level < 10 IU/mL at baseline was the strongest predictor of HBsAg loss. However, the rate of HBsAg loss was only 7.4 per 100 persons per year and it occurred in a mean period of 5.8 ± 4.2 years. Although half of the subjects included in this study had HBsAg < 10 IU/mL and undetectable HBV DNA, 80% (8/10) of them achieved HBsAg loss after 72 wk of IFN treatment, suggesting that PEG-IFN alfa-2a treatment can make inactive carriers achieve HBsAg clearence in a short-term period compared with spontaneous HBsAg loss occurring in the nature history. Although Chen et al[35] reported in a case-control study that the positive predictive value of HBsAg level of 200 IU/mL in predicting HBsAg loss occurring within 1 year was 36%, their study design was different from ours. The aim of their study was to observe the difference in HBsAg decrease between 46 patients who underwent spontaneous HBsAg loss and 46 patients who had no HBsAg loss during the same observation course. The aim of our study was to compare the rate of HBsAg clearance in patients treated with PEG-IFN alfa-2a compared with untreated patients, and the result showed that the rate of HBsAg clearance was significantly higher in patients treated with PEG-IFN alfa-2a than in untreated patients. The results of our study suggested that inactive carriers can receive PEG-IFN alfa-2a therapy to increase the probability of HBsAg clearance and shorten the time compared with that occurring spontaneously.
In conclusion, our study demonstrated that treatment with PEG-IFN alfa-2a produced a high rate of HBsAg loss/seroconversion in inactive carriers with low HBsAg levels. However, whether inactive carriers with HBsAg levels more than 100 IU/mL could benefit from PEG-IFN alfa-2a treatment needs further study.
ACKNOWLEDGMENTS
We are grateful to staff for effectively facilitating the present project.
COMMENTS
Background
Although inactive hepatitis B surface antigen (HBsAg) carriers often have no liver inflammation and are not recommended to undergo treatment, they may develop hepatitis relapse or revert back to HBeAg-positive chronic hepatitis, and cirrhosis and hepatocellular carcinoma (HCC) may still develop in some inactive HBsAg carriers in a long-term follow-up period. In contrast, no cirrhosis or HCC occurred in patients with HBsAg loss after interferon (IFN) treatment. So, HBsAg loss is generally considered to be the ultimate goal of therapy, indicating a complete response to treatment and the resolution of the disease. It was suggested that inactive HBsAg carriers could get benefits from IFN treatment if HBsAg loss was achieved after treatment.
Research frontiers
HBsAg loss is the goal and ideal end-point of treatment in chronic hepatitis B. The spontaneous rate of HBsAg loss in inactive carriers was only 0.5%-2.5% per year, and HBsAg clearance occurred in a mean period of 86.6 ± 29 mo (range, 26-115) after the initial visit. Even in patients with an HBsAg level < 10 IU/mL, a mean period of 5.8 ± 4.2 years is required to achieve HBsAg clearance.
Innovations and breakthroughs
In contrast to chronic hepatitis B, in which a low rate of HBsAg loss is achieved after IFN treatment, inactive HBsAg carriers with HBsAg < 100 IU/mL could obtain a high rate of HBsAg loss after PEG-IFN treatment in a shorter period than that occurring spontaneously.
Applications
Inactive HBsAg carriers will benefit from PEG-IFN treatment, if HBsAg loss can be achieved after a short period of PEG-IFN therapy.
Terminology
Inactive HBsAg carriers are patients who were HBsAg-positive, with low hepatitis B virus (HBV) replication and no liver inflammation. HBsAg loss was defined as an HBsAg concentration < 0.05 IU/mL, and seroconversion defined as an HBsAg concentration < 0.05 IU/mL and an anti-HBs level ≥ 10 mIU/L. HBsAg loss often indicates recovery from HBV infection.
Peer-review
The manuscript entitled “Hepatitis B surface antigen clearance in inactive hepatitis B surface antigen carriers treated with peginterferon alfa-2a” discusses a possible application of an IFN therapy in inactive HBsAg carriers with a very low HBsAg level. The authors report that in their study the HBsAg disappeared in 65% of treated patients. This result seems to be very good, taking into account that usually HBsAg clearance is rarely observed.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Beijing Ditan Hospital Institutional Review Board.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: There was no conflict of interest and this study was carried out as a part of our routine work.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 12, 2016
First decision: March 9, 2016
Article in press: May 9, 2016
P- Reviewer: Belopolskaya M, Charuworn P, Lee HC S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu SQ
References
- 1.Hou JL, lai W. [The guideline of prevention and treatment for chronic hepatitis B: a 2015 update] Zhonghua Ganzangbing Zazhi. 2015;23:888–905. doi: 10.3760/cma.j.issn.1007-3418.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fattovich G, Olivari N, Pasino M, D’Onofrio M, Martone E, Donato F. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut. 2008;57:84–90. doi: 10.1136/gut.2007.128496. [DOI] [PubMed] [Google Scholar]
- 5.Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116:829–834. doi: 10.1016/j.amjmed.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 6.McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759–768. doi: 10.7326/0003-4819-135-9-200111060-00006. [DOI] [PubMed] [Google Scholar]
- 7.Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 8.Chu CM, Liaw YF. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol. 2009;104:1693–1699. doi: 10.1038/ajg.2009.187. [DOI] [PubMed] [Google Scholar]
- 9.Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, Su J, Sun CA, Liaw YF, Chen CJ. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747–1754. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 10.Moucari R, Korevaar A, Lada O, Martinot-Peignoux M, Boyer N, Mackiewicz V, Dauvergne A, Cardoso AC, Asselah T, Nicolas-Chanoine MH, et al. High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: a long-term follow-up study. J Hepatol. 2009;50:1084–1092. doi: 10.1016/j.jhep.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology. 2002;123:1084–1089. doi: 10.1053/gast.2002.36026. [DOI] [PubMed] [Google Scholar]
- 12.Manno M, Cammà C, Schepis F, Bassi F, Gelmini R, Giannini F, Miselli F, Grottola A, Ferretti I, Vecchi C, et al. Natural history of chronic HBV carriers in northern Italy: morbidity and mortality after 30 years. Gastroenterology. 2004;127:756–763. doi: 10.1053/j.gastro.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up. Hepatology. 2007;45:1187–1192. doi: 10.1002/hep.21612. [DOI] [PubMed] [Google Scholar]
- 14.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 15.Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sprinzl MF, Russo C, Kittner J, Allgayer S, Grambihler A, Bartsch B, Weinmann A, Galle PR, Schuchmann M, Protzer U, et al. Hepatitis B virus-specific T-cell responses during IFN administration in a small cohort of chronic hepatitis B patients under nucleos(t)ide analogue treatment. J Viral Hepat. 2014;21:633–641. doi: 10.1111/jvh.12189. [DOI] [PubMed] [Google Scholar]
- 17.Li HM, Wang JQ, Wang R, Zhao Q, Li L, Zhang JP, Shen T. Hepatitis B virus genotypes and genome characteristics in China. World J Gastroenterol. 2015;21:6684–6697. doi: 10.3748/wjg.v21.i21.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei DH, Liu HZ, Huang AM, Liu XL, Liu JF. A new trend of genotype distribution of hepatitis B virus infection in southeast China (Fujian), 2006-2013. Epidemiol Infect. 2015;143:2822–2826. doi: 10.1017/S0950268815000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan HL, Wong VW, Tse AM, Tse CH, Chim AM, Chan HY, Wong GL, Sung JJ. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol. 2007;5:1462–1468. doi: 10.1016/j.cgh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Wursthorn K, Lutgehetmann M, Dandri M, Volz T, Buggisch P, Zollner B, Longerich T, Schirmacher P, Metzler F, Zankel M, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44:675–684. doi: 10.1002/hep.21282. [DOI] [PubMed] [Google Scholar]
- 21.Chan HL, Thompson A, Martinot-Peignoux M, Piratvisuth T, Cornberg M, Brunetto MR, Tillmann HL, Kao JH, Jia JD, Wedemeyer H, et al. Hepatitis B surface antigen quantification: why and how to use it in 2011 - a core group report. J Hepatol. 2011;55:1121–1131. doi: 10.1016/j.jhep.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Sonneveld MJ, Hansen BE, Piratvisuth T, Jia JD, Zeuzem S, Gane E, Liaw YF, Xie Q, Heathcote EJ, Chan HL, et al. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology. 2013;58:872–880. doi: 10.1002/hep.26436. [DOI] [PubMed] [Google Scholar]
- 23.Piratvisuth T, Marcellin P, Popescu M, Kapprell HP, Rothe V, Lu ZM. Hepatitis B surface antigen: association with sustained response to peginterferon alfa-2a in hepatitis B e antigen-positive patients. Hepatol Int. 2013;7:429–436. doi: 10.1007/s12072-011-9280-0. [DOI] [PubMed] [Google Scholar]
- 24.Liaw YF, Jia JD, Chan HL, Han KH, Tanwandee T, Chuang WL, Tan DM, Chen XY, Gane E, Piratvisuth T, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology. 2011;54:1591–1599. doi: 10.1002/hep.24555. [DOI] [PubMed] [Google Scholar]
- 25.Marcellin P, Bonino F, Yurdaydin C, Hadziyannis S, Moucari R, Kapprell HP, Rothe V, Popescu M, Brunetto MR. Hepatitis B surface antigen levels: association with 5-year response to peginterferon alfa-2a in hepatitis B e-antigen-negative patients. Hepatol Int. 2013;7:88–97. doi: 10.1007/s12072-012-9343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.26 Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, Dauvergne A, Asselah T, Boyer N, Bedossa P, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49:1151–1157. doi: 10.1002/hep.22744. [DOI] [PubMed] [Google Scholar]
- 27.Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Luo K, Wang Y, Hadziyannis S, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009;49:1141–1150. doi: 10.1002/hep.22760. [DOI] [PubMed] [Google Scholar]
- 28.Chu CM, Liaw YF. Spontaneous relapse of hepatitis in inactive HBsAg carriers. Hepatol Int. 2007;1:311–315. doi: 10.1007/s12072-007-9002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 30.Huo TI, Wu JC, Lee PC, Chau GY, Lui WY, Tsay SH, Ting LT, Chang FY, Lee SD. Sero-clearance of hepatitis B surface antigen in chronic carriers does not necessarily imply a good prognosis. Hepatology. 1998;28:231–236. doi: 10.1002/hep.510280130. [DOI] [PubMed] [Google Scholar]
- 31.Brunetto MR, Oliveri F, Colombatto P, Moriconi F, Ciccorossi P, Coco B, Romagnoli V, Cherubini B, Moscato G, Maina AM, et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139:483–490. doi: 10.1053/j.gastro.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 32.Lampertico P, Viganò M, Di Costanzo GG, Sagnelli E, Fasano M, Di Marco V, Boninsegna S, Farci P, Fargion S, Giuberti T, et al. Randomised study comparing 48 and 96 weeks peginterferon α-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut. 2013;62:290–298. doi: 10.1136/gutjnl-2011-301430. [DOI] [PubMed] [Google Scholar]
- 33.Chan HL, Wong GL, Tse CH, Chan HY, Wong VW. Viral determinants of hepatitis B surface antigen seroclearance in hepatitis B e antigen-negative chronic hepatitis B patients. J Infect Dis. 2011;204:408–414. doi: 10.1093/infdis/jir283. [DOI] [PubMed] [Google Scholar]
- 34.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, et al. Determinants of spontaneous surface antigen loss in hepatitis B e antigen-negative patients with a low viral load. Hepatology. 2012;55:68–76. doi: 10.1002/hep.24615. [DOI] [PubMed] [Google Scholar]
- 35.Chen YC, Jeng WJ, Chu CM, Liaw YF. Decreasing levels of HBsAg predict HBsAg seroclearance in patients with inactive chronic hepatitis B virus infection. Clin Gastroenterol Hepatol. 2012;10:297–302. doi: 10.1016/j.cgh.2011.08.029. [DOI] [PubMed] [Google Scholar]


