Abstract
Background:
Carum carvi L. (caraway), known as black zeera in Iran, has been indicated for the treatment of epilepsy in Iranian folk medicine. This study evaluated whether the aqueous extract and essential oil of caraway seeds have anticonvulsant effects in mice.
Methods:
The anticonvulsant effects of the aqueous extract (200, 400, 800, 1600, and 3200 mg/kg, i.p.) and essential oil (25, 50, 100, 200, and 400 mg/kg, i.p.) of caraway were assessed using pentylenetetrazol (PTZ; 95 mg/kg i.p.) induced convulsions. Diazepam (3 mg/kg) was used as positive control. The latency time before the onset of myoclonic, clonic, and tonic convulsions and the percentage of mortality were recorded. In addition, the effect of caraway on neuromuscular coordination was evaluated using the rotarod performance test.
Results:
The extract and essential oil dose-dependently increased the latency time to the onset of myoclonic (ED50, 1257 and 62.2 mg/kg, respectively) and clonic (ED50, 929 and 42.3 mg/kg, respectively) seizures. The extract and essential oil of caraway prevented the animals from tonic seizure with ED50s of 2142.4 and 97.6 mg/kg, respectively. The extract and essential oil of caraway protected 28.6 and 71.4% of the animals from PTZ-induced death, respectively, and had no significant effect on neuromuscular coordination.
Conclusion:
This study showed that the aqueous extract and essential oil of caraway had anticonvulsant properties. However, the essential oil was more potent and effective than was the aqueous extract as an anticonvulsant. Additionally, the anticonvulsant effect of caraway was not due to a muscle relaxant activity. These findings support the acclaimed antiepileptic effect of caraway in folk medicine and propose its potential use in petit mal seizure in humans.
Keywords: Carum, Anticonvulsants, Pentylenetetrazole, Seizures, Mice
What’s Known
Previous studies have revealed the different properties and potential medical effects of caraway, including its central anti-stress and nootropic effects.
In Iranian ancient and folk medicine, it was believed that caraway seeds possessed antiepileptic effects. Carvone, a chemical constituent of caraway seeds, exhibited anticonvulsant activity in animal models of seizure.
What’s New
The aqueous extract and essential oil of caraway possessed dose-dependent anticonvulsive properties. The essential oil was a more effective and potent anticonvulsant than the aqueous extract. The essential oil and aqueous extract of caraway had no muscle-relaxant activity.
The maximum non-lethal dose of the aqueous extract and essential oil of caraway were 3200 mg/kg and 400 mg/kg, respectively.
Introduction
Epilepsy is a serious brain disorder that could affect anyone at any age. Epilepsy, if symptomatic, could decrease life expectancy by about 18 years.1 There are many antiepileptic drugs available worldwide; however, one-third of those who develop epilepsy unfortunately continue to experience uncontrolled seizures.1 Discovery of new drugs is the only hope for most of these individuals. Additionally, most of the current antiepileptic drugs have major side effects, which underscores the need for the discovery of newer drugs with lesser side effects.
Ethnomedicine, known as the consumption of plants by humans for medical purposes, goes back to at least 60,000 years ago.2 Plants have had a great contribution to the production of more than 7,000 compounds in the pharmaceutical industry in developed countries.3 Thus, the extract and essential oil of plants are an excellent source for finding new anticonvulsant drugs with higher activity and/or lower toxicity and for developing complementary remedy for epilepsy. Several plant species indicated to possess antiseizure properties in various folkloric medicines have exhibited anticonvulsant effects in animal models.4 Carum carvi L. (family: Apiaceae), known as caraway (black zeera in Iran), has been vastly used since ancient times for different purposes, including food flavoring and folklore therapy.5 In Iranian folk medicine, caraway has been indicated for gastrointestinal upsets (carminative, spasmolytic, and anti-flatulence), fever, colds and coughs, menstrual disorders, breast-milk stimulation, and pain relief.6,7 Previous studies have revealed different properties and potential medical effects of caraway such as its antimicrobial,8 antidiabetic,9 diuretic,10 antiulcerogenic,11,12 estrogenic,4 renoprotective (against diabetic nephropathy),13 and hypolipidemic14 effects. In Iranian ancient and folk medicine, it is believed that caraway seeds possess antiepileptic effects.7,15 There are also social beliefs that caraway is an effective remedy for epilepsy due to its warm and dry nature. Experimental studies have reported the central effects of caraway including antistress (adaptogenic) and nootropic activity.16 In addition, several species belonging to Apiaceae family, including Cuminum cyminum Linn.,17 Ferula gumosa Boiss.,18 Heracleum crenatifolium,19 Pimpinella anisum L. (anise),20 and Carum copticum21, have been shown to exhibit anticonvulsant activity. Furthermore, carvone, a chemical constituent of caraway seeds, has previously been demonstrated to exhibit anticonvulsant activity in animal models of seizure.22 Moreover, caraway is thought to exert anti-inflammatory and antioxidant23 activities, indicated to be beneficial in the treatment of epilepsy. Based on this evidence, the present study sought to evaluate the anticonvulsant effects of the essential oil and aqueous extract of caraway in an animal model of petit mal seizure to confirm its acclaimed ethnomedical use. The animal model of pentylenetetrazol (PTZ)-induced convulsions was used in this study. It is the principal, commonly used, and reliable animal model with a high predictive validity for evaluating potential anticonvulsant agents effective against absence seizure in humans.24
Materials and Methods
Experimental Animals
International standard guidelines for the care and use of animals were thoroughly observed in this study. The study was approved by the local Ethics Committee of Shiraz University of Medical Sciences (SUMS) for animal care and was conducted from May 2013 to July 2014 at the Department of Pharmacology, SUMS. All the experiments were performed between 9:00 a.m. and 13:00 p.m.
Adult male albino mice (25-35 g) were obtained from the Animal House of SUMS. The mice were housed under standard conditions throughout the study (12:12 h light/dark cycle and controlled temperature [22ºC±2]). The animals had free access to food and water and were given at least one week to acclimatize to laboratory conditions.
Drugs
Diazepam hydrochloride (10 mg/2 mL) from Darou Pakhsh Pharmaceutical Company (Iran) was employed as positive control. It was diluted to 3 mg/10 mL with water before use. Different concentrations of the aqueous extract and essential oil of caraway were freshly prepared on test days by serial dilution from a stock solution of 320 mg/mL in water and 40 mg/mL in olive oil, respectively. All the drugs were administered intraperitoneally (i.p.) in a volume of 0.1 mL/10 g body weight of mice.
Preparation of The Aqueous Extract
Dried caraway seeds were purchased from an herbal store in Shiraz and authenticated by the Department of Pharmacognosy at SUMS (herbarium # PM-673).
The aqueous extract (5.5% w/w) was obtained by macerating powdered caraway seeds in distilled water for 24 hours. After the filtration, the filtered extract was concentrated over water bath and brought to dryness using a desiccator.
Preparation of the Essential Oil
The essential oil (2.2% w/w) with a pale-yellowish color and a fresh pleasant odor was obtained from powdered caraway seeds by water distillation using a Clevenger-type apparatus.25
Phytochemical Screening of Plant Preparations
The aqueous extract was screened for the presence of tannins, alkaloids, saponins, flavonoids, terpenoids, and anthraquinones using standard procedures.26 The essential oil was analyzed for the detection of carvone by thin-layer chromatography on silica gel 60 F254 (layer thickness, 250 μm) using Toluene: EtOAc (93:7). The spots were visualized by UV illumination and vanillin/H2SO4 reagent.27
Assessment of the Acute Toxicity of the Aqueous Extract and Essential Oil of Caraway
The mice (n, 5/group) were intraperitoneally administered different doses of the aqueous extract (1600, 3200, 3600, 4000, or 5000 mg/kg) and essential oil (200, 400, 600, 800, or 1200 mg/kg) and were observed for 24 hours for any possible mortality. The highest dose that caused no death up to 24 hours was considered as the maximum nonlethal dose. Doses of the aqueous extract and essential oil used in this study were chosen based on their respective maximum nonlethal dose.
Assessment of the Effect of the Aqueous Extract of Caraway on Pentylenetetrazol-Induced Seizure
Forty-nine adult male albino mice were randomly divided into 7 groups (n, 7/group) and were intraperitoneally administered different doses of the aqueous extract (200, 400, 800, 1600, or 3200 mg/kg), water (vehicle), or diazepam (3 mg/kg) 30 minutes before the intraperitoneal injection of 95 mg/kg of PTZ. The dosage of diazepam was based on the previous studies in our laboratory.28
Assessment of the Effect of the Essential Oil of Caraway on Pentylenetetrazol-Induced Seizure
Forty-nine adult male albino mice were randomly divided into 7 groups (n, 7/group) and were intraperitoneally administered different doses of the essential oil (25, 50, 100, 200, or 400 mg/kg) or olive oil (vehicle) 30 minutes before the intraperitoneal injection of PTZ (95 mg/kg).
Pentylenetetrazol-Induced Seizure
Thirty minutes after the administration of the different doses of the aqueous extract or the essential oil, vehicles, or diazepam, each mouse was intraperitoneally injected PTZ (95 mg/kg). The mice were closely monitored during a 30-minute period following the injection of PTZ, and the latency times to the onset of myoclonic, clonic, and tonic convulsions were recorded.28 The dose at which caraway exhibited 50% of its maximal effect in delaying the onset time to myoclonic and clonic seizures (ED50) was determined by GraphPad Prism, version 5. In addition, the numbers of death/group were recorded up to 24 hours after PTZ injection. The ED50s, median effective doses of caraway protecting 50% of the mice against PTZ-induced tonic seizure or death, were determined via the Litchfield and Wilcoxon method28 using PHARM/PCS software, version 4.
Assessment of the Effect of Caraway on Neuromuscular Coordination
The effect of the caraway on coordinated motor movements was assessed using the rotarod performance test.28 Twenty-four hours prior to the test, the mice were trained to remain on the rotarod (diameter, 3 cm; 20 rpm) for more than 60 seconds. On the next day, the mice were tested on the rotarod (UGO Basile, Italy, model 7600) before and 30 minutes after the intraperitoneal administration of the different doses of the aqueous extract (200-3200 mg/kg; n, 6/group) or essential oil (25-400 mg/kg; n, 6/group), diazepam (3 mg/kg; n, 6), water (n, 6), or olive oil (n, 6). The stay time on the rotarod was recorded for each mouse for a maximum of 300 seconds.
Statistical Analysis
The results were expressed as mean ± standard error of the mean. The data were normally distributed based on the Kolmogorov–Smirnov test. The data from the PTZ experiments were analyzed using the one-way analysis of variance, followed by the Dunnett t test. The numbers of the animals protected from tonic convulsion or death were compared using the Fisher exact test. The data from the rotarod experiments were analyzed by analysis of covariance with time spent on the rotarod before injection as a covariate. The linear regression analysis was used to evaluate the dose-dependent effects of the extract and essential oil. Statistical Package for the Social Sciences (SPSS), version 18, was used for data analysis. A P value <0.05 was considered statistically significant.
Results
Acute Toxicity of Caraway
The maximum nonlethal dose of the aqueous extract and essential oil were 3200 mg/kg and 400 mg/kg, respectively, which were used as the highest tolerable doses of the aqueous extract and essential oil in this study.
Phytochemical Screening
Phytochemical screening showed that the aqueous extract contained tannins, alkaloids, and terpenoids. Carvone, the main constituent to identify and standardize the caraway oil,27 showed a hot red spot in the middle of the thin-layer chromatogram (Rf, 0.5).
Effect of the Aqueous extract of Caraway on Pentylenetetrazol-Induced Seizure
The aqueous extract at doses of 800, 1600, and 3200 mg/kg significantly increased the latency time to the onset of myoclonic seizure by 221.2, 370.6, and 378.5% (F(5,41), 40.8; P<0.001) and clonic seizure by 310, 394.6, and 401.9% (F(5,41), 36.9; P<0.001) in comparison to the control group (figure 1). The effect of the aqueous extract on delaying myoclonic (slope, 0.052±0.006; P<0.001), clonic (slope, 0.062±0.008; P<0.001), and tonic (slope, 0.219±0.028; P<0.001) seizures was dose-dependent with an ED50 of 1257, 929, and 2142 mg/kg, respectively. The aqueous extract at doses of 1600 and 3200 mg/kg significantly protected 42.86 and 71.43% of the mice from tonic seizure (P<0.001) (table 1). The effect of the aqueous extract on preventing PTZ-induced death was incomplete; even at the highest tolerable dose (i.e. 3200 mg/kg) it could not protect half of the animals from death (P=0.373) (table 1). Diazepam (3 mg/kg) protected all the animals (100%) from PTZ-induced seizure and death (data not shown).
Figure 1.
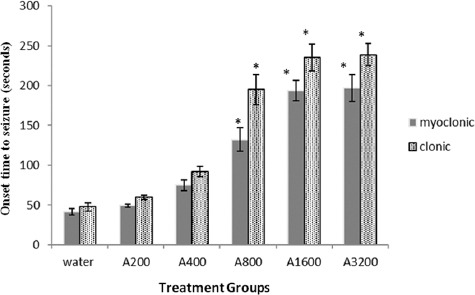
Shows effect of the aqueous extract of caraway against pentylenetetrazol-induced seizure. Bars represent mean ± standard error of the mean of the latency times to the onset of myoclonic and clonic seizures for 7mice/group following the administration of different doses of the aqueous extract of caraway (200-3200 mg/kg; A200-A3200) or water (control). *Indicates significance (P<0.001) in comparison to the control.
Table 1.
Effect of the aqueous extract and essential oil of caraway on the onset time to tonic seizure and prevention of pentylenetetrazol-induced tonic seizure and death
| Treatment groups | Onset time to tonic seizure (sec.) | Prevented from tonic seizure (N) | Prevented from death (N) |
|---|---|---|---|
| Water | 63.4±5.1 (7) | 0 | 0 |
| Aqueous extract | |||
| 200 mg/kg | 69±4.5 (7) | 0 | 0 |
| 400 mg/kg | 115±3.3 (7) | 0 | 0 |
| 800 mg/kg | 268.3±22.0 (7) | 0 | 1 |
| 1600 mg/kg | 389.2±76.7 (4) | 3 | 2 |
| 3200 mg/kg | 739.5±460.5 (2) | 5 | 2 |
| Vehicle | 382.6±68.9 (7) | 0 | 0 |
| Essential oil | |||
| 25 mg/kg | 644.2±250.7 (6) | 1 | 1 |
| 50 mg/kg | 607±127.1 (5) | 2 | 2 |
| 100 mg/kg | 626±107 (2) | 5 | 5 |
| 200 mg/kg | 580.3±309.9 (3) | 4 | 4 |
| 400 mg/kg | 960±360 (2) | 5 | 2 |
Data are mean±standard error of the mean of the latency time to tonic seizure and the numbers (N) of the animals protected from tonic seizure and death in the groups (n, 7) receiving different doses of the essential oil (25-400 mg/kg) or the aqueous extract (200-3200 mg/kg) of caraway, water, or olive oil. Numbers in the parentheses indicate the number of the animals that exhibited tonic seizure in each group
Effect of the Essential Oil of Caraway Against Pentylenetetrazol-Induced Seizure
The essential oil at doses of 50, 100, 200, and 400 mg/kg significantly increased the onset time to myoclonic seizure by 124, 282.3, 359, and 272.2% (F(5,41), 18.79; P<0.001) and clonic seizure by 176.2, 294, 354.9, and 275.6% (F(5,41), 25.18; P<0.001) in comparison to the control group (figure 2). The essential oil at 25mg/kg increased latency to clonic seizure by 92% in comparison to the control group, but it did not reach the significance level (P=0.080) (figure 2). The effect of the essential oil on myoclonic (slope, 0.255±0.054; P<0.001) and clonic (slope, 0.298±0.065; P<0.001) seizures was dose-dependent with an ED50 of 62.19 and 42.3 mg/kg, respectively.
Figure 2.
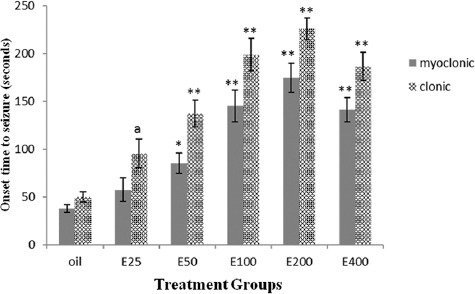
Shows effect of the essential oil of caraway against pentylenetetrazol-induced seizure. Bars represent mean ± standard error of the mean of the latency times to the onset of myoclonic and clonic seizures for 7 mice/group following the administration of different doses of the essential oil of caraway (25-400 mg/kg; E25-E400) or olive oil (vehicle). aP=0.080, *P=0.048, and **P<0.001 compared to the vehicle.
The essential oil at doses of 100 and 400 mg/kg significantly protected the animals from tonic seizure and death (P=0.021) (table 1). The essential oil at 200 mg/kg protected 57.1% of the animals from tonic seizure and death, but it did not reach the significance level (P=0.07). The dose at which the essential oil protected half of the animals from tonic seizure and death (ED50) was 97.6 and 316.4 mg/kg, respectively.
There were no significant differences in latencies to the onset of myoclonic and clonic seizures and the percentage of the animals showing tonic seizure and death between the water and olive oil groups.
Effect of the Aqueous Extract and Essential Oil of Caraway on Neuromuscular Coordination
The independent t-test showed that diazepam at 3 mg/kg caused a significant reduction in the number of seconds that the mice spent on the rotarod, compared to the control group (P=0.020) (figure 3). The aqueous extract at doses of 200, 400, 800, 1600, and 3200 mg/kg decreased the stay time on the rotarod by 16.4, 23.6, 21.5, 13.6, and 19% from baseline, respectively (figure 3). However, these changes were not statistically significant in comparison to the control group.
Figure 3.
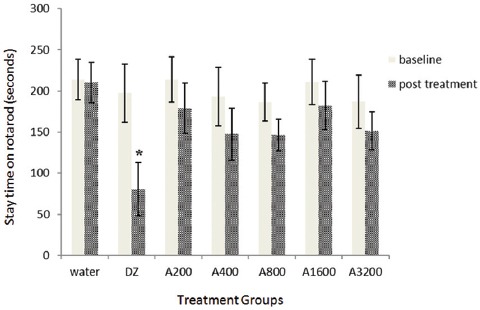
Shows effect of the aqueous extract of caraway on neuromuscular coordination. Bars represent mean ± standard error of the mean of stay time on the rotarod (seconds) for 7 mice before (baseline) and after the administration of different doses of the aqueous extract of caraway (200-3200 mg/kg; A200-A3200), diazepam (3 mg/kg, DZ), or water (vehicle).
The essential oil at doses of 25, 50, 100, 200, and 400 mg/kg reduced the stay time on the rotarod by 12.3, 22.9, 15.4, 29.3, and 23.2% from baseline, respectively (figure 4), which was not significantly different from the control group.
Figure 4.
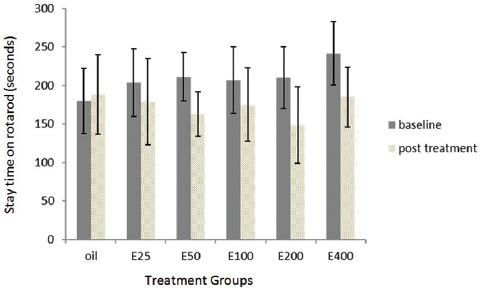
Shows effect of the essential oil of caraway on neuromuscular coordination. Bars represent mean ± standard error of the mean of stay time on the rotarod (seconds) for 7 mice before (baseline) and after the administration of different doses of the essential oil of caraway (25-400 mg/kg; E25-E400) or olive oil (vehicle).
Discussion
The present study showed that both the aqueous extract and the essential oil of caraway seeds had anticonvulsive properties. However, our findings indicated that the essential oil was more potent than was the aqueous extract as an anticonvulsant. As compounds effective in the PTZ test seem to be effective in absence seizure in humans,29 caraway may have a potential clinical use in absence seizure.
In the current study, the aqueous extract prevented PTZ-induced seizure in a dose-dependent manner, but it could not fully protect the animals from PTZ-induced death. Since no other studies have evaluated the effect of caraway on seizure, it is not possible to compare our results. Nonetheless, the aqueous extract of C. cumin (green zeera), a member of the Apiaceae family, has exhibited antiepileptic activities in a dose range17 of 400-4000 mg/kg using PTZ-induced seizure, which is in agreement with the current finding. Nevertheless, the extract of caraway seems to have higher efficacy than does that of cumin on delaying clonic seizure (402% vs. 330%) and preventing death (28.6% vs. 0%). The aqueous extract of C. copticum, another member of Apiaceae family, also attenuated seizure in a PTZ-kindling model.21 Although C. copticum exerted anticonvulsant property at lower doses (200-600 mg/kg) than did caraway, this might be due to the different models of seizures and animals (rats vs. mice) used in the two studies and/or the presence of different phytochemicals in these extracts.
The essential oil exerted anticonvulsant activity in a dose-dependent manner in the present study. Chiming in with our result, a previous study showed that the fruit essential oil of cumin attenuated PTZ-induced seizures in mice.30 The caraway and cumin oils have a similar minimum effective and maximum nonlethal dose. However, it appears that the caraway oil is more effective than is cumin oil in delaying clonic seizure (275.6% vs. 223.6%) and protecting mice from death (72% vs. 0%). These similarities and differences between the essential oil of caraway and cumin may be related to common as well as dissimilar phytochemical constituents present in these plants,25 which may be responsible for their anticonvulsant activities. In concordance with the results of the current study, the essential oils of the other species of Apiaceae family exhibited anticonvulsant properties against PTZ-induced seizure. Anise oil (0.25-1 mL/kg) supressed PTZ-induced tonic seizure and death in mice.31 In addition, anise oil (1-3 mL/kg) prolonged seizure attacks and decreased the duration and amplitude of epileptiform burst discharges induced by PTZ injection in rats.20 Nevertheless, the reported LD50 (0.93 mL/kg) and ED50 (0.52 mL/kg) of anise oil in mice31 indicate that this oil has a low therapeutic index (1.79) and that its reported anticonvulsant doses in mice are within a toxic range. Similarly, the essential oil of F. gumosa (2.5 mL/kg) showed anticonvulsant effect against PTZ-induced seizure, but its effective dose was toxic and close to its LD50 value (2.62 mL/kg).18 Conversely, caraway oil at its anticonvulsant doses did not show any neurotoxicity in the rotarod performance test, and its effective doses were much lower (0.058-0.46 mL/kg) than were those of anise31 and ferula18 oils. Taken together, it seems that caraway oil has higher efficacy, more potency, and less toxicity against PTZ-induced seizure than do the essential oils of the other plants from Apiaceae family studied thus far. Therefore, caraway oil seems to have a potential value as an anticonvulsant in the future. Certainly, more studies using different models of seizures are needed to further characterize the anticonvulsant potential and spectrum of the activity of caraway oil.
The findings of this study indicated that caraway oil was more potent in delaying myoclonic and clonic seizures and more effective in protecting animals from PTZ-induced death than was the aqueous extract of caraway. This may be due to the type and/or amounts of chemical compositions in the essential oil of caraway. Future studies for further evaluating the anticonvulsant potential of caraway should focus on this fraction.
The phytoconstituent(s) and mechanism(s) responsible for the anticonvulsant activity of caraway are not clear. The essential oil of caraway has multiple compounds, including carvone, carvacrol, carvenone, limonene, linalool, γ-terpinene, α-pinene, linalool, and p-cymene.25 One or a combination of these compounds may contribute to the anticonvulsant effect of caraway oil. In a study, (S)-(+)-carvone (200 mg/kg) was reported to increase the latency of convulsions induced by PTZ and picrotoxin.22 Another study showed the anticonvulsant ability of cyanocarvone in the mouse hippocampus, probably through increasing the activity of acetylcholinesterase enzyme.32 Alternatively, a blockade of voltage-gated Na+ channels resulting in reduced isolated nerve excitability and the modulation of the GABAergic system have been proposed as mechanisms underlying the anticonvulsant effect of a natural α,β-epoxy-carvone-compound.33 This evidence suggests that carvone, detected in caraway oil in the present study, may partly be responsible for the anticonvulsant activity of caraway. It is noteworthy that the antiseizure effect of caraway oil in the present study started at 50 mg/kg, which is lower than the effective dose of carvone (200 mg/kg). This suggests that the anticonvulsant activity of caraway oil is probably mediated by a synergy between various phytochemicals present in the essential oil acting through different mechanisms. This is supported by the findings that other constituents present in caraway oil such as limonene (200 mg/kg),34 linalool (200 and 300 mg/kg),35 and carvacrol (200 mg/kg)36 are all bioactive in a PTZ model of seizure but at doses higher than the minimum effective dose of caraway oil. The anticonvulsant property of linalool has been attributed to its direct interaction with the NMDA receptor complex and also to the modulation of acetylcholine mechanism.35 Limonene seems to act with a mechanism similar to benzodiazepines, which is the potentiation of GABAergic neurotransmission.34 Taken together, it can be suggested that various compounds via different mechanisms of actions collectively contribute to the antiseizure effect of caraway oil.
Phytochemical analysis confirmed the presence of terpenoids in the aqueous extract in the present study. In accordance with this finding, water-soluble carvone derivatives and their glucosides were found in caraway extract.37 Terpenoids and plants containing these compounds were shown to exert anticonvulsant activity.38 Therefore, terpenoids may in part underlie the antiseizure action of caraway extract via different mechanisms,38 as was described above.
The presence of alkaloids was detected in the aqueous extract of caraway in this study. Many kinds of alkaloids have shown anticonvulsant effects by such diverse mechanisms as modulating neurotransmitter systems, blocking calcium influx, blocking sodium channels, and anti-inflammatory and antioxidant activities.38 In addition, the antiepileptic properties of plant species such as Aconitum species, Piper species, and Rauwolfia serpentine have been attributed to their alkaloid compounds.38 Therefore, alkaloids may be another phytochemical contributing to the anticonvulsant activity of caraway extract.
In summary, as a wide range of compounds exist in caraway seeds, it is difficult to ascribe the anticonvulsant property of caraway to a particular compound or to a specific mechanism of action. Certainly, the phytoconstituent(s) responsible for the observed anticonvulsant property of caraway and the relevant mechanisms should be identified in future studies.
Conclusion
This study showed that both the aqueous extract and the essential oil of caraway possessed dose-dependent anticonvulsive properties. The essential oil was a more effective and potent anticonvulsant than was the aqueous extract; thus, it may have a potential clinical use in absence seizure in humans. Our findings support the acclaimed anticonvulsant effect of caraway in folkloric medicine; however, the responsible phytochemical(s) and the underlying mechanism of its action should be clarified in future studies.
Acknowledgement
The present article was extracted from a thesis written by Alireza Showraki and was financially supported by Shiraz University of Medical Sciences (Grant # 90-3766). The authors thank Mrs. Maryam Mojahed for her assistance in plant preparations and phytochemical screening.
Conflict of Interest: None declared.
References
- 1.Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 2.Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environmental Health Perspectives. 2001;109:69. doi: 10.2307/3434847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askew M, Janick J, Whipkey A. Interactive European network for industrial crops and their applications. Summary Report for the European Union 1996. 2000 [Google Scholar]
- 4.de Almeida RN, Agra Mde F, Maior FN, de Sousa DP. Essential oils and their constituents: anticonvulsant activity. Molecules. 2011;16:2726–42. doi: 10.3390/molecules16032726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johri RK. Cuminum cyminum and Carum carvi: An update. Pharmacogn Rev. 2011;5:63–72. doi: 10.4103/0973-7847.79101. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zargari A. Medicinal plants. 5th ed. Tehran: Tehran University Publications; 1990. [Google Scholar]
- 7.Mirheidar H. Carum carvi. Application of Plants in Prevention and Treatment of Illnesses. Tehran: Office of Islamic Culture Publications; 1992. [Google Scholar]
- 8.Iacobellis NS, Lo Cantore P, Capasso F, Senatore F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J Agric Food Chem. 2005;53:57–61. doi: 10.1021/jf0487351. [DOI] [PubMed] [Google Scholar]
- 9.Eddouks M, Lemhadri A, Michel JB. Caraway and caper: potential anti-hyperglycaemic plants in diabetic rats. J Ethnopharmacol. 2004;94:143–8. doi: 10.1016/j.jep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Lahlou S, Tahraoui A, Israili Z, Lyoussi B. Diuretic activity of the aqueous extracts of Carum carvi and Tanacetum vulgare in normal rats. J Ethnopharmacol. 2007;110:458–63. doi: 10.1016/j.jep.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Khayyal MT, el-Ghazaly MA, Kenawy SA, Seif-el-Nasr M, Mahran LG, Kafafi YA, et al. Antiulcerogenic effect of some gastrointestinally acting plant extracts and their combination. Arzneimittelforschung. 2001;51:545–53. doi: 10.1055/s-0031-1300078. [DOI] [PubMed] [Google Scholar]
- 12.Nariman F, Eftekhar F, Habibi Z, Massarrat S, Malekzadeh R. Antibacterial Activity of Twenty Iranian Plant Extracts Against Clinical Isolates of Helicobacter pylori. Iran J Basic Med Sci. 2009;12:105–11. [Google Scholar]
- 13.Sadiq S, Nagi AH, Shahzad M, Zia A. The reno-protective effect of aqueous extract of Carum carvi (black zeera) seeds in streptozotocin induced diabetic nephropathy in rodents. Saudi J Kidney Dis Transpl. 2010;21:1058–65. [PubMed] [Google Scholar]
- 14.Saghir MR, Sadiq S, Nayak S, Tahir MU. Hypolipidemic effect of aqueous extract of Carum carvi (black Zeera) seeds in diet induced hyperlipidemic rats. Pak J Pharm Sci. 2012;25:333–7. [PubMed] [Google Scholar]
- 15.Gorji A, Khaleghi Ghadiri M. History of epilepsy in Medieval Iranian medicine. Neurosci Biobehav Rev. 2001;25:455–61. doi: 10.1016/S0149-7634(01)00025-2. [DOI] [PubMed] [Google Scholar]
- 16.Koppula S, Kopalli SR, Sreemantula S. Adaptogenic and nootropic activities of aqueous extracts of Carum carvi Linn (caraway) fruit: an experimental study in Wistar rats. AJMH. 2009;21:72. [Google Scholar]
- 17.Hosseinzadeh H, Ramezani M, Fadishei M, Basirat M. Anticonvulsant effects of Cuminium cyminum L. seeds extracts and essential oil in mice. Journal of Medicinal Plants. 2002:1. [Google Scholar]
- 18.Sayyah M, Kamalinejad M, Bahrami Hidage R, Rustaiyan A. Antiepileptic potential and composition of the fruit essential oil of Ferula gummosa boiss. Iran Biomed J. 2001;5:69–72. [Google Scholar]
- 19.Tosun F, Kızılay ÇA, Erol K, Kılıç FS, Kürkçüoğlu M, Başer KHC. Anticonvulsant activity of furanocoumarins and the essential oil obtained from the fruits of Heracleum crenatifolium. Food Chem. 2008;107:990–3. doi: 10.1016/j.foodchem.2007.08.085. [DOI] [Google Scholar]
- 20.Karimzadeh F, Hosseini M, Mangeng D, Alavi H, Hassanzadeh GR, Bayat M, et al. Anticonvulsant and neuroprotective effects of Pimpinella anisum in rat brain. BMC Complement Altern Med. 2012;12:76. doi: 10.1186/1472-6882-12-76. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezvani ME, Roohbakhsh A, Mosaddegh MH, Esmailidehaj M, Khaloobagheri F, Esmaeili H. Anticonvulsant and depressant effects of aqueous extracts of Carum copticum seeds in male rats. Epilepsy Behav. 2011;22:220–5. doi: 10.1016/j.yebeh.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 22.de Sousa DP, de Farias Nobrega FF, de Almeida RN. Influence of the chirality of (R)-(-)- and (S)-(+)-carvone in the central nervous system: a comparative study. Chirality. 2007;19:264–8. doi: 10.1002/chir.20379. [DOI] [PubMed] [Google Scholar]
- 23.Agrahari P, Singh DK. A review on the pharmacological aspects of Carum carvi. Journal of Biology and Earth Sciences. 2014;4:M1–M13. [Google Scholar]
- 24.Loscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–68. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Jalali-Heravi M, Zekavat B, Sereshti H. Use of gas chromatography-mass spectrometry combined with resolution methods to characterize the essential oil components of Iranian cumin and caraway. J Chromatogr A. 2007;1143:215–26. doi: 10.1016/j.chroma.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 26.Edeoga H, Okwu D, Mbaebie B. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685–8. [Google Scholar]
- 27.Salehi Surmaghi MH, Amin GhR, Kaveh Sh. Carvi fructus. Iranian herbal pharmacopeia, editors: Iranian herbal pharmacopeia scientific committee. 1st ed. Tehran: Iranian Ministry of Health & Medical Education Publications; 2002. pp. 419–24. [Google Scholar]
- 28.Keshavarz M, Showraki A, Emamghoreishi M. Anticonvulsant Effect of Guaifenesin against Pentylenetetrazol-Induced Seizure in Mice. Iran J Med Sci. 2013;38:116–21. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 29.Uma Devi P, Pillai KK, Vohora D. Modulation of pentylenetetrazole-induced seizures and oxidative stress parameters by sodium valproate in the absence and presence of N-acetylcysteine. Fundam Clin Pharmacol. 2006;20:247–53. doi: 10.1111/j.1472-8206.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 30.Sayyah M, Mahboubi A, Kamalinejad M. Anticonvulsant effect of the fruit essential oil of Cuminum cyminum in mice. Pharm Biol. 2002;40:478–80. doi: 10.1076/phbi.40.6.478.8446. [DOI] [Google Scholar]
- 31.Pourgholami MH, Majzoob S, Javadi M, Kamalinejad M, Fanaee GH, Sayyah M. The fruit essential oil of Pimpinella anisum exerts anticonvulsant effects in mice. J Ethnopharmacol. 1999;66:211–5. doi: 10.1016/S0378-8741(98)00161-5. [DOI] [PubMed] [Google Scholar]
- 32.Costa DA, de Oliveira GA, Lima TC, dos Santos PS, de Sousa DP, de Freitas RM. Anticonvulsant and antioxidant effects of cyano-carvone and its action on acetylcholinesterase activity in mice hippocampus. Cell Mol Neurobiol. 2012;32:633–40. doi: 10.1007/s10571-012-9812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Almeida RN, de Sousa DP, Nobrega FF, Claudino Fde S, Araujo DA, Leite JR, et al. Anticonvulsant effect of a natural compound alpha, beta-epoxy-carvone and its action on the nerve excitability. Neurosci Lett. 2008;443:51–5. doi: 10.1016/j.neulet.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 34.Viana GS, do Vale TG, Silva CM, Matos FJ. Anticonvulsant activity of essential oils and active principles from chemotypes of Lippia alba (Mill.) N.E. Brown. Biol Pharm Bull. 2000;23:1314–7. doi: 10.1248/bpb.23.1314. [DOI] [PubMed] [Google Scholar]
- 35.de Sousa DP, Nobrega FF, Santos CC, de Almeida RN. Anticonvulsant activity of the linalool enantiomers and racemate: investigation of chiral influence. Nat Prod Commun. 2010;5:1847–51. [PubMed] [Google Scholar]
- 36.Quintans-Júnior LJ, Guimarães AG, Araújo BE, Oliveira GF, Santana MT, Moreira FV, et al. Carvacrol,(-)-borneol and citral reduce convulsant activity in rodents. Afr J Biotechnol. 2013;9:6566–72. [Google Scholar]
- 37.Matsumura T, Ishikawa T, Kitajima J. Water-soluble constituents of caraway: carvone derivatives and their glucosides. Chem Pharm Bull (Tokyo) 2002;50:66–72. doi: 10.1248/cpb.50.66. [DOI] [PubMed] [Google Scholar]
- 38.Zhu HL, Wan JB, Wang YT, Li BC, Xiang C, He J, et al. Medicinal compounds with antiepileptic/anticonvulsant activities. Epilepsia. 2014;55:3–16. doi: 10.1111/epi.12463. [DOI] [PubMed] [Google Scholar]


