Abstract
Aspirin is an anti-inflammatory drug, peroxyl radical scavenger, and antioxidant agent that inhibits phospholipases, nitric oxide synthetases, and cyclooxygenase enzymes. The existing literature contains no studies on the effects of various doses of aspirin on spinal cord injury (SCI). Therefore, we sought to investigate the putative effects of aspirin on experimental SCI.
The weight-drop injury model was used to produce SCI in 100 albino Wistar rats. The animals were allocated to five groups: a control group, where the rats did not undergo any surgical or medical intervention except for anesthesia; a sham-treated group, where laminectomy was performed without SCI and no further therapy was administered; and three other groups, where the rats with SCI received low-dose aspirin [20 mg/kg], high-dose aspirin [80 mg/kg], and a vehicle, respectively. Half of the rats were sacrificed 24 hours later, and their spinal cords were excised for biochemical studies. The other rats were subjected to Basso, Beattie, and Bresnahan (BBB) locomotor rating scale scoring once a week for 6 consecutive weeks.
Aspirin decreased lipid peroxidation following SCI as the mean (± standard error) catalase level was significantly higher in the high-dose aspirin group (46.10±12.01) than in the sham-treated group (16.07±2.42) and the vehicle-treated group (15.31±3.20) (P<0.05; P<0.05, respectively). Both of the groups treated with high-dose and low-dose aspirin demonstrated a higher mean BBB score than did the control group (P<0.001) and the sham-treated group (P<0.001).
Our data provide evidence in support of the potential effects of aspirin in biochemical and neurobehavioral recovery after SCI.
Keywords: Aspirin, Antioxidant, Spinal cord injury
What’s Known
Inflammation is a known sequel of spinal cord injury (SCI).
Various anti-inflammatory agents have been trialed as a treatment for SCI.
The existing literature contains no studies on the effects of various doses of aspirin on SCI.
What’s New
Neurobehavioral and biochemical assessment of rats with SCI treated with aspirin was performed.
The results provide evidence in support of the potential effects of aspirin in biochemical and neurobehavioral recovery after SCI.
Introduction
Annual crude incidence rates of traumatic spinal cord injury (SCI) vary from 12.1 to 57.8 per million.1 These regional differences in the prevalence rates of SCI across the globe notwithstanding, there has been a trend toward a rise in the prevalence rates over the last decades.2 Traumatic SCI can precipitate serious biochemical and pathological events that result in tissue necrosis and functional deficits. The earlier biochemical reactions are hydrolysis of fatty acids from membrane phospholipids, production of biologically active eicosanoids, and peroxidation of lipids with the formation of reactive oxygen species. The latter is the main agent responsible for cellular damage.3 Injuries to the spinal cord cause tissue damage through both primary and secondary mechanisms; the outcome of the secondary injury may be amenable to therapeutic modulation. With the development of experimental SCI models and recent advances in SCI research, various treatment strategies are being evaluated.
Locomotor assessment is a measure of functional recovery after SCI and serves as a tool for evaluating the therapeutic efficacy of different agents. The Basso, Beattie, Bresnahan (BBB) locomotor rating scale is a semiquantitative scale based on the locomotor response of rats. This scale has values ranging from 0 to 21. The 21-point open-field locomotion score was developed in order to study the sequence of locomotor recovery patterns.4 In animal models, any treatment that may raise the BBB scores may be considered neuroprotective.
Anti-inflammatory and/or immunosuppressive drugs such as octreotide and melatonin;5 indomethacin;6 selenium;7 resveratrol and methylprednisolone;8 magnesium;9 erythropoietin;10 combination of aspirin, dipyridamole, and steroids;11 and ibuprofen and naproxen12 have been trialed as treatments for SCI. Aspirin is known as an anti-inflammatory agent that inhibits phospholipases, nitric oxide (NO) synthetases, and cyclooxygenase enzymes.
Given the current dearth of data on the effects of various doses of aspirin on SCI, we performed neurobehavioral and biochemical assessment of spinal cord injured rats treated with aspirin, which is an inhibitor of membrane lipid peroxidation, a peroxyl radical scavenger and an antioxidant substance.
Materials and Methods
Animals
All the surgical interventions and postoperative animal care in the present study were performed in accordance with the Guidelines and Policies for Rodent Survival Surgery, provided by the Canadian Council on Animal Care.
In this study, adult male albino Wistar rats (n, 100) (Pasteur Institute, Iran) weighting between 200 and 250 grams were used. The rats were kept in cages at room temperature (18 to 21 °C) and fed with rat chow and water. All the experiments were approved by the Animal Experimentation Ethics Committee, affiliated to The Neuroscience Research Center, Kerman University of Medical Sciences (Registration # EC/KNRC/85-51). The study was carried out in 2013, and the subjects were randomly divided into five groups of A, B, C, D, and E. Each group consisted of 20 rats. Sample size was calculated based on similar studies.13 In Group A (control), the rats did not undergo any surgical or medical intervention except for anesthesia. In Group B, the rats were given a vehicle comprising alcohol, propylene glycol, and polyethylene glycol 400 (Merck Co., Darmstadt, Germany). The animals in this group received no therapy but were subjected to laminectomy and cord injury. Thereafter, the vehicle was administrated, and spinal tissue samples were taken. Anesthesia was induced with ketamine hydrochloride (50 mg/kg), and xylazine hydrochloride (5 mg/kg) was injected intraperitoneally. Laminectomy was carried out at the caudal portion of the 7th and the total 8th thoracic (T) spinal levels. The dura mater was opened in midline, and then a 1.5 cm long sample of the spinal cord was obtained for biochemical analysis. In Group C (high-dose aspirin), laminectomy and cord injury were performed, and the rats were subsequently treated with a high dose of aspirin (80 mg/kg) (Darou Pakhsh, Iran). In Group D (low-dose aspirin), laminectomy and cord injury were carried out, and the rats were thereafter treated with a low dose of aspirin (20 mg/kg). The rats in Group C and Group D received aspirin within the first hour and on the 6th and 24th hour post injury. In Group E (sham), laminectomy was performed without SCI, and spinal cord tissue samples were taken. Aspirin and the vehicle were administrated intraperitoneally. Half of the rats in each group were sacrificed on the day after the final aspirin dose. The spinal cord samples, obtained for biochemical analysis, were wrapped in aluminum foil and stored at -70 °C. The other rats were sacrificed in the 6th week just after neurobehavioral evaluation (BBB locomotor rating scale). In groups B, C, D, and E, laminectomy was conducted at the caudal portion of T7 and all of T8 (partial T7 and total T8 laminectomy) spinal levels, leaving the dura mater intact. SCI was performed in groups B, C, and D through the weight-drop method.14 A 10-gram rod was dropped onto the laminectomized (T8 segment) cord from a height of 50 millimeters, and the impounder was left for 20 seconds before being withdrawn to produce a moderate contusion. Immediately after SCI, all the injured rats were paraplegic with no observable hind-limb movement.
Biochemical Investigations
Catalase levels were assayed based on the rate of hydrogen peroxide (H2O2) absorbance at 240 nanometers according to Doğruer et al.15 Decomposition of one micromole of hydrogen peroxide per minute (µM H2O2/min) at 25 ºC, which produces lysate by catalyzation, was used to define catalase activity.
Total protein concentration in cell lysate was determined using bovine serum albumin as the standard.16 The specific activity was expressed as units per milligram of cellular proteins.
Behavioral Tests
To determine whether aspirin improved recovery of function after SCI, we employed the BBB locomotor rating scale.4 Neurobehavioral assessment was undertaken by two observers, blinded to the experimental groups. The rats were assessed prior to injury to ensure that there were no baseline deficits.
The two examiners, who were blinded to the treatments of each animal, participated in all the open-field tests and were positioned in front of each other to observe both sides of the rat. The rats were assessed individually for scale validation and inter-rater reliability testing. Scoring discrepancies were discussed by the evaluators at the time of testing, and averages were taken. The BBB scores were generated for each hind limb, with the average of the two limbs (rounded down) tabulated at 1st, 2nd, 3rd, 4th, 5th, and 6th post-injury weeks.
Statistical Analysis
The biochemical data were compared between the groups using the analysis of variance (ANOVA). In cases where the P value was less than 0.05, the groups were compared using the post-hoc honestly significant difference (HSD) test. The locomotor function according to the above scale was analyzed statistically utilizing the two-way repeated measures ANOVA to compare the continuous variables between the groups over the study period (i.e. 6 weeks). A p value less than 0.05 was considered statistically significant. Statistical Package for the Social Sciences (SPSS), version 17.0 (SPSS, Chicago, IL, U.S.A.), was employed for the statistical analyses.
Results
Findings of the Biochemical Investigation
Aspirin decreased lipid peroxidation following SCI as the mean catalase level was higher in the high-dose aspirin group than in the sham-treated group and the vehicle-treated group (P<0.05; P<0.05, respectively). The mean±standard error (SE) of the catalase level was significantly higher in the high-dose aspirin group (46.10±12.01) than in the control group (26.67±2.38), sham-treated group (16.07±2.42), and vehicle-treated group (15.31±3.20) group (P<0.05) (figure 1).
Figure 1.
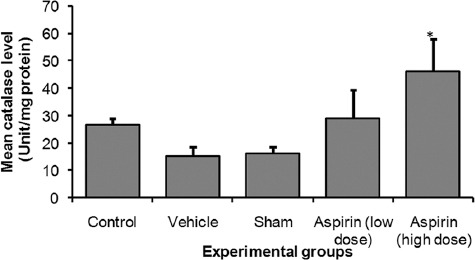
Shows mean catalase level of control and experiments rats’ spinal. *significant at p <0.05 vs. all other groups except for low dose aspirin
Findings of the Functional Recovery Test
Both of groups treated with high-dose aspirin (mean±SE =20.86±0.14) and low-dose aspirin (mean±SE =20.57±0.20) demonstrated a higher mean BBB score than the control group (mean±SE =12.00±0.22) and the sham-treated group (mean±SE=12.00±0.32) (P<0.001).
There was a main effect for group (P<0.001), and so was there a main effect for time (P<0.001). Additionally, there was a significant interaction between group and time (P<0.001). According to the HSD test, the high-dose and low-dose aspirin groups demonstrated significantly higher mean BBB scores than did the control, vehicle-treated, and sham-treated groups throughout the 6 weeks of the study (P<0.001) (table 1 or figure 2).
Table 1.
Mean (±standard error) Basso, Beattie, Bresnahan (BBB) locomotor rating scale over 6 weeks* In adult male albino Wistar rats
| Group | 1st week | 2nd week | 3rd week | 4th week | 5th week | 6th week |
|---|---|---|---|---|---|---|
| Control | 12.00±0.22 | 12.14±0.26 | 12.29±0.18 | 12.29±0.18 | 12.29±0.18 | 12.29±0.18 |
| Vehicle | 12.25±0.25 | 12.25±0.25 | 12.75±0.25 | 12.75±0.25 | 13.00±0.41 | 13.25±0.48 |
| Sham | 12.00±0.32 | 12.20±0.37 | 12.40±0.24 | 12.80±0.37 | 13.40±0.51 | 14.00±0.45 |
| Low-dose aspirin* | 20.57±0.20 | 20.71±0.18 | 20.71±0.18 | 20.71±0.18 | 20.71±0.18 | 20.71±0.18 |
| High-dose aspirin* | 20.86±0.14 | 20.86±0.14 | 20.86±0.14 | 20.86±0.14 | 20.86±0.14 | 20.86±0.14 |
There was a significant difference at a P value less than 0.001 for high-dose and low-dose aspirin versus the other three groups over the 6 weeks; there was a significant interaction between group and time (P<0.001)
Figure 2.
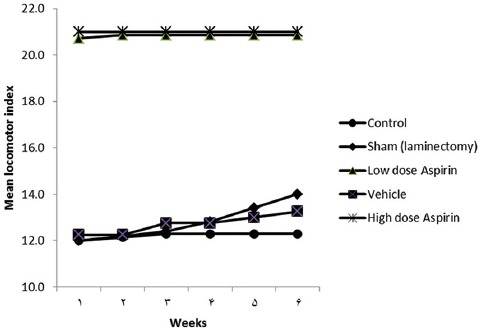
Mean (± Standard Error) Basso, Beattie, Bresnahan (BBB) Locomotor Rating Scale over 6 Weeks* in Adult Male Albino Wistar Rats is Depicted. *There was a significant difference at a P value less than 0.001 for high-dose and low-dose aspirin versus the other three groups over the 6 weeks; there was a significant interaction between group and time (P<0.001).
Discussion
In the present study, we demonstrated the potential effects of aspirin on biochemical and neurobehavioral recovery leading to partial functional recovery in an animal model of SCI. Traumatic and ischemic injuries to the central nervous system, including the spinal cord, cause tissue damage through both direct (primary) and indirect (secondary) mechanisms. Secondary injury is caused by the activation of endogenous substances.12 The spinal cord and brain are particularly vulnerable to free radical oxidation following hypoxic or traumatic insults because of their high lipid content and poor iron-binding capacity.17 After SCI, signals emit from cells that aggregate to the injured terrain and from in-situ non-neuronal cells. Both of neuronal and non-neuronal cells have undergone reactive changes. These changes, collectively called the “inflammatory response,” are potentially self-limiting and set the stage for recovery by removing obstructive debris, by loosening extracellular matrix so that the cells and substances can move freely, and by facilitating defenses against destructive microorganisms. Nonetheless, components of the inflammatory response are also destructive in an injured spinal cord and trigger a progressive tissue destruction that often results in permanent structural and functional damage.12
Under normal conditions, superoxide (O2−) anions are generated during mitochondrial electron transport. There is also a balance between the antioxidants and oxidants produced by aerobic cellular systems. One of the antioxidant defense systems is O2− dismutase, which eliminates O2− by converting it to H2O2 which is reduced to water by cytosolic antioxidants such as catalases.18 Concentrations of the products of lipid peroxidation (e.g., malondialdehyde), together with catalases, can be measured to monitor the degree of oxidation/peroxidation status. Catalase activity was increased in our aspirin-treated groups in a dose-dependent manner. The results of a study by Grosser and Schroder19 (2003) showed that endothelial NO synthetase was a site of action for aspirin and that the NO/cGMP system assumed a crucial function in mediating the cytoprotective action of aspirin. Moreover, NO-regulated antioxidant proteins such as hemeoxygenase-1 contribute to endothelial protection by aspirin19.
It has been shown that aspirin increases endothelial resistance to oxidative damage.20 Accordingly, in the present study, aspirin may have affected catalase activity through the aforementioned mechanisms.
When the locomotor scores of the experimental groups were compared, no statistically significant differences could be observed in the first 4 postoperative weeks between the high-dose aspirin-treated group and the vehicle-treated group. However, the differences between these groups were statistically significant from the 5th week to the end of the study period. These differences were observed at the 6th week between the vehicle-treated group and the low-dose aspirin-treated group. The difference between the time-related motor scores of the trauma and vehicle-treated groups was not statistically significant, but the locomotor (BBB) recovery of the aspirin-treated groups was observed during the study period.
It seems that aspirin exerted its effects on injury response within days of the SCI in the rats in the present study. Therefore, if this is true, the improved percentage of the white matter seen at the epicenter in the aspirin-treated group could present at any time following the acute injury phase, which would include the 6 weeks.
Our neurobehavioral results demonstrated that the spinal cord injured rats, which received aspirin, had higher scores than did the normal rats. Although the underlying mechanism of this finding is unknown, it seems that aspirin improved the rats’ motor function through its various effects. This finding should encourage researchers to undertake an investigation concerning the effects of aspirin on the sensory/motor or neurobehavioral function of normal rats.
There are several limitations to our study. First, because we did not know the exact effects of aspirin on SCI, we examined only low and high doses. Second, dose-response assessment in pharmacological investigations and also in those similar to the current study is crucial. Third, in terms of administration timing, we should have taken into account pre-treatment and various post-injury periods. SCI involves multiple pathological mechanisms and biochemical responses; thus, no single agent is likely to provide complete neuroprotection from secondary injuries. Combination drug therapy may become a feasible option for the future treatment of SCI.
Conclusion
The results of the present study showed that aspirin led to partial functional recovery in an animal model of SCI. The biochemical and neurobehavioral improvements observed in this study indicate that aspirin treatment may be of benefit in experimental SCI. Future studies should be carried out to determine the precise protective mechanisms. Axonal and tissue preservation by immunohistochemistry (i.e., NeuN counts or fluorogold tract tracing), protein quantification (i.e., NF200, MAP2, CNPase western blot analysis), and cavity formation/tissue preservation imaging (i.e., luxol fast blue–hematoxylin eosin stain) are recommended.
Acknowledgement
This research was funded by Kerman Neuroscience Research Center. The authors are also grateful to Ehsan Mehrabi Kermani for editing the English text.
Conflict of Interest: None declared.
References
- 1.van den Berg ME, Castellote JM, Mahillo-Fernandez I, de Pedro-Cuesta J. Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology. 2010;34:184–92. doi: 10.1159/000279335. discussion 92. [DOI] [PubMed] [Google Scholar]
- 2.Yip PK, Malaspina A. Spinal cord trauma and the molecular point of no return. Mol Neurodegener. 2012;7:6. doi: 10.1186/1750-1326-7-6. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saunders RD, Dugan LL, Demediuk P, Means ED, Horrocks LA, Anderson DK. Effects of methylprednisolone and the combination of alpha-tocopherol and selenium on arachidonic acid metabolism and lipid peroxidation in traumatized spinal cord tissue. J Neurochem. 1987;49:24–31. doi: 10.1111/j.1471-4159.1987.tb03388.x. [DOI] [PubMed] [Google Scholar]
- 4.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 5.Erol FS, Kaplan M, Tiftikci M, Yakar H, Ozercan I, Ilhan N, et al. Comparison of the effects of octreotide and melatonin in preventing nerve injury in rats with experimental spinal cord injury. J Clin Neurosci. 2008;15:784–90. doi: 10.1016/j.jocn.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Pantovic R, Draganic P, Erakovic V, Blagovic B, Milin C, Simonic A. Effect of indomethacin on motor activity and spinal cord free fatty acid content after experimental spinal cord injury in rabbits. Spinal Cord. 2005;43:519–26. doi: 10.1038/sj.sc.3101763. [DOI] [PubMed] [Google Scholar]
- 7.Yeo JE, Kim JH, Kang SK. Selenium attenuates ROS-mediated apoptotic cell death of injured spinal cord through prevention of mitochondria dysfunction;in vitro and in vivo study. Cell Physiol Biochem. 2008;21:225–38. doi: 10.1159/000113764. [DOI] [PubMed] [Google Scholar]
- 8.Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Kocak A, et al. Effects of resveratrol and methylprednisolone on biochemical, neurobehavioral and histopathological recovery after experimental spinal cord injury. Acta Pharmacol Sin. 2006;27:1317–25. doi: 10.1111/j.1745-7254.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 9.Gok B, Okutan O, Beskonakli E, Kilinc K. Effects of magnesium sulphate following spinal cord injury in rats. Chin J Physiol. 2007;50:93–7. [PubMed] [Google Scholar]
- 10.Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev. 2004;27:113–20. doi: 10.1007/s10143-003-0300-y. [DOI] [PubMed] [Google Scholar]
- 11.Fujita Y, Shingu T, Kurihara M, Miyake H, Kono T, Tsujimura M, et al. Evaluation of a low dose administration of aspirin, dipyridamol and steroid. Therapeutic effects on motor function and protective effects on Na+-K+-activated ATPase activity against lipid peroxidation in an experimental model of spinal cord injury. Paraplegia. 1985;23:56–7. doi: 10.1038/sc.1985.9. [DOI] [PubMed] [Google Scholar]
- 12.Kwon BK, Okon E, Hillyer J, Mann C, Baptiste D, Weaver LC, et al. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J Neurotrauma. 2011;28:1545–88. doi: 10.1089/neu.2009.1149. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charan J, Kantharia N. How to calculate sample size in animal studies? Journal of Pharmacology & Pharmacotherapeutics. 2013;4:303. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000;17:1–17. doi: 10.1089/neu.2000.17.1. [DOI] [PubMed] [Google Scholar]
- 15.Dogruer ZN, Unal M, Eskandari G, Pata YS, Akbas Y, Cevik T, et al. Malondialdehyde and antioxidant enzymes in children with obstructive adenotonsillar hypertrophy. Clin Biochem. 2004;37:718–21. doi: 10.1016/j.clinbiochem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Berker KI, Ozdemir Olgun FA, Ozyurt D, Demirata B, Apak R. Modified Folin-Ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants. J Agric Food Chem. 2013;61:4783–91. doi: 10.1021/jf400249k. [DOI] [PubMed] [Google Scholar]
- 17.Rangan U, Bulkley GB. Prospects for treatment of free radical-mediated tissue injury. Br Med Bull. 1993;49:700–18. doi: 10.1093/oxfordjournals.bmb.a072641. [DOI] [PubMed] [Google Scholar]
- 18.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosser N, Schroder H. Aspirin protects endothelial cells from oxidant damage via the nitric oxide-cGMP pathway. Arterioscler Thromb Vasc Biol. 2003;23:1345–51. doi: 10.1161/01.ATV.0000083296.57581.AE. [DOI] [PubMed] [Google Scholar]
- 20.He Z, Liao Q, Xu X, Luo C, Zhou T, Li Q, et al. A study of the acting mechanism of aspirin for resistance to oxidative damage. Hua Xi Yi Ke Da Xue Xue Bao. 2001;32:413–6. [PubMed] [Google Scholar]


