Abstract
Purpose
To determine the effects of bevacizumab on patient-reported outcomes (PROs; secondary end point) in the AURELIA trial.
Patients and Methods
Patients with platinum-resistant ovarian cancer were randomly assigned to chemotherapy alone (CT) or with bevacizumab (BEV-CT). PROs were assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Ovarian Cancer Module 28 (EORTC QLQ-OV28) and Functional Assessment of Cancer Therapy–Ovarian Cancer symptom index (FOSI) at baseline and every two or three cycles (8/9 weeks) until disease progression. The primary PRO hypothesis was that more patients receiving BEV-CT than CT would achieve at least a 15% (≥ 15-point) absolute improvement on the QLQ-OV28 abdominal/GI symptom subscale (items 31-36) at week 8/9. Patients with missing week 8/9 questionnaires were included as unimproved. Questionnaires from all assessments until disease progression were analyzed using mixed-model repeated-measures (MMRM) analysis. Sensitivity analyses were used to determine the effects of differing assumptions and methods for missing data.
Results
Baseline questionnaires were available from 89% of 361 randomly assigned patients. More BEV-CT than CT patients achieved a ≥ 15% improvement in abdominal/GI symptoms at week 8/9 (primary PRO end point, 21.9% v 9.3%; difference, 12.7%; 95% CI, 4.4 to 20.9; P = .002). MMRM analysis covering all time points also favored BEV-CT (difference, 6.4 points; 95% CI, 1.3 to 11.6; P = .015). More BEV-CT than CT patients achieved ≥ 15% improvement in FOSI at week 8/9 (12.2% v 3.1%; difference, 9.0%; 95% CI, 2.9% to 15.2%; P = .003). Sensitivity analyses gave similar results and conclusions.
Conclusion
Bevacizumab increased the proportion of patients achieving a 15% improvement in patient-reported abdominal/GI symptoms during chemotherapy for platinum-resistant ovarian cancer.
INTRODUCTION
The prognosis is poor for women with platinum-resistant ovarian cancer (defined as relapse within 6 months of platinum-based chemotherapy), with reported tumor response rates of 10% to 15%, median progression-free survival (PFS) of 3 to 4 months, and median overall survival of 9 to 12 months in most phase III trials.1 The goals of treatment in this setting are to improve symptoms, delay progression, and prolong survival.2 However, there is a paucity of data to show whether chemotherapy improves symptoms in women with platinum-resistant ovarian cancer.
In the randomized open-label phase III AURELIA trial, adding bevacizumab to single-agent chemotherapy significantly improved the primary end point of PFS (hazard ratio, 0.48; 95% CI, 0.38 to 0.60; P < .001; median, 6.7 months with bevacizumab-containing therapy v 3.4 months with chemotherapy alone) and the secondary end point of objective response rate (30.9% v 12.6%, respectively, P < .001).3 There was no significant difference in overall survival at the final analysis (hazard ratio, 0.85; 95% CI, 0.85 to 1.08; P = .174; median, 16.6 months v 13.3 months, respectively). Consistent with the Gynecologic Cancer Intergroup consensus recommendations for trials in recurrent ovarian cancer,4 AURELIA included extensive evaluation of patient-reported outcomes (PROs) as a secondary objective. Our a priori hypotheses were that, despite relatively low objective tumor response rates, chemotherapy would improve disease-related symptoms in some women, and that adding bevacizumab would be associated with greater improvements, particularly in women with abdominal symptoms and/or ascites at baseline. PROs were assessed with three instruments: European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire C30 (EORTC QLQ-C30),5 EORTC QLQ Ovarian Cancer Module 28 (OV28),6 and the eight-item Functional Assessment of Cancer Therapy–Ovarian Cancer Symptom Index (FOSI).7 These instruments have demonstrated reliability and validity, are commonly used in clinical trials, and provide complementary and corroborative information about quality of life (QoL) in advanced ovarian cancer.8 The QLQ-OV28 includes seven questions about abdominal/GI symptoms, which are predominant and burdensome particularly in this patient population.9 The eight-item FOSI includes items assessing worry about future deterioration, contentment with QoL, and lack of energy, in addition to five GI symptoms.7
Few studies of QoL in recurrent ovarian cancer have shown substantial differences between treatments. This is possibly because studies rarely include prespecified PRO objectives pertinent to the treatment setting and/or agent under evaluation, but also because global QoL scales are probably relatively insensitive to such differences. To gain greater insight into the effects of bevacizumab and chemotherapy on QoL in this setting, we developed a priori hypotheses focusing on patients' ratings of abdominal/GI symptoms in AURELIA.
PATIENTS AND METHODS
Study Design
After investigators' selection of single-agent chemotherapy (weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan), patients were randomly assigned to receive chemotherapy alone or chemotherapy combined with bevacizumab (15 mg/kg every 3 weeks or 10 mg/kg every 2 weeks) until progressive disease (PD), unacceptable toxicity, or withdrawal of patient consent (Fig 1).3
Fig 1.
CONSORT diagram. BEV-CT, chemotherapy with bevacizumab; CT, chemotherapy; PRO, patient-reported outcome; PD, progressive disease.
PRO Assessment and End Points
PRO questionnaires were completed at baseline and then every two cycles (or three cycles for patients receiving three-weekly regimens) until the cycle in which PD was determined. PRO hypotheses were developed before the database was locked for the primary PFS analysis and were prespecified in a statistical analysis plan. Week 8/9 was predefined as the primary analysis time point, with week 16/18 as a secondary analysis time point. Because the median PFS with chemotherapy for platinum-resistant ovarian cancer is approximately 12 weeks, it was anticipated that disease progression would lead to missing questionnaires in more than half of the patients at week 16/18, and more than three quarters of the patients at week 30.
The primary PRO hypothesis was that a higher proportion of women in the bevacizumab group would experience a ≥ 15% improvement in an abdominal/GI symptom subscale comprising items 31 to 36 of the EORTC QLQ-OV28; item 37 was deliberately excluded because heartburn/indigestion was judged to be a less specific symptom of ovarian cancer. However, a posthoc sensitivity analysis included items 31 to 37 (as defined in the QLQ-OV28 scoring system6).
Prespecified secondary PRO hypotheses were that similar proportions of women in the two treatment groups would experience ≥ 15% improvements in FOSI and in the QLQ-C30 physical, role, emotional, social functioning, and global health-related QoL subscales, because these scales assess a wider range of concerns less likely to be differentially affected by the addition of bevacizumab.
Statistical Analyses
Analyses were based on the intent-to-treat (ITT) population, with patients analyzed within the treatment group to which they were randomly assigned. The scoring manuals from the developers of QLQ-OV28, FOSI, and QLQ-C30 were applied when calculating scores for each of the instruments and (where applicable) their subscales, except for the items included within the QLQ-OV28 abdominal/GI symptom score, described in the PRO Assessment and End Points section. The PRO hypotheses were not covered by the statistical testing strategy or sample size calculations for the main trial analysis, which focused on PFS.
Only questionnaires completed until PD were included in the main analyses, consistent with the protocol-specified PRO collection up to but not beyond PD. Questionnaires completed after PD were excluded based on the medical assumption that these patients were unlikely to be benefiting from their study treatment, may have been receiving another treatment, and were therefore not relevant to the intended comparison of chemotherapy alone versus bevacizumab plus chemotherapy. However, posthoc sensitivity analyses were performed to determine the impact of questionnaires completed after PD. Compliance was calculated at each time point using the number of patients alive and progression-free as the denominator.
For the primary hypothesis, a responder (improvement) analysis approach was adopted, with improvement defined as a 15-point (15%) absolute increase in the 100-point scale. This threshold was chosen to represent a meaningful improvement, rather than the more conventional 10-point increase used to define a minimum clinically important difference,10,11 because a more stringent response definition was considered preferable, especially in an open-label trial. However, sensitivity analyses included improvement defined as a 10% increase. In accordance with published recommendations,10–12 proportions were calculated including all patients with baseline questionnaires and counting those patients with missing questionnaires at a subsequent time point as not having improved. In a posthoc sensitivity analysis, only patients whose postbaseline questionnaires were missing because of PD, death, patient too ill, or (in the chemotherapy-alone group) switch to bevacizumab monotherapy were considered as unimproved, with other patients whose questionnaires were missing postbaseline excluded. In addition, we did complete-case responder analyses (excluding all patients with missing postbaseline questionnaires irrespective of the reason), although these may be considered too conservative because they exclude patients with early PD.
For each treatment group, the difference between treatment groups in the proportion of patients meeting the criteria for improvement is presented with 95% CIs with the Hauck-Anderson continuity correction. P values for these between-treatment group comparisons were obtained from Fisher's exact test.
Statistical analyses for secondary PRO hypotheses and posthoc sensitivity analyses were similar to those for the primary PRO hypothesis; the primary analysis was based on a 15% absolute increase in the total scale (equating to a five-point increase on a 32-point scale [FOSI] or a 15-point increase on a 100-point scale [QLQ-C30]), with a 10% definition of response used for sensitivity analyses.
A linear mixed-model repeated-measures (MMRM) analysis,13 adjusting for score at baseline, time, and a treatment-by-time interaction, was used to estimate the treatment effect over time. This analysis was based on continuous PRO variables rather than the responder approach described for the primary hypothesis. Patient was defined as a random variable and the covariance structure was assumed to be unstructured. Estimates of the least-squares means for treatment effects within and between treatment groups were reported with corresponding 95% CIs.
Prespecified subgroup analyses were performed in patients with ascites at baseline (who typically have significantly impaired QoL) and patients with sufficient symptoms at baseline to allow detection of a 15% improvement in a given scale or subscale (defined as a baseline score ≥ 15% of the total scale or subscale). This equates to a score of ≥ 15 points on the 100-point QLQ-OV28 scale or less than 27 points on the 32-point FOSI scale (in which a higher summary score represents a less symptomatic patient). P values are provided to aid interpretation but must be interpreted conservatively to account for the multiple scales, time points, and hypotheses. We considered P values of .01 to .05 to reflect modest evidence of a difference and P values less than .01 to reflect moderate evidence of a difference. The PRO analyses were performed by Parexel International GmbH (Berlin, Germany).
RESULTS
Patient Population
There were no substantial differences in baseline characteristics between the PRO-evaluable and ITT populations (Table 1). Mean scores for each PRO scale at baseline were similar between treatment groups (Appendix Table A1 [online-only]). Reasons for missing data are listed in Appendix Table A2.
Table 1.
Baseline Characteristics and Selected Chemotherapy of the PRO-Evaluable Population (abdominal/GI symptoms) and the Intent-to-Treat Population
| Characteristic | PRO-Evaluable Population |
Intent-to-Treat Population |
||||||
|---|---|---|---|---|---|---|---|---|
| Chemotherapy Alone (n = 162) |
Bevacizumab Plus Chemotherapy (n = 155) |
Chemotherapy Alone (n = 182) |
Bevacizumab Plus Chemotherapy (n = 179) |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Age, years | ||||||||
| Median | 61 | 62 | 61 | 62 | ||||
| Range | 32-84 | 25-80 | 25-84 | 25-80 | ||||
| Origin of cancer: ovary | 140 | 86 | 143 | 92 | 157 | 86 | 167 | 93 |
| Serous/adenocarcinoma at diagnosis | 138 | 85 | 135 | 87 | 152 | 84 | 156 | 87 |
| Histologic grade at diagnosis | ||||||||
| 1 | 8 | 5 | 8 | 5 | 9 | 5 | 10 | 6 |
| 2 | 40 | 25 | 49 | 32 | 48 | 26 | 53 | 30 |
| 3 | 95 | 59 | 77 | 50 | 105 | 58 | 94 | 53 |
| Two prior chemotherapy regimens | 72 | 44 | 65 | 42 | 78 | 43 | 72 | 40 |
| Platinum-free interval < 3 months*† | 38 | 23 | 42 | 27 | 46 | 25 | 50 | 28 |
| ECOG PS | ||||||||
| 0 | 89 | 55 | 95 | 61 | 99 | 54 | 107 | 60 |
| 1 | 59 | 36 | 47 | 30 | 69 | 38 | 58 | 32 |
| 2 | 11 | 7 | 11 | 7 | 11 | 6 | 12 | 7 |
| Missing | 3 | 2 | 2 | 1 | 3 | 2 | 2 | 1 |
| Measurable disease | 158 | 98 | 152 | 98 | 144 | 79 | 143 | 80 |
| Ascites | 49 | 30 | 50 | 32 | 54 | 30 | 59 | 33 |
| Selected chemotherapy | ||||||||
| Weekly paclitaxel | 46 | 28 | 48 | 31 | 55 | 30 | 60 | 34 |
| Pegylated liposomal doxorubicin | 59 | 36 | 57 | 37 | 64 | 35 | 62 | 35 |
| Topotecan | 57 | 35 | 50 | 32 | 63 | 35 | 57 | 32 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; PRO, patient-reported outcome.
Stratification factor.
From last platinum to subsequent disease progression.
Questionnaire Completion Compliance
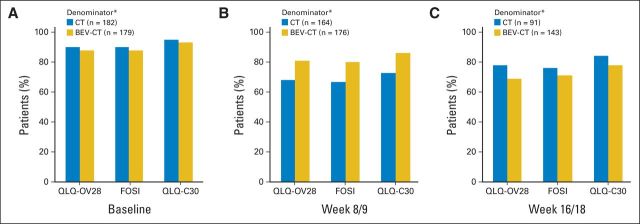
Baseline questionnaires were available from 89% of patients for QLQ-OV28 and FOSI and 94% of patients for QLQ-C30 (Fig 2). Compliance was somewhat higher in the bevacizumab arm at week 8/9 and in the chemotherapy arm at week 16/18 for all three questionnaires.
Fig 2.
Compliance for the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire–Ovarian Cancer Module 28 (QLQ-OV28), Functional Assessment of Cancer Therapy–Ovarian Cancer Symptom Index (FOSI), and EORTC QLQ Cancer Module 30 (C30) questionnaires. (A) Baseline; (B) week 8/9; (C) week 16/18. (*) Denominator (patients known to be progression free) excludes patients whose disease progressed or who died or were lost to follow-up at least 14 days before the scheduled assessment date. BEV, bevacizumab; CT, chemotherapy.
Abdominal/GI Symptoms (QLQ-OV28)
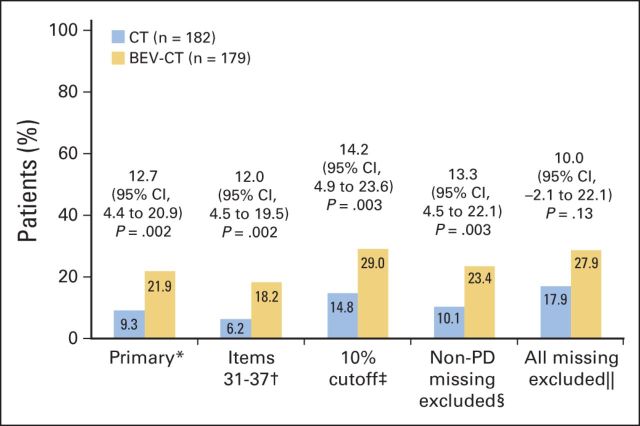
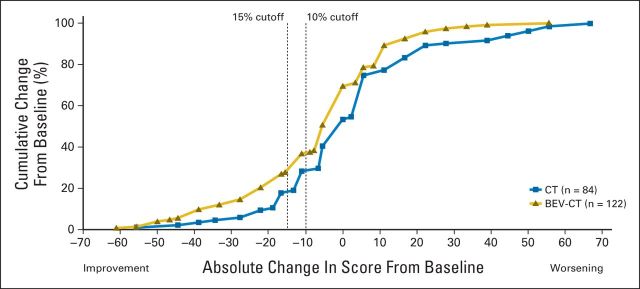
At week 8/9, significantly more patients assigned to the bevacizumab plus chemotherapy arm reported a ≥ 15% improvement in abdominal/GI symptoms; 34 (21.9%) of 155 patients assigned to bevacizumab versus 15 (9.3%) of 162 patients assigned to chemotherapy alone (difference, 12.7% favoring bevacizumab; 95% CI, 4.4 to 20.9%; P = .002). Sensitivity analyses yielded similar results and conclusions (Fig 3). As expected, the complete-case analysis excluding patients with early progression showed a smaller effect size, wider CIs, and a higher P value (difference, 10.0%; 95% CI, −2.1% to 22.1%; P = .13). The cumulative distribution plot (Appendix Fig A1 [online-only]) shows that the observed difference was largely independent of the chosen cutoff. Similar analyses in the subgroup of 233 patients (65% of the ITT population) with sufficient symptoms at baseline to allow detectable improvement also favored the bevacizumab arm. A ≥ 15% improvement was observed in 34 (29.6%) of 115 of patients assigned to bevacizumab versus 15 (12.7%) of 118 patients assigned to chemotherapy alone (difference, 16.9%; 95% CI, 6.1% to 27.6%; P = .002).
Fig 3.
Primary and sensitivity analyses of the primary hypothesis (≥ 15% improvement in abdominal/GI symptoms [European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Ovarian Cancer Module 28]). 95% Pearson-Clopper CIs with the Hauck-Anderson continuity correction for the difference between arms. (*) 15% cutoff, items 31 to 36, missing questionnaires counted as no improvement. (†) 15% cutoff, items 31 to 37, missing questionnaires counted as no improvement. (‡) 10% cutoff, items 31 to 36, missing questionnaires counted as no improvement. (§) 15% cutoff, items 31 to 36, questionnaires missing for reasons other than disease progression (PD) were not included in denominator. (‖) 15% cutoff, items 31 to 36, all missing questionnaires excluded from denominator. BEV, bevacizumab; CT, chemotherapy.
A second subgroup analysis included only the 99 PRO-evaluable patients with ascites at baseline (27% of the ITT population), who were expected to have considerable abdominal pain/GI symptoms. This subgroup showed a greater treatment effect with a ≥ 15% improvement in 44.0% of the bevacizumab arm versus 4.1% of the chemotherapy-alone arm (difference, 39.9%; 95% CI, 23.9% to 55.9%; P < .001).
At week 16/18, significantly more patients assigned to bevacizumab than chemotherapy showed a ≥ 15% improvement in abdominal/GI symptoms (15.5% v 5.6%, respectively; difference, 9.9%; 95% CI, 2.9% to 17.0%; P = .005), consistent with week 8/9 findings. Sensitivity analyses again gave similar results and conclusions.
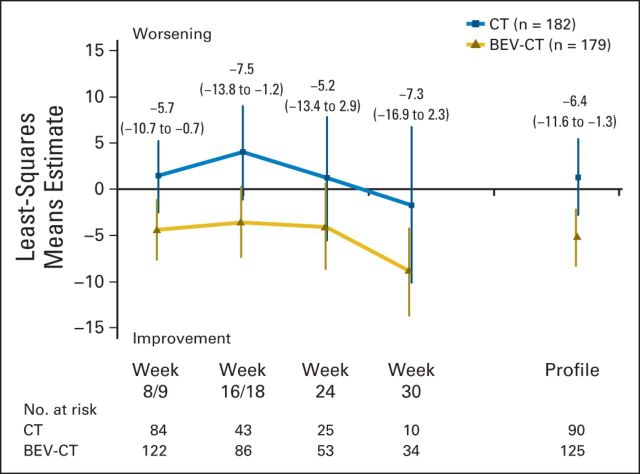
To summarize the effects of chemotherapy with or without bevacizumab over all time points until PD or death, whichever occurred first, MMRM analysis was performed (Fig 4). The profile showed a 6.4-point difference favoring the bevacizumab arm (95% CI, 1.3 to 11.6; P = .015). The week 8/9 estimate corroborated the responder analyses findings.
Fig 4.
Mixed-model repeated-measures analyses for the abdominal/GI subscale of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Ovarian Cancer Module. Estimates for the between-treatment group comparisons for each time point and for the entire profile were obtained. The estimates are presented with the corresponding 95% CIs in parentheses. BEV, bevacizumab; CT, chemotherapy.
Secondary Hypotheses
Significantly more patients assigned to bevacizumab and chemotherapy than to chemotherapy alone showed a ≥ 15% improvement in FOSI score at week 8/9 (12.2% v 3.1%, respectively; difference, 9.0%; 95% CI, 2.9% to 15.2%; P = .003). Sensitivity analyses gave similar results and conclusions.
Within the subgroup of 267 patients with a baseline FOSI score less than 27 points (74% of the overall population), a ≥ 15% absolute improvement was achieved at week 8/9 in 19 (14.6%) of 130 patients assigned to bevacizumab versus five (3.6%) of 137 assigned to chemotherapy alone (difference, 11.0%; 95% CI, 3.7% to 18.2%; P = .002). In the subgroup of 99 patients with ascites at baseline, a ≥ 15% improvement was observed in 21.6% versus 2.1%, respectively (difference, 19.5%; 95% CI, 6.3% to 32.6%; P = .004).
At week 16/18, the proportion of patients with a ≥ 15% improvement in FOSI also favored the bevacizumab treatment group (9.0% v 1.3%, respectively; difference, 7.7%; 95% CI, 2.6 to 12.9%; P = .002).
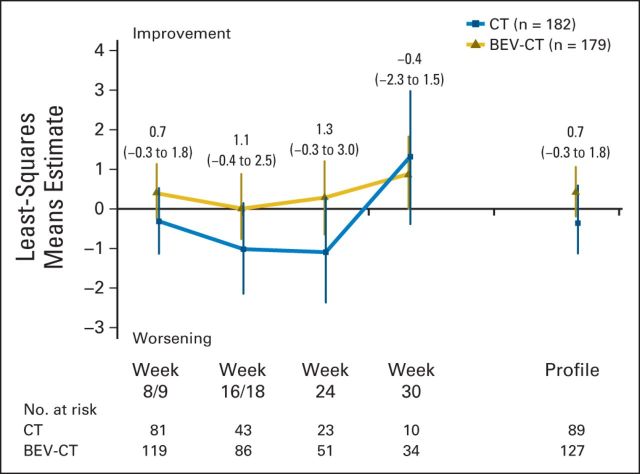
Figure 5 shows the effects of chemotherapy with or without bevacizumab on the FOSI over all time points until PD or death, whichever occurred first. The corresponding MMRM analysis for the FOSI did not show an important treatment effect either overall or at week 8/9 (estimated between-treatment group difference, 0.7; 95% CI, −0.3 to 1.8; P = .21).
Fig 5.
Mixed-model repeated-measures analysis for the Functional Assessment of Cancer Therapy–Ovarian Cancer Symptom Index. Estimates for the between-treatment group comparison for each time point and for the entire profile were obtained. The estimates are presented with the corresponding 95% CIs in parentheses. BEV, bevacizumab; CT, chemotherapy.
Figure 6 shows findings for the QLQ-C30 at week 8/9 with subscales for physical, role and social function, and global health/QoL favoring the bevacizumab group. There was no difference between the treatment groups for the emotional function subscale. Sensitivity analyses again gave similar results and conclusions although, as expected, the complete-case analysis excluding patients with early PD showed smaller effect sizes, wider CIs, and higher P values except for the physical function subscale (Fig 6B). Differences between treatment groups at week 8/9 persisted (and in the case of emotional function subscale, became apparent) at week 16/18 for all function subscales and global health/QoL. The MMRM analysis of the EORTC QLQ-C30 global health/QoL subscale showed no significant difference between treatment groups (Appendix Table A3).
Fig 6.
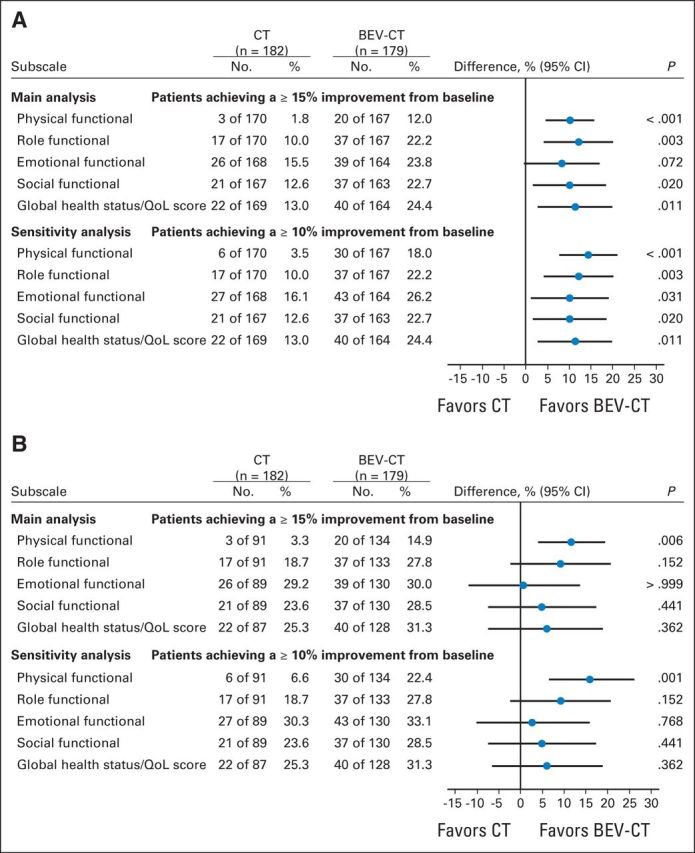
Comparison of proportions of patients achieving improvement from baseline in European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Cancer Module (week 8/9). (A) Proportions were calculated including all patients with baseline questionnaires and counting those missing questionnaires at a subsequent time point as unimproved. (B) Patients with missing questionnaires after baseline were not included in the denominator. BEV, bevacizumab; CT, chemotherapy; QoL, quality of life.
DISCUSSION
These analyses of PROs, a secondary end point of AURELIA, show for the first time in platinum-resistant ovarian cancer that treatment can improve symptoms, one of the main treatment goals for these patients. Bevacizumab improved abdominal/GI symptoms, as demonstrated by a significantly higher proportion of patients achieving the predefined 15% improvement in abdominal/GI symptoms using a responder-analysis approach. This finding was corroborated by sensitivity analyses using alternative PROs, assumptions, and statistical methods. Effects on a broader range of QoL concerns either favored the bevacizumab group or were no different. Together, these findings suggest that the beneficial effects of bevacizumab on ovarian cancer symptoms were not outweighed by any additional toxicity detrimentally affecting QoL.
Subgroup analyses focusing on patients with an abdominal/GI symptom score ≥ 15 at baseline showed a higher proportion with benefit from bevacizumab, as expected. The pronounced treatment benefit in the predefined subgroup analysis of patients with ascites at baseline is of special interest and clinical importance given the impact of abdominal/GI symptoms on QoL in this population.
MMRM analyses showed that trends in QoL favoring the bevacizumab arm became less apparent at successive assessments, as expected. Differential treatment effects in cancer-related symptoms are likely to diminish over time because improvements usually occur within the first few cycles. Subsequent assessments of QoL come from patients whose disease has not yet progressed and who generally have few cancer-related symptoms. Furthermore, each successive assessment includes fewer patients and thus estimation of treatment effects is less precise.
The most likely area of controversy for the present analyses is the handling of missing data, which is a major challenge in PRO analysis, particularly in a setting such as platinum-resistant ovarian cancer, in which disease progresses rapidly yet the importance of PROs is of utmost importance. In AURELIA, as in most oncology trials, PROs were collected only until PD and therefore the numbers of questionnaires diminish rapidly and to a greater extent in the chemotherapy-alone arm. Before knowing the primary outcome of the trial, we chose a responder analysis as most appropriate and reliable for AURELIA, in accordance with expert recommendations.10,11 Responder analyses, as used for assessing objective tumor response, offer a valid and reliable means of detecting the subset of patients with a proven improvement of a given magnitude (in the case of the present analysis, 15%) from all those with a baseline assessment, while providing a logical and conservative solution to dealing with patients missing subsequent assessments by assuming that they have not had a ≥ 15% improvement. We recognize two potential criticisms of this approach. First, we assumed that patients with missing questionnaires after baseline were unlikely to achieve symptomatic benefit at the time point of the missing questionnaire. Statistically, this may seem a strong assumption. However, medically we considered it unlikely that a patient with a missing questionnaire (particularly because of PD) was experiencing substantial symptomatic benefit at that time. At the opposite extreme, the complete-case analysis is open to greater criticism because it implies the assumption that patients whose disease progresses early are equally likely to benefit from treatment as those whose disease does not progress early. Results of sensitivity analyses supported our findings and conclusions. Secondly, the higher rate of progression in the chemotherapy-alone group, which resulted in more missing postbaseline questionnaires at each time point, means that the effects of bevacizumab on PFS are reiterated in the PROs analyses. This is clinically reasonable and unavoidable because the effects of anticancer treatment on symptoms are mechanistically related to, associated with, and therefore confounded by effects on tumor response and time to PD. These effects are overlapping and complementary, not independent.
The 15% cutoff to define response was deliberately chosen to reflect a meaningful improvement rather than the more commonly used (but less stringent) 10% cutoff to define a minimal clinically important difference. Results and conclusions were independent of the cutoff. Results of the responder analyses for the primary end point (abdominal/GI symptoms) were corroborated by the MMRM analysis approach, which reflects average treatment effects over the entire course of treatment until disease progression, albeit showing that the differences between groups diminished over time, as expected.
The absolute proportions of women in AURELIA who achieved a ≥ 15% improvement in their abdominal/GI symptoms may seem small (22% of women in the bevacizumab plus chemotherapy arm v 9% in the chemotherapy-alone arm). Importantly, symptoms at baseline were not mandatory for enrolment onto the trial and therefore the relatively modest absolute percentages reflect the large proportion (35%) of women in AURELIA without substantial symptoms at baseline, as well as the stringent criterion we used to define improvement and the poor prognosis of women with platinum-resistant ovarian cancer. Predefined analyses showed greater benefits in the clinically relevant subgroups of women with ascites and/or symptoms at baseline.
The results of these prespecified QoL analyses indicate that the benefits of bevacizumab in AURELIA extended beyond the prolongation of PFS to include greater improvements in abdominal/GI symptoms and other aspects of QoL, supporting a role for bevacizumab with chemotherapy in the treatment of women with platinum-resistant ovarian cancer.
Supplementary Material
Acknowledgment
We thank the Independent Data Monitoring Committee for AURELIA: J.B. Vermorken (Chair), V. Gebski, and M. Friedlander. Support for third-party writing assistance for this article, supplied by Jennifer Kelly (Medi-Kelsey Ltd, Ashbourne, United Kingdom), was provided by F. Hoffmann-La Roche.
Appendix
The authors are grateful to the following participating investigators. Group d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens (GINECO): J.-C. Barats, C. Becuwe, D. Berton-Rigaud, H. Bourgeois, D. Coeffic, P. D'Agostino, V. Delecroix, N. Dohollou, M. Fabbro, A. Floquet, P. Follana, J.F. Geay, L. Gladieff, A.-C. Hardy-Bessard, C. Lahmar, D. Lebrun-Jezekova, E. Legouffe, M. Leheurteur, A. Lesoin, B. Levaché, A. Lortholary, F. Mefti, J. Meunier, M.A. Mouret-Reynier, I. Ray-Coquard, J. Salvat, S. Scholl, F. Selle, B. Weber; Arbeitsgemeinschaft Gynäkologische Onkologie Ovarian Cancer Study Group (AGO-OVAR): M. Beckmann, A. Burges, T. Fehm, G. Gebauer, B. Gerber, L. Hanker, P. Harter, R. Kimmig, J. Kosse, R. Kreienberg, H.-J. Leuck, W. Meier, J.P. Scharf, J. Sehouli, M. Thill, C. Uleer, P. Wimberger; Grupo Español de Investigaciön en Cáncer de Ovario (GEICO): I. Bover, A. de Juan, I. Diaz, E. Garcia, Y. Garcia, A. Gonzalez, A. Herrero, B. Ojeda, E. Ortega, M.-J. Rubio, A. Santaballa; Nordic Society of Gynaecological Oncology (NSGO): M. Anttila, I. Baasland, K. Boman, H. Havsteen, N. Keldsen, G. Kristensen, G.-B. Nyvang, P. Rosenberg, B. Tholander; Multicenter Italian Trials in Ovarian Cancer (MITO): P.-P. Benedetti, E. Breda, L. Frigerio, A. Oaknin, A. Poveda, F. Raspaglisi, A. Savarese; Belgian Gynaecological Oncology Group (BGOG): V. D'Hondt, M. Huizing, I. Vergote, P. Vuylsteke; Dutch Gynaecological Oncology Group (DGOG): H.J. Bloemendal, G.-J. Creemers, M. De Jong, M. Los; Hellenic Cooperative Oncology Group (HECOG): F. Zagouri; Portugal: D. Pereira, F. Vaz; Turkey: A. Ayhan, E. Buyukunal, H. Onat, O. Ozyilkan; Bosnia and Herzegovina: A. Pasic, Z. Vranjes.
Table A1.
Mean PRO Scores at Baseline by Treatment Arm
| PRO Scale | Chemotherapy Alone |
Bevacizumab Plus Chemotherapy |
||||
|---|---|---|---|---|---|---|
| No. of Patients | Mean | SD | No. of Patients | Mean | SD | |
| EORTC QLQ-OV28* | ||||||
| Abdominal/GI | 162 | 29.6 | 22.13 | 155 | 32.3 | 22.92 |
| Peripheral neuropathy | 161 | 23.4 | 30.13 | 155 | 31.9 | 33.17 |
| Hormonal | 158 | 25.5 | 27.88 | 155 | 19.1 | 24.16 |
| Body image | 155 | 30.3 | 30.89 | 154 | 30.5 | 30.42 |
| Attitudes toward disease/treatment | 153 | 59.7 | 27.25 | 153 | 59.9 | 25.05 |
| Other chemotherapy-related adverse effects | 160 | 20.2 | 16.27 | 155 | 20.2 | 16.10 |
| Single-symptom scale | ||||||
| Heartburn | 159 | 14.3 | 24.44 | 154 | 18.4 | 26.69 |
| Hair loss | 155 | 14.4 | 33.77 | 150 | 20.7 | 39.32 |
| Upset by hair loss† | 38 | 51.8 | 41.52 | 55 | 41.2 | 36.83 |
| Food and drink taste different from usual | 157 | 13.4 | 25.84 | 154 | 13.4 | 25.14 |
| Sexual function | 146 | 9.8 | 16.46 | 148 | 10.5 | 17.09 |
| Sexual enjoyment‡ | 38 | 42.1 | 29.70 | 44 | 33.3 | 29.64 |
| Dry vagina‡ | 38 | 39.5 | 38.64 | 47 | 31.9 | 37.40 |
| EORTC QLQ-C30 | ||||||
| Global health status/QoL | 169 | 61.1 | 22.80 | 164 | 56.8 | 21.53 |
| Physical function | 170 | 76.8 | 21.41 | 167 | 75.1 | 21.20 |
| Role function | 170 | 71.5 | 30.52 | 167 | 70.0 | 29.85 |
| Emotional function | 168 | 64.5 | 24.01 | 164 | 66.9 | 22.56 |
| Cognitive function | 168 | 83.8 | 19.93 | 164 | 85.6 | 20.85 |
| Social function | 167 | 72.8 | 30.69 | 163 | 71.5 | 28.19 |
| Fatigue | 170 | 36.1 | 26.76 | 166 | 37.3 | 27.25 |
| Nausea and vomiting | 170 | 8.8 | 18.59 | 166 | 8.7 | 18.14 |
| Pain | 171 | 30.2 | 27.89 | 166 | 27.7 | 28.35 |
| Dyspnea | 169 | 23.7 | 29.86 | 167 | 23.6 | 30.02 |
| Insomnia | 168 | 36.7 | 32.55 | 167 | 34.7 | 32.18 |
| Appetite loss | 170 | 19.4 | 29.61 | 166 | 21.3 | 31.17 |
| Constipation | 166 | 18.1 | 28.09 | 166 | 19.7 | 29.83 |
| Diarrhea | 166 | 14.5 | 24.17 | 161 | 8.5 | 18.74 |
| Financial difficulties | 166 | 14.5 | 26.05 | 162 | 16.5 | 27.36 |
| FOSI | ||||||
| Summary | 159 | 22.3 | 4.79 | 156 | 22.6 | 5.00 |
Abbreviations: C30, Cancer Module; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; FOSI, Functional Assessment of Cancer Therapy–Ovarian Cancer Symptom Index; OV28, Ovarian Cancer Module; PRO, patient-reported outcome; QoL, quality of life; SD, standard deviation.
Scored using the scoring manual available at the time of the trial design.
To be answered only by patients who had any hair loss.
To be answered only by patients who were sexually active.
Table A2.
Reasons for Missing Data
| Reason for Missing Data | EORTC QLQ-OV28 Abdominal/GI Symptoms |
FOSI |
||
|---|---|---|---|---|
| Chemotherapy Alone | Bevacizumab Plus Chemotherapy | Chemotherapy Alone | Bevacizumab Plus Chemotherapy | |
| Week 8/9 | ||||
| Total No. of Patients | 78 | 33 | 78 | 37 |
| Progressive disease | 58 | 19 | 56 | 18 |
| Death | 5 | 4 | 5 | 4 |
| Treatment switch | 1 | 0 | 1 | 0 |
| Other reasons | 14 | 10 | 16 | 15 |
| Week 16/18 | ||||
| Total No. of Patients | 119 | 69 | 116 | 70 |
| Progressive disease | 99 | 43 | 96 | 44 |
| Death | 6 | 6 | 6 | 6 |
| Switch | 3 | 0 | 3 | 0 |
| Other reasons | 11 | 20 | 11 | 20 |
Abbreviations: EORTC QLQ-OV28, Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Ovarian Cancer Module; FOSI, Functional Assessment of Cancer Therapy–Ovarian Cancer Symptom Index.
Table A3.
Mixed-Model Repeated-Measures Analysis of Change From Baseline in EORTC QLQ-C30 Global Health Status/QoL by Treatment Arm
| Time Point | Chemotherapy Alone (n = 182) |
Bevacizumab Plus Chemotherapy (n = 179) |
Difference |
|||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | LS Means Estimate | 95% CI | No. of Patients | LS Means Estimate | 95% CI | LS Means Estimate | 95% CI | |
| Week 8/9 | 87 | 0.9 | −3.03 to 4.89 | 128 | 0.5 | −2.81 to 3.77 | −0.4 | −5.62 to 4.73 |
| Week 16/18 | 44 | −0.8 | −5.81 to 4.20 | 91 | 1.2 | −2.50 to 4.82 | 2.0 | −4.25 to 8.19 |
| Week 24 | 26 | −4.0 | −10.06 to 2.09 | 53 | −0.3 | −4.61 to 4.05 | 3.7 | −3.77 to 11.18 |
| Week 30 | 10 | −4.6 | −13.21 to 3.92 | 37 | −1.2 | −5.89 to 3.42 | 3.4 | −6.33 to 13.16 |
| Profile | 95 | −2.1 | −6.32 to 2.07 | 133 | 0.0 | −3.07 to 3.14 | 2.2 | −3.08 to 7.40 |
Abbreviations: EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire, Cancer Module; LS, least-squares; QoL, quality of life.
Fig A1.
Cumulative distribution function plot of change from baseline in abdominal/GI symptoms (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Ovarian Cancer Module) at week 8/9 (intent-to-treat population). BEV, bevacizumab; CT, chemotherapy.
Footnotes
See accompanying editorial on page 1287 and article on page 1302; listen to the podcast by Dr Iasonos at www.jco.org/podcasts
Supported by F. Hoffmann-La Roche, Basel, Switzerland (AURELIA trial).
Presented in part at the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013, and at the 20th Annual Meeting of the International Society for Quality of Life Research, Miami, FL, October 9-12, 2013.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00976911.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Ulrich Freudensprung, F. Hoffman-La Roche (C); Vesna Sneller, F. Hoffmann-La Roche (C); Gill Hales, F. Hoffmann-La Roche (C) Consultant or Advisory Role: Martin R. Stockler, Roche (C); Felix Hilpert, Roche (C); Michael Friedlander, Roche (C); Chee Khoon Lee, Roche (U); Florence Joly, Roche (C); Mansoor Raza Mirza, Roche (C) Stock Ownership: Gill Hales, F. Hoffmann-La Roche Honoraria: Martin R. Stockler, Roche; Felix Hilpert, Roche; Florence Joly, Roche; José Angel Arranz, Roche; Mansoor Raza Mirza, Roche; Eric Pujade-Lauraine, Roche Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: Michael Friedlander, Roche
AUTHOR CONTRIBUTIONS
Conception and design: Martin R. Stockler, Felix Hilpert, Chee Khoon Lee, Gill Hales, Eric Pujade-Lauraine
Provision of study materials or patients: Felix Hilpert, Florence Joly, Eric Pujade-Lauraine
Collection and assembly of data: Felix Hilpert, Nikolaus de Gregorio, José Angel Arranz, Mansoor Raza Mirza, Eric Pujade-Lauraine
Data analysis and interpretation: Martin R. Stockler, Felix Hilpert, Michael Friedlander, Madeleine T. King, Lari Wenzel, Florence Joly, Roberto Sorio, Ulrich Freudensprung, Vesna Sneller, Eric Pujade-Lauraine
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Naumann RW, Coleman RL. Management strategies for recurrent platinum-resistant ovarian cancer. Drugs. 2011;71:1397–1412. doi: 10.2165/11591720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Friedlander M, Butow P, Stockler M, et al. Symptom control in patients with recurrent ovarian cancer: Measuring the benefit of palliative chemotherapy in women with platinum refractory/resistant ovarian cancer. Int J Gynecol Cancer. 2009;19(suppl 2):S44–S48. doi: 10.1111/IGC.0b013e3181bf7fb8. [DOI] [PubMed] [Google Scholar]
- 3.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 4.Friedlander M, Trimble E, Tinker A, et al. Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer. 2011;21:771–775. doi: 10.1097/IGC.0b013e31821bb8aa. [DOI] [PubMed] [Google Scholar]
- 5.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 6.Greimel E, Bottomley A, Cull A, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-OV28) in assessing the quality of life of patients with ovarian cancer. Eur J Cancer. 2003;39:1402–1408. doi: 10.1016/s0959-8049(03)00307-1. [DOI] [PubMed] [Google Scholar]
- 7.Beaumont J, Yount S, Lalla D, et al. Validation of the Functional Assessment of Cancer Therapy-Ovarian (FACT-O) Symptom Index (FOSI) in a phase II clinical trial of pertuzumab in patients with advanced ovarian cancer. J Clin Oncol. 2007;25(suppl):663s. abstr 16021. [Google Scholar]
- 8.Luckett T, King M, Butow P, et al. Assessing health-related quality of life in gynecologic oncology: A systematic review of questionnaires and their ability to detect clinically important differences and change. Int J Gynecol Cancer. 2010;20:664–684. doi: 10.1111/IGC.0b013e3181dad379. [DOI] [PubMed] [Google Scholar]
- 9.Cella D, Paul D, Yount S, et al. What are the most important symptom targets when treating advanced cancer? A survey of providers in the National Comprehensive Cancer Network (NCCN) Cancer Invest. 2003;21:526–535. doi: 10.1081/cnv-120022366. [DOI] [PubMed] [Google Scholar]
- 10.Osoba D, Bezjak A, Brundage M, et al. Analysis and interpretation of health-related quality-of-life data from clinical trials: Basic approach of The National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer. 2005;41:280–287. doi: 10.1016/j.ejca.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Brundage M, Osoba D, Bezjak A, et al. Lessons learned in the assessment of health-related quality of life: Selected examples from the NationalCancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:5078–5081. doi: 10.1200/JCO.2007.11.4645. [DOI] [PubMed] [Google Scholar]
- 12.Sloan JA, Dueck AC, Erickson PA, et al. Analysis and interpretation of results based on patient-reported outcomes. Value Health. 2007;10(suppl 2):S106–S115. doi: 10.1111/j.1524-4733.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui O. MMRM versus MI in dealing with missing data: A comparison based on 25 NDA data sets. J Biopharm Stat. 2011;21:423–436. doi: 10.1080/10543401003777995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.