Abstract
Treatment of older adults with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) is challenging because of disease morbidity and associated treatments. Both diseases represent a genetically heterogeneous group of disorders primarily affecting older adults, with treatment strategies ranging from supportive care to hematopoietic stem-cell transplantation. Although selected older adults can benefit from intensive therapies, as a group they experience increased treatment-related morbidity, are more likely to relapse, and have decreased survival. Age-related outcome disparities are attributed to both tumor and patient characteristics, requiring an individualized approach to treatment decision making beyond consideration of chronologic age alone. Selection of therapy for any individual requires consideration of both disease-specific risk factors and estimates of treatment tolerance and life expectancy derived from evaluation of functional status and comorbidity. Although treatment options for older adults are expanding, clinical trials accounting for the heterogeneity of tumor biology and aging are needed to define standard-of-care treatments for both disease groups. In addition, trials should include outcomes addressing quality of life, maintenance of independence, and use of health care services to assist in patient-centered decision making. This review will highlight available evidence in treatment of older adults with AML or MDS and unanswered clinical questions for older adults with these diseases.
ACUTE MYELOID LEUKEMIA
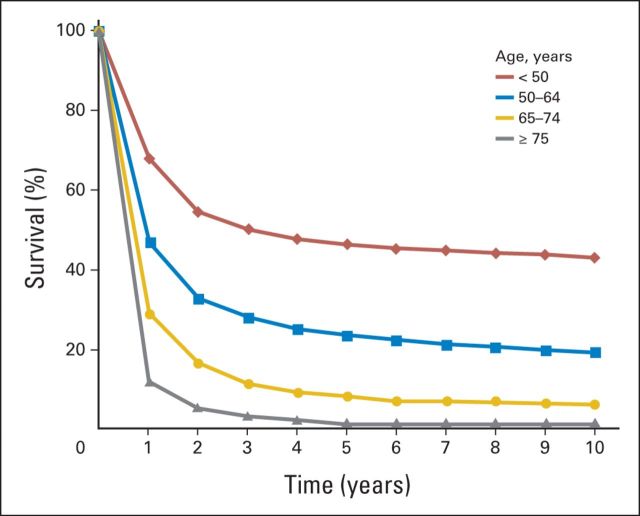
Median age at diagnosis of acute myeloid leukemia (AML) ranges between 68 and 72 years; approximately one third of newly diagnosed patients are age ≥ 75 years.1 There is no consensus regarding optimal therapy for older adults (often defined as those age ≥ 60 years).2,3 Survival is age dependent, with lower rates for older adults (Fig 1).1 Clinical trial and observational data show that for older adults, chemotherapy can provide a survival advantage over supportive care, even among selected patients age > 80 years.4–7 However, concerns regarding efficacy and toxicity of therapy have resulted in < 40% of older adults receiving chemotherapy for AML in the United States.5 Survival has improved over time, although the magnitude of improvement declines with age.1,5,8,9 Age is a surrogate measure for both changes in tumor biology (conferring treatment resistance) and patient characteristics (decreasing treatment tolerance).10 Understanding which patients are likely to benefit from aggressive therapies versus low-intensity therapies or supportive care is critical. Individualized decision making based on evolving stratification of tumor and patient characteristics, along with frank discussions with patients, can help inform the tailoring of treatment and supportive care.
Fig 1.
Relative survival by time and age for acute myeloid leukemia based on SEER data.
Tumor Biology
AML is a different disease in older patients. One reason is the aging of the hematopoietic stem cell (HSC), caused by DNA damage, telomere shortening, and oxidative stress.11–13 Recipient age has had a dramatic influence on HSC homing and seeding efficiency in murine experiments. Gene expression profiling has revealed that HSC aging is accompanied by the systemic downregulation of genes mediating lymphoid specification and function and upregulation of genes involved in specifying myeloid fate and function.12,13 A study in 273 older patients with AML demonstrated that leukemic blasts were more likely to be CD34/CD33 positive or CD34/CD33 negative, correlating with poor overall survival (OS).14
Cytogenetic abnormalities are the most important prognostic factor in AML. Older patients with AML have more poor-risk karyotypes (eg, −7, 7q−, −5, 5q−; abnormalities of 11q, 17p, and Inv3; and complex karyotypes involving ≥ three chromosomes) and fewer good-risk karyotypes [eg, inv(16), t(16;16), t(8;21), or t(15;17)].15,16 In an analysis of 1,065 older adults treated in clinical trials, the proportions with favorable, intermediate, and adverse cytogenetics were 7.3%, 79.1%, and 13.6%, associated with 5-year OS rates of 34%, 13%, and 2%, respectively.16 Molecular mutations and gene deregulation also play a role in prognosis. In a comparison of 425 younger and older patients, older patients had a higher probability of RAS, Src, and tumor necrosis factor pathway activation, which may have contributed to their worse survival.17 In addition, mutations in FLT3-ITD, NPM1, and CEPBA in patients with normal karyotypes affect prognosis. In 99 older FLT3-negative patients, presence of the NPM1 mutation was associated with a higher complete remission (CR) rate (NPM1 negative, 40.5% v NPM1 positive, 80.0%; P = .03) but not with disease-free survival (DFS) or OS. Meanwhile, presence of the FLT3 mutation was associated with worse OS, regardless of NPM1 status (FLT3 positive, 210 v FLT3 negative, 634 days; P = .03).18
There are several reasons for poor response rates to chemotherapy among older patients. Leukemic blasts from older patients are less likely to undergo apoptosis after treatment19 and have higher expression of the MDR1 gene.20 MDR1 encodes a membrane transporter protein responsible for drug efflux and resistance and is implicated in apoptosis inhibition.21 Finally, the bone marrow microenvironment is a dynamic network of growth factors, cytokines, chemokines, and stromal cells that affects AML and patients alike.22 Thus, the biology of AML in older patients is complex and leads to worse outcomes. Use of evolving risk stratification schema based on genetic and epigenetic data in clinical trials may provide an important advance in individualized treatment.23,24
Treatment Trials in Older Patients
Most clinical trials in AML have enrolled patients age 60 to 80 years with adequate performance status (PS; Eastern Cooperative Oncology Group [ECOG] PS 0 to 2). Median survival in clinical trials has historically been < 1 year, with improvements seen in more recent trials.10 In general, older adults are less likely to achieve CR and remain relapse free, and their 30-day mortality rates range from 10% to 30%.10,25 In one analysis of trial data (N = 968) using intensive induction, CR rates were 64%, 46%, 39%, and 33% in patients age < 56, 56 to 65, 66 to 75, and ≥ 75 years, respectively. Among those achieving remission, DFS was 21.6, 7.4, 8.3, and 8.9 months, respectively; OS for all participants was 18.8, 9.0, 6.9, and 3.5 months, respectively.10
Induction
Standard induction therapy for nonacute promyelocytic AML typically includes cytarabine and an anthracycline administered for 7 and 3 days, respectively (7 + 3). This approach improved survival compared with supportive care (median, 5 v 3 months) for adults age ≥ 65 years with no increased time spent hospitalized.7 Since this landmark trial was published, many subsequent trials have shown only incremental improvements in outcomes. Using different induction regimens to improve the balance between benefit and toxicity has not consistently improved efficacy, safety, health care services use, or quality of life (QOL). Strategies pursued have included dose attenuation,26 anthracycline substitution,27–29 addition of sorafenib,30 growth factors,28,31 and modulation of multidrug resistance32 (Table 1).
Table 1.
Selected Randomized Trials of Induction Chemotherapy for Older Patients With AML
| Chemotherapy Regimen* | Year Published | Age Range (years) | No. of Patients | CR (%) | OS |
Induction Death Rate (%) | Comments | |
|---|---|---|---|---|---|---|---|---|
| Median (months) | P | |||||||
| Intensive v supportive care | ||||||||
| Löwenberg et al7 | 1989 | 65-85 | < .05 | No difference in days hospitalized | ||||
| Cytarabine, daunorubicin, and vincristine | 31 | 58 | 5.3 | 9.7 | ||||
| Supportive care | 29 | 0 | 2.8 | NA | ||||
| Type of anthracycline/dose intensification | ||||||||
| Löwenberg et al27 | 1998 | 61-88 | .23 | |||||
| Cytarabine plus daunomycin | 242 | 38 | 9.0 | 6.0 | ||||
| Cytarabine plus mitoxantrone | 247 | 47 | 9.7 | 6.0 | ||||
| Pautas et al29 | 2010 | 50-70 | No difference | .16 | Median OS of 17 months for entire study cohort | |||
| Cytarabine plus daunorubicin 80 mg/m2 | 156 | 70 | 8 | |||||
| Cytarabine plus idarubacin 12 mg/m2 × 3 days | 155 | 83 | 3 | |||||
| Cytarabine plus idarubacin 12 mg/m2 × 4 days | 157 | 78 | 6 | |||||
| Löwenberg et al33 | 2009 | 60-83 | No difference | .16 | OS benefits suggested in patients age 60-65 years | |||
| Cytarabine plus daunorubicin 45 mg/m2 | 411 | 54 | 11 | |||||
| Cytarabine plus daunorubicin 90 mg/m2 | 402 | 64 | 12 | |||||
| Dose-attenuated induction | ||||||||
| Tilly et al26 | 1990 | 65-83 | .12 | |||||
| Rubidazone plus cytarabine | 46 | 52 | 12.8 | 31 | ||||
| Low-dose cytarabine | 41 | 32 | 8.8 | 10 | ||||
| Growth factor support | ||||||||
| Stone et al31 | 1995 | 60-80† | .10 | |||||
| Cytarabine plus daunorubicin | 195 | 54 | 9.4 | 16 | ||||
| Cytarabine, daunorubicin, and GM-CSF | 193 | 51 | 9.4 | 20 | ||||
| MDR1 modulation | ||||||||
| Baer et al32 | 2002 | 60-84 | No difference | .48 | ||||
| Cytarabine, daunorubicin, and etoposide | 61 | 46 | 20 | |||||
| Cytarabine, daunorubicin, etoposide, and PSC-833 | 59 | 39 | 44 | |||||
| Addition of sorafenib | ||||||||
| Serve et al30 | 2013 | 61-80 | No difference | .88 | FLT3-ITD positive, 14% | |||
| Cytarabine plus daunorubicin 60 mg/m2 | 97 | 60 | 7 | |||||
| Cytarabine, daunorubicin 60 mg/m2, and sorafenib | 104 | 48 | 17 | |||||
| Addition of gemtuzumab ozogamicin | ||||||||
| Castaigne et al34 | 2012 | 50-70 | < .05 | |||||
| Cytarabine plus daunorubicin | 139 | 75‡ | 11 | 4 | ||||
| Cytarabine, daunorubicin, and gemtuzumab | 139 | 81‡ | 28 | 6 | ||||
| Burnett et al35 | 2012 | 51-84 | .05 | Improved 3-year survival: 25% v 20% | ||||
| Daunorubicin, cytarabine, or clofarabine | 556 | 58 | Improved | 9 | ||||
| Daunorubicin, cytarabine, or clofarabine plus gemtuzumab | 559 | 65 | 8 | |||||
| Lower-intensity therapy | ||||||||
| Kantarjian et al36 | 2012 | 64-91 | .2 | Poor- or intermediate-risk cytogenetics only; ECOG PS 0-2 with minimal comorbid conditions | ||||
| Supportive care or low-dose cytarabine | 243 | 8 | 5 | 8 | ||||
| Decitabine | 242 | 18 | 8 | 9 | ||||
| Burnett et al37 | 2007 | 51-90 | < .05 | No specific fitness criteria except comorbidity if age < 70 years | ||||
| Low-dose cytarabine ± ATRA | 103 | 18 | Improved | 26 | ||||
| Hydroxyurea ± ATRA | 99 | 1 | 26 | |||||
| Burnett et al38 | 2013 | 51-90 | No difference | .7 | ||||
| Clofarabine | 200 | 22 | 18 | |||||
| Low-dose cytarabine | 206 | 12 | 13 | |||||
Abbreviations: AML, acute myelogenous leukemia; ATRA, all-trans-retinoic acid; CR, complete remission; ECOG, Eastern Cooperative Oncology Group; GM-CSF, granulocyte macrophage colony-stimulating growth factor; NA, not applicable; OS, overall survival; PS, performance status.
One limitation in translating clinical trial data into best practice is lack of consistency in patient populations recruited and in drug doses used, making comparisons of results between trials challenging. To date, there are no older patient–specific dosing recommendations for many drugs based on physiologic changes of aging beyond adjustments for creatinine clearance when appropriate, cardiac function (ie, anthracyclines), and unacceptable toxicity risk (ie, high-dose cytarabine).
Age ≥ 80 years, 4%.
Rates represent CR with incomplete platelet count recovery.
Improved response rates and survival have been reported in some recent trials. For example, 90 mg/m2 of daunorubicin improved CR rates compared with 45 mg/m2 (64% v 54%) for older adults receiving 7 + 3,33 with no significant increased toxicity. Although OS did not improve in the entire cohort, a subset of patients age 60 to 65 years seemed to benefit. No randomized data are available to compare 90- with often-used 60-mg/m2 dosing. Two studies showed improvements in survival without significant toxicity by adding low-dose gemtuzumab ozogamicin to intensive induction for patients age > 50 years.34,35 At present, gemtuzumab is currently unavailable in the United States.
Lower-intensity regimens have also been tested.36–40 Off-label use of DNA hypomethylating agents (eg, azacitidine and decitabine) has increased in recent years.5 A randomized trial comparing decitabine with treatment choice (supportive care or low-dose cytarabine) for adults age ≥ 65 years with intermediate or poor risk cytogenetics found improved CR rates (18% v 8%) favoring decitabine, with nonsignificant improvement in survival.36 In subset analyses of patients with low–blast count (20% to 30%) AML included in a myelodysplastic syndromes (MDS) trial, azacitidine improved survival compared with conventional care (low-dose cytarabine, best supportive care, or 7 + 3).40 Low-dose cytarabine improved survival among patients not fit for intensive therapy compared with supportive care alone, although fitness was not defined for patients age > 70 years.37 The role of lower-intensity regimens is under active investigation; to date, none have been shown to be superior to intensive induction in randomized trials.
Postremission Therapy
Optimal strategies for postremission (consolidation) therapy in older patients with AML are unclear. The average adult harbors an estimated 1 × 109 leukemia cells at the time of remission. A Cancer and Leukemia Group B trial that randomly assigned patients in remission to maintenance or observation was stopped early after 100% of the observation arm relapsed in a median of 4.1 months.41 In younger adults, high-dose cytarabine consolidation is superior to standard dose; however, this benefit is not seen in patients age > 60 years, as a result of increased toxicity.42 Intermediate doses of cytarabine (1 to 1.5 gm/m2) can be safely administered to older patients and have seemed superior to standard doses in retrospective studies.43 There is no evidence defining optimal duration or intensity of consolidation, although a clear association between dose-intensity and increased toxicity has been seen.42,44 Allogeneic HSC transplantation (HSCT) remains a standard approach to improve long-term survival for younger patients with poor-risk AML. Advances in supportive care and use of reduced-intensity conditioning (RIC) regimens have resulted in a trend toward increased use of HSCT in adults age 60 to 70 years.45 An analysis of > 1,000 patients age 40 to 79 years (11% age ≥ 65 years) who received RIC for AML consolidation or MDS therapy showed no association between age and nonrelapse mortality, relapse, DFS, or OS.46 Although this therapy may be feasible for selected older adults with good PS and minimal comorbidity, it is unclear if it is superior to conventional approaches for survival and QOL.47
A major concern in selecting postremission treatments for older adults is the higher likelihood that patients will no longer be candidates for effective treatments postinduction because of declines in functional status or acquired comorbidities. In trials, up to 20% of older adults who achieve remission do not receive any consolidation therapy.44 Limitations to delivery of postremission therapy may contribute to age-related outcome disparity.
Individualizing Patient Assessment
Summary of aggregate clinical trial data does not adequately determine which older adults are likely to benefit from specific therapies, given the complexity of tumor and patient characteristics underlying treatment responsiveness and tolerance. Individualized decision making is critical. Prognostic models have been developed from clinical trial data to predict outcomes for older adults (Table 2).3,48–50 Using algorithms derived from these risk stratification models, estimates of early mortality (16% to 71%3), CR (12% to 91%48), and 3-year survival (3% to 40%49) range widely among older adults treated intensively. Each algorithm provides a useful foundation for improving risk stratification at the time of treatment. However, each model relies on chronologic age as a surrogate for measureable patient-specific factors that vary among individuals of similar age (ie, comorbidity, physical function, cognition, and psychological state). Systematic measurement of patient-specific factors can help discriminate among fit, vulnerable, and frail patients for a given treatment. Although randomized data for comprehensive patient-assessment strategies are lacking, there is supportive evidence available.
Table 2.
Predictors of Outcome for Older Patients Receiving Induction Chemotherapy for AML
| Study | No. of Patients | Treatment | Tumor Characteristics | Clinical Variables | Patient Characteristics | Outcome |
|---|---|---|---|---|---|---|
| Analyses from clinical trials | ||||||
| Kantarjian et al3 | 446 | Intensive | Complex karyotype | Creatinine > 1.3 mg/dL | Age > 80 years; ECOG PS > 1 | 8-week mortality |
| Krug et al48 | 1,406 | Intensive | Secondary AML/or prior hematologic disease; molecular/cytogenetic risk | Body temperature, hemoglobin, platelets, LDH, fibrinogen | Age | 60-day mortality; CR |
| Röllig et al49 | 909 | Intensive | Karyotype; NPM1-mutated CD34 expression > 10% | WBC > 20/μL; LDH > 700 U/L | Age > 65 years | Survival |
| Wheatley et al50 | 2,208 | Intensive | Cytogenetic risk group; secondary AML | WBC | Age, ECOG PS | 1-year survival |
| Geriatric assessment | ||||||
| Deschler et al51 | 107 | Nonintensive | Bone marrow blast percentage; cytogenetic risk group | Impaired ADLs; KPS < 80; high fatigue score, HCT-CI ≥ 3 | Survival | |
| Klepin et al52 | 74 | Intensive | Cytogenetic risk group; prior MDS | Hemoglobin | Cognitive impairment (3MS < 77); impaired physical performance (SPPB < 9) | Survival |
| Sherman et al53 | 101 | Mixed | Adverse cytogenetics; secondary AML | HCT-CI > 1; difficulty with strenuous activity; pain (more often v less); ECOG PS > 1 | Survival |
Abbreviations: 3MS, modified Mini–Mental State Exam; ADL, activity of daily living; AML, acute myeloid leukemia; CR, complete remission; ECOG, Eastern Cooperative Group; HCT-CI, hematopoietic cell transplantation comorbidity index; KPS, Karnofsky performance status; LDH, lactate dehydrogenase; MDS, myelodysplastic syndromes; PS, performance status; SPPB, Short Physical Performance Battery.
Comorbidity is common among patients with AML. In studies of older adults, comorbidity burden (Charlson comorbidity index > 1 and hematopoietic cell transplantation comorbidity index [HCT-CI] > 2) is associated with lower remission rates, increased early mortality, and decreased survival.51,54–58 For example, among 177 patients age ≥ 60 years who received induction, HCT-CI score was 0 (no major comorbidity) in 22%, 1 to 2 in 30%, and ≥ 3 in 48%, corresponding with early death rates (3%, 11%, and 29%) and OS (45, 31, and 19 weeks, respectively).51 Current evidence supports pretreatment comorbidity assessment using the Charlson comorbidity index or HCT-CI. The prognostic implications of individual comorbid conditions are not well studied.
It is clear that functional status also influences treatment tolerance. The relationship between ECOG PS at diagnosis, age, and 30-day mortality during intensive induction is dramatic. Trial data show similar 30-day mortality (11% to 15%) for patients age 56 to 65, 66 to 75, and > 75 with ECOG PS 0, contrasted with rates of 29%, 47%, and 82%, respectively, for baseline ECOG PS 3.10 Fit older adults, even those age > 75 years, may tolerate induction chemotherapy similar to those in middle age, but the negative prognostic implications of poor PS increase with age. Although ECOG scores are useful in identifying frail patients (ECOG PS > 2), physiologic reserve capacity varies widely among older adults with ECOG PS 0 to 2 because of the subjectivity of the scale. Further refinement is needed to identify vulnerable adults. In fact, studies have shown that assessment of self-reported activities of daily living (ADLs) and objectively measured physical performance (testing composed of walking speed, chair stands, and balance) are predictive of survival after accounting for PS.51,52,59
Pretreatment assessment of older adults needs to take into account the complexity of variables that may differ from patient to patient. One such method is geriatric assessment (GA). Pretreatment GA is feasible51,60 and suggests that chronologic age may not be a robust predictor of outcome after accounting for function, comorbidity, and symptoms53 (Table 2). In a prospective study of adults age > 60 years treated intensively, pretreatment GA detected significant impairments even among those with ECOG PS 0 to 1: cognitive impairment, 24%; depression, 26%; distress, 50%; ADL impairment, 34%; impaired physical performance, 31%; and comorbidity, 40%.60 Importantly, most patients in the study were impaired in one (92.6%) or more (63%) measured characteristics. The additive effects of multiple impairments may be more important than individual conditions, and the implications likely differ by treatment intensity. GA has identified impaired cognition, impaired physical performance, ADL impairment, and symptoms (eg, fatigue and pain) as independent predictors of worse survival. The utility of GA is currently under investigation in cooperative group treatment trials. Ultimately, understanding specific patient vulnerabilities may help to predict tolerance and response to standard therapies, inform adaptive clinical trial design for specific patient subgroups, and identify targets for intervention to improve treatment tolerance such as exercise for physical impairment.61,62
Proposed Approach to Treatment of Older Adults
Treatment recommendations for older adults with AML need to be individualized based on tumor biology and patient characteristics. Although validation is needed, available data can begin to differentiate fit, vulnerable, and frail patients when considering intensive therapy (Table 3). Frail older adults, particularly those age > 75 years, are at high risk for toxicity with therapy.10 Best evidence suggests frail older adults with unfavorable tumor biology are unlikely to tolerate or benefit from aggressive treatment. In the absence of clinical trials, supportive care or lower-intensity therapy should be pursued. Fit patients are most likely to benefit from curative therapies, and strong consideration should be given to intensive induction regardless of age. Evidence suggests that for fit patients, older age is associated with QOL and physical function similar to those of younger patients during and after intensive chemotherapy.63 Optimal treatment for the large population of older adults who fall between these two extremes is unclear, and clinical trials are needed. In practice, consideration should be given to enhanced supportive care for vulnerable patients targeting modifiable risk factors (ie, early physical therapy for patients with impaired physical performance). Ultimately, decisions for patients should be determined through patient-centered discussions with frank consideration of best available data interpreted through individualized assessment and patients' values and goals of care.
Table 3.
Proposed Risk Stratification and Treatment Considerations for AML Induction Based on Patient Characteristics
| Patient Risk Category | Characteristic | Treatment Considerations* |
||
|---|---|---|---|---|
| General | Favorable Tumor Biology† | Intermediate or Unfavorable Tumor Biology‡ | ||
| Frail | ECOG PS ≥ 3; major comorbidity (HCT-CI > 2); impairment in ADLs | High treatment-related mortality (particularly for those age > 75 years); clinical trials targeting frail patients are needed | Consider lower-intensity therapy (HMAs, low-dose cytarabine); patients with poor PS (particularly age 60-75 years) but without end-stage comorbidity may consider intensive treatment if risks and benefits are consistent with goals of care | Consider best supportive care, including palliative care consultation if available, versus lower-intensity therapy (HMAs, low-dose cytarabine) |
| Vulnerable | ECOG PS 0-2; absence of major comorbidity (HCT-CI ≤ 2); impairment in IADLs; impaired physical performance (SPPB < 9); impaired cognition (3MS < 77); high symptom burden (fatigue, pain) | Outcomes for this subgroup are inadequately defined in clinical trials; in nonrandomized studies, this group has been at risk for shorter survival compared with fit patients; clinical trials are needed to validate definitions of vulnerability and test treatment and supportive care strategies to improve outcomes in this group | Consider intensive therapy | Consider intensive therapy if risks and benefits are consistent with goals of care versus lower-intensity therapies (HMAs, low-dose cytarabine); consider enhanced supportive care targeting vulnerabilities (eg, early physical therapy for impaired mobility) |
| Fit | ECOG PS 0-1; HCT-CI < 1; absence of risk factors for frail and vulnerable patients | Best evidence suggests fit older adults derive benefit from aggressive therapy; future clinical trials should compare investigational therapies with standard intensive treatment in fit older adults | Intensive therapy should be offered | Consider intensive treatment with possible RIC allogeneic HSCT if risks and benefits are consistent with goals of care versus lower-intensity therapies (HMAs, low-dose cytarabine) |
Abbreviations: 3MS, modified Mini–Mental State Exam; ADL, activity of daily living; AML, acute myeloid leukemia; ECOG, Eastern Cooperative Oncology Group; HCT-CI, hematopoietic cell transplantation comorbidity index; HMA, hypomethylating agent; HSCT, hematopoietic stem-cell transplantation; IADL, instrumental activity of daily living; PS, performance status; RIC, reduced-intensity conditioning; SPPB, Short Physical Performance Battery.
Clinical trials preferred.
Favorable tumor biology: inv(16), t(16;16), t(8;21), or t(15;17).
Intermediate-risk tumor biology: normal cytogenetics, +8 alone, t(9;11), or other nondefined. Unfavorable: complex (≥ three clonal abnormalities), −5, 5q−, −7, 7q−, or abnormalities of 11q, inv(3), t(3;3), or t(6;9). In normal cytogenetic category, NPM1 mutation in absence of FLT3-ITD or isolated biallelic CEBPA mutation confers better risk versus presence of FLT3-ITD, which confers worse risk.
Future Directions and Unresolved Issues
Although there are more questions than answers when considering best practices for older adults with AML, significant advances are being made in understanding the heterogeneity of both tumor biology and patient characteristics that influence outcomes. Major unanswered questions include: Is intensive therapy better than nonintensive therapy for fit older adults? Can therapy be directed by tumor biology and patient characteristics? What consolidation strategies maximize disease control and QOL? Can trials be designed for vulnerable or frail patients? Answers to these questions will require creative trial design accounting for the following: the role of therapies targeting biologically defined subsets of disease and patients; the interaction between tumor biology and the physiologic changes of aging; and novel outcomes capturing QOL, functional independence, patient-reported outcomes, and health care services use.
MDS
MDS are a heterogeneous group of disorders characterized by ineffective hematopoiesis and cytopenias. MDS can be indolent or progress to bone marrow failure or AML. With an estimated 3-year survival rate of 45%,64 MDS are associated with significant morbidity, impaired QOL, and high health care services use. Of the estimated 15,000 to 20,000 US patients diagnosed annually, > 80% are age > 70 years.1 Incidence of MDS will rise as the population ages, making these disorders an important public health concern. Treatment strategies range from supportive care to stem-cell transplantation, requiring a careful analysis of risks to lifespan and QOL versus the risk-benefit ratio of intervention.
Disease Risk Stratification
Several well-validated prognostic schemes are used to classify patients into higher- and lower-risk groups. The most common—the International Prognostic Scoring System (IPSS)—was derived from 816 patients, 75% of whom were age > 60 years. The IPSS incorporates blast percentage, number of cytopenias, and cytogenetics; patients are placed into four risk categories: low, intermediate-1, intermediate-2, and high.65 These categories have median survivals ranging from 5.7 years (low-risk group) to 0.4 years (high-risk group). Additional factors provide additive prognostic value to the IPSS, including multilineage dysplasia and severe anemia or transfusion dependency. Several of these variables are incorporated into the WHO prognostic scoring system,66 which divides patients into five risk categories (very low, low, intermediate, high, very high). This system can be used both at diagnosis and after progression. A revised IPSS was developed67 using data from 7,012 patients (median age, 71 years), which differs by further subdividing cytogenetic abnormalities, better quantifying cytopenias, and increasing the weight of higher blast percentages. It divides patients into five risk categories, from very low to very high, with median survivals from 8.8 (very low) to 0.8 years (very high). Age was a prognostic factor for survival but not for progression to AML and had more impact in lower- versus higher-risk groups. This system has been validated, and its prognostic discrimination seems superior to the IPSS and WHO prognostic scoring system.68 Importantly, it retains its prognostic ability among patients treated with disease-modifying agents.68,69
Patient Risk Stratification
Selection of optimal therapy for older patients with MDS depends on both assessment of patients' overall fitness and disease risk stratification. Unfortunately, optimal evidence-based strategies to define fitness for specific MDS therapies are scarce. Factors that affect life expectancy and treatment tolerance (eg, comorbidity, functional status, cognition, and mood) vary significantly among patients of similar age. We know most about comorbid conditions, which occur in > 50% of patients with MDS.70,71 Higher comorbidity burden is associated with shortened survival, independent of age and disease risk stratification.70,71 Specific conditions associated with shortened survival include cardiac disease, hepatic disease, severe pulmonary disease, renal disease, and solid tumors.70
GA may be useful to predict both life expectancy and treatment tolerance. A multisite study investigating the predictive utility of pretreatment GA among older adults treated nonintensively for MDS (n = 51) or AML (n = 69) found that requiring assistance with ADLs, Karnofsky PS < 80%, and high fatigue rating were independently associated with shortened survival after accounting for age and tumor characteristics.51 These characteristics may also increase vulnerability to toxicity. Issues related to physical and cognitive function and social support may be critical when considering therapies of high intensity, high frequency, or long duration.
Therapy
Treatment recommendations for MDS have evolved to target higher-risk MDS and subgroups defined by cytogenetic abnormalities. An integrated approach using disease and patient risk stratifications, as well as patient preference, is essential to optimize a risk-adapted strategy. Current guidelines recommend classifying patients into relatively low-risk (IPSS low or intermediate-1) and higher-risk groups (IPSS intermediate-2 or high). Treatment goals for lower-risk patients include minimizing disease morbidity (maximizing QOL and independence); goals for higher-risk patients include altering the disease course. Further classification based on patient characteristics is necessary to individualize therapy (Tables 4 and 5).
Table 4.
Selected Randomized Treatment Trials for MDS
| Trial | No. of Patients | Disease Characteristics | Patient Characteristics | Positive Outcome | Toxicity |
|---|---|---|---|---|---|
| Silverman et al80 | |||||
| Azacitidine 75 mg/m2 subcutaneously × 7 days every 4 weeks | 99 | IPSS intermediate-1, intermediate-2, or high | Median age, 68 years (range, 31-92); ECOG PS 0-2; creatinine ≤ 1.5 mg/dL | Response rate, 23% v 5%; time to AML or death, 21 v 13 months; AML transformation, 15% v 38%; improved QOL (physical function, symptoms, psychological state) | Grade 3-4 myelosuppression, 43% to 58%; infection, 20% |
| Supportive care | 92 | ||||
| Fenaux et al73 | |||||
| Azacitidine 75 mg/m2 subcutaneously × 7 days every 4 weeks | 179 | IPSS intermediate-2 or high, 87% | Median age, 69 years (range, 38-88); ECOG PS 0-2 | Median OS, 24.5 v 15 months; | Myelosuppression |
| Conventional care (supportive, low-dose cytarabine, intensive chemotherapy) | 179 | ||||
| Kantarjian et al74 | |||||
| Decitabine 15 mg/m2 IV every 8 hours for 3 days every 6 weeks | 89 | IPSS intermediate or high | Median age, 70 years (range, 65-76)*; ECOG PS 0-1 | Response rate, 17% v 0%; improved QOL (global health, fatigue, dyspnea) | Dose reductions or delays, 35%; grade 4 myelosuppression, > 50% |
| Supportive care | 81 | ||||
| Kantarjian et al75 | |||||
| Decitabine 20 mg/m2 IV for 5 days | 64 | FAB MDS or CMML; IPSS intermediate or high | Median age, 65 years; creatinine < 2 mg/dL | CR rate higher with 5-day IV schedule, 39% v 21% v 24% | Myelosuppression-associated hospitalization, 18% |
| Decitabine 20 mg/m2 subcutaneously for 5 days | 14 | ||||
| Decitabine 10 mg/m2 IV for 10 days every 4 weeks | 17 | ||||
| Fenaux et al76 | |||||
| Lenalidomide 10 mg per day on days 1-21 | 69 | MDS with del5q31; IPSS low or intermediate-1; RBC transfusion dependence | Median age, 69 years (range, 38-86); creatinine < 2 mg/dL | RBC transfusion independence ≥ 26 weeks, 56.1% v 42.6% v 5.9%; RBC transfusion independence > 8 weeks associated with decreased risk of death and AML progression | Myelosuppression in first two cycles; DVT in 10-mg group, 5.8% |
| Lenalidomide 5 mg per day on days 1-28 | 69 | ||||
| Placebo on 28-day cycle | 67 |
Abbreviations: AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; CR, complete remission; DVT, deep venous thrombosis; ECOG, Eastern Cooperative Oncology Group; FAB, French-American-British; IPSS, International Prognostic Scoring System; IV, intravenous; MDS, myelodysplastic syndromes; PS, performance status; QOL, quality of life.
Age range for decitabine arm.
Table 5.
Treatment Considerations for Older Patients With MDS Based on Disease and Patient Risk Stratification
| Disease Characteristic* | Goal of Therapy | Patient Characteristics | Treatment Considerations | Comments |
|---|---|---|---|---|
| Very low risk, low risk, or asymptomatic | Improve QOL | Any | Observation | Absence of evidence to support improved QOL or survival with early therapy |
| Very low risk, low risk, intermediate risk, or symptomatic | ||||
| 5q− deletion | Improve QOL | Any | Lenolidomide | RCT evidence for decreased transfusion requirements; dose adjust for renal function; tolerance and benefits of lenolidomide understudied in vulnerable and frail patients |
| Absence of 5q− with erythropoietin level < 500 mU/mL | Improve QOL | Any | Erythropoeitin ± GCSF; consider lenolidomide | Time-limited trial; discontinue if no response in 8 weeks; some data for non-5q disease suggest significant response rate; would consider especially if isolated anemia |
| Good performance status and minimal comorbidity | Consider hypomethylating agents | Absence of RCT data for very low– and low-risk patients; observational data suggest potential benefit | ||
| Intermediate risk, high risk, and very high risk | Delay progression; extend life | Any age, good performance status, absence of major comorbidity | Hypomethylating agents | RCT evidence supports improvements in survival, progression to AML, symptoms, and QOL; strongest evidence for 7-day azacitidine |
| Cure | Age 60-75 years, excellent performance status, absence of major comorbidity | Consider referral for RIC HSCT v hypomethylating agents; comprehensive geriatric assessment may help inform fitness | Observational studies support feasibility and potential survival advantage for fit older patients with high-risk disease; RCT data lacking | |
| Delay progression; extend life | Poor performance status and/or major comorbidity | Consider hypomethylating agents v supportive care | Absence of RCT evidence in frail patients; however, given potential for survival advantage and improved QOL, would discuss azacitidine treatment |
Abbreviations: AML, acute myeloid leukemia; GCSF, granulocyte colony-stimulating factor; HSCT, hematopoietic stem-cell transplantation; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndromes; QOL, quality of life; RCT, randomized controlled trial; RIC, reduced-intensity conditioning.
Revised IPSS.
Supportive Care
Supportive care is essential for all patients. Supportive care includes red-cell and platelet transfusions, antibiotics for infections, growth factors for selected patients, and iron chelation. Patients with symptomatic anemia may benefit from epoetin alfa or longer-acting darbepoetin alfa (response rates approximately 50%).77 Patients most likely to respond have low or intermediate-1 IPSS scores, serum erythropoietin level < 200 mU/mL, transfusion requirement < 2 units of red cells per month, and shorter interval between diagnosis and treatment.78,79 Granulocyte colony-stimulating factor may act synergistically with epoetin.79,80 Growth factors for symptomatic anemia can improve QOL and decrease transfusions without increased risk of AML progression.81,82 Improvement in survival is suggested for low-risk patients in nonrandomized studies.79,80
Over time, most patients become transfusion dependent, increasing the risk of iron overload, which negatively affects survival. Guidelines recommend iron chelation for patients with lower-risk MDS, ongoing transfusion dependence, and anticipated survival > 1 year.81
Hypomethylating Agents
MDS are associated with abnormal methylation of the genome, which worsens with disease progression. Azacitidine and decitabine inhibit DNA methyltransferases and have activity in MDS.72–74 Only azacitidine (administered at 75 mg/m2 subcutaneously for 7 days every 28 days) showed a survival benefit in randomized trials of patients with high-risk MDS.72,73 Treatment of the high-risk group doubled 2-year survival compared with conventional therapy (51% v 26%).73 Treatment with azacitidine also improved fatigue, dyspnea, self-reported physical functioning, and psychological distress, after controlling for the number of transfusions received.83
Azacitidine showed a similar survival advantage in patients age > 75 years.84 Thus, age is not a predictor in a prognostic scoring system derived from patients with high-risk MDS treated with azacitidine.85 Furthermore, the 24-month survival benefit in a population study of MDS supports the generalizability of benefit outside of clinical trials.86 A registry study comparing differing azacitidine schedules for patients of all risk groups age ≥ 75 years further illustrates benefits (transfusion independence, 40%) and complications (cycles delayed, 29%; hospitalized for infection, 47%).87 Decitabine also decreased transfusion requirements and symptoms, although no definitive survival benefit was shown in a randomized trial.74,75
Challenges for older adults using hypomethylating agents include the 7-day scheduling for azacitidine, considered optimal based on available evidence but challenging logistically. The primary toxicity of these agents is myelosuppression, which often worsens before response occurs. Finally, duration of treatment can be challenging; median duration in clinical trials was > 6 months for all patients and > 12 months for responders.40,73 However, hypomethylating agents can alter the natural history of MDS, and they improve survival and QOL, particularly among patients with good PS and high-risk disease.
Immunomodulatory Agents
In an initial phase II study, lenalidomide used in transfusion-dependent patients at low or intermediate-1 risk with 5q deletion reduced transfusion requirements in 76% of participants, with a median time to response of 4.6 weeks.88 The primary toxicity was myelosuppression, which required dose adjustment in 84% of participants. A subsequent dose-response placebo-controlled randomized trial in older patients (median age, 69 years) confirmed achievement of transfusion independence (rates of 56%, 43%, and 6% for 10-mg, 5-mg, and placebo groups, respectively), documented cytogenetic responses, and suggested that treatment improved QOL.76,89 However, dose reductions (52% to 55%) and interruptions (29% to 46%) were common with both doses. Although a 10-mg starting dose seems optimal, 5 mg is also active and may be more appropriate for many older adults, given the need for dose adjustment for mild impairment in renal function (creatinine clearance < 60 mL/minute). Population data from Medicare claims also show reduced transfusion rates consistent with clinical trial data, supporting generalizability.90 It also has efficacy in patients with low-risk MDS without 5q deletion as well as those with high-risk disease with 5q deletion.91,92 Lenalidomide can be considered in all patients with transfusion-dependent MDS and 5q deletion as well as older patients with transfusion-dependent low-risk MDS without 5q deletion.
HSCT
Currently, the only curative therapy for MDS is allogeneic HSCT. Transplantation-related mortality increases with age and comorbidities. However, with RIC regimens, HSCT is feasible in selected patients age > 60 years.46,93,94 When MDS outcomes were analyzed in patients undergoing RIC HSCT (n = 535; age 40 to 78 years), age was not a significant predictor of outcome.46 The 2-year survival rate for patients age ≥ 65 years (n = 55) was 38%, 57% of whom had high-risk disease. HSCT for selected older patients can result in appreciable survival rates, even for those with high-risk disease. However, most data for older adults in this context refer to patients age < 70 years, with minimal data for those age > 75 years, excluding > half of patients diagnosed with MDS worldwide.
At present, HSCT is reserved for fit patients (good PS and minimal comorbidity) with higher-risk disease. A recent nonrandomized analysis of 514 patients age 60 to 70 years with de novo MDS compared RIC HSCT with nontransplantation strategies and suggested that life expectancy was improved with transplantation in patients at intermediate-2 or high risk according to IPSS (36 v 28 months) but not for those at low or intermediate-1 risk (38 v 77 months).95 These findings highlight the critical importance of balancing risks of disease versus treatment. Referral for transplantation evaluation should be discussed with fit older patients (best evidence for those age 60 to 70 years) with high-risk disease who wish to pursue aggressive, potentially curative therapies. The real-world applicability of transplantation in older adults will depend on refined evidence-based definitions of fitness and collection of outcomes to inform treatment decisions, including QOL, health care services use, and functional independence.
Unresolved Clinical Questions
Despite recent advances in therapy for MDS, many unresolved questions remain concerning optimal treatment of older adults. For example, optimal treatment strategies for patients with impaired functional status and multimorbidity have not been addressed in clinical trials. Trials targeting less fit patients are needed, as are rigorous categorizations of fitness, vulnerability, and frailty for each treatment modality. In the noncurative setting, the optimal duration of therapies to balance disease control, QOL, and functional independence is unclear. The optimal time to begin treatment in lower-risk patients is also unclear. The role of transplantation needs to be further defined; evidence remains confounded by lack of randomized controlled trials, and additional outcomes (eg, functional independence, health care services use, symptoms, and treatment satisfaction) are inconsistently captured to best inform decision making at the time of treatment.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Heidi D. Klepin
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Surveillance, Epidemiology, and End Results program. SEER Cancer Statistics Review 1975-2009. http://seer.cancer.gov/archive/csr/1975_2009_pops09/
- 2.Juliusson G. Most 70- to 79-year-old patients with acute myeloid leukemia do benefit from intensive treatment. Blood. 2011;117:3473–3474. doi: 10.1182/blood-2010-11-321737. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 5.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica. 2012;97:1916–1924. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetzler M, Mrózek K, Kohlschmidt J, et al. Intensive induction is effective in select octogenarian acute myeloid leukemia patients: Prognostic significance of karyotype and selected molecular markers used in the European LeukemiaNet classification. Haematologica. 2014;99:308–313. doi: 10.3324/haematol.2013.092072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Löwenberg B, Zittoun R, Kerkhofs H, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: A randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7:1268–1274. doi: 10.1200/JCO.1989.7.9.1268. [DOI] [PubMed] [Google Scholar]
- 8.Shah A, Andersson TM, Rachet B, et al. Survival and cure of acute myeloid leukaemia in England, 1971-2006: A population-based study. Br J Haematol. 2013;162:509–516. doi: 10.1111/bjh.12425. [DOI] [PubMed] [Google Scholar]
- 9.Thein MS, Ershler WB, Jemal A, et al. Outcome of older patients with acute myeloid leukemia: An analysis of SEER data over 3 decades. Cancer. 2013;119:2720–2727. doi: 10.1002/cncr.28129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamminga LM, de Haan G. Cellular memory and hematopoietic stem cell aging. Stem Cells. 2006;24:1143–1149. doi: 10.1634/stemcells.2005-0345. [DOI] [PubMed] [Google Scholar]
- 12.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plesa C, Chelghoum Y, Plesa A, et al. Prognostic value of immunophenotyping in elderly patients with acute myeloid leukemia: A single-institution experience. Cancer. 2008;112:572–580. doi: 10.1002/cncr.23219. [DOI] [PubMed] [Google Scholar]
- 15.Farag SS, Archer KJ, Mrózek K, et al. Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: Results from Cancer and Leukemia Group B 8461. Blood. 2006;108:63–73. doi: 10.1182/blood-2005-11-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): Analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 17.Rao AV, Valk PJ, Metzeler KH, et al. Age-specific differences in oncogenic pathway dysregulation and anthracycline sensitivity in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:5580–5586. doi: 10.1200/JCO.2009.22.2547. [DOI] [PubMed] [Google Scholar]
- 18.Scholl S, Theuer C, Scheble V, et al. Clinical impact of nucleophosmin mutations and Flt3 internal tandem duplications in patients older than 60 yr with acute myeloid leukaemia. Eur J Haematol. 2008;80:208–215. doi: 10.1111/j.1600-0609.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- 19.Garrido SM, Cooper JJ, Appelbaum FR, et al. Blasts from elderly acute myeloid leukemia patients are characterized by low levels of culture- and drug-induced apoptosis. Leuk Res. 2001;25:23–32. doi: 10.1016/s0145-2126(00)00083-7. [DOI] [PubMed] [Google Scholar]
- 20.Leith CP, Kopecky KJ, Chen IM, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: A Southwest Oncology Group study. Blood. 1999;94:1086–1099. [PubMed] [Google Scholar]
- 21.Smyth MJ, Krasovskis E, Sutton VR, et al. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc Natl Acad Sci U S A. 1998;95:7024–7029. doi: 10.1073/pnas.95.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nipp RD, Rao AV. Performance status in elderly patients with acute myeloid leukemia: Exploring gene expression signatures of cytokines and chemokines. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/glt039. [epub ahead of print on June 19, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcucci G, Yan P, Maharry K, et al. Epigenetics meets genetics in acute myeloid leukemia: Clinical impact of a novel seven-gene score. J Clin Oncol. 2014;32:548–556. doi: 10.1200/JCO.2013.50.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mrózek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30:4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 26.Tilly H, Castaigne S, Bordessoule D, et al. Low-dose cytarabine versus intensive chemotherapy in the treatment of acute nonlymphocytic leukemia in the elderly. J Clin Oncol. 1990;8:272–279. doi: 10.1200/JCO.1990.8.2.272. [DOI] [PubMed] [Google Scholar]
- 27.Löwenberg B, Suciu S, Archimbaud E, et al. Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy: The value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly—Final report: European Organisation for the Research and Treatment of Cancer and the Dutch-Belgian Hemato-Oncology Cooperative Hovon Group. J Clin Oncol. 1998;16:872–881. doi: 10.1200/JCO.1998.16.3.872. [DOI] [PubMed] [Google Scholar]
- 28.Goldstone AH, Burnett AK, Wheatley K, et al. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: The results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 29.Pautas C, Merabet F, Thomas X, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: Results of the ALFA-9801 study. J Clin Oncol. 2010;28:808–814. doi: 10.1200/JCO.2009.23.2652. [DOI] [PubMed] [Google Scholar]
- 30.Serve H, Krug U, Wagner R, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: Results from a randomized, placebo-controlled trial. J Clin Oncol. 2013;31:3110–3118. doi: 10.1200/JCO.2012.46.4990. [DOI] [PubMed] [Google Scholar]
- 31.Stone RM, Berg DT, George SL, et al. Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia: Cancer and Leukemia Group B. N Engl J Med. 1995;332:1671–1677. doi: 10.1056/NEJM199506223322503. [DOI] [PubMed] [Google Scholar]
- 32.Baer MR, George SL, Dodge RK, et al. Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: Cancer and Leukemia Group B Study 9720. Blood. 2002;100:1224–1232. [PubMed] [Google Scholar]
- 33.Löwenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 34.Castaigne S, Pautas C, Térre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 35.Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30:3924–3931. doi: 10.1200/JCO.2012.42.2964. [DOI] [PubMed] [Google Scholar]
- 36.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 38.Burnett AK, Russell NH, Hunter AE, et al. Clofarabine doubles the response rate in older patients with acute myeloid leukemia but does not improve survival. Blood. 2013;122:1384–1394. doi: 10.1182/blood-2013-04-496596. [DOI] [PubMed] [Google Scholar]
- 39.Burnett AK, Russell NH, Culligan D, et al. The addition of the farnesyl transferase inhibitor, tipifarnib, to low dose cytarabine does not improve outcome for older patients with AML. Br J Haematol. 2012;158:519–522. doi: 10.1111/j.1365-2141.2012.09165.x. [DOI] [PubMed] [Google Scholar]
- 40.Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 41.Cassileth PA, Harrington DP, Hines JD, et al. Maintenance chemotherapy prolongs remission duration in adult acute nonlymphocytic leukemia. J Clin Oncol. 1988;6:583–587. doi: 10.1200/JCO.1988.6.4.583. [DOI] [PubMed] [Google Scholar]
- 42.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia: Cancer and Leukemia Group B. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 43.Hassanein M, Atenafu EG, Schuh AC, et al. High-dose cytarabine-based consolidation shows superior results for older AML patients with intermediate risk cytogenetics in first complete remission. Leuk Res. 2013;37:556–560. doi: 10.1016/j.leukres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Stone RM, Berg DT, George SL, et al. Postremission therapy in older patients with de novo acute myeloid leukemia: A randomized trial comparing mitoxantrone and intermediate-dose cytarabine with standard-dose cytarabine. Blood. 2001;98:548–553. doi: 10.1182/blood.v98.3.548. [DOI] [PubMed] [Google Scholar]
- 45.Hahn T, McCarthy PL, Jr, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31:2437–2449. doi: 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farag SS, Maharry K, Zhang MJ, et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60-70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant. 2011;17:1796–1803. doi: 10.1016/j.bbmt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krug U, Röllig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: A Web-based application for prediction of outcomes. Lancet. 2010;376:2000–2008. doi: 10.1016/S0140-6736(10)62105-8. [DOI] [PubMed] [Google Scholar]
- 49.Röllig C, Thiede C, Gramatzki M, et al. A novel prognostic model in elderly patients with acute myeloid leukemia: Results of 909 patients entered into the prospective AML96 trial. Blood. 2010;116:971–978. doi: 10.1182/blood-2010-01-267302. [DOI] [PubMed] [Google Scholar]
- 50.Wheatley K, Brookes CL, Howman AJ, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145:598–605. doi: 10.1111/j.1365-2141.2009.07663.x. [DOI] [PubMed] [Google Scholar]
- 51.Deschler B, Ihorst G, Platzbecker U, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98:208–216. doi: 10.3324/haematol.2012.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121:4287–4294. doi: 10.1182/blood-2012-12-471680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherman AE, Motyckova G, Fega KR, et al. Geriatric assessment in older patients with acute myeloid leukemia: A retrospective study of associated treatment and outcomes. Leuk Res. 2013;37:998–1003. doi: 10.1016/j.leukres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Etienne A, Esterni B, Charbonnier A, et al. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109:1376–1383. doi: 10.1002/cncr.22537. [DOI] [PubMed] [Google Scholar]
- 55.Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 56.Djunic I, Virijevic M, Novkovic A, et al. Pretreatment risk factors and importance of comorbidity for overall survival, complete remission, and early death in patients with acute myeloid leukemia. Hematology. 2012;17:53–58. doi: 10.1179/102453312X13221316477651. [DOI] [PubMed] [Google Scholar]
- 57.Savic A, Kvrgic V, Rajic N, et al. The hematopoietic cell transplantation comorbidity index is a predictor of early death and survival in adult acute myeloid leukemia patients. Leuk Res. 2012;36:479–482. doi: 10.1016/j.leukres.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 58.Breccia M, Frustaci AM, Cannella L, et al. Comorbidities and FLT3-ITD abnormalities as independent prognostic indicators of survival in elderly acute myeloid leukaemia patients. Hematol Oncol. 2009;27:148–153. doi: 10.1002/hon.889. [DOI] [PubMed] [Google Scholar]
- 59.Wedding U, Röhrig B, Klippstein A, et al. Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol. 2006;132:665–671. doi: 10.1007/s00432-006-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klepin HD, Geiger AM, Tooze JA, et al. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. J Am Geriatr Soc. 2011;59:1837–1846. doi: 10.1111/j.1532-5415.2011.03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alibhai SM, O'Neill S, Fisher-Schlombs K, et al. A clinical trial of supervised exercise for adult inpatients with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leuk Res. 2012;36:1255–1261. doi: 10.1016/j.leukres.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klepin HD, Danhauer SC, Tooze JA, et al. Exercise for older adult inpatients with acute myelogenous leukemia: A pilot study. J Geriatr Oncol. 2011;2:11–17. doi: 10.1016/j.jgo.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohamedali H, Breunis H, Timilshina N, et al. Older age is associated with similar quality of life and physical function compared to younger age during intensive chemotherapy for acute myeloid leukemia. Leuk Res. 2012;36:1241–1248. doi: 10.1016/j.leukres.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 64.Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 65.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 66.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–3510. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 67.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Voso MT, Fenu S, Latagliata R, et al. Revised International Prognostic Scoring System (IPSS) predicts survival and leukemic evolution of myelodysplastic syndromes significantly better than IPSS and WHO Prognostic Scoring System: Validation by the Gruppo Romano Mielodisplasie Italian Regional Database. J Clin Oncol. 2013;31:2671–2677. doi: 10.1200/JCO.2012.48.0764. [DOI] [PubMed] [Google Scholar]
- 69.Mishra A, Corrales-Yepez M, Ali NA, et al. Validation of the revised International Prognostic Scoring System in treated patients with myelodysplastic syndromes. Am J Hematol. 2013;88:566–570. doi: 10.1002/ajh.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Della Porta MG, Malcovati L, Strupp C, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica. 2011;96:441–449. doi: 10.3324/haematol.2010.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Naqvi K, Garcia-Manero G, Sardesai S, et al. Association of comorbidities with overall survival in myelodysplastic syndrome: Development of a prognostic model. J Clin Oncol. 2011;29:2240–2246. doi: 10.1200/JCO.2010.31.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 73.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 75.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 76.Fenaux P, Giagounidis A, Selleslag D, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with low-/intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–3776. doi: 10.1182/blood-2011-01-330126. [DOI] [PubMed] [Google Scholar]
- 77.Moyo V, Lefebvre P, Duh MS, et al. Erythropoiesis-stimulating agents in the treatment of anemia in myelodysplastic syndromes: A meta-analysis. Ann Hematol. 2008;87:527–536. doi: 10.1007/s00277-008-0450-7. [DOI] [PubMed] [Google Scholar]
- 78.Hellström-Lindberg E, Gulbrandsen N, Lindberg G, et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: Significant effects on quality of life. Br J Haematol. 2003;120:1037–1046. doi: 10.1046/j.1365-2141.2003.04153.x. [DOI] [PubMed] [Google Scholar]
- 79.Park S, Grabar S, Kelaidi C, et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: The GFM experience. Blood. 2008;111:574–582. doi: 10.1182/blood-2007-06-096370. [DOI] [PubMed] [Google Scholar]
- 80.Jädersten M, Malcovati L, Dybedal I, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol. 2008;26:3607–3613. doi: 10.1200/JCO.2007.15.4906. [DOI] [PubMed] [Google Scholar]
- 81.Greenberg PL, Attar E, Bennett JM, et al. Myelodysplastic syndromes: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2013;11:838–874. doi: 10.6004/jnccn.2013.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jädersten M, Montgomery SM, Dybedal I, et al. Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood. 2005;106:803–811. doi: 10.1182/blood-2004-10-3872. [DOI] [PubMed] [Google Scholar]
- 83.Kornblith AB, Herndon JE, 2nd, Silverman LR, et al. Impact of azacytidine on the quality of life of patients with myelodysplastic syndrome treated in a randomized phase III trial: A Cancer and Leukemia Group B study. J Clin Oncol. 2002;20:2441–2452. doi: 10.1200/JCO.2002.04.044. [DOI] [PubMed] [Google Scholar]
- 84.Seymour JF, Fenaux P, Silverman LR, et al. Effects of azacitidine compared with conventional care regimens in elderly (≥ 75 years) patients with higher-risk myelodysplastic syndromes. Crit Rev Oncol Hematol. 2010;76:218–227. doi: 10.1016/j.critrevonc.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Itzykson R, Thépot S, Quesnel B, et al. Long-term outcome of higher-risk MDS patients treated with azacitidine: An update of the GFM compassionate program cohort. Blood. 2012;119:6172–6173. doi: 10.1182/blood-2012-04-422204. [DOI] [PubMed] [Google Scholar]
- 86.Wang R, Gross CP, Frick K, et al. The impact of hypomethylating agents on the cost of care and survival of elderly patients with myelodysplastic syndromes. Leuk Res. 2012;36:1370–1375. doi: 10.1016/j.leukres.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xicoy B, Jiménez MJ, García O, et al. Results of treatment with azacitidine in patients aged ≥ 75 years included in the Spanish Registry of Myelodysplastic Syndromes. Leuk Lymphoma. doi: 10.3109/10428194.2013.834532. [epub ahead of print on September 16, 2013] [DOI] [PubMed] [Google Scholar]
- 88.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 89.Revicki DA, Brandenburg NA, Muus P, et al. Health-related quality of life outcomes of lenalidomide in transfusion-dependent patients with low- or intermediate-1-risk myelodysplastic syndromes with a chromosome 5q deletion: Results from a randomized clinical trial. Leuk Res. 2013;37:259–265. doi: 10.1016/j.leukres.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 90.Zeidan AM, Gore SD, McNally DL, et al. Lenalidomide performance in the real world: Patterns of use and effectiveness in a Medicare population with myelodysplastic syndromes. Cancer. 2013;119:3870–3878. doi: 10.1002/cncr.28298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adès L, Boehrer S, Prebet T, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: Results of a phase 2 study. Blood. 2009;113:3947–3952. doi: 10.1182/blood-2008-08-175778. [DOI] [PubMed] [Google Scholar]
- 92.Sibon D, Cannas G, Baracco F, et al. Lenalidomide in lower-risk myelodysplastic syndromes with karyotypes other than deletion 5q and refractory to erythropoiesis-stimulating agents. Br J Haematol. 2012;156:619–625. doi: 10.1111/j.1365-2141.2011.08979.x. [DOI] [PubMed] [Google Scholar]
- 93.Brunner AM, Kim HT, Coughlin E, et al. Outcomes in patients age 70 or older undergoing allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2013;19:1374–1380. doi: 10.1016/j.bbmt.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 94.Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109:1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 95.Koreth J, Pidala J, Perez WS, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: An international collaborative decision analysis. J Clin Oncol. 2013;31:2662–2670. doi: 10.1200/JCO.2012.46.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]