Case Report
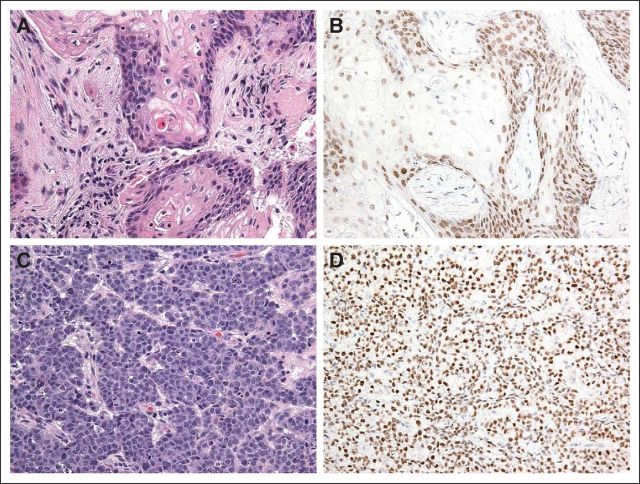
A 13-year-old previously healthy girl presented with a 2-month history of persistent cough and intermittent fever and a 1-month history of left knee pain. She had been treated by her primary care physician with a course of oral and intravenous antibiotics for a right-sided pneumonia with transient improvement in symptoms. She had a history of fracture of the right ankle after mild trauma 4 months before presentation. Plain radiographs of the left knee revealed lytic lesions in the tibia and femur suspicious for osteomyelitis (Fig 1A). She underwent curettage of the left tibial lesion, which demonstrated cords of bland basal cells with extensive squamous differentiation without malignant cytological features such as mitosis or apoptosis (Fig 2A). Pathology diagnosis was adamantinoma, and she was referred to our institution for further treatment.
Fig 1.

Fig 2.
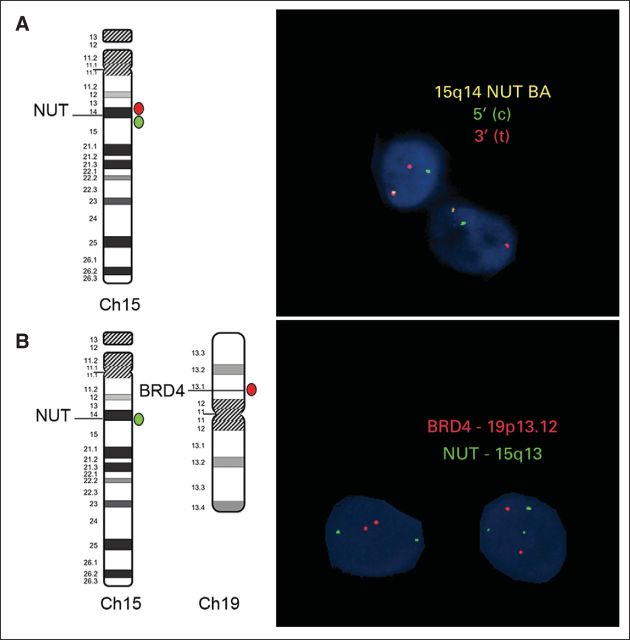
On presentation, the patient had a persistent cough, chest pain, and dyspnea. Air entry to the right lung was decreased. Chest x-ray showed a massive right-sided pleural effusion with a right hilar mass. The chest computed tomography (CT) scan showed a large heterogeneous right hilar mass measuring 8 cm × 6.7 cm with extension to the right mainstem bronchus and right lower lobe lung collapse (Fig 1B). Positron emission tomography (PET) -CT demonstrated avid fluoroudeoxyglucose (FDG) uptake in the region of the right hilum corresponding to the patient's primary lesion. Foci of increased uptake were also noted within several lymph node stations including right supraclavicular, anterior mediastinal, right mammary, and right subphrenic. Multifocal osseous metastatic disease was also noted within the vertebral bodies, right clavicle, humeri, femurs, tibias, and right talus (Fig 3A). She underwent a video-assisted thoracoscopic surgical procedure, and multiple parietal pleural deposits were biopsied. Pathologic examination of pleural deposits showed an undifferentiated malignant tumor composed of cords and nests of small- to medium-sized cells with round to oval hyperchromatic nuclei, inconspicuous nucleoli, and scant eosinophilic cytoplasm. Numerous mitosis and apoptosis were seen (Fig 2C). Immunohistochemistry staining demonstrated that the tumor cells from both left tibial lesion and pleural deposits were positive for cytokeratin AE1/AE3, focally positive for cytokeratin 7, and stained negative for CD45, CD99, S-100, alfa-fetoprotein, placental alkaline phosphatase, inhibin, desmin, CK20, CD117, chromogranin, Epstein-Barr virus–encoded RNA, terminal deoxynucleotidyl tranferase, CD3, synaptophysin, and calretinin. Tumor cells retained integrase interacter 1. Malignant cells were also found in the pleural fluid. Bone marrow biopsy showed involvement with a poorly differentiated epithelial tumor composed of nests and sheets of moderate to large cells with round vesicular nuclei, prominent nucleoli, and moderate amounts of eosinophilic cytoplasm. On the basis of her clinical presentation, we suspected nuclear protein in testis (NUT) midline carcinoma (NMC). The immunohistochemistry for the NUT antigen performed at the Brigham and Women's hospital showed expression in tumor cells from both left tibial lesion (Fig 2B) and pleural deposits (Fig 2D). The interphase fluorescence in situ hybridization study performed on the pleural biopsy specimen revealed that 84% of cells had NUT rearrangement (Fig 4A), as indicated by splitting of rhodamine-labeled (red) and fluorescein-5-isothiocyanate–labeled (green) bacterial artificial chromosome probes flanking the NUT gene at 15q14. The classic BRD4-NUT fusion [t(15;19)]) was absent (Fig 4B), as indicated by lack of fusion of the rhodamine-labeled bacterial artificial chromosome probe covering the BRD4 gene, with the fluorescein-5-isothiocyanate–labeled probe covering the NUT gene. Nevertheless, three green signals were detected in this assay, demonstrating the splitting of the probe covering NUT. In additional fluorescence in situ hybridization studies (not shown), the BRD3 gene at 9q34 was also found not to be fused to NUT. Therefore, the final diagnosis was metastatic NUT variant midline carcinoma.
Fig 3.
Fig 4.
Treatment was initiated with paclitaxel 250 mg/m2 on day 1 and cisplatin 50 mg/m2 on days 1 and 2, every 3 weeks. At the end of first cycle, PET-CT demonstrated a decrease in size of the patient's right hilar mass lesion and its FDG avidity. Multiple lymph nodes seen previously decreased in size, with stable to decreased FDG uptake. Osseous metastatic disease also showed a decrease in FDG uptake (Fig 3B). Vorinostat 180 mg/m2 once daily orally on days 1 through 5 and on days 8 through 12 was added to the chemotherapy regimen from the second cycle onward. The adverse effects observed included nausea, vomiting, weight loss, and grade 3 thrombocytopenia. After three cycles of chemotherapy, her cough and bone pain resolved. PET-CT revealed further improvement in measurable disease, with a barely visible right hilar mass and resolution of lymphadenopathy (Fig 3C). At the end of third cycle, she underwent thoracocentesis and biopsy of the right hilar mass, which revealed continued presence of viable cancer cells.
She received three additional chemotherapy cycles. Although she remained asymptomatic clinically, the PET CT performed at the end of six cycles demonstrated disease progression, as evidenced by newly enlarged left supraclavicular lymph nodes, as well as enlarged and metabolically active nodes in the mediastinal, hilar, right cardiophrenic, and retroperitoneal regions. Multifocal lesions were seen within the liver parenchyma. In addition, there were multiple new foci of osseous metastatic disease (Fig 3D). Subsequently, she received cyclophosphamide 240 mg/m2 and topotecan 0.7 mg/m2 daily for 5 days. Her disease continued to progress rapidly, and she developed worsening malignant ascites. She died 2 weeks after disease progression, and 6 months after original diagnosis.
Discussion
NMC is a rare, aggressive, and genetically defined poorly differentiated carcinoma characterized by chromosomal rearrangement of the NUT gene on chromosome 15.1 NMC was first described in children, but it has since been reported in patients of all ages.2 The term midline is used because of NMC's tendency to arise from midline anatomic sites, most commonly in the head neck and trunk (73%) and in the respiratory tract (43%). It is usually locally invasive and widely metastatic at diagnosis.3
In 75% of patients, the BRD4-NUT protein is the result of the fusion of the NUT gene on chromosome 15q14 with the BRD4 on chromosome 19p13.4 In the remaining patients, the NUT gene is fused with BRD3 on chromosome 9q34 or a variant partner gene. These tumors are termed NUT variant.1 The BRD-NUT fusion protein binds to acetylated histones and, through a poorly understood mechanism, leads to globally decreased histone acetylation and decreased expression of genes that are needed for squamous differentiation.2,5 The diagnosis of NMC depends on the demonstration of the NUT rearrangement. Immunohistochemistry with anti NUT antibody is 100% specific and 87% sensitive for the diagnosis.6
Outcome data were recently published from the NUT midline cancer registry for 54 patients. The overall 2-year progression free survival and overall survival were 9% and 19%, respectively. Progression-free survival and overall survival were significantly higher in patients with localized disease and in those who underwent radiation therapy or gross total resection. The various chemotherapy regimens—whether they were platinum based, anthracycline based, a combination—did not seem to make a significant difference to the outcome.7 There have been case reports of the successful treatment of patients with localized NMC using platinum drugs and taxanes.8–10 Studies have shown that the repression of histone acetylation by BRD4-NUT can be reversed with histone deacetylase inhibitors, leading to squamous differentiation and arrested growth in vitro and in animal models. Response to vorinostat has been reported in a case of a child with refractory disease.11 Vorinostat has demonstrated synergistic anticancer effects in combination with taxanes and platinum compounds.12 On the basis of this information, we added vorinostat to the chemotherapy regimen in our patient. The initial response was encouraging, and this regimen was well tolerated.
NMC generally presents as a poorly differentiated or undifferentiated carcinoma.1–7 The unusual degree of squamous differentiation in the tibial metastasis which was absent in the primary (lung) has not been previously described in NMC. The presence of NUT staining in the tibial lesion confirmed that this was indeed a metastasis and not a separate entity. We hypothesize that this differentiation could be a result of either the local tumor microenvironment or a subclone that had an increased capacity for differentiation. Further investigation into the molecular mechanisms of this rare and unique malignant neoplasm in the future may broaden our understanding of oncogenesis and its regulation.
Acknowledgment
Supported by Samuel Waxman Cancer Research Foundation (C.A.F.).
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
REFERENCES
- 1.French CA. NUT midline carcinoma. Cancer Genet Cytogenet. 2010;203:16–20. doi: 10.1016/j.cancergencyto.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol. 2012;7:247–265. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 3.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22:4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 4.French CA, Miyoshi I, Kubonishi I, et al. BRD4-NUT fusion oncogene: A novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–307. [PubMed] [Google Scholar]
- 5.French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: A family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 6.Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–991. doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18:5773–5779. doi: 10.1158/1078-0432.CCR-12-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mertens F, Wiebe T, Adlercreutz C, et al. Successful treatment of a child with t(15;19)-positive tumor. Pediatr Blood Cancer. 2007;49:1015–1017. doi: 10.1002/pbc.20755. [DOI] [PubMed] [Google Scholar]
- 9.Fujioka N, French CA, Cameron MJ, et al. Long-term survival of a patient with squamous cell carcinoma harboring NUT gene rearrangement. J Thorac Oncol. 2010;5:1704–1705. doi: 10.1097/JTO.0b013e3181ebaa20. [DOI] [PubMed] [Google Scholar]
- 10.Engleson J, Soller M, Panagopoulos I, et al. Midline carcinoma with t(15;19) and BRD4-NUT fusion oncogene in a 30-year-old female with response to docetaxel and radiotherapy. BMC Cancer. 2006;6:69. doi: 10.1186/1471-2407-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz BE, Hofer MD, Lemieux ME, et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res. 2011;71:2686–2696. doi: 10.1158/0008-5472.CAN-10-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramalingam SS, Maitland ML, Frankel P, et al. Carboplatin and paclitaxel in combination with either vorinostat or placebo for first-line therapy of advanced non–small-cell lung cancer. J Clin Oncol. 2010;28:56–62. doi: 10.1200/JCO.2009.24.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]