Abstract
Exercise is commonly recommended to counteract aging-related muscle weakness. While numerous exercise intervention studies on the elderly have been performed, few have included elite senior athletes, such as those who participate in the National Senior Games. The extent to which participation in highly competitive exercise affects muscle strength is unknown, as well as the extent to which such participation mitigates any aging-related strength losses. The purpose of this study was to examine isometric thigh muscle strength in selected athletes of the National Senior Games and healthy noncompetitive controls of similar age, as well as to investigate strength changes with aging in both groups. In all, 95 athletes of the Games and 72 healthy controls participated. Of the senior athletes, 43 were runners, 12 cyclists, and 40 swimmers. Three trials of isometric knee flexion and extension strength were collected using a load cell affixed to a custom-designed chair. Strength data were normalized to dual-energy x-ray absorptiometry-obtained lean mass of the leg. A 3-factor multivariate analysis of variance (group × gender × age group) was performed, which included both the extension and flexion variables ([alpha] = 0.05). Athletes exhibited 38% more extension strength and 66% more flexion strength than the controls (p < 0.001). Strength did not decrease with advancing age in either the athletes or the controls (p = 0.345). In conclusion, senior athletes who participate in highly competitive exercise have greater strength than healthy aged-matched individuals who do not. Neither group displayed the expected strength losses with aging. Our subject cohorts, however, were not typical of those over age 65 years because individuals with existing health conditions were excluded from the study.
Keywords: elderly, isometric, knee, quadriceps, hamstrings
Introduction
Age-related decline of the neuromuscular system is recognized as a major cause of impairment of physical performance and loss of independence in the elderly (5). Epidemiological data support the concept that neuromuscular decline is associated with an increased risk of morbidity, disability, and mortality (1). Quadriceps strength in particular is associated with functional independence and is predictive of mortality and long-term survival in clinical populations (4,8,10,16,22). Because older individuals constitute the fastest growing portion of the American population (2), a reduction of the typical neuromuscular decline associated with aging could have a large impact on society.
Aging-related neuromuscular impairment is associated with a significant decline in muscle strength, secondary to a loss of muscle mass, that typically becomes functionally important after a person reaches age 60 (6). Type II muscle fibers are most affected, with loses demonstrated in both the number of fibers and the size of the remaining fibers (15). When normalized to muscle mass, the age-associated decreases in strength appear to be similar between the genders, although women may experience strength losses earlier than men (6).
Exercise is a prophylactic agent against age-related weakness. Exercise interventions on the elderly have elicited positive musculoskeletal adaptations, including enzymatic alterations and improved contractile and neural properties, in addition to increased muscle mass and strength (7,9,12,17,21,24). While these intervention studies have shown that a portion of the lost muscle mass and function can be regained through exercise, improvements are relatively small in absolute terms, and the capacity for recovery may be limited (9,21).
The National Senior Games Association is a nonprofit organization dedicated to motivating older adults to lead healthy lifestyles. The National Senior Games, also known as the “Senior Olympics,” is the largest multisport event in the world for senior athletes. All events are divided into 5-year age categories so that the participants compete against other athletes approximately the same age. Approximately 250,000 individuals participate in the community-level games. The competitors who make it to the National Senior Games represent the upper 5% of older competitive athletes in the United States. The National Senior Olympians comprise a unique study cohort because of their history of success at highly competitive level over a number of years. Study of the senior athletes has the potential to provide more information on the benefits of exercise than what can be learned from exercise interventions. Comparisons between the National Senior Olympians and healthy controls can provide insight into the effects of long-term intense exercise on the mitigation of the neuromuscular decline associated with aging.
The goal of this investigation was to examine the effects of highly competitive exercise, gender, and age on the strength of the knee flexors and extensors. Specifically, we aimed to determine if thigh muscular strength: (a) differs between elite senior athletes and healthy community-dwelling controls, (b) declines between 5-year age groups, and (c) differs between genders in our study cohort. This study is unique in that data are reported on a large group of very elite elderly athletes, all of whom reported participation in highly competitive exercise for the last 20 years. Other studies which have examined exercise on the strength of older individuals are generally short-term interventions, and few have included women (2,7,12,17,27).
Methods
Experimental Approach to the Problem
This study is a cross-sectional comparison of 2 cohorts: a group of elite master endurance athletes and a control group of healthy individuals of the same age range. Because our specific aim is to determine if thigh muscle strength differs between athletes and controls, gender, and between 5-year age categories, we recruited a large sample (n = 167) of individuals of both genders who are aged between 60 and 93 years. Our independent variables are (a) athlete or control group, (b) gender, and (c) 5-year age group. Our dependent variables are isometric strength of the knee extensors and knee flexors. A 3-factor multivariate analysis of variance (MANOVA) (group × gender × age group) was performed, which included both the extension and flexion variables ([alpha] = 0.05).
Subjects
Ninety-five elite senior athletes aged 65 years or older were recruited from the 2005 Summer National Senior Games. They competed in one of the following sports: running events longer than 400 m (n = 43), cycling events longer than 5 K (n = 12), and any swimming event (n = 40). These events were chosen because data were collected simultaneously with another study, an aim of which was to investigate the magnitude of lower extremity loading (i.e., high-, medium-, and low-impact sports) on bone mineral density (25).
Healthy control subjects (n = 72) aged 65 years or older were recruited from the greater Pittsburgh metropolitan area via the research registry of the University of Pittsburgh's Claude D. Pepper Older Americans Independence Center. The Pepper Center is a federally funded research center focused on keeping elders independently living and functioning. We did not control for the activity level of this group; therefore, their self-reported activity levels ranged from sedentary to active (i.e., exercising more than 3 days per week for more than 1 hour per day). However, none were active in running, cycling, or swimming. Subject demographics are detailed in Table 1.
Table 1.
| Group | Age (y)* | Height (cm) | Mass (kg)* | Men:Women |
|---|---|---|---|---|
| Senior Athlete | 72.6 ± 6.4 | 168.7 ± 8.6 | 72.6 ± 13.5 | 57:38 |
| Control | 75.4 ± 5.6 | 170.8 ± 25.5 | 79.5 ±11.7 | 46:26 |
Control participants were significantly older and heavier than the athletes (p < 0.05).
We wanted to look at the effects of highly competitive exercise on strength. Considering the age range of our participants, disease or injury could have been a strong confounding factor. Therefore, we recruited as healthy a subject population as possible in both the athlete and control groups. Subjects were excluded if they had a history of any of the following: chronic obstructive pulmonary disease, myocardial infarction, or coronary artery disease; cerebral vascular accident or a history of transient ischemic attacks; rheumatoid arthritis, gout, or osteoarthritis severe enough to limit activity; use of a cane or walker; history of osteoporosis or bisphosphonate therapy; current use of antidepressant drugs or any drugs that may interfere with neurological, musculoskeletal, or cognitive function; history of insulin-dependent diabetes mellitus, or neurological or rheumatological disorders that might interfere with sensory input; fracture, ligament reconstruction, or sprain within the past 12 months; and any other disease, injury, or disorder that may affect strength. Athletes were excluded from the study if they were competitively active in any other sport other than running, cycling, or swimming or if they competed in more than one of these sports.
Participants were asked whether they were right or left leg dominant, with leg dominance being defined as the leg they would use to kick a ball. In total, 89 of the athletes declared themselves right leg dominant, 3 left leg dominant, and 3 stated that they had no preference; 70 of the 72 controls stated that they were right leg dominant and 2 said they were left leg dominant.
Procedures
Informed consent from the University of Pittsburgh Institutional Review Board was obtained from all the subjects prior to their participation. Data collection occurred at the University of Pittsburgh Clinical and Translational Research Center (CTRC). We did not control for the subjects' hydration status at the time of testing. Because of the strong inverse association between quadriceps strength and morbity/mortality (4,22), we assessed the strength of the thigh musculature. Isometric knee extension and flexion strength (100 Hz) was measured with a tension/compression load cell (model 3132; Lebow Products, Inc., Troy, MI, USA). The load cell was attached to an adjustable bar which was secured to a custom-designed aluminum chair. Pilot testing in our laboratory has determined that the output of the chair has an intraclass correlation coeffient of 0.907 with that of a Biodex dynamometer. All measurements were taken on the subject's left side. The data were collected in conjunction with another study, the purpose of which was to examine the magnitude of lower extremity loading on bone mineral density in senior athletes. It is our standard clinical practice to assess bone density on the left side because it is typically the nondominant side. Because we hope to look at the relationship between strength and bone mineral density in a forthcoming paper, we measured the strength on the subjects' left side.
Subjects were placed in a comfortable seated position on the chair and secured using canvas straps to minimize extraneous movements. The hip and knee were positioned in 90° and 45° of flexion, respectively. The load cell was positioned just proximal to the malleoli. The distance from the knee to the load cell was recorded in order to calculate torque. Subjects were asked to position their arms either crossed at the chest level or resting on their lap to avoid bracing or pulling on the chair. A photograph of a subject in the athlete group performing the knee flexion strength test is shown in Figure 1.
Figure 1.
After gravity effect torque was calculated, subjects performed 3 repetitions of maximal isometric knee extension or flexion lasting 5 seconds each. The order in which flexion or extension was tested was counterbalanced; 30 seconds of rest was provided between contractions. Similarly, subjects performed 3 repetitions, each lasting 5 seconds, of maximal isometric knee flexion or extension.
Subjects were instructed to continue breathing during the tests and to not hold their breath in order to prevent against doing the Valsalva maneuver. During the test, subjects were encouraged to “push” for the extension trials and to “pull” for the flexion trials. The test administrator loudly repeated the word push or pull each second for a total of 5 seconds.
Lean mass of the left leg (in kg) was measured with a Hologic QDR-4500A (Hologic, Inc., Bedford, MA, USA) dual-energy x-ray absorptiometer (DXA) used in fast-array mode. A licensed DXA technician performed the scans. Subjects were positioned supine with the lower extremities in slight external rotation and ankle in maximum plantar flexion. The Hologic software Version 12.4 defined the leg as the segment distal to the femoral neck. Bone mineral density scans were also made of the total body, spine, and forearm, the data of which are presented elsewhere (20,25).
Strength data were analyzed using Matlab 7.1 (Mathworks, Inc., Natick, MA, USA). Raw data were filtered with a fourth-order low-pass Butterworth filter with a cutoff frequency of 25 Hz. The cutoff frequency was based on a residual analysis described by Winter (26). Torque was calculated as the product of the force in the load cell times the distance from the knee to the adjustable bar. Peak torque was recorded for each trial of knee extension and flexion. The 3 trials per subject were averaged to yield representative values. The average peak torque was then divided by the lean mass of the leg to yield a strength measurement in N·m·kg−1. Isometric peak torque has an intraclass correlation coefficient of greater than 0.89 (3).
Subjects were asked to complete a physical activity questionnaire to determine lifetime and current physical activity (14). The questionnaire was handed to the subjects and they were instructed to complete it before leaving the testing center. Upon retrospect, we learned that the questionnaire was too detailed and complex for a subject to complete alone. It would have been better if done in interview format. Due to a lack of manpower and the desire to test as many subjects as possible during the 2-week window of the National Senior Games, we did not read through the surveys while the subjects were still in our laboratory. After the games were over, it was apparent that the survey data were not usable. From the surveys, we have confirmed that the athletes were active for more than 20 years, but we cannot provide more information on activity with confidence.
Statistical Analyses
To determine the effect of age on strength, all subjects were categorized into 5-year age groups such that individuals aged 65–69 years constituted one group (n = 51), as did those aged 70–74 years (n = 45) and 75–79 years (n = 41). Those subjects aged 80 years and older (n = 31) were categorized into a single group.
The knee flexion and extension peak torque data obtained in this study had an observed bivariate correlation of 0.667 (p < 0.01). Because of this high correlation, these 2 factors were considered simultaneously as dependent measures in a 3-factor MANOVA to determine if strength differences existed between the athlete and control groups, genders, and age categories. Our sample size of 167 participants assured that the power of the statistical test exceeded 80%. Tukey post hoc tests were performed when appropriate ([alpha] = 0.05).
Three subjects (athlete group, 2 men, 1 woman, 2 in 65–69 age group, 1 in 70- to 74-year age group) were outliers because of very high values of their strength variables (z-score > 3.29). A sensitivity analysis was performed with the subjects included and then excluded from the statistics. The inclusion of the subjects did not change the outcome of the study, and as we have no reason to believe that the data are not valid, the subjects were kept in the MANOVA.
Results
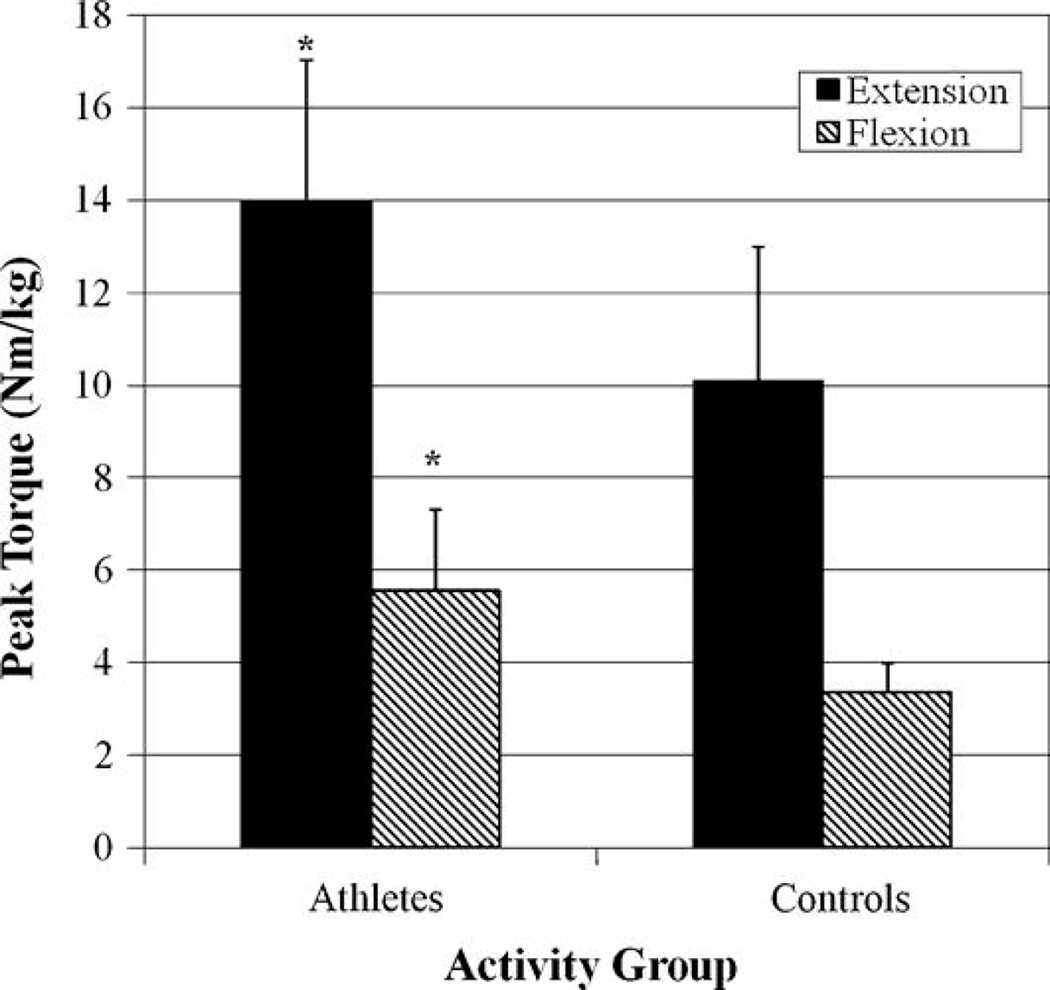
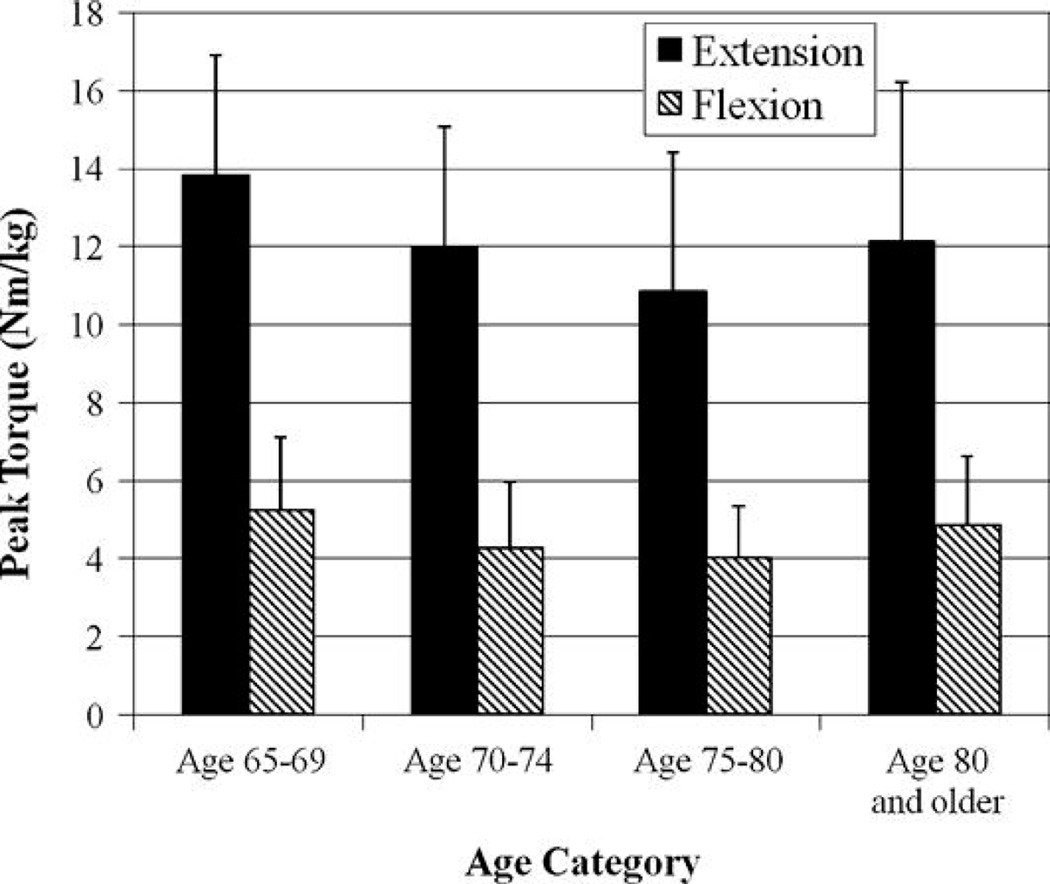
Our subjects ranged in age from 65 to 93 years. Controls were significantly older and heavier (p < 0.05) than the athletes. Our purpose was to determine the effects of competitive exercise at an elite level (i.e., athlete or control group), gender, and age on the strength of the thigh musculature. Athletes were stronger than controls (p < 0.001, Figure 2) and men were stronger than women (p < 0.05) for both knee extension (man = 12.7 ± 3.1 N·m·kg−1, woman = 11.5 ± 4.1 N·m·kg−1) and flexion strength (man = 4.8 ± 1.8 N·m·kg−1, woman = 4.4 ± 1.7 N·m·kg−1). No differences were noted between age categories (p = 0.345, Figure 3). The 2-way interaction of age category by gender was not significant (p = 0.164) nor were activity group by age category (p = 0.175) or activity group by gender (p = 0.221). The 3-way interaction was not significant (p = 0.296).
Figure 2.
Figure 3.
Discussion
Our purpose was to examine the effects of competitive exercise, gender, and age on isometric knee flexion and extension strength. We determined that thigh muscle strength was significantly greater in elite athletes and in men in our combined subject population. Aging was not associated with significant losses in strength in our combined sample of athletes and controls.
Athletes had an average of 66% greater isometric knee flexion strength and 38% greater isometric extension strength than did the control group. This is not surprising as the participants in the “athlete” group represent an elite group of competitive individuals who, by virtue of qualifying for the National games, are in the upper 5% of participants at the community level. What is notable about our study is not this finding because it was expected, but rather, that we have quantified the knee flexion and extension strength of these elderly endurance athletes compared to their healthy control contemporaries. Other researchers have reported that exercise results in positive adaptation of skeletal muscle, including increased strength, mass, and protein synthesis (9,24), and exercise is often considered a prophylactic agent against the age-related decline in physical function. Trappe et al. (24) noted a 53% increase in knee extension strength after 6 months of a resistance training in older men. Conversely, Puggaard noted no increases in isometric thigh muscle strength after an 8-month aerobic training intervention in 75- and 85-year-old women (17). The current study differs from previous studies because we primarily examined the effect of highly competitive endurance training, not resistance training, on strength, and we also did not perform an intervention but rather studied a people with a lifestyle of intense training.
Most of the research on the effects of exercise in the elderly has involved intervention studies. The current study is unique in that it is not an intervention study; rather, we report on elite master endurance athletes compared to healthy controls of the same age range. However, several authors have reported data on master athletes compared to controls, although the bulk of this research has reported data on men only. Klitgaard et al. (13) reported that elderly swimmers had greater isometric strength than age-matched control subjects. Similarly, Sipila and Suominen (19) reported greater knee extension and flexion strength in men athletes aged 70–81 years compared to an age-matched population sample.
All the athletes in the current study were competitors in endurance events of running, cycling, or swimming in the National Senior Games. However, we were unable to assess the amount of resistance exercise they did as part of their normal training routine. Nor did we record the amount of endurance and/or resistance exercise done by individuals in the control group. Rather, the groups were simply defined as (a) both “healthy” and (b) one group was comprised of elite competitive athletes and those in the other group were not elite competitive. Ettinger et al. (7) reported no differences in both isometric knee flexion at extension strength between older individuals receiving either an aerobic exercise or resistance exercise intervention. When compared to a control group, Ettinger et al. (7) reported increased knee flexion strength but no differences in knee extension strength.
When comparing strength differences between genders in the combined sample of athletes and controls in the current study, we found that men were approximately 8 and 10% stronger than women in isometric knee flexion and extension, respectively. This finding was expected because hormonal and genetic factors favor greater muscle mass, and therefore strength, in men. Others have also documented increased strength in men (18,11).
Interestingly, we did not find the hypothesized decrease in thigh muscle strength in older individuals as compared to the youngest tested age group of 65–69 years. This is true for both the combined sample of athletes and controls and when athletes were considered alone. Klitgaard et al. (13) reported that elderly swimmers who have been competitive for 12–17 years (mean age: 69 years) and young subjects (mean age: 28 years) displayed similar isometric knee extension strength. However, exercise intervention studies have not reported such results; rather, older individuals still did not have as much strength as individuals aged between 20 and 30 years (8,23).
In Figure 3, a U-shaped pattern is noted in both the extension and flexion data such that the individuals aged 80 years and older are stronger than those aged 75–79 years. The strength of the oldest group of participants is slightly greater than those aged 70–74 years (extension: 70–74 years = 11.95 ± 3.10 N·m·kg−1, 80+ years = 12.10 ± 4.12 N·m·kg−1; flexion: 70–74 years = 4.25 ± 1.71 N·m·kg−1, 80+ years = 4.86 ± 1.77 N·m·kg−1). This apparent increase in strength may be due to a natural attrition of people not able to participate in studies as they age. It may be that only the healthiest of the old are still independently mobile and able to transport themselves into the city to participate in the study. Some who may still be able to participate in their 70s may have a genetic predisposition to disease and will not be able to participate in their 80s, so that those who participate in their 80s are quite healthy.
Members of our research group have previously reported that the body mass index of each of the age groups were as follows: 60–69 years = 25.9± 3.9 kg·m−2, 70–74 years = 27.1 ± 3.5 kg·m−2, 75–79 years = 27.7 ± 4.8 kg·m−2, and 80+ years = 26.4 ± 3.6 kg·m−2 (20). Thus, those participants aged 80 years and above were leaner than those in their 70s. Our strength data are normalized to the lean mass of the left leg. However, the lack of significance of the age category variable is not entirely due this normalization. When the raw torque data are compared between the age groups, the U-shaped pattern is still apparent in both the flexion and extension data.
Torque data have been reported in numerous formats in the literature. They may be reported as the raw torque value in Nm, not normalized to anything. Normalization is typically used to allow for better or more representative comparisons between groups. As such, torque data may be normalized to body weight or to both body weight and height. They may be normalized to lean mass of the body or to lean mass of the leg. We chose to normalize strength to lean leg mass because we though it would provide a better indication of muscle quality.
It should be noted that the lean mass of the entire leg, not just the thigh, was used to normalize the knee flexion and extension torque to allow for better comparisons between subjects. We did not collect another measure of thigh mass, such as a circumference. Nor do we have the lean mass of the thigh. We do not have a measure of the cross-sectional area of the hamstrings and quadriceps alone, such as would be obtained from an magnetic resonance imaging. Due to software constraints of the Hologic DXA system, we only have access to the entire lean mass of the leg. While this does not allow us to do a direction normalization of Nm of torque to hamstrings or quadriceps size, it does provide much more insight into muscle quality than if the data would have been presented as Nm alone.
There are several limitations to this study that are not mentioned above. The study was cross-sectional in nature; therefore, we do not know how a person's strength would change as they aged from 65 years to older than 80. The lifetime physical activity of both the athlete and control subjects is unknown. The hydration status of each subject is unknown. The control group was very healthy and not necessarily sedentary. The activity level of the control participants ranged from sedentary to active in sports other than running, cycling, and swimming.
Future studies should assess the total current and historical physical activity to gain perspective of how activity level influences strength. Also, future research endeavors should obtain true quadriceps and hamstring cross-sectional areas on senior athletes to examine the quality of the muscle tissue.
Practical Applications
This study reveals that in our cohort of healthy older individuals, the expected strength losses associated with aging were not found. This is particularly true for the Senior Olympians. Continual participation in high intensity endurance training may have a protective effect on the loss of muscle strength that accompanies the aging process. While this study provides basic information about quadriceps and hamstrings strength in elite senior athletes and healthy controls, further research is needed to examine why outcomes were different than hypothesized. Because data on the training routines are unfortunately lacking in this study, critical information may be missing.
Acknowledgments
We would like to acknowledge the University of Pittsburgh CTRC for supporting this research as well as the University of Pittsburgh Claude D. Pepper Older Americans Independence Center for allowing us to recruit our healthy control subjects through its research registry (grant P30 AG024827-01AG). Additionally, this study was performed with the permission of the National Senior Games Association. We would also thank Megan Miller, CCRC, Karen Vujevich, CRNP, and Julie Wagner, PAC, MPA, for their assistance during data collection, and Dr. Susan Sereika, of the University of Pittsburgh School of Nursing, for her assistance in performing the statistical analyses.
Footnotes
The study was conducted at the University of Pittsburgh Clinical and Translational Research Center.
References
- 1.Baumgartner R, Koehler K, Gallagher D. Epidomiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 2.Bulbulian R, Hargan M. The effect of activity history and current activity on static and dynamic postural balance in older adults. Physiol Behav. 2000;70:319–325. doi: 10.1016/s0031-9384(00)00272-9. [DOI] [PubMed] [Google Scholar]
- 3.Callaghan MJ, McCarthy CJ, Oldham JA. Electromyographic fatigue characteristics of the quadriceps in patellofemoral pain syndrome. Man Ther. 2001;6:27–33. doi: 10.1054/math.2000.0380. [DOI] [PubMed] [Google Scholar]
- 4.Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, Morton DJ, Wingard DL, Barrett-Connor E. Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med. 2003;25:226–231. doi: 10.1016/s0749-3797(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 5.Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2:21–29. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 6.Doherty TJ. The influence of aging and sex on skeletal muscle mass and strength. Curr Opin Clin Nutr Metab Care. 2001;4:503–508. doi: 10.1097/00075197-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger WH, Jr, Burns R, Messier SP, Applegate W, Rejeski WJ, Morgan T, Shumaker S, Berry MJ, O'Toole M, Monu J, Craven T. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST) JAMA. 1997;277:25–31. [PubMed] [Google Scholar]
- 8.Felson DT, Niu J, McClennan C, Sack B, Aliabadi P, Hunter DJ, Guermazi A, Englund M. Knee buckling: Prevalence, risk factors, and associated limitations in function. Ann Intern Med. 2007;147:534–540. doi: 10.7326/0003-4819-147-8-200710160-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hawkins SA, Wiswell RA, Marcell TJ. Exercise and the master athlete-A model of successful aging? J Gerontol A Biol Sci Med Sci. 2003;58:1009–1011. doi: 10.1093/gerona/58.11.m1009. [DOI] [PubMed] [Google Scholar]
- 10.Hulsmann M, Quittan M, Berger R, Crevenna R, Springer C, Nuhr M, Mortl D, Moser P, Pacher R. Muscle strength as a predictor of long-term survival in severe congestive heart failure. Eur J Heart Fail. 2004;6:101–107. doi: 10.1016/j.ejheart.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Ivey FM, Tracy BL, Lemmer JT, NessAiver M, Metter EJ, Fozard JL, Hurley BF. Effects of strength training and detraining on muscle quality: Age and gender comparisons. J Gerontol A Biol Sci Med Sci. 2000;55:B152–B157. doi: 10.1093/gerona/55.3.b152. discussion B158–B159. [DOI] [PubMed] [Google Scholar]
- 12.Jadelis K, Miller ME, Ettinger WH, Jr, Messier SP. Strength, balance, and the modifying effects of obesity and knee pain: Results from the Observational Arthritis Study in Seniors (OASIS) J Am Geriatr Soc. 2001;49:884–891. doi: 10.1046/j.1532-5415.2001.49178.x. [DOI] [PubMed] [Google Scholar]
- 13.Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, Schnohr P, Saltin B. Function, morphology and protein expression of ageing skeletal muscle: A cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand. 1990;140:41–54. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- 14.Kriska A, Knowler W, LaPorte R, Drash A, Wing R, Blair S, Bennett P, Kuller L. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 15.Lexell J, Downham D. What is the effect of ageing on type 2 muscle fibers? J Neurol Sci. 1992;107:250–251. doi: 10.1016/0022-510x(92)90297-x. [DOI] [PubMed] [Google Scholar]
- 16.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 17.Puggaard L. Effects of training on functional performance in 65, 75 and 85 year-old women: Experiences deriving from community based studies in Odense, Denmark. Scand J Med Sci Sports. 2003;13:70–76. doi: 10.1034/j.1600-0838.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 18.Seger JY, Thorstensson A. Muscle strength and myoelectric activity in prepubertal and adult males and females. Eur J Appl Physiol Occup Physiol. 1994;69:81–87. doi: 10.1007/BF00867932. [DOI] [PubMed] [Google Scholar]
- 19.Sipila S, Suominen H. Ultrasound imaging of the quadriceps muscle in elderly athletes and untrained men. Muscle Nerve. 1991;14:527–533. doi: 10.1002/mus.880140607. [DOI] [PubMed] [Google Scholar]
- 20.Stone B, Perera S, Velez N, Zhang A, Miller M, Greenspan S. Proceedings of Annual meeting of the American Geriatrics Society. Seattle, WA: 2007. Predicting skeletal integrity in master athletes and non-athlete controls; pp. S196–S197. [Google Scholar]
- 21.Suominen H. Muscle training for bone strength. Aging Clin Exp Res. 2006;18:85–93. doi: 10.1007/BF03327422. [DOI] [PubMed] [Google Scholar]
- 22.Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, Moore AJ, Moxham J, Polkey MI. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62:115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trappe S, Williamson D, Godard M. Maintenance of whole muscle strength and size following resistance training in older men. J Gerontol A Biol Sci Med Sci. 2002;57:B138–B143. doi: 10.1093/gerona/57.4.b138. [DOI] [PubMed] [Google Scholar]
- 24.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552:47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velez N, Zhang A, Miller M, Perera S, Greenspan S. The effect of high impact exercise on skeletal integrity in master athletes. J Am Geriatr Soc. 2006;54:S193–S194. doi: 10.1007/s00198-008-0590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winter DA, Sidwall HG, Hobson DA. Measurement and reduction of noise in kinematics of locomotion. J Biomech. 1974;7:157–159. doi: 10.1016/0021-9290(74)90056-6. [DOI] [PubMed] [Google Scholar]
- 27.Wong AM, Lin YC, Chou SW, Tang FT. Coordination exercise and postural stability in elderly people: Effect of Tai Chi Chaun. Arch Phys Med Rehabil. 2001;82:608–612. doi: 10.1053/apmr.2001.22615. [DOI] [PubMed] [Google Scholar]