Abstract
A long-standing challenge in synthetic chemistry is the development of the transamidation reaction. This process, which involves the conversion of one amide to another, is typically plagued by unfavourable kinetic and thermodynamic factors. Although some advances have been made with regard to the transamidation of primary amide substrates, secondary amide transamidation has remained elusive. Here we present a simple two-step approach that allows for the elusive overall transformation to take place using non-precious metal catalysis. The methodology proceeds under exceptionally mild reaction conditions and is tolerant of amino-acid-derived nucleophiles. In addition to overcoming the classic problem of secondary amide transamidation, our studies expand the growing repertoire of new transformations mediated by base metal catalysis.
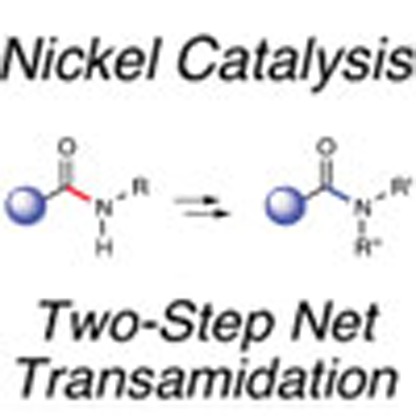 Transamidation reactions are kinetically and thermodynamically challenging because of the stability of the amide starting materials. Here, the authors show a two-step process—activation of a secondary amide, followed by nickel-catalysed C–N bond cleavage—that allows mild and high yielding transamidation.
Transamidation reactions are kinetically and thermodynamically challenging because of the stability of the amide starting materials. Here, the authors show a two-step process—activation of a secondary amide, followed by nickel-catalysed C–N bond cleavage—that allows mild and high yielding transamidation.
Within the realm of chemical synthesis, our ability to interconvert functional groups remains an important endeavour with tremendous impact1,2. One particularly interesting transformation is the transamidation reaction (Fig. 1a)3,4,5. In addition to being conceptually curious, the reaction could serve as a practical means to prepare amides. Transamidation, however, has proven difficult to achieve both synthetically and enzymatically6. Primary amide transamidation is the most common7,8. In contrast, the transamidation of secondary amides, which are ubiquitous in peptides, has remained an unsolved problem. Over the past few decades, only two methodological advances have been described: Bertrand's usage of excess AlCl3 to promote the transamidation of several unfunctionalized substrates, described in 1994 (ref. 9), and the 2003 report by Gelman and Stahl of a dimeric aluminium complex to promote secondary amide transamidation, which yields equilibrium mixtures10. A simple and efficient method to achieve secondary amide transamidation has eluded discovery.
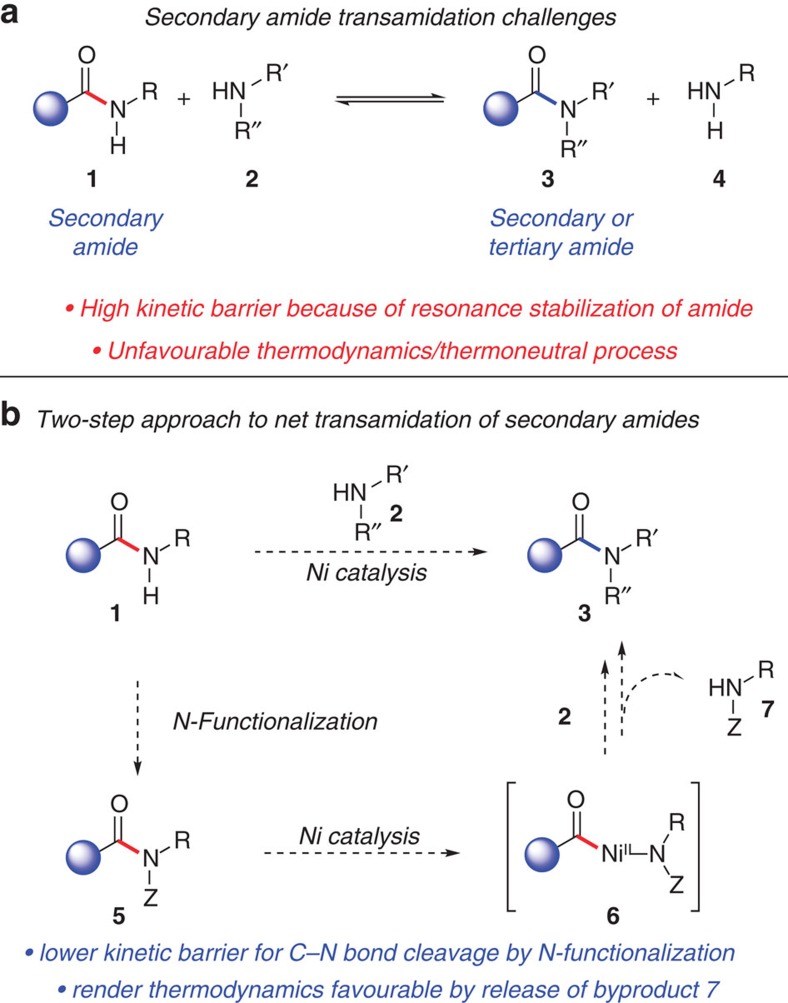
Figure 1. Development of Ni-catalysed secondary amide transamidation.
(a) An illustration of the challenges currently faced with secondary amide transamidation. (b) The two-step approach to achieve net transamidation of secondary amides via N-functionalization and C–N bond activation.
Two main challenges must be overcome to achieve the transamidation of secondary amides. First, amides are notoriously stable because of resonance effects11,12. One must therefore surmount the high kinetic barrier for acyl C–N bond cleavage. The other difficulty pertains to thermodynamics, as the conversion of a secondary amide to another amide is effectively a thermoneutral process, aside from specialized cases. Indeed, the state-of-the-art methodology to achieve the transamidation of secondary amides gives equilibrium mixtures of substrate and product amides10,12.
To overcome the kinetic and thermodynamic challenges associated with secondary amide transamidation, we pursued the two-step approach outlined in Fig. 1b. It was envisioned that substrate 1 would first undergo N-functionalization with an electron-withdrawing group as a means to weaken the amide C–N bond13,14. From ‘activated' species 5, oxidative addition using an appropriate nickel15,16,17 catalyst would give acyl metal species 6. This step would rely on the recently disclosed nickel-catalysed amide bond cleavage that has only seen limited use in esterification and the Suzuki–Miyaura coupling.18,19 Interception of this intermediate with primary or secondary amines 2 would furnish the desired product 3. The reaction would be driven thermodynamically by the liberation of the N-functionalized amine 7, which would be less nucleophilic than amine 2 undergoing transamidation.
In this manuscript, we demonstrate the success of our two-step approach to achieve the net transamidation of secondary amides. The methodology utilizes non-precious metal catalysis and proceeds under exceptionally mild reaction conditions. Moreover, the chemistry is tolerant of amino-acid-derived nucleophiles. These studies provide the most general solution to the classic challenge of secondary amide transamidation available to-date.
Results
Discovery of the transamidation reaction
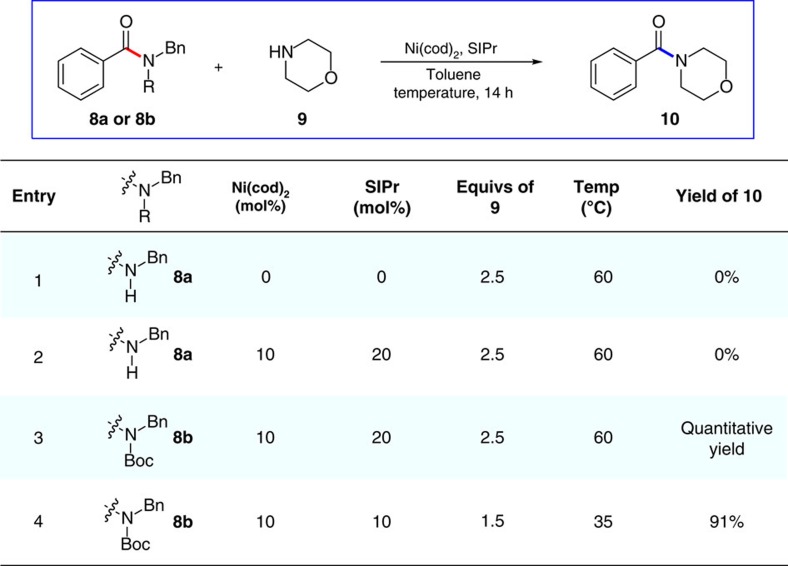
Our studies commenced by surveying the reaction of simple benzamides and morpholine using nickel catalysis (see Supplementary Table 1 for full details). A small sampling of control experiments and key results are shown in Fig. 2. As expected, the attempted transamidation of secondary amide 8a with morpholine (9) fails in the absence (entry 1) or presence of nickel/SIPr (entry 2). However, after Boc activation of the substrate, we were delighted to find that 8b underwent the nickel-catalysed reaction with 9 to deliver morpholino amide 10 quantitatively (entry 3). Of note, no reaction takes place if the nickel and SIPr are excluded from the reaction, thus highlighting the unique ability of nickel catalysis to facilitate the net transamidation reaction. We also examined less forcing conditions. It was ultimately found that exposure of 8b to 1.5 equivalents of amine 9 at 35 °C furnished amide 10 in 91% isolated yield (entry 4). These exceptionally mild reaction conditions were deemed suitable for exploration of the substrate scope.
Figure 2. Key experiments and reaction discovery.
Failed reaction using substrate 8a in the absence of catalyst/ligands or activating group (entries 1 and 2), initial promising result (entry 3) and optimized reaction conditions (entry 4). For entries 1–3, yields shown were determined by 1H NMR analysis with an internal standard. For entry 4, the yield shown reflects the average of two isolation experiments.
Scope of the methodology
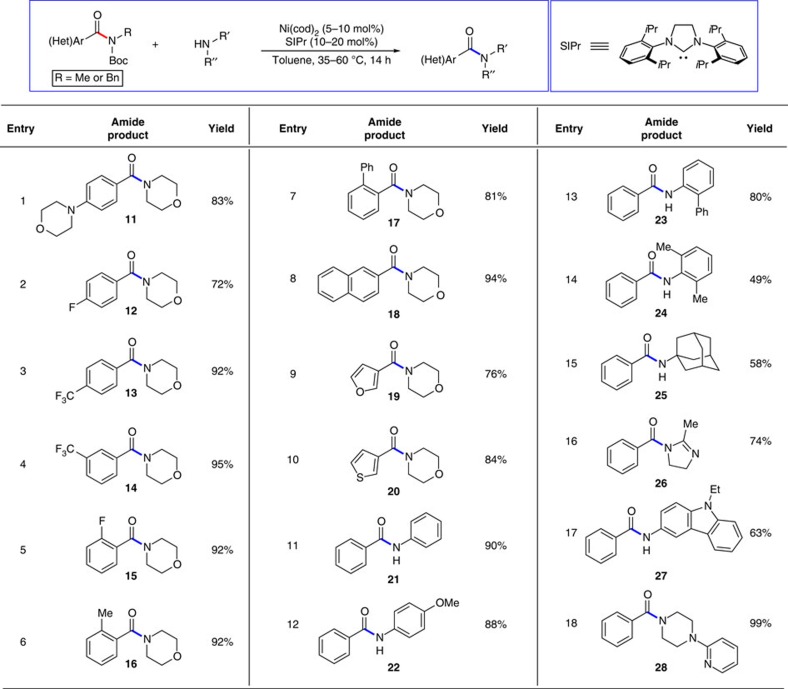
A variety of readily accessible Boc-activated secondary amides were examined. As shown in Fig. 3, considerable variation is tolerated with regard to the benzamide substrate and the amine nucleophile. Substrates bearing electron-donating or -withdrawing groups can be utilized, as shown by the formation of 11–15 (entries 1–5). Of note, CF3 and F substituents, often encountered in medicinally relevant scaffolds20,21, posed no difficulty (entries 2–5). Another interesting aspect of the reaction scope is that ortho substitution is well tolerated (entries 5–7), suggesting that the methodology does not suffer from steric hindrance. The extended aromatic naphthyl unit could also be employed to give 18 (entry 8), in addition to the furan and thiophene heterocycles to furnish 19 and 20, respectively (entries 9 and 10). Substrates derived from aliphatic amides do not undergo the nickel-catalysed transamidation under our typical reaction conditions, which may ultimately provide opportunities for achieving site-selective reactions of polyamide substrates. With regard to the amine reactant, various alternatives to morpholine can be employed. For example, anilines may be utilized, as shown by the formation of secondary amides 21–24 (entries 11–14). Moreover, the use of adamantyl-1-amine furnished amide 25 in 58% yield (entry 15). The formation of 24 and 25 (entries 14 and 15) again highlights the tolerance of this methodology towards steric demands. Finally, several heterocyclic amine nucleophiles were tested and found to undergo the nickel-catalysed reaction. The successful use of an amidine, carbazole and pyridine-appended piperazine to provide 26–28, respectively (entries 16–18), demonstrates the tolerance of the nickel catalyst system towards multiple basic heteroatoms.
Figure 3. Scope of methodology.
The scope of the transamidation methodology was evaluated with respect to the Boc-activated amide substrate (entries 1–10), and with respect to the amine nucleophile using 8b (entries 11–18). Reactions were carried out using Ni(cod)2 (5–10 mol%), SIPr (10–20 mol%), substrate (1.00 equiv), amine (1.5–2.5 equiv) and toluene (1.0 M) at 35–60 °C for 14 h. Yields shown reflect the average of two isolation experiments.
Discussion
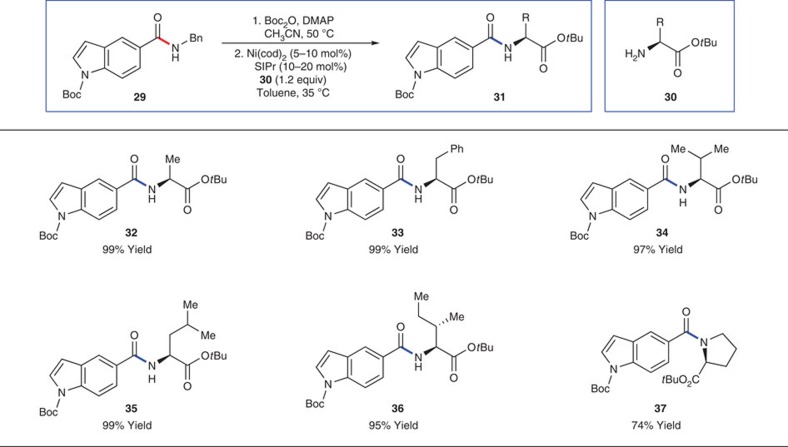
The results above demonstrate the feasibility of the second part of our two-step reaction design. As a critical final evaluation of this chemistry, we performed the net transamidation of a secondary amide substrate using a variety of amino-acid-derived nucleophiles (Fig. 4). Thus, indolylamide 29 readily underwent Boc activation in quantitative yield under standard conditions. Next, exposure of this intermediate to a variety of optically pure amino esters gave the corresponding secondary amides in excellent yields over the two-step process. Nucleophiles derived from alanine, phenylalanine, valine, leucine, isoleucine and proline could all be utilized, leading to secondary amides 32–37. The mild nature of this two-step process should not be overlooked. In all cases, the N-Boc of the indole and the ester of the amine nucleophile were not disturbed. Moreover, each product was obtained in >99% ee, suggesting that no appreciable loss of stereochemical fidelity occurred. This is notable since each nucleophile and product contains an epimerizable stereocentre.
Figure 4. The two-step approach for transamidation with amino-acid derivatives.
The scope of the transamidation was evaluated with various amino-acid derivatives to give products 32–37. Amide 29 was first Boc-activated with Boc2O (3.0 equiv), 4-(dimethylamino)pyridine (30 mol%) and CH3CN (0.20 M) at 50 °C for 19 h. The transamidation reactions were then carried out using Ni(cod)2 (5–10 mol%), SIPr (10–20 mol%), substrate (1.00 equiv), amine (1.2 equiv) and toluene (1.0 M) at 35 °C for 14 h. Yields shown reflect the average of two isolation experiments.
In summary, we have developed a two-step approach to effect the transamidation of secondary amides. Our strategy relies on a simple Boc-activation step of the secondary amide substrates, followed by an uncommon nickel-catalysed activation of an acyl C–N bond. The methodology proceeds under mild conditions, is tolerant of variation in both reaction partners and proceeds in the presence of basic heteroatoms and several heterocyclic scaffolds. Moreover, ester functional groups and epimerizable stereocentres found in amino-acid derivatives easily withstand the reaction conditions. To test the scalability of this method, a gram-scale coupling of the indole substrate with the phenylalanine amino ester derivative gave the desired transamidated product in excellent yield. Future efforts focused on ligand development are expected to fuel further breakthroughs in reaction discoveries, much like the evolution witnessed in recent decades pertaining to Pd- and Ni-catalysed couplings. Nonetheless, these studies provide the most general solution to the classic challenge of secondary amide transamidation available to-date, while also expanding the growing repertoire of new transformations mediated by base metal catalysis.
Methods
General
1H NMR (500 MHz), 13C NMR (125 MHz) and 19F NMR (282 MHz) spectra were recorded on Bruker spectrometers. High resolution mass spectrometry was performed on Thermo Scientific Exactive Mass Spectrometer with DART ED-CUBE. Infrared spectra were recorded on a Perkin-Elmer UATR Two Fourier transform infrared spectrometer. Determination of enantiopurity was carried out on a Mettler Toledo supercritical fluid chromatography using a Daicel ChiralPak OJ-H column. Supplementary Figs 1–46 for the NMR spectra and HPLC chromatograms, Supplementary Table 1 for the optimization of reaction and Supplementary Methods for the characterization data can be found in the Supplementary Information.
General procedure Ni-catalysed transamidation reaction
A 1-dram vial containing a magnetic stir bar was flame-dried under reduced pressure, and then allowed to cool under N2. The vial was charged with substrate SI-7 (0.200 mmol, 1.0 equiv) and the free-based amino ester (0.240 mmol, 1.2 equiv). The vial was flushed with N2, then taken into a glove box and charged with Ni(cod)2 (0.010–0.020 mmol, 5–10 mol%) and SIPr (0.020–0.040 mmol, 10–20 mol%). Subsequently, toluene (0.20 ml, 1.0 M) was added. The vial was sealed with a Teflon-lined screw cap, removed from the glove box and stirred at 35 °C for 14 h. After cooling to 23 °C, the mixture was diluted with hexanes (0.5 ml) and filtered over a plug of silica gel (10 ml of ethyl acetate eluent). The volatiles were removed under reduced pressure, and the crude residue was purified by preparative thin-layer chromatography.
Gram-scale transamidation procedure
A scintillation vial containing a magnetic stir bar was flame-dried under reduced pressure, and then allowed to cool under N2. The vial was charged with SI-7 (2.22 mmol, 1.0 equiv) and the free-based amino ester SI-28 (2.66 mmol, 1.2 equiv). The vial was flushed with N2, then taken into a glove box and charged with Ni(cod)2 (0.111 mmol, 5 mol%) and SIPr (0.222 mmol, 10 mol%). Subsequently, toluene (2.22 ml, 1.0 M) was added. The vial was sealed with a Teflon-lined screw cap, removed from the glove box and stirred at 35 °C for 14 h. After cooling to 23 °C, the mixture was diluted with hexanes (5.0 ml) and filtered over a plug of silica gel (100 ml of EtOAc eluent). The volatiles were removed under reduced pressure, and the crude residue was purified via flash chromatography (20:1 hexanes:EtOAc→15:1→5:1) to yield amide product 33 (quant. yield).
Additional information
How to cite this article: Baker, E. L. et al. A two-step approach to achieve secondary amide transamidation enabled by nickel catalysis. Nat. Commun. 7:11554 doi: 10.1038/ncomms11554 (2016).
Supplementary Material
Supplementary Figures 1-46, Supplementary Table 1, Supplementary Methods and Supplementary References
Acknowledgments
We are grateful to Boehringer Ingelheim, DuPont, Bristol-Myers Squibb, the Camille and Henry Dreyfus Foundation, the A. P. Sloan Foundation, the S. T. Li Foundation and the University of California, Los Angeles. E.L.B. acknowledges a National Science Foundation graduate fellowship (DGE-1144087) and Y.Z. thanks the UCLA CSST Program for financial support. These studies were also supported by shared instrumentation grants from the NSF (No. CHE-1048804) and the National Center for Research Resources (No. S10RR025631).
Footnotes
Author contributions Authorship was assigned on the basis of the contributions of each author to the work described in the manuscript (detailed below) following a discussion involving all authors. N.K.G. wrote the manuscript and all authors contributed to the reading and editing of the manuscript. E.L.B., M.M.Y., Y.Z. and S.M.A. conducted the chemical reactions described in the manuscript. E.L.B., M.M.Y. and S.M.A. compiled the Supplementary Information.
References
- Corey E. J. & Cheng X.-M. The Logic of Chemical Synthesis Wiley-VCH (1995). [Google Scholar]
- Hudlicky T. & Reed J. W. The Way of Synthesis: Evolution of Design and Methods for Natural Products Wiley-VCH (2007). [Google Scholar]
- González-Rosende M. E., Castillo E., Lasri J. & Sepúlveda-Arques J. Intermolecular and intramolecular transamidation reactions. Prog. React. Kinet. Mech. 29, 311 (2004). [Google Scholar]
- Pattabiraman V. R. & Bode J. W. Rethinking amide bond synthesis. Nature 480, 471–479 (2011). [DOI] [PubMed] [Google Scholar]
- Loomis W. D. & Stumpf P. K. in Transamination and Transamidation (Nitrogen Metabolism) eds Allen E. K.et al. 249–250Springer Berlin Heidelberg (1958). [Google Scholar]
- Sergeeva M. V., Mozhaev V. V., Rich J. O. & Khmelnitsky Y. L. Lipase-catalyzed transamidation of non-activated amides in organic solvent. Biotechnol. Lett. 22, 1419–1422 (2000). [Google Scholar]
- Dineen T. A., Zajac M. A. & Myers A. G. Efficient transamidation of primary carboxamides by in situ activation with N,N-dialkylformamide dimethyl acetals. J. Am. Chem. Soc. 128, 16406–16409 (2006). [DOI] [PubMed] [Google Scholar]
- Allen C. L., Atkinson B. N. & Williams J. M. J. Transamidation of primary amides with amines using hydroxylamine hydrochloride as an inorganic catalyst. Angew. Chem. Int. Ed. 51, 1383–1386 (2012). [DOI] [PubMed] [Google Scholar]
- Bon E., Bigg D. C. H. & Bertrand G. Aluminum chloride-promoted transamidation reactions. J. Org. Chem. 59, 4035–4036 (1994). [Google Scholar]
- Eldred S. E., Stone D. A., Gellman S. H. & Stahl S. S. Catalytic transamidation under moderate conditions. J. Am. Chem. Soc. 125, 3422–3423 (2003). [DOI] [PubMed] [Google Scholar]
- Greenberg A., Breneman C. M. & Liebman J. F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science Wiley-VCH (2002). [Google Scholar]
- Pauling L., Corey R. B. & Branson H. R. The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl Acad. Sci. USA 37, 205–211 (1951). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsen S. K., May P. D. & Summers J. B. Di-tert-butyl N-acylimidodicarbonates as isolable acylating agents: mild conversion of primary carboxamides to substituted amides. J. Org. Chem. 56, 5482–5485 (1991). [Google Scholar]
- Mucsi Z., Chass G. A. & Csizmadia I. G. Amidicity change as a significant driving force and thermodynamic selection rule of transamidation reactions. A synergy between experiment and theory. J. Phys. Chem. B 112, 7885–7893 (2008). [DOI] [PubMed] [Google Scholar]
- Tasker S. Z., Standley E. A. & Jamison T. F. Recent advances in homogeneous nickel catalysis. Nature 509, 299–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesganaw T. & Garg N. K. Ni- and Fe-catalyzed cross-coupling reactions of phenol derivatives. Org. Process Res. Dev. 17, 29–39 (2013). [Google Scholar]
- Rosen B. M. et al. Nickel-catalyzed cross-couplings involving carbon-oxygen bonds. Chem. Rev. 111, 1346–1416 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hie L. et al. Conversion of amides to esters by the nickel-catalysed activation of amide C–N bonds. Nature 524, 79–83 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weires N. A., Baker E. L. & Garg N. K. Nickel-catalysed Suzuki-Miyaura coupling of amides. Nat. Chem. 8, 75–79 (2016). [DOI] [PubMed] [Google Scholar]
- Gillis E. P., Eastman K. J., Hill M. D., Donnelly D. J. & Meanwell N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decase (2001–2011). Chem. Rev. 114, 2432–2506 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-46, Supplementary Table 1, Supplementary Methods and Supplementary References