Abstract
Disseminated tumor cells (DTCs) in the bone marrow (BM) and circulating tumor cells (CTCs) in blood of breast cancer patients (pts) are known to correlate with worse outcome. Here we demonstrate a different prognostic value of DTCs and CTCs and explain these findings by early clodronate intake. CTCs (n = 376 pts) were determined using the AdnaTest BreastCancer (Qiagen Hannover GmbH, Germany) and DTCs (n = 525 pts) were analyzed by immunocytochemistry using the pan-cytokeratin antibody A45-B/B3. Clodronate intake was recommended in case of DTC-positivity. CTCs were detected in 22% and DTCs in 40% of the pts, respectively. DTCs were significantly associated with nodal status (p = 0.03), grading (p = 0.01), lymphangiosis (p = 0.03), PR status (p = 0.02) and clodronate intake (p < 0.0001), no significant associations were demonstrated for CTCs. CTCs significantly correlated with reduced PFS (p = 0.0227) and negative prognostic relevance was predominantly related to G2 tumors (p = 0.044), the lobular (p = 0.024) and the triple-negative subtype (p = 0.005), HR-negative pts (p = 0.001), postmenopausal women (p = 0.013) and patients who had received radiation therapy (p = 0.018). No prognostic significance was found for DTCs. Therefore early clodronate intake can improve prognosis of breast cancer patients and CTCs might be a high risk indicator for the onset of metastasis not limited to bone metastasis.
Despite major improvements in diagnosis and treatment, about 30% of primary breast cancer patients show a relapse of the disease years after first diagnosis which is explained by micrometastatic spread to the bone marrow (BM) by disseminated tumor cells (DTCs) being present in up to 40% of the patients1. After the publication of three large cohorts of primary breast cancer patients, the presence and persistence of DTCs during recurrence–free follow-up has been widely accepted as an independent prognostic marker with regard to increased risk for progression free survival (PFS) and overall survival (OS)2,3,4. However, although DTCs have even been used as a monitoring tool for treatment in primary breast cancer BM aspiration is very invasive and less accepted by patients due to pain and discomfort5. In this regard, increasing evidence suggested that circulating tumor cells (CTCs) in blood could be a useful biomarker to estimate risk for recurrence in breast cancer. The prognostic significance of these cells has already been demonstrated by different groups6,7,8 and very recently, the independent prognostic relevance of CTCs, both before and after adjuvant chemotherapy, has been demonstrated in an impressive cohort of 2026 primary breast cancer patients participating in a large prospective clinical trial9.
Phenotyping of both cell types in primary breast cancer has demonstrated that a proportion of DTCs and CTCs are present in a non-proliferative state and have stem-cell like characteristics, which may explain resistance to conventional chemotherapeutic drugs10,11,12,13. Furthermore, a discordant receptor status between the primary tumor and these cells with regard to the human epidermal growth factor receptor 2 (HER2) and the estrogen receptor (ER) has been demonstrated which might lead to uneffective trastuzumab and/or antihormonal treatment14,15,16. Thus, alternative, chemotherapy-as well as receptor independent therapies are urgently needed to eradicate minimal residual disease.
One approach targeting these cells might be the use of bisphosphonates (BPs). In this regard, the intake of clodronate has been shown to increase OS, reduce the frequency of skeletal complications as well as the incidence and number of new bony and visceral metastases in women with breast cancer who were at high risk for distant metastases17,18. Applying zoledronic acid (ZOL) in patients undergoing adjuvant hormonal therapy (ABCSG-12, ZO-FAST) resulted in a prolonged PFS19,20,21. In contrast, the AZURE trial (ZOL added to adjuvant therapy), only showed an improved OS for patients who were postmenopausal for at least 5 years22. However, analysing the data of 18206 women with BP intake over a period of two to five years, a highly significant reduction in local and distant recurrence, bone recurrence and breast cancer mortality was confirmed for postmenopausal women, whereas no effect could be shown for premenopausal women23.
Up to now, four small pilot studies have demonstrated that ZOL as well as clodronate contributed to eradicate DTCs, even after years of first diagnosis24,25,26,27. In a cohort of 394 patients, after a seven year follow-up, we recently demonstrated that DTCs were not associated with worse prognosis and speculated that this result was associated with the recommendation of early clodronate intake in case of DTC-positivity28. However, clodronate intake could only be confirmed in about 46% of DTC-positive patients whereas in the other patients, clodronate intake was unknown.
In the current study, we evaluated DTCs and CTCs in a cohort of 525 primary breast cancer patients diagnosed between 2004 and 2009 with a median follow up of five years and interviewed all DTC-positive patients for clodronate intake. Here we demonstrate a different prognostic value of DTCs and CTCs with regard to PFS which probably can be explained by early intake of clodronate according to DTC status at primary diagnosis.
Materials
Patient population and patient characteristics
The study was conducted at the Department of Gynecology and Obstetrics in Essen. In total, 525 primary, non-metastatic breast cancer patients with first diagnosis between Feb 2004 and Dec 2009, in an adjuvant setting, have been evaluated. Patients’ characteristics at the time of diagnosis are shown in Table 1.
Table 1. Clinical data of patients.
| Total | Total | CTC pos(%) | p-value | Total | DTC pos(%) | p-value |
|---|---|---|---|---|---|---|
| 376 | 84 (22) | 525 | 211 (40) | |||
| Median age | 60 years (range 27–86 years) | |||||
| Tumor size | ||||||
| pT1 | 252 | 51 (20) | 0.37 | 329 | 127 (39) | 0.07 |
| pT2-4 | 121 | 83 (43) | ||||
| pT5 | 3 | 1 (33) | ||||
| Nodal Status | ||||||
| Node negative | 256 | 55 (21) | 0.53 | 349 | 129 (37) | 0.03 |
| Node positive | 119 | 29 (24) | 174 | 82 (47) | ||
| Histology | ||||||
| Ductal | 289 | 65 (22) | 0.06 | 397 | 164 (41) | 0.54 |
| Lobular | 47 | 15 (32) | 70 | 24 (34) | ||
| Others | 40 | 4 (10) | 58 | 23 (40) | ||
| Grading | ||||||
| I | 75 | 13 (17) | 0.45 | 94 | 25 (27) | 0.01 |
| II | 205 | 50 (24) | 277 | 118 (43) | ||
| III | 96 | 21 (22) | 153 | 68 (44) | ||
| Lymphangiosis | ||||||
| Negative | 307 | 69 (22) | 0.92 | 418 | 159 (38) | 0.03 |
| Positive | 65 | 15 (23) | 103 | 51 (50) | ||
| Haemangiosis | ||||||
| Negative | 371 | 84 (23) | 0.99 | 510 | 205 (40) | 0.88 |
| Positive | 1 | 0 (0) | 8 | 3 (38) | ||
| ER Status | ||||||
| Negative | 60 | 14 (23) | 0.85 | 94 | 44(47) | 0.14 |
| Positive | 315 | 70 (22) | 430 | 166 (39) | ||
| PR Status | ||||||
| Negative | 74 | 16 (22) | 0.86 | 136 | 66 (49) | 0.02 |
| Positive | 301 | 68 (23) | 388 | 144 (37) | ||
| Her2 Status | ||||||
| Negative | 312 | 68 (21) | 0.22 | 438 | 170 8399 | 0.19 |
| Positive | 52 | 15 (29) | 84 | 39 (46) | ||
| Menopausal Status | ||||||
| Premenopausal | 45 | 11 (24) | 0.75 | 72 | 35 (49) | 0.30 |
| Perimenopausal | 44 | 8 (18) | 67 | 26 (39) | ||
| Postmenopausal | 287 | 65 (23) | 386 | 150 (39) | ||
| Immunhistochemical Subtype | ||||||
| (ER−, PR−, Her2−) | 40 | 9 (23) | 0.47 | 61 | 27 (44) | 0.22 |
| (ER−, PR−, Her2+) | 12 | 2 (17) | 24 | 14 (58) | ||
| (ER+ and/ or PR+, Her2−) | 281 | 59 (21) | 377 | 143 (38) | ||
| (ER+ and/ or PR+, Her2+) | 41 | 13 (32) | 61 | 25 (41) | ||
| Bone marrow Status | ||||||
| Negative | 243 | 59 (24) | 0.25 | |||
| Positive | 131 | 25 (19) | ||||
| Bisphosphonate Intake | ||||||
| No | 254 | 58 (23) | 0.69 | 342 | 43 (13) | <0.0001 |
| Yes | 119 | 25 (21) | 179 | 164 (92) | ||
Study design
We conducted a prospective study, which we analyzed retrospectively at a single institution to determine the prognostic value of DTCs in the BM and CTCs in blood of patients with primary, non-metastatic breast cancer in an adjuvant setting. The median follow-up time was 58.6 months (range: 0 to 117.8 months).
Therapeutic strategy for BP intake: Since 1997, all DTC-positive patients, presenting with primary diagnosis of breast cancer in our clinic, were recommended an additional therapy with oral clodronate (2 × 520 mg per day for at least two years), based on the publication of Diel et al.17, in the New Engl J Med17. In a first analysis of 400 patients, diagnosed between 1998 and 2003, no prognostic impact of DTCs could be documented28 and a small pilot study demonstrated a positive effect of ibandronate treatment (50 mg per day) on the eradication of DTCs, still present 2–10 years after primary diagnosis25. Since the intake of clodronate was only known for a subgroup of patients and, furthermore, depended on the doctor in charge outside our clinic, we here enquired whether our recommendation had been followed interviewing patients (doctors) to evaluate the impact of clodronate on the eradication of DTCs. 15 DTC negative patients were treated with ZOL according to ABCSG-12 data and ZO-FAST study19,21.
Eligibility criteria
The eligibility criteria were: histologically proven breast cancer, BM aspiration at time of primary diagnosis, no severe uncontrolled co-morbidities or medical conditions, no further malignancies at present or in history, completion of adjuvant treatment according to guidelines29 including adjuvant chemotherapy (anthracyclines, 5-fluorouracil, taxanes, cyclophosphamide), anti-hormonal therapy in case of hormone responsive tumors (tamoxifen or an aromatase inhibitor), trastuzumab in case of HER2-positivity (after FDA approval in November 2006) and radiotherapy. Patients treated with neoadjuvant chemotherapy were excluded.
Selection and detection of DTCs
Between 10 and 20 ml BM were aspirated from the anterior iliac crests of all primary breast cancer patients at the beginning of surgery of the primary tumor, before start of any therapy and processed within 24 hours. BM tumor cell isolation and detection have been described elsewhere15. Briefly, BM cells were isolated from heparinized BM (5000 U/ml BM) by Ficoll-Hypaque density gradient centrifugation (density 1.077 g/mol; Pharmacia, Freiburg, Germany) at 400x g for 30 min. Slides were analyzed for DTCs by immunocytochemistry using the pan-cytokeratin antibody A45-B/B3. Microscopic evaluation of the slides was carried out using the ARIOL system (Applied Imaging).
Selection, detection and evaluation of CTCs
Two × 5 ml EDTA blood were collected from 376 patients for the isolation of CTCs before the application of therapeutic substances and before surgery with an S-Monovette® (Sarstedt AG & Co.) and stored at 4 °C until further examination. The samples were processed immediately or latest 4 hours after blood withdrawal. CTCs were analyzed with the AdnaTest BreastCancer (AdnaGen AG, Langenhagen, Germany). Establishment and validation of this assay has been described in detail elsewhere12,15. Briefly, all samples underwent immunomagnetic enrichment using the AdnaTest BreastCancerSelect followed by RNA isolation and subsequent gene expression analysis [EpCAM (GA733-2), MUC-1, HER2] by reverse transcription and Multiplex-PCR in separated tumor cells using the AdnaTest BreastCancerDetect. The AdnaTest BreastCancer for the evaluation of CTCs is considered positive if a PCR fragment of at least one tumor associated transcript is clearly detected. Visualization of the PCR fragments was carried out with a 2100 Bioanalyzer using the DNA 1000 LabChips (Agilent Technologies) and the Expert Software Package (version B.02.03.SI307) both Böblingen, Germany. Peaks with a concentration of >0.15 ng/μl are positive for the transcripts GA733-2, MUC1 and HER2. Peaks that are not detected at the above setting are negative (concentration of <0.15 ng/μl). The primers generate fragments of the following sizes: GA 733-2: 395 base pairs (bp), MUC1: 293 bp, HER2: 270 bp and actin: 114 bp.
Immunohistochemical analysis of the primary tumor
For each of the 525 patients, the tumor type, TNM-staging and grading were assessed in the Departments of Pathology of our University Hospital.
Statistical analysis
Relationship of DTC/CTC positivity with presented parameters has been assessed by logistic regression modelling. Time related event data (PFS/OS) has been evaluated by applying uni- and bivariable Cox-Regression models. Univariable models have been applied to all presented parameters, providing hazard ratio estimates and their 95% confidence intervals [CIs]. Bivariable models were presented including DTCs/CTCs and one additional parameter as well as their interaction term. Besides assessment of statistical significance of bivariable model related parameters’ effects, pairwise subgroups’ specific hazard ratio estimates have been presented directly allowing for quantification of related to parameter constellation in-/decrease in hazard of event.
Study approval/Informed consent/Accordance
All specimens were obtained after written informed consent from all subjects prior to inclusion in the study and collected using protocols approved by the clinical Ethic committee of the University Hospital Essen (05/2856). All methods were carried out in accordance with the approved guidelines.
Results
Patients’ characteristics
Clinical data are shown in detail in Table 1. A total of 525 patients were included into the study. The median age of the patients was 60 years, range 27 to 86 years. 329/525 (63%) patients had T1 tumors, 349/525 (66%) were node-negative, most of the patients had a ductal carcinoma and a predominantly poor or moderately differentiated tumor. 64% of the patients were lymph node negative, ER and PR positivity was observed in 82% (430/525) and 74% (388/525) of the tumors, respectively. In 16% (84/525) of the cases, HER2 was overexpressed. Classifying tumors in subtypes based on their ER, PR and HER2 expression, 72% (377/525) of the tumors were ER and/or PR positive and HER2-negative, 12% (61/525) were triple-negative (ER−/PR−/ HER2−) and 5% (24/525) of the tumors only expressed HER2 (ER−/PR−/HER2+).
After a median follow-up time of 58.6 months (range: 0 to 117.8 months), the OS rate was 92% (483/525 patients) and relapses occurred in 12% (65/525 patients) of cases.
Correlation of DTCs and CTCs with established prognostic markers and outcome
As apparent from Table 1, the detection rate for CTCs was 22% (84/376 patients) and DTCs were detected in 211/525 patients (40%). Whereas no significant associations with clinical parameters were found for CTCs, DTCs were significantly associated with nodal status (p = 0.03), grading (p = 0.01), lymphangiosis (p = 0.03), PR status (p = 0.02) but not with CTC-status (p = 0.25).
CTCs significantly correlated with reduced PFS (p = 0.023; Fig. 1) and its negative prognostic relevance was predominantly related to the lobular subtype (p = 0.024), G2 tumors (p = 0.044), the triple-negative subtype (p = 0.005), postmenopausal women (p = 0.013) and patients who had received radiation therapy (p = 0.018) (Table 2). Although no significant correlations with regard to OS could be demonstrated for CTCs alone (p = 0.267; Fig. 1), within triple-negative patients as well as within HR-negative patients, CTC-positive patients had a significantly shorter OS compared to CTC-negative subjects (p = 0.024) (Table 2).
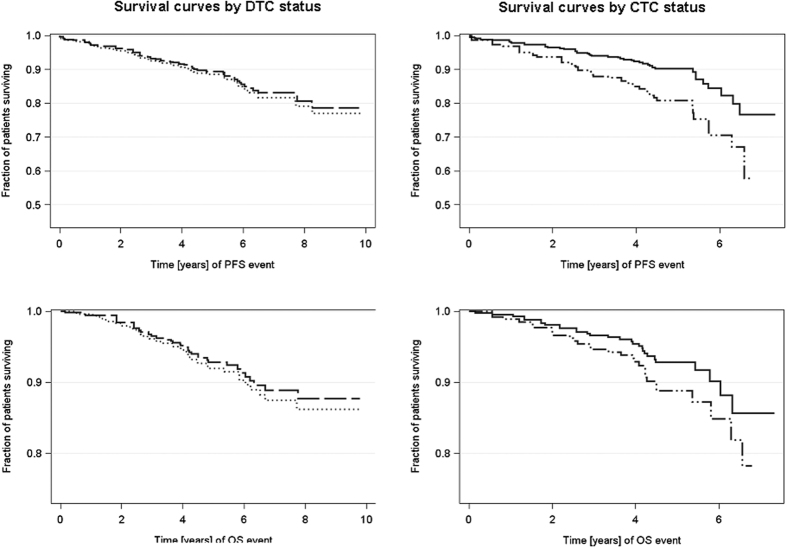
Figure 1. Prognostic significance of CTCs and DTCs with regard to PFS and OS.
Kaplan-Meier curves were drawn in order to compare PFS and OS with regard to CTCs in blood and DTCs in the BM. Whereas no significant associations could be shown for DTCs with regard to PFS [P = 0.7215; HR 0.916 (0.564–1.487)] and OS [P = 0.6953; HR 0.885 (0.481–1.630)], CTCs significantly correlated with PFS [P = 0.0227; HR 2.058 (1.106–3.828)] but not with OS [P = 0.267; HR1.588 (0.702–3.595)]. Univariable Cox Regression model-estimated survival curves. Dotted line = DTC negative patients [n = 314], longdashed line = DTC positive patients [n = 211], solid line = CTC negative patients [n = 292], mediumdashdotdot’ed line = CTC positive patients [n = 84].
Table 2. Univariable Analysis and Bivariable Cox Regressions.
| Univariable Analysis and Bivariable Cox Regressions | CTCs | DTCs | ||
|---|---|---|---|---|
| Univariable Analysis | PFS | OS | PFS | OS |
| CTCs | 2.058 [1.106–3.828); p = 0.023 | 1.588 [0.702–3.595], p = 0.2671 | – | – |
| DTCs | – | – | 0.916 [0.564–1.487], p = 0.722 | 0.885 [0.481–1.630], p = 0.695 |
| Bivariable Analysis | ||||
| Histology | ||||
| Ductal Subtype | 1.728 [0.822–3.634], p = 0.149 | 1.405 [0.505–3.905], p = 0.515 | 0.802 [0.459–1.402], p = 0.439 | 0.688 [0.326–1.452], p = 0.326 |
| Lobular Subtype | 4.621 [1.227–17.405], p = 0.024 | 3.540 [0.705–17.787], p = 0.125 | 2.011 [0.539–7.502], p = 0.298 | 3.114 [0.570–17.027], p = 0.190 |
| Grading | ||||
| G1 | 1.281 [0.143–11.477], p = 0.825 | 2.588 [0.234–28.567], p = 0.438 | 2.324 [0.469–11.521], p = 0.302 | 2.426 [0.342–17.235], p = 0.376 |
| G2 | 2.353 [1.023–5.410], p = 0.044 | 1.540 [0.542–4.379], p = 0.418 | 0.695 [0.347–1.392], p = 0.305 | 0.490 [0.201–1.193], p = 0.116 |
| G3 | 1.929 [0.679–5.482], p = 0.218 | 1.329 [0.274–6.435], p = 0.724 | 0.915 [0.434–1.928], p = 0.815 | 1.335 [0.496–3.596], p = 0.567 |
| IHC-Subtype | ||||
| ER+ and or PR+/HER2− | 2.096 [0.949–4.628], p = 0.067 | 1.466 [0.472–4.553]; p = 0.508 | 1.126 [0.611–2.074], p = 0.704 | 1.039 [0.454–2.377], p = 0.929 |
| ER+ and or PR+/HER2+ | n.a., no events CTC+ | n.a., no events CTC+ | 0.454 [0.088–2.345], p = 0.346 | 0.752 [0.125–4.510], p = 0.755 |
| HER2+/ER−/PR− | 3.671 [0.694–19.420], p = 0.126 | 1.128 [0.12–10.206], p = 0.915 | 0.123 [0.032–0.481], p = 0.003 | 0.056 [0.007–0.465], p = 0.008 |
| Triple-negative | 8.565 [1.897–38.661], p = 0.005 | 7.924 [1.315–47.769], p = 0.024 | 1.660 [0.446–6.185], p = 0.450 | 2.229 [0.533–9.331], p = 0.272 |
| Radiotherapy | ||||
| (yes) | 2.434 [1.165–5.087],p = 0.018 | 2.121 [0.860–5.231], p = 0.103 | 0.937 [0.526–1.671], p = 0.827 | 1.165 [0.590–2.300], p = 0.660 |
| (no) | 1.059 [0.205–5.467], p = 0.945 | n.a., no events CTC+ | 2.113 [0.596–7.494], p = 0.247 | 0.894 [0.149–5.356], p = 0.903 |
| Hormone Receptor | ||||
| (pos) | 1.529 [0.707–3.304], p = 0.281 | 1.066 [0.353–3.217], p = 0.910 | 0.950 [0.526–1.715], p = 0.865 | 0.829 [0.375–1.835], p = 0.644 |
| (neg) | 6.910 [2.188–21.823], p = 0.001 | 4.310 [1.150–16.123], p = 0.030 | 0.708 [0.293–1.709], p = 0.442 | 0.889 [0.333–2.370], p = 0.814 |
| Menopausal status | ||||
| Post | 2.270 [1.183–4.453], p = 0.014 | 1.850 [0.802–4.266], p = 0.149 | 0.722 [0.410–1.270], p = 0.258 | 0.850 [0.433–1.667], p = 0.636 |
| Prä | 1.187 [0.123–11.453], p = 0.882 | n.a., no events CTC+ | 2.271 [0.601–8.576], p = 0.226 | 1.194 [0.199–7.165], p = 0.847 |
n.a. not applicable.
Hazard ratio (p-value); presented HR are estimated for DTC+/CTC + cases within respective subgroup [=row] from Cox regression model.
In contrast to CTCs, no prognostic significance could be shown for DTCs with regard to PFS (p = 0.722) and OS (0.695) (Fig. 1). Although no significant correlations with regard to outcome could be demonstrated for DTCs alone, within the subgroup of HER2-positive patients, DTC-negative patients had a significantly shorter PFS (p = 0.003) and OS (p = 0.008) compared to DTC-positive patients.
Effect of bisphosphonate intake on outcome
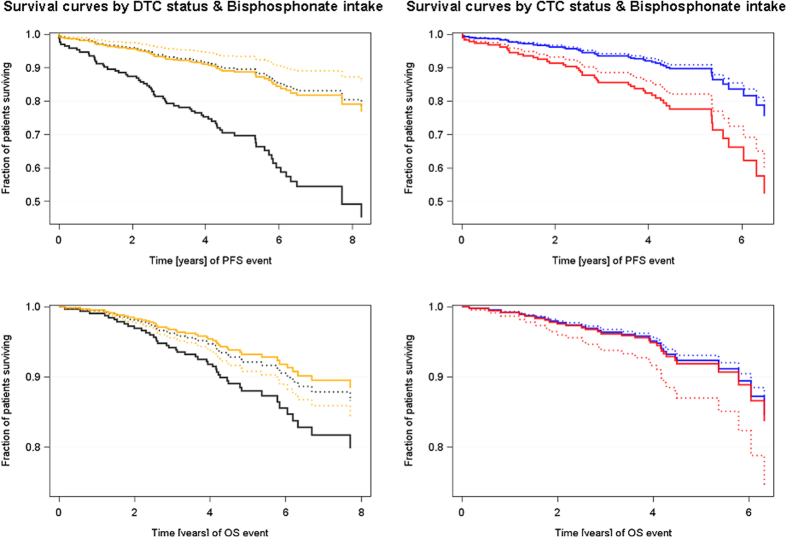
Table 1 illustrates BP intake of the patients. Among the 211 DTC-positive patients, 207 could be interviewed for BP intake. The group of 179 patients who had received BP therapy consisted of 164 DTC-positive patients who took clodronate (2 × 520 mg per day) for the duration of two years and 15 postmenopausal DTC-negative patients who had received ZOL (4 mg, twice a year) for the duration of three years. Among the 342 patients who had not taken BPs, 43 were DTC-positive. When the effect of BP intake was correlated with outcome (Fig. 2), patients were stratified into four groups: DTC-positive/BP yes (164 patients), DTC-positive/BP no (43 patients), DTC-negative/BP no (314 patients) and DTC-negative/BP (ZOL) yes (n = 15). As shown in Fig. 2, no significant differences with regard to PFS were found between the first three groups whereas DTC-negative patients who had received ZOL seemed to have a worse PFS when compared to DTC-negative patients taking no BPs (p = 0.014) as well as to DTC-positive patients who took BPs (p = 0.025). No significant differences with regard to OS were found between the four groups analyzed. When PFS and OS was evaluated in pre- and postmenopausal patients comparing those exposed to BPs to those not exposed to BPs, no differential effect of BPs according to menopausal status could be obtained (Supplementary Table 1a–d).
Figure 2. Influence of Bisphosphonates intake on PFS and OS.
Kaplan-Meier curves were drawn in order to compare PFS and OS with regard to BP intake in four study groups. DTC-negative patients who had received ZOL seemed to have a worse PFS when compared to DTC-negative patients taking no BPs [p = 0.014; HR 3.268 (1.277–8.364)] as well as to DTC-positive patients who took clodronate [p = 0.025; HR 0.332 (0.126–0.873)]. No significant differences with regard to OS were found between the four groups analyzed. CTC-positive patients who had received clodronate, had a significantly shorter PFS than CTC-negative patients receiving no BPs [p = 0.040; HR 2.629 (1.049–6.591]. No differences in all four groups were shown for OS. Bivariable Cox Regression model-estimated survival curves. Black dotted line = DTC negative patients without BPs [n = 299], black solid line = DTC negative patients with BPs (zoledronic acid) [n = 15], orange dotted line = DTC positive patients without BPs [n = 43], orange solid line = DTC positive patients with BPs [n = 164], blue dotted line = CTC negative patients without BPs [n = 196], blue solid line = CTC negative patients with BPs [n = 94], red dotted line = CTC positive patients without BPs [n = 58], red solid line = CTC positive patients with BPs [n = 25].
When this patient stratification was applied for CTCs with regard to PFS, CTC-positive patients who had received BPs, had a significantly shorter PFS than CTC-negative patients receiving no BPs (p = 0.040). Notably, only 34% of the CTC-positive patients were also DTC-positive. No differences in all four groups were shown for OS.
Discussion
The presence of DTCs in the BM and CTCs in blood of breast cancer patients were shown to provide independent prognostic information in a variety of studies and can be regarded as an early indicator of therapy failure2,3,4,9. In our current study, contradictory to previous findings of other groups, we demonstrated no prognostic value of DTCs with regard to PFS and OS and explain these findings by early clodronate intake, according to the presence of DTCs at primary diagnosis. In contrast, CTCs were significantly associated with reduced PFS. Thus, we assume that CTCs might be a high risk indicator for the onset of metastasis not limited to bone metastasis.
The limitation of this study is that we did not perform a randomized study. However, one has to consider that the evaluation of DTCs is a special offer, not belonging to daily clinical routine. Thus, offering this method and leaving DTC-positive patients untreated would have been rejected by our local ethic committee and furthermore, would have resulted in no commitment to this study by the patients.
We detected DTCs in 211 of 525 (40%) of our patients which was significantly associated with positive nodal status, higher grading, lymphangiosis and positive PR status but could not show any prognostic significance with regard to PFS. Although no significant correlations with regard to OS could be demonstrated for DTCs alone, in the subgroup of HER2-positive and DTC-negative patients had a significantly shorter PFS (p = 0.003) and OS (p = 0.008) compared to DTC-positive patients which might be explained by the fact that trastuzumab has not been available for most of our study patients during that time. However, in contrast to DTC negative patients the DTC positives received BPs which might explain their better outcome. The positivity rate of DTCs in our patients is in concordance with what previous authors have already reported, with a diagnostic rate of 20% to 40% regardless of nodal status1,30. The association between DTC status and standard prognostic markers has been discussed controversially. Although most research groups reported a positive association between DTCs and pathological stage and tumor grade, others have failed to document any correlation of DTCs with established clinical prognostic markers15,31,32,33. The fact that we could not demonstrate any prognostic significance between DTCs and PFS as well as OS is in contrary to a variety of published studies. While Braun et al., confirmed in his large pooled analysis that DTC detection at the time of primary diagnosis independently predicted an unfavorable outcome with level 1 evidence, others have confirmed that the persistence of DTCs in the BM after adjuvant treatment was an independent marker for reduced PFS and OS2,3,16.
The detection rate for CTCs was 22% (84/376 patients) and was not associated with any other clinicopathological factor. In contrast to DTCs, CTCs significantly correlated with reduced PFS and negative prognostic relevance was predominantly related to the lobular subtype, G2 tumors, the triple-negative subtype, postmenopausal women, HR-negative patients and patients who had received radiation therapy. As already documented for DTCs, the detection rate for CTCs is in accordance with a variety of other published studies using molecular and immunocytological methods34,35. Furthermore, the prognostic significance of these cells in primary breast cancer before and after adjuvant treatment has already been demonstrated6,7,8,9.
Only a few research groups compared the relationship between the presence of DTCs in the BM and CTCs in blood, generally showing a weak correlation. Whereas one study documented a significant congruence between DTCs and CTCs36, interestingly, most other groups propose that the prognosis of women with primary breast cancer depends on DTCs rather than CTCs and that CTCs seem to be less prognostic37,38,39. In our study, no significant correlation between DTCs and CTCs was found but, in contrast to other studies, CTCs rather than DTCs were significantly prognostic for early relapse. These findings underline our assumption that CTCs, probably circulating from reservoirs in lung or liver, might be a high risk indicator for already ongoing metastasis not limited to bone metastasis. In this regard, Deng et al. analyzed PI3K mutations in DTCs, CTCs and metastases from breast cancer patients and found that single cell analysis of CTCs can reveal genotypic heterogeneity that may change over time, and can show mutational discordance with DTCs and distant metastases13. Furthermore, using comparative genomic hybridization and next generation sequencing for the comparison of primary tumors, metastases and CTCs, Heitzer et al., demonstrated that most mutations initially found only in CTCs were also present at subclonal level in the primary tumors and metastases from the same patient40.
However, although tumor cell dissemination to the bone occurs in about 30% of the patients, only a minority of DTC-positive patients will develop distant metastasis in the course of the disease. Unfortunately, it is currently not predictable which of these cells will evolve into metastasis. It has been shown that DTCs show a clinically significant biological heterogeneity41. By use of comparative genomic hybridization, Mathiesen et al., demonstrated that the frequency of copy number changes of the DTCs revealed similarities with primary breast tumors in more than two third of the analyzed DTCs and similar aberration patterns were visible in DTCs collected at diagnosis and at 3 years relapse-free survival42. Whole-genome amplification and subsequent next generation sequencing of two primary breast tumors and corresponding DTCs recently showed comparable whole- arm gains or losses but also clear differences between the primary tumor and DTCs indicating that DTCs underwent further evolution43.
In addition, we and others recently demonstrated that a subset of DTCs/CTCs show stem cell character or undergoes epithelial mesenchymal transition (EMT) which might explain why several treatment options are not able to eradicate these cells10,11,12. The fact that the negative prognostic impact of CTCs in our study was mostly related to the triple-negative subtype and patients who had received radiation therapy is in good concordance with our previous studies and can be explained by resistance formation due to metabolism changes in EMT or stem cell like CTCs, demonstrating that CTCs show EMT and tumor stem cell characteristics12,15. Thus, down regulating targets like ER, PR and HER2, once in circulation, probably makes targeted therapy less effective. Preliminary data of a subgroup of our patients strengthens the assumption that stem cell like CTCs are present among CTCs detected in these patients. Whether these cells predict worse outcome has to be further analyzed in a bigger patient cohort.
The presence as well as the persistence of DTCs and CTCs after therapy clearly indicates a rationale for testing of alternative or secondary treatment options. In this regard, some efforts, including additional chemo, -antibody or BP therapy, have already been published.
Chemotherapy
In a Norwegian study, patients were treated with docetaxel 100 mg/m2, 3qw, 6 courses, if DTC were present 6 months after having completed anthracycline-based chemotherapy. As a consequence, most of the patients experienced disappearance of DTCs with a change to DTC-negativity in 79% of the cases5. The success of a switch from anthracycline to docetaxel even if not targeted, might be explained by the different intracellular targets. Whereas anthracycline acts as DNA damaging agent and mRNA synthesis blocker, taxols mitostatic effects result from interactions with microtubuli ant prevents cell division. However, since this study was no randomized trial, other treatments like endocrine and or trastuzumab treatment might have eradicated these cells.
Targeted therapy
A variety of studies including ours have indicated that HER2 expression on both DTCs and CTCs differed from HER2 expression in the primary tumor and that the expression of HER2 on DTCs and CTCs was correlated with poor prognosis16,44. Due to this disconcordance, a recently published study indicated that only a minority of patients with HER2-positive DTCs were treated with trastuzumab16. In this regard, two pilot studies showed that trastuzumab is able to eliminate these cells45,46. In the present study, especially for the triple negative subtype, we found a negative correlation for the presence of CTCs and PFS. These findings raise the question, whether a further molecular characterization with regards to ER, PR and HER2 expression on CTCs might be able to provide predictive information of a potential phenotype switch compared to the primary tissue, as already shown by our group15 that could lead new therapeutic opportunities.
Bisphosphonates
Since all targeted therapies can only eliminate DTCs/CTCs presenting the target of interest, receptor independent therapies are highly appreciated to eliminate the residual disease. In this regard, the intake of BPs has been discussed to be successful in eradicating DTCs. In a cohort of 394 patients, diagnosed between 1998 and 2003, we recently demonstrated that DTCs were not associated with worse prognosis and speculated that this result was associated with the recommendation of early clodronate intake in case of DTC-positivity at primary diagnosis28. In four small pilot studies, ZOL as well as ibandronate were shown to contribute to eradication of DTCs, even after years of first diagnosis. Rack et al., demonstrated that the administration of 4 mg ZOL per month for the duration of 6 months in patients with persisting DTCs resulted in an elimination of DTCs in 87% of the patients24 and Solomayer et al. showed a positive effect of ZOL concerning the change of DTCs in the BM of patients with primary non-metastatic breast cancer resulting in a higher number of DTC-negative BM cases in the ZOL group as compared to the control group after 12 months26. In the study by Banys et al., primary, DTC-positive breast cancer patients were randomized to treatment with ZOL plus adjuvant systemic therapy or adjuvant systemic therapy alone showing that all ZOL treated patients were DTC-negative after 24 months and that patients with persisting DTCs 12 months after first diagnosis had a significantly shorter OS27. We demonstrated that DTCs present in the BM 2–10 years after first diagnosis were successfully eliminated by ibandronate treatment (50 mg per day) for 12 months in all patients25. In a retrospective single-center analysis with 3141 early stage breast cancer patients, Hartkopf et al. confirmed the prognostic impact of DTCs with regard to DFS and OS and further showed that application of BP treatment was significantly associated with increased DFS and OS in DTC-positive patients, whereas no effect was seen in DTC negative patients4. However, there were some differences with regard to our study. The decision, to use BPs was either due to enrolment in studies that included BP therapy or based on the decision of the treating physician. Furthermore, they stated that BPs were mostly used in postmenopausal, hormone receptor positive and DTC positive patients. In our study, BPs were offered in case of DTC-positivity, independent of menopausal status. They further showed that cytotoxic treatment before BM sampling was the strongest risk factor for DTC detection whereas the remaining factors had no impact on DTC status in the multivariate analysis. It is very important to know that these results were due to the inclusion of 391 neoadjuvant patients who were excluded in our study and published separately recently47.
However, although these studies impressively documented the antineoplastic effect of BPs on DTCs, big clinical BP studies, especially the AZURE trial, showing an improved survival only for patients who were postmenopausal for at least five years, dampened the euphoria that BPs might be the therapy of choice for eliminating minimal residual disease in all breast cancer patients22. With regard to clodronate, the NSABP B-34 trial (patients randomized to receive clodronate, 1.600 mg or placebo for three years) and the GAIN trial (patients randomized to receive oral ibandronate, 50 mg daily for two years) have confirmed the restricted benefit of BPs48,49. Apart from the ABCSG 12 and the ZOFAST trial, the majority of studies, addressing the antineoplastic effect of BPs, included very heterogenous patient populations, different BPs with different durations of treatment which made difficulties in interpreting the results19,20,50,51.
In our patient cohort, no significant differences for DTCs in pre-and postmenopausal women were observed with regard to PFs and OS. This might be due to the small number of patients in these subgroups. Interestingly, a negative prognostic impact with regard to PFS could be demonstrated for CTC-positive, postmenopausal women when compared with CTC-negative women.
Nevertheless, the benefit of BPs in the pre- or perimenopausal setting is very questionable. The meta-analysis of the Early Breast Cancer Trialists´ Colloborative Group had influence on clinical practice, especially for postmenopausal women who showed highly significant reductions in recurrence, distant recurrence, bone recurrence and breast cancer mortality resulting in the decision to treat this patient group with BPs23. According to the results of the ABCSG-12 trial, only premenopausal women receiving LHRH antagonists or developing complete ovarian suppression following adjuvant chemotherapy had a benefit from BPs19. In our study, the number of patients/events per sub group was too small to draw any conclusions on the missing differential effect of BPs according to menopausal status on PFS and OS.
In conclusion, the limitation of this study is that we did not perform a randomized study. However, one has to consider that the evaluation of DTCs is a special offer, not belonging to daily clinical routine. Thus, offering this method and leaving DTC-positive patients untreated would have been rejected by our local ethic committee and furthermore, would have resulted in no commitment to this study by the patients. Nevertheless, although our control group of 43 patients is too small to draw the final conclusion that the lack of prognostic value of DTCs is likely to be secondary to BPs, we here demonstrate that DTC-positive patients, receiving clodronate at primary diagnosis, have the same prognosis as patients with no DTCs, independent of their menopausal status. Further prospective studies with higher patient numbers will have to answer that question. Interestingly, CTCs might be a high risk indicator for the onset of metastasis not limited to bone metastasis.
Additional Information
How to cite this article: Kasimir-Bauer, S. et al. Different prognostic value of circulating and disseminated tumor cells in primary breast cancer: Influence of bisphosphonate intake? Sci. Rep. 6, 26355; doi: 10.1038/srep26355 (2016).
Supplementary Material
Footnotes
S.K.-B. is a consultant for QIAGEN, Hannover GmbH, Germany.
Author Contributions All authors have made substantive intellectual contributions to this study and have given final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content. S.K.-B. Study design, supervision, data collection, data analysis, wrote the main manuscript K.R. data collection B.A. did BM aspirations and blood sampling, included patients into the study A.-K.B. wrote the main manuscript, data analysis S.W. statistics, figures T.K. statistics, figures R.K. did BM aspirations and blood sampling, included patients into the study O.H. Study design, supervision, data collection, did BM aspirations and blood sampling, included patients into the study, wrote the main manuscript.
References
- Banys M., Krawczyk N. & Fehm T. The role and clinical relevance of disseminated tumor cells in breast cancer. Cancers 6, 143–152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005). [DOI] [PubMed] [Google Scholar]
- Janni W. et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse – a European pooled analysis. Clin. Cancer Res. 17, 2967–2976 (2011). [DOI] [PubMed] [Google Scholar]
- Hartkopf A. D. et al. Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients-results from a large single-centre analysis. Eur J Cancer 50, 2550–9 (2014). [DOI] [PubMed] [Google Scholar]
- Synnestvedt M. et al. Disseminated tumor cells as selection marker and monitoring tool for secondary adjuvant treatment in early breast cancer. Descriptive results from an intervention study. BMC Cancer 12, 616 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatiadis M. et al. Molecular detection and prognostic value of circulating cytokeratin-19 messenger RNA-positive and HER2 messenger RNA-positive cells in the peripheral blood of women with early-stage breast cancer. Clin. Breast Cancer 7, 883–889 (2007). [DOI] [PubMed] [Google Scholar]
- Xenidis N. et al. Cytokeratin-19 mRNA-Positive Circulating Tumor Cells After Adjuvant Chemotherapy in Patients With Early Breast Cancer. J. Clin. Oncol. 27, 2177–2184 (2009). [DOI] [PubMed] [Google Scholar]
- Lucci A. et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 13, 688–695 (2012). [DOI] [PubMed] [Google Scholar]
- Rack B. et al. SUCCESS Study Group. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J. Natl. Cancer Inst 106, 10.1093/jnci/dju066 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic M. et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin. Cancer Res. 12, 5615–5621 (2006). [DOI] [PubMed] [Google Scholar]
- Reuben J. M. et al. Primary breast cancer patients with high risk clinicopathologic features have high percentages of bone marrow epithelial cells with ALDH activity and CD44(+)CD24(lo) cancer stem cell phenotype. Eur. J. Cancer 47, 1527–1536 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasimir-Bauer S., Hoffmann O., Wallwiener D., Kimmig R. & Fehm T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 14, 15 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G. et al. Single cell mutational analysis of PIK3CA in circulating tumor cells an metastases in breast cancer reveals heterogeneity, discordance and mutation persistence in cultured disseminated tumor cells from bone marrow. BMC Cancer 14, 456 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehm T. et al. ERalpha-status of disseminated tumour cells in bone marrow of primary breast cancer patients. Breast Cancer Res. 10, 76 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehm T. et al. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 11, 59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartkopf A. D. et al. The HER2 status of disseminated tumor cells in the bone marrow of early breast cancer patients is independent from primary tumor and predicts higher risk of relapse. Breast Cancer Res. Treat. 138, 509–517 (2012). [DOI] [PubMed] [Google Scholar]
- Diel I. J. et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N. Engl. J. Med. 339, 357–363 (1998). [DOI] [PubMed] [Google Scholar]
- Diel I. J. et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases in bone marrow: a long term follow-up. Ann. Oncol. 9, 2007–2011 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnant M. et al. Austrian Breast and Colorectal Cancer Study Group, Vienna, Austria. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 12, 631–641 (2011). [DOI] [PubMed] [Google Scholar]
- Gnant M. et al. Austrian Breast and Colorectal Cancer Study Group, Vienna, Austria. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann. Oncol. 26, 313–320 (2015). [DOI] [PubMed] [Google Scholar]
- Coleman R. et al. Zoledronic acid (zoledronate) for postmeopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60 months results. Ann. Oncol. 24, 398–405 (2013). [DOI] [PubMed] [Google Scholar]
- Coleman R. et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol 15, 997–1006 (2014). [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomized trials. Lancet 386, 1353–1361 (2015). [DOI] [PubMed] [Google Scholar]
- Rack B. et al. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res. 30, 1807–1813 (2010). [PubMed] [Google Scholar]
- Hoffman, et al. Effect of ibandronate on disseminated tumor cells in the bone marrow of patients with primary breast cancer: A pilot study. Anticancer Res. 10, 3623–3628 (2011). [PubMed] [Google Scholar]
- Solomayer E. F. et al. Influence of zoledronic acid on disseminated tumor cells in primary breast cancer patients. Ann. Oncol. 23, 2271–2277 (2012). [DOI] [PubMed] [Google Scholar]
- Banys M. et al. Influence of zoledronic acid on disseminated tumor cells in bone marrow and survival: results of a prospective clinical trial. BMC Cancer 13, 480 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann O. et al. Disseminated tumor cells in the bone marrow of primary, non-metastatic breast cancer patients are not associated with worse outcome: A seven year follow-up study. Archives of Gynecology and Obstetrics 292, 1117–1125 (2015). [DOI] [PubMed] [Google Scholar]
- AGO-Empfehlungen gynäkologische Onkologie Kommission Mamma http://www.ago-online.de/de/infothek-fuer-aerzte/leitlinienempfehlungen/mamma (2006) (Date of access: 21/05/2009).
- Pantel K. & Alix-Panabières C. Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep. 3, 584 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidard F. C. et al. Disseminated tumor cells of breast cancer patients: a strong prognostic factor for distant and local relapse. Clin. Cancer Res. 14, 3306–3311 (2008). [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S. et al. Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer 116, 3330–3337 (2010). [DOI] [PubMed] [Google Scholar]
- Farmen R. K. et al. Bone marrow cytokeratin 19 mRNA level is an independent predictor of relapse-free survival in operable breast cancer patients. Breast Cancer Res. Treat. 108, 251–258 (2008). [DOI] [PubMed] [Google Scholar]
- Lianidou E. S., Mavroudis D. & Georgoulias V. Clinical challenges in the molecular characterization of circulating tumour cells in breast cancer. Br. J. Cancer 108, 2426–2432 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosse S. A., Gorges T. M. & Pantel K. Biology, Detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 7, 1–11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindlbeck C. et al. Comparison of circulating tumor cells (CTC) in peripheral blood and disseminated tumor cells in the bone marrow (DTC-BM) of breast cancer patients. J Cancer Res. Clin. Oncol. 139, 1055–1062 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoy I. H. et al. Real-time RT-PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br. J. Cancer 94, 672–680 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedswang G. et al. Comparison of the clinical significance of occult tumor cells in blood and bone marrow in breast cancer. Int. J. Cancer 118, 2013–2019 (2006). [DOI] [PubMed] [Google Scholar]
- Bidard F. C. et al. Disseminated tumor cells of breast cancer patients: a strong prognostic factor for distant and local relapse. Clin. Cancer Res. 14, 3306–3311 (2008). [DOI] [PubMed] [Google Scholar]
- Heitzer E. et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 73, 2965–2975 (2013). [DOI] [PubMed] [Google Scholar]
- Synnestvedt M. et al. Disseminated tumour cells in the bone marrow in early breast cancer: morphological categories of immunocytochemically positive cells have different impact on clinical outcome. Breast Cancer Res. Treat. 138, 485–497 (2013). [DOI] [PubMed] [Google Scholar]
- Mathiesen R. R. et al. High-resolution analyses of copy number changes in disseminated tumor cells of patients with breast cancer. Int. J. Cancer 131, 405–415 (2012). [DOI] [PubMed] [Google Scholar]
- Møller E. K. et al. Next-generation sequencing of disseminated tumor cells. Front. Oncol. 3, 320 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S. et al. ErbB2 overexpression on occult metastatic cells in bone marrow predicts poor clinical outcome of stage I-III breast cancer patients. Cancer Res. 61, 1890–1895 (2001). [PubMed] [Google Scholar]
- Rack B. et al. Trastuzumab clears HER2/neu-positive isolated tumor cells from bone marrow in primary breast cancer patients. Arch. Gynecol. Obstet. 285, 485–492 (2012). [DOI] [PubMed] [Google Scholar]
- Georgoulias V. et al. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy- resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann. Oncol. 23, 1744–1750 (2012). [DOI] [PubMed] [Google Scholar]
- Kasimir-Bauer S. et al. Does primary systemic therapy eradicate disseminated and circulating tumor cells? Breast Cancer Res. 18, 20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A. H. et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 13, 734–742 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möbus V., Diel I. J.& Harbeck H. GAIN study: A phase III trial to compare ETC vs EC-TX and ibandronate vs observation in patients with node-positive primary breast cancer -1st interim efficacy analysis. Cancer Res. 71, 2–4 (2012). [Google Scholar]
- Smith I. E. Do BIG1-98 and ZOFAST demand a change in guidelines for endocrine therapy? Breast Cancer Res. 11, 18 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim H. A., Kamal N. S. & Malak R. A. Bisphosphonates in the adjuvant treatment of young women with breast cancer: the estrogen rich is a poor candidate! J. Thorac. Dis. 5, 27–35 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.