Abstract
Genes acquired by horizontal transfer are increasingly being found in animal genomes. Understanding their origin and evolution requires knowledge about the phylogenetic relationships from both source and recipient organisms. We used RNASeq data and respective assembled transcript libraries to trace the evolutionary history of polygalacturonase (pectinase) genes in stick insects (Phasmatodea). By mapping the distribution of pectinase genes on a Polyneoptera phylogeny, we identified the transfer of pectinase genes from known phasmatodean gut microbes into the genome of an early euphasmatodean ancestor that took place between 60 and 100 million years ago. This transfer preceded the rapid diversification of the suborder, enabling symbiont-free pectinase production that would increase the insects’ digestive efficiency and reduce dependence on microbes. Bacteria-to-insect gene transfer was thought to be uncommon, however the increasing availability of large-scale genomic data may change this prevailing notion.
Herbivores depend on plant cell wall degrading enzymes, such as cellulases and pectinases, to break down ingested matter. Any enzyme not produced endogenously (from the organisms’ own genomes) might be provided by symbionts residing in the gut1. However, occasionally horizontal gene transfer of enzymes from a microbe to an animal organism enables symbiont-independent digestion in the host2,3,4. While pectinase enzymes [endo- and exo-polygalacturonases] appear to rarely occur in animals, fungal and bacterial pectinase genes have been found as part of the genome of leaf beetles (Chrysomelidae) and weevils (Curculionidae), suggesting horizontal gene transfer events. These genes of putative microbial origin are expressed endogenously and have been experimentally shown to actually degrade pectin2,5,6.
Recently, multiple transcripts for highly expressed polygalacturonase (pectinase) genes were found in the midgut transcriptomes of six species of stick insects7 (Phasmatodea). The sequences showed high similarity to microbial pectinase genes, however the same genes were also found in genomic DNA from microbe-free brain tissue of these insect species7. This confirms that these pectinases and their eukaryote-specific signal peptides are endogenously transcribed by the insects and do not represent microbial contamination. This fact is further corroborated by their presence in multiple species reared on different diets and the fact that the phasmatodean digestive tract is unsuited for microbial fermentation8. Additionally, the reproductive physiology and behavior of Phasmatodea9 preclude vertical transmission of obligate symbionts due to the absence of egg-smearing, coprophagy, or adult-nymph trophyllaxis10, and both culturing and metagenomics assays have demonstrated an absence of symbiotic microbes8,11.
Therefore, it was assumed that these genes were acquired via horizontal gene transfer7, but definite evidence for this hypothesis was lacking and unanswered associated questions remained: What was the source of these genes? What was/is its ecological relationship to Phasmatodea? What are these enzymes’ activities? How did this gene family evolve after the transfer? Additionally, the timing of this putative horizontal gene transfer is undetermined. This is largely due to the paucity of molecular data for Phasmatodea—including the suborder Timematodea, which is the sister group of all remaining stick and leaf insects or Euphasmatodea12—and for closely related insect orders such as grasshoppers (Orthoptera), webspinners (Embioptera), or roaches (Blattodea)13.
Identifying a horizontal gene transfer requires data from the source and the recipient and/or its descendants. Additionally, outgroup taxa are needed to ensure the absence of the target gene, to determine that the horizontal gene transfer occurred within the group of interest or its common ancestor. Therefore, such findings are limited by the diversity of published sequences14,15. To address the questions outlined above, we combined biochemical analyses of these individual phasmatodean enzymes along with identification of these molecules within potential, microbial sources and potential insect hosts. To this end, we used assembled RNASeq data of phasmatodean and other polyneopteran insects sampled by the 1KITE consortium, (1K Insect Transcriptome Evolution Project, http://www.1kite.org).
Results
Activity of Individual Phasmatodea Pectinases
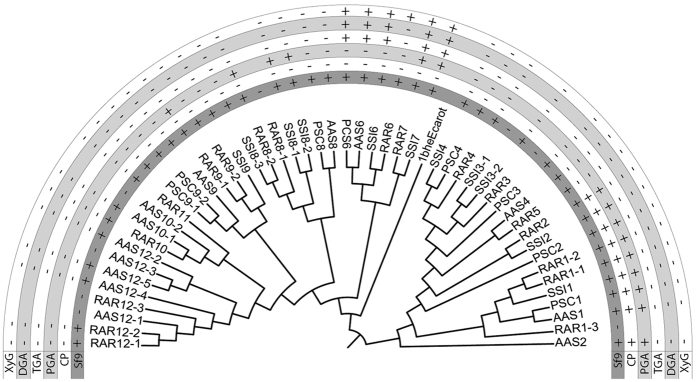
Gut extracts from all six species with published transcriptomes7 could degrade citrus pectin and polygalacturonic acid (PGA) (Fig. 1). From these six species we obtained full length ORFs for 93 pectinase enzymes, including some pectinase genes the original six transcriptomes missed (Table S1). From the four species chosen for downstream expression (comprising 50 enzymes in total), we successfully amplified 44 enzymes, transformed E. coli with 43, and expressed 42 in Sf9 cells (Figure S1, Table S1). Enzyme activity as tested with thin layer chromatography (TLC) (Figures S2–S6) and agarose diffusion assays (Figures S7 and S8) mostly correlated with the gene clustering (Fig. 2): enzymes with the same activity profiles formed monophyletic clades, such that the activity of an enzyme could be predicted by its position in the tree. Several enzymes could degrade citrus pectin and/or PGA to dimers and bigger oligomers, meaning they are endo-active polygalacturonases. Most enzymes in one monophyletic group could degrade PGA and the oligomers into galacturonic acid. This implies that they are exo-active enzymes. Enzymes in this clade were also active on xylogalacturonan. Other enzymes showed no detectable activity on any substrate, including enzymes known to be highly expressed such as PSC3, which is the most highly expressed pectinase and 10th most highly expressed enzyme in the Peruphasma schultei anterior midgut7.
Figure 1. Substrate tests of stick insect gut extracts.
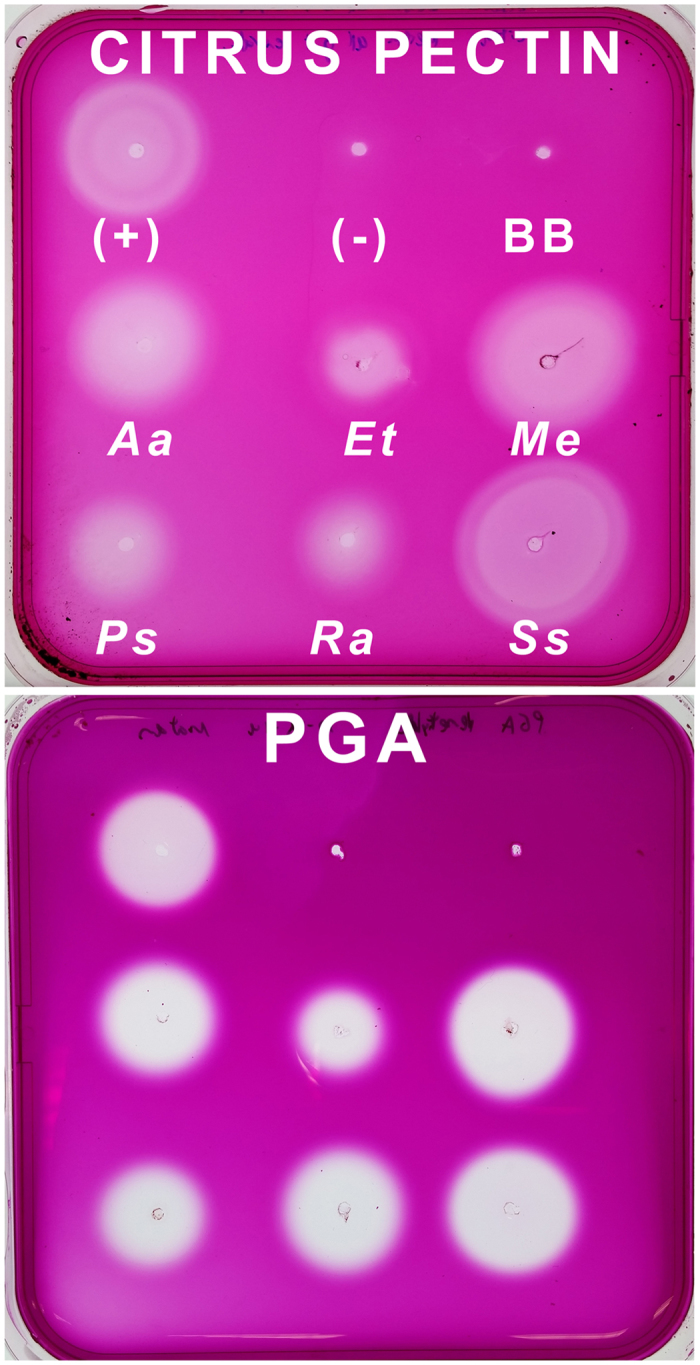
The positive control was the gut extract of the beetle Phaedon cochleariae (Chrysomelidae, Coleoptera). Blackberry leaves were used to control for background enzymes in the diet, although guts were purged of contents before use in this assay. Aa = Aretaon asperrimus. BB = Blackberry leaves. Et = Extatosoma tiaratum. Me = Medauroidea extradentata. PGA = Polygalacturonic acid. Ps = Peruphasma schultei. Ra = Ramulus artemis. Ss = Sipyloidea sipylus.
Figure 2. Maximum Likelihood tree and enzymatic activities of pectinases from four phasmatodean species.
The outgroup is a bacteria, Erwinia carotovora. A “+” in the Sf9 row means the gene was successfully amplified, transformed, and expressed into Sf9 cells, enabling later substrate tests. For the other rows, “+” means positive enzymatic activity and “−” means no activity detected when the expressed enzyme was tested on the given substrate. CP = citrus pectin, DGA = digalacturonic acid, PGA = polygalacturonic acid, TGA = trigalacturonic acid, XyG = xylogalacturonan.
Evidence for Horizontal Gene Transfer
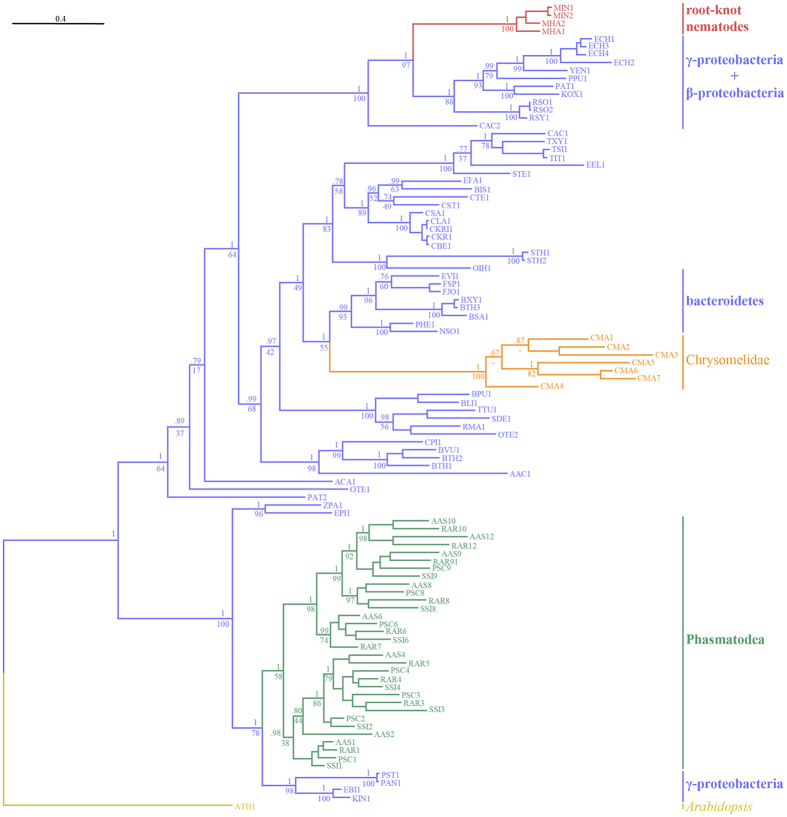
The Bayesian analysis for insect, nematode, and microbial GH28 pectinases converged after 300,000 generations with an average standard deviation of split frequencies of 0.014. The alpha-shape parameter of the gamma distribution was 1.8. Due to the bootstrap algorithm in RAxML, the Maximum Likelihood (ML) analysis converged after 450 bootstrap replicates. The Le and Gascuel model (LG) was determined as best-scoring evolutionary model; the estimated alpha-shape parameter of the gamma distribution was 1.7. The ML and Bayesian phylogenetic analyses converged, providing trees with almost the same topology. The Bayesian topology was chosen as reference topology, and the more conservative16,17 bootstrap support values obtained from the ML analysis were indicated on the corresponding branches (Fig. 3).
Figure 3. Phylogeny of the GH28 proteins.
To minimize the size of the tree, only pectinases from the four exemplar species of Phasmatodea used in the expression assays are included in this figure, with one representative from genes with multiple alleles. Gene tree topology obtained from the Bayesian analysis, (posterior probability support at nodes) chosen as a reference topology. Bootstrap values obtained from the ML analysis are indicated at the corresponding nodes below posterior probability values. The tree is represented as a cladogram with branch lengths and rooted with the A. thaliana sequence (GenBank Accession Number NP_850359.1) as outgroup. Branches are colored in violet if derived from bacterial sequences, in red if derived from nematode sequences, in orange if derived from beetle sequences, in green if derived from Phasmatodea sequences, and in yellow for the plant sequence outgroup. Species name abbreviations are indicated in Table S2.
This consensus pectinase phylogeny shows that genes of the GH28 family pectinases originated independently three times within the studied Metazoa. Root-knot nematode pectinases18 (Meloidogyne sp.) form a highly supported, monophyletic clade (posterior probability [PP]: 1.0, bootstrap support [BS]: 100) and are nested within gamma-proteobacteria, beta-proteobacteria, and Firmicutes (PP: 1.0, BS: 97). Pectinase genes identified in the leaf beetle Callosobruchus maculatus5 (Chrysomelidae, Coleoptera) form a monophyletic clade with maximal PP and BS support that clusters with Bacteroidetes (PP: 1.0, BS: 55). Finally, the phasmatodean pectinase genes form a third, distinct and well-supported clade (PP: 1.0, BS: 58), with two monophyletic sub-clades within. Their closest related sequences are a group of gamma-proteobacteria, including the genera Pantoea, Klebsiella, and Enterobacter (PP: 1.0, BS: 78).
Pectinases in the Polyneoptera
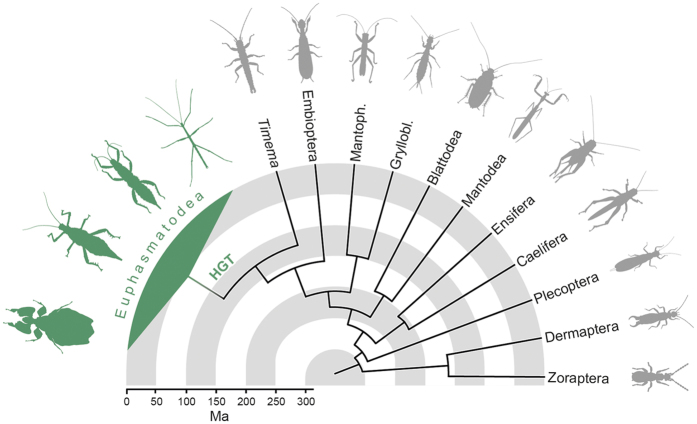
From the 1KITE transcriptome assemblies, we found pectinase transcripts in all phasmatodean insects in which relevant parts of the midgut were present, except Timema cristinae (Fig. 4, Table S3). To verify this finding, we also mined the draft genome for this species19, and found it also lacks pectinase genes. This confirms that pectinase genes are indeed probably absent in Timema. In addition, we could not identify any pectinases in the transcriptome assemblies of any other polyneopteran insects analyzed (Fig. 4, Table S3), implying that pectinases are either not expressed or were missing due to a very low expression level of respective genes, though the latter is unlikely for digestive enzymes at the sequence depth (~2.5 Gbases) used to generate these transcriptomes20.
Figure 4. Presence of endogenous pectinases in polyneopteran species.
The horizontal gene transfer (HGT) occurred after Euphasmatodea and Timematodea [identified by the single representative genus, Timema] diverged. Polyneopteran phylogenetic relationships and divergence times (in millions of years ago) are based on Misof et al.20 and Bradler et al.12.
Discussion
Although most herbivorous insects depend on symbionts in their digestive system to supply the required cellulase and/or pectinases enzymes to fully digest the plant cell walls, many do produce a subset of the necessary enzymes endogenously. It was long assumed that any enzymes not provided by symbionts are ancestral in insects21. This had been confirmed for GH9 cellulase, which is endogenously produced in various insect lineages21 including Phasmatodea7,22 and has even been hypothesized to be ancestral to all Metazoa23. In contrast, endogenous pectinase genes are only found in a few, distantly related insect lineages such as several groups of beetles5,24,25, leafhoppers26, aphids27, and phasmids7. In the present study, we identified, isolated, and successfully expressed multiple, endogenous, phasmatodean pectinase genes. They are found in representatives of all major phasmatodean lineages with the exception of Timematodea, which is the sister group to Euphasmatodea12,19. The pectinase genes are also absent in closely related groups such as grasshoppers, termites, and roaches (Fig. 4, Table S3; for a detailed discussion of the phylogeny of this lineage, see Beutel et al.13). The presence of endogenous pectinase is thus an evolutionary novelty for Euphasmatodea (i.e. all phasmids except Timematodea). Based on this finding and the fact that the most similar homologues to the phasmatodean pectinase genes are found in gamma-proteobacteria (Fig. 3), we conclude that the phasmatodean genes have been acquired from bacteria by horizontal gene transfer. A similar origin was assumed in beetles5 and nematodes15. However, our analysis indicates that phasmatodean pectinase genes are not closely related to those found in beetles or nematodes (Fig. 3). This implies several independent horizontal transfers for these genes in insects and in Ecdysozoa. Gamma-proteobacteria are the predominant microbes in the phasmatodean digestive tract8,11, which could suggest a symbiotic relationship, except that representatives of this group such as Enterobacter were also isolated from the rearing environment of Phasmatodea8 and were most likely transients obtained with the food. We therefore cannot clarify whether the genes identified in Euphasmatodea had been acquired from true symbionts or from microbes that occasionally occurred in the ancestral euphasmatodean digestive system. The alternative scenario, that all ecdysozoan or insect pectinase genes were inherited from a common ancestor and have been lost or are unexpressed in all other clades, is considerably less parsimonious. Furthermore, this alternative hypothesis would imply monophyly of ecdysozoan pectinases, which is not the case as they form at least three distinct clusters interspersed by bacterial homologs (Fig. 3).
The phylogeny of the euphasmatodean pectinase genes (Figs 2 and 3) suggests that originally only one or two genes or gene copies were transferred from bacteria. Prior to a later diversification of Euphasmatodea, these genes underwent further duplication and subfunctionalization28. Some copies retained the ancestral endo- and/or exo-acting activities, respectively capable of breaking down the polymers or smaller oligomers of galacturonic acid. Other gene copies most likely lost the ancestral pectinolytic functions, since we did not observe any activity on the substrates tested. Nevertheless, the high expression levels for these enzymes7 suggests they still have a (yet unknown) function. All active enzymes examined retained their canonical active sites, which are common to polygalacturonases29. Moreover, some inactive enzymes also retained active sites, so their inability to degrade the substrates provided remains to be explored (Table S1). For example, surrounding amino acid substitutions may modify the structure of the protein and prevent the active sites from being accessible to the substrate.
Phasmid pectinase enzyme activities were susceptible to the degree of methylation of the polygalacturonic acid substrate, raising the question of whether or not some need a pectin methylesterase enzyme (PME) to efficiently break down pectin. Bacterial pectinases in particular cannot degrade highly methylated pectins and depend on synergistic PME activity30, so finding these patterns in horizontally acquired pectinases in Phasmatodea is logical. However, a search for transcripts homologous to known PMEs (GenBank Accession No’s: AAM39440.1, ADO57389.1, AAK81304.1, AEE33008.1) was negative for all phasmatodean lineages. The same was observed for nematodes15. The possibility remains in Phasmatodea that other genes, perhaps even some of the otherwise inactive pectinases, have developed PME activity as a neofunctionalization. Alternatively, either symbiotic microbes in the phasmatodean gut are producing PMEs or the ingested plants’ own PMEs are still active in the stick insect gut. These hypotheses are presently being tested.
Timematodea contain only a single genus, Timema, with 21 described species found in the western United States31. Euphasmatodea split from Timematodea, which lack pectinase genes, between 125 (±60) and 103 (±19) million years ago12,20. The more than 3,000 described euphasmatodean species started to rapidly diversify from a common ancestor around 61 (±14) million years ago12,32. Thus, the horizontal gene transfer most likely took place within this range of ~40 million years between the split of Phasmatodea and the diversification of Euphasmatodea (Fig. 4). It remains an intriguing question whether the acquisition of endogenous pectinase genes may have influenced or even enabled this strong and rapid diversification of Euphasmatodea. This hypothesis was also proposed by Kirsch et al.5, who assumed that the acquisition of pectinase genes was a key event in the evolution of various herbivorous beetle groups. The alternative hypothesis—that the pectinase transfer occurred in an ancestor of all Phasmatodea and was subsequently lost in Timematodea—is less parsimonious but otherwise cannot be ruled out.
It is noteworthy that all insect clades for which pectinases have been identified are associated with a strictly plant-based diet. In sucking insects such as aphids27 and leafhoppers26, pectinases enzymes are involved in plant penetration33 and softening of the plant before oviposition34. Chewing insects like beetles and most likely phasmids use the enzymes for digestion of ingested plant matter21. Further studies may reveal whether such evolutionary events as horizontal gene transfer were present for other organisms that evolved similar dietary specialization.
The horizontal gene transfer from a transient microbe into its commensal host, as suggested here, demonstrates the various, unpredictable, and exciting pathways evolution can take. Our results show that pectinase genes were transferred from microbes to ecdysozoans several times independently, indicating that these cross-domain horizontal gene transfers may be much more common than previously thought15,35. If this can be verified in future research, the historical and present role of symbionts or transients in driving evolutionary diversification in their hosts must be reconsidered.
Materials and Methods
Whole midgut agarose diffusion assay for substrate activity
To test if Phasmatodea guts were pectinolytic, we filled square Petri-dishes with 0.1% solutions of either citrus pectin (Sigma) or polygalacturonic acid (PGA) (Megazyme) in 0.4% agarose and 50 mM citrate-phosphate buffer (pH 5.0). We made wells in the plates and filled them with 5 μL of macerated, whole midguts cleared of their contents and dissected from the aforementioned six species with the published midgut transcriptomes7: Aretaon asperrimus (Heteropteryginae), Peruphasma schultei (Pseudophasmatinae), Sipyloidea sipylus (Necrosciinae), and Extatosoma tiaratum (Lanceocercata: Extatosomatinae), Medauroidea extradentata, and Ramulus artemis (Clitumninae). We used pectinases from Aspergillus niger (Sigma) as positive control. Plates were incubated upside-down at 40 °C overnight, stained for one hour in 0.01% Ruthenium Red (Colour Index No. 77800) on a shaker at 20 rpm, and destained in diH2O. Enzyme activity was detectable as clearings in the stained gel.
Creating cDNA libraries and cloning of full length genes
Amino acid sequences from known pectinases of the glycoside hydrolase (GH) family 28 (www.cazy.org) were retrieved from GenBank (Accession Numbers: JQ728556.1, Y17906.1, EU450666.1). We used the tBLASTn algorithm36 with an e-value cutoff of 1E-10 to mine for homologous sequences to these from the six published phasmatodean midgut transcriptomes (Genbank Accession No. PRJNA238833 & PRJNA221630). For incomplete transcripts, we designed specific primers for 5′- and 3′-Rapid Amplification of cDNA Ends (RACE) PCR using the Primer3 program v0.4.0 (http://bioinfo.ut.ee/primer3-0.4.0/primer3/). From living, cultured specimens of these six species, we dissected the anterior midgut, removed the gut contents, and stored the tissue in RNAlater® solution (Qiagen). After maceration in a frozen Tissue Lyser, RNA was extracted using the innuPREP RNA MiniKit (Analytik-Jena) and purified with theRNeasy® MinElute® cleaning kit (Qiagen) following the manufacturers’ protocols. From the RNA, we synthesized cDNA and performed RACE PCR as needed with the SMARTer RACE cDNA Amplification Kit (BD Contech) following the manufacturer’s instructions. PCR products were cloned into One Shot® Top10 Chemically Competent E. coli cells with the pCR™4-TOPO/TA® Vector (Invitrogen), and subsequently sequenced by the Sanger method using M13 forward and reverse primers on an ABI 3730 xl automatic DNA sequencer (PE Applied Biosystems). Once we obtained complete open reading frames (ORFs) for every pectinase gene, they were converted to amino acid sequences and checked for eukaryote-specific signal peptides using the SignalP 4.1 server (http://www.cbs.dtu.dk/services/SignalP/). We annotated the sequences accordingly and deposited them in GenBank (Accession Numbers in Table S1).
Multiple alignment and inference of pectinase gene tree
For a general pectinase tree, the phasmid proteins were combined with those of bacteria, nematodes, and beetles as identified using an NCBI database search for glycoside hydrolase (GH) family 28 enzymes, which have a conserved, GH28 pectinolytic domain (Table S2), with Arabidopsis thaliana (GenBank Accession Number NP_850359.1) as outgroup37. These GH28 sequences were aligned using MAFFT38 with the L-INS-i option optimized for alignment of protein sequences with one conserved domain and allowing for long gaps whenever necessary. The alignment was pruned using trimAL39 to remove all positions containing more than 70% of gaps. The pruned alignment was used to infer gene trees with both, Bayesian and Maximum Likelihood (ML) methods. For the Bayesian tree we used MrBayes40 3.2.6 with an estimated gamma distribution among site rate variation (ASRV), along with a mixture of evolutionary models. A total of 300,000 generations were performed on 8 Monte Carlo Markov Chains (MCMC). We discarded a burn-in of 25% of sampled generations for inference of a consensus tree and calculation of posterior probabilities. The ML phylogeny was obtained using RAxML41 8.1.24 using the automated selection (AUTO) of the best fitting evolutionary model and an estimated gamma distribution of ASRV. The autoMRE option (default 0.03) was used to automatically conduct bootstopping, indicating that enough bootstrap replicates had been sampled to obtain bootstrap value convergence among the tree topologies. In both cases, the A. thaliana GH28 sequence37 was used as outgroup to root the trees. The trees were visualized and exported as figures using FigTree software (http://tree.bio.ed.ac.uk/software/figtree/).
Expression of pectinase genes in specific insect tissue
We chose four exemplar species, one from each taxonomic family, for downstream analysis: A. asperrimus, P. schultei, R. artemis, and S. sipylus. We designed gene-specific forward and reverse primers (Table S1) to amplify the complete ORF of each putative enzyme from the cDNA, and cloned them into Top10 cells with the pIB/V5-His TOPO/TA® vector (Invitrogen). We included Kozak sequences (RCCATGG) at the 3′ end of the forward primers and did not include the stop codon in the reverse primers. Colony PCR or direct sequencing was done to ensure the genes were cloned in the correct direction, then we extracted the plasmids with a GeneJET™ Plasmid Miniprep Kit (Thermo Scientific) and transfected them into Sf9 cells (Invitrogen) using the reagent FuGENE HD (Promega). Culture medium was harvested after 72 hours incubation at 27 °C and centrifuged, and the supernatant tested for successful expression via Western Blot (Figure S1) with anti-V5-HRP antibody (Invitrogen). Plate assays for substrate activity were performed on the individual enzymes following the same protocol as the whole gut extracts.
Thin Layer Chromatography (TLC) assays for substrate activity
Enzyme solutions were dialyzed in three baths of 50 mM citrate-phosphate buffer pH 5.0 at 4 °C using Slide-A-Lyzer Dialysis Cassettes (Thermo Scientific) with 10 KDa cutoffs, desalted in ZebaTM Desalt Spin Columns (Thermo Scientific) with 7 KDa cutoffs, and stored at 4 °C until use. 10 μL of desalted enzyme were combined in microcentrifuge tubes with 2 μL 0.2 M citrate phosphate buffer (pH 5.0) and 8 μL of the following ratios of 1% w/v substrate stock solutions and diH2O: 4:4 citrus pectin (Sigma), 4:4 soy- or potato- rhamnogalacturonan (Megazyme), 4:4 demethylated polygalacturonic acid (PGA) from citrus (Sigma), 1:7 trigalacturonic acid (TGA) (Megazyme), 2:6 digalacturonic acid (DGA) (Megazyme), and 4:4 xylogalacturonan produced following the protocol published by Beldman et al.25. We used pectinases from Aspergillus niger (Sigma) as positive control. The tubes were incubated for 16 hours at 40 °C, then spotted onto TLC plates (silica gel 60, 20 × 10 cm, Merck) and developed with 9:3:1:4 of ethyl acetate:acetic acid:formic acid:water. We used as reference standards 2 μg each of galacturonic acid, DGA, and TGA, as well as xylose mono-, di-, and trimers and galactose as needed (Megazyme). The dried plates were sprayed with 0.2% (w/v) orcinol in 9:1 methanol/sulfuric acid, and subsequently heated with a heat gun until spots appeared.
Timing the origin of the phasmatodean pectinases
As we did for the six transcriptomes, we mined the transcriptomes of 38 phasmatodean species, which broadly represent all recognized major lineages32, and 16 representative polyneopteran outgroups (GenBank Accession No: PRJNA183205, Table S3) for target genes. Digestive enzymes in stick insects are expressed in the anterior midgut7, which starts approximately at the thorax/abdomen border8. For most of the studied phasmids, the transcriptome was not taken from the entire animal but rather from the head and parts of the thorax. Table S3 provides a detailed list of which body parts were used for respective species. To ensure that the relevant midgut tissue had been included, we also mined for endogenous insect cellulase enzymes23 from GH family 9, which are also highly and differentially expressed in the anterior midgut7,22. Phasmids should have more than five genes from this group7. We therefore excluded in the present study all phasmatodean transcriptomes with five or fewer GH9 cellulase genes, which suggested the transcriptome did not include midgut tissue. For all outgroup taxa, the entire animal was used to generate the transcriptomes, ensuring the presence of all digestive tissue. To date the origin of the horizontal gene transfer we mapped the presence of endogenous pectinase genes on the dated phylogenies of Misof et al.20 and Bradler et al.12.
Ethics
All methods were carried out in accordance with local guidelines for animal research. All experimental protocols were approved by the Max Planck Institute for Chemical Ecology in accordance with these guidelines.
Data accessibility
Phasmatodea pectinase sequences are available under GenBank Accession Number’s KT921897-KT921989. 1KITE RNASeq data (BioProject PRJNA183205) is available from the Consortium upon request, with the Accession Numbers for published individual transcriptomes in Table S3.
Additional Information
How to cite this article: Shelomi, M. et al. Horizontal Gene Transfer of Pectinases from Bacteria Preceded the Diversification of Stick and Leaf Insects. Sci. Rep. 6, 26388; doi: 10.1038/srep26388 (2016).
Supplementary Material
Acknowledgments
Phasmatodea tissue was provided by Lynn Kimsey and Steve Heydon of the Bohart Museum of Entomology (USA), Benyo Andras of the Budapest Zoo (Hungary) and from private collectors Pedro Roldan Duran, Sacha Eilmus, Corinna Krempl, and Manuel Wagner (Germany). Phasmatodea transcriptomes were provided by Brian Johnson of the University of California, Davis (USA). Thanks to Roy Kirsch for his input and advising, and Bianca Wurlitzer and Domenica Schnabelrauch for technical assistance (Max Planck Institute for Chemical Ecology). We are grateful for receiving data of unpublished transcriptomes from 1KITE (Polyneoptera subgroup) and thank Sabrina Simon and Karen Meusemann (1KITE consortium, http://www.1kite.org) for providing the transcript libraries and useful comments and help during manuscript preparation. M.S. and Y.P. were funded by the National Science Foundation (USA) Postdoctoral Research Fellowship in Biology, Grant No. DBI-1402883, the Max Planck Society (Germany), and by funds from the University of California, Davis (USA). Funding support for the 1KITE Project and authors B.W. and X.Z. include: China National GeneBank and BGI-Shenzhen, China; German Research Foundation (NI 1387/ 1-1; MI 649/6, MI 649/10, RE 345/1-2, BE1789/8-1, BE 1789/10-1, STA 860/4, Heisenberg grant WA 1496/8-1); Austria Science Fund FWF; NSF (DEB 0816865); Ministry of Education, Culture, Sports, Science and Technology of Japan Grant-in-Aid for Young Scientists (B 22770090); Japan Society for the Promotion of Science (P14071); Deutsches Elektronen-Synchrotron (I-20120065); Paul Scherrer Institute (20110069); Schlinger Endowment to CSIRO Ecosystem Sciences; Heidelberg Institute for Theoretical Studies; University of Memphis-FedEx Institute of Technology; and Rutgers University.
Footnotes
Author Contributions M.S. and Y.P. conceived of the study, carried out lab work, performed data analysis, carried out sequence alignments, participated in the design of the study, and drafted the manuscript. S.B. and B.W. (1KITE member) contributed in writing the manuscript. E.G.J.D. performed the phylogenetic analyses of the pectinase genes. X.Z. (1KITE member) with the BGI team provided transcriptomes from 1KITE project. D.H. provided funding and resources. All authors approved this manuscript.
References
- Fischer R., Ostafe R. & Twyman R. M. Cellulases from Insects. Adv Biochem Eng Biotechnol 136, 51–64, doi: 10.1007/10_2013_206 (2013). [DOI] [PubMed] [Google Scholar]
- Pauchet Y. & Heckel D. G. The genome of the mustard leaf beetle encodes two active xylanases originally acquired from bacteria through horizontal gene transfer. Proc Biol Sci 280, 20131021, doi: 10.1098/rspb.2013.1021 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuna R. et al. Adaptive horizontal transfer of a bacterial gene to an invasive insect pest of coffee. Proc Natl Acad Sci USA 109, 4197–4202, doi: 10.1073/pnas.1121190109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boto L. Horizontal gene transfer in the acquisition of novel traits by metazoans. Proc Biol Sci 281, 20132450, doi: 10.1098/rspb.2013.2450 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch R. et al. Horizontal gene transfer and functional diversification of plant cell wall degrading polygalacturonases: Key events in the evolution of herbivory in beetles. Insect Biochem Mol Biol 52C, 33–50, doi: 10.1016/j.ibmb.2014.06.008 (2014). [DOI] [PubMed] [Google Scholar]
- Shen Z. et al. Polygalacturonase from Sitophilus oryzae: possible horizontal transfer of a pectinase gene from fungi to weevils. J Insect Sci 3, 24, doi: 10.1093/jis/3.1.24 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelomi M., Jasper W. C., Atallah J., Kimsey L. S. & Johnson B. R. Differential expression of endogenous plant cell wall degrading enzyme genes in the stick insect (Phasmatodea) midgut. BMC Genomics 15, 917, doi: 10.1186/1471-2164-15-917 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelomi M., Sitepu I. R., Boundy-Mills K. L. & Kimsey L. S. Review of the Gross Anatomy and Microbiology of the Phasmatodea Digestive Tract. Journal of Orthoptera Research 24, 29–40, doi: 10.1665/034.024.0105 (2015). [DOI] [Google Scholar]
- Severin H. P. & Severin H. C. The Mechanisms in the Hatching of the Walking Stick, Diapheromera femorata Say. In Stick Insects of the Continental United States and Canada: Species and Early Studies. (ed Chad Arment) (Coachwhip Publications, 2006). [Google Scholar]
- Kikuchi Y. & Fukatsu T. Insect-bacterium mutualism without vertical transmission. In Insect symbiosis Vol. 3 Contemporary topics in entomology series (eds Kostas Bourtzis & Thomas A. Miller) (CRC Press, 2003). [Google Scholar]
- Shelomi M., Lo W. S., Kimsey L. S. & Kuo C. H. Analysis of the gut microbiota of walking sticks (Phasmatodea). BMC Research Notes 6, 368, doi: 10.1186/1756-0500-6-368 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradler S., Cliquennois N. & Buckley T. R. Single origin of the Mascarene stick insects: ancient radiation on sunken islands? BMC Evolutionary Biology 15, 196, doi: 10.1186/s12862-015-0478-y (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutel R. G., Wipfler B., Gottardo M. & Dallai R. Polyneoptera or “Lower Neoptera” - New Light on Old and Difficult Phylogenetic Problems. Atti Accademia Nazionale Italiana di Entomologia 61, 113–142 (2013). [Google Scholar]
- Pauchet Y., Wilkinson P., Chauhan R. & ffrench-Constant R. H. Diversity of beetle genes encoding novel plant cell wall degrading enzymes. PLoS One 5, e15635, doi: 10.1371/journal.pone.0015635 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp A., Boschetti C., Perry M., Tunnacliffe A. & Micklem G. Expression of multiple horizontally acquired genes is a hallmark of both vertebrate and invertebrate genomes. Genome biology 16, 50, doi: 10.1186/s13059-015-0607-3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erixon P., Svennblad B., Britton T. & Oxelman B. Reliability of Bayesian posterior probabilities and bootstrap frequencies in phylogenetics. Syst Biol 52, 665–673 (2003). [DOI] [PubMed] [Google Scholar]
- García-Sandoval R. Why some clades have low bootstrap frequencies and high Bayesian posterior probabilities. Israel Journal of Ecology & Evolution 60, 41–44 (2014). [Google Scholar]
- Danchin E. G. et al. Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematodes. Proceedings of the National Academy of Sciences 107, 17651–17656, doi: 10.1073/pnas.1008486107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Carrasco V. et al. Stick insect genomes reveal natural selection’s role in parallel speciation. Science 344, 738–742, doi: 10.1126/science.1252136 (2014). [DOI] [PubMed] [Google Scholar]
- Misof B. et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767, doi: 10.1126/science.1257570 (2014). [DOI] [PubMed] [Google Scholar]
- Calderón-Cortés N., Quesada M., Watanabe H., Cano-Camacho H. & Oyama K. Endogenous Plant Cell Wall Digestion: A Key Mechanism in Insect Evolution. Annual Review of Ecology, Evolution, and Systematics 43, 45–71, doi: 10.1146/annurev-ecolsys-110411-160312 (2012). [DOI] [Google Scholar]
- Shelomi M., Watanabe H. & Arakawa G. Endogenous cellulase enzymes in the stick insect (Phasmatodea) gut. J. Insect Physiol. 60, 25–30, doi: 10.1016/j.jinsphys.2013.10.007 (2014). [DOI] [PubMed] [Google Scholar]
- Davison A. & Blaxter M. Ancient Origin of Glycosyl Hydrolase Family 9 Cellulase Genes. Mol Biol Evol 22, 1273–1284, doi: 10.1093/molbev/msi107 (2005). [DOI] [PubMed] [Google Scholar]
- Evangelista D. E., de Paula F. F. P., Rodrigues A. & Henrique-Silva F. Pectinases From Sphenophorus levis Vaurie, 1978 (Coleoptera: Curculionidae): Putative Accessory Digestive Enzymes. Journal of Insect Science 15, doi: 10.1093/jisesa/ieu168 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D.-S. et al. A culture-based study of the bacterial communities within the guts of nine longicorn beetle species and their exo-enzyme producing properties for degrading xylan and pectin. Journal of Microbiology (Seoul, Korea) 45, 394–401 (2007). [PubMed] [Google Scholar]
- Hail D., Lauzìere I., Dowd S. E. & Bextine B. Culture independent survey of the microbiota of the glassy-winged sharpshooter (Homalodisca vitripennis) using 454 pyrosequencing. Environmental Entomology 40, 23–29, doi: 10.1603/EN10115 (2011). [DOI] [PubMed] [Google Scholar]
- McAllan J. & Adams J. B. The significance of pectinase in plant penetration by aphids. Canadian Journal of Zoology 39, 305–310, doi: 10.1139/z61-034 (1961). [DOI] [Google Scholar]
- Lo N., Tokuda G. & Watanabe H. In Biology of Termites: A Modern Synthesis (eds David Edward Bignell, Yves Roisin & Nathan Lo) 51–67 (Springer, 2011). [Google Scholar]
- Pickersgill R., Smith D., Worboys K. & Jenkins J. Crystal structure of polygalacturonase from Erwinia carotovora ssp. carotovora. J Biol Chem 273, 24660–24664, doi: 10.1074/jbc.273.38.24660 (1998). [DOI] [PubMed] [Google Scholar]
- André-Leroux G., Tessier D. & Bonnin E. Action pattern of Fusarium moniliforme endopolygalacturonase towards pectin fragments: Comprehension and prediction. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics 1749, 53–64, doi: 10.1016/j.bbapap.2005.02.008 (2005). [DOI] [PubMed] [Google Scholar]
- Law J. H. & Crespi B. J. The evolution of geographic parthenogenesis in Timema walking-sticks. Mol Ecol 11, 1471–1489, doi: 10.1046/j.1365-294X.2002.01547.x (2002). [DOI] [PubMed] [Google Scholar]
- Bradler S., Robertson J. A. & Whiting M. F. A molecular phylogeny of Phasmatodea with emphasis on Necrosciinae, the most species-rich subfamily of stick insects. Systematic Entomology 39, 205–222, doi: 10.1111/syen.12055 (2014). [DOI] [Google Scholar]
- Cherqui A. & Tjallingii W. F. Salivary proteins of aphids, a pilot study on identification, separation and immunolocalisation. J. Insect Physiol. 46, 1177–1186, doi: 10.1016/S0022-1910(00)00037-8 (2000). [DOI] [PubMed] [Google Scholar]
- Boyd D. W., Cohen A. C. & Alverson D. R. Digestive enzymes and stylet morphology of Deraeocoris nebulosus (Hemiptera: Miridae), a predacious plant bug. Annals of the Entomological Society of America 95, 395–401, doi: 10.1603/0013-8746(2002)095[0395:DEASMO]2.0.CO;2 (2002). [DOI] [Google Scholar]
- Moran Y., Fredman D., Szczesny P., Grynberg M. & Technau U. Recurrent horizontal transfer of bacterial toxin genes to eukaryotes. Mol Biol Evol 29, 2223–2230, doi: 10.1093/molbev/mss089 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C. et al. BLAST+: architecture and applications. BMC bioinformatics 10, 421, doi: 10.1186/1471-2105-10-421 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X. et al. Sequence and analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature 402, 761–768, doi: 10.1038/45471 (1999). [DOI] [PubMed] [Google Scholar]
- Katoh K. & Standley D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772–780, doi: 10.1093/molbev/mst010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutiérrez S., Silla-Martínez J. M. & Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973, doi: 10.1093/bioinformatics/btp348 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic biology 61, 539–542, doi: 10.1093/sysbio/sys029 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690, doi: 10.1093/bioinformatics/btl446 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Phasmatodea pectinase sequences are available under GenBank Accession Number’s KT921897-KT921989. 1KITE RNASeq data (BioProject PRJNA183205) is available from the Consortium upon request, with the Accession Numbers for published individual transcriptomes in Table S3.