Abstract
It is widely accepted that cyanobacteria-dependent oxygen that was released into Earth's atmosphere ca. 2.5 billion years ago sparked the evolution of the aerobic metabolism and the antioxidant system. In modern aerobes, enzymes such as superoxide dismutases (SODs), peroxiredoxins (PXs), and catalases (CATs) constitute the core of the enzymatic antioxidant system (EAS) directed against reactive oxygen species (ROS). In many anaerobic prokaryotes, the superoxide reductases (SORs) have been identified as the main force in counteracting ROS toxicity. We found that 93% of the analyzed strict anaerobes possess at least one antioxidant enzyme, and 50% have a functional EAS, that is, consisting of at least two antioxidant enzymes: one for superoxide anion radical detoxification and another for hydrogen peroxide decomposition. The results presented here suggest that the last universal common ancestor (LUCA) was not a strict anaerobe. O2 could have been available for the first microorganisms before oxygenic photosynthesis evolved, however, from the intrinsic activity of EAS, not solely from abiotic sources. Key Words: Archaea—Atmospheric gases—Evolution—H2O2 resistance—Oxygenic photosynthesis. Astrobiology 16, 348–358.
1. Introduction
For its dual role in biological systems, oxygen has rightly been called a double-edged molecular sword. It is necessary for aerobic metabolism, and its deficiency hinders normal growth and development. But at the same time the reduction of molecular oxygen in biological systems results in the formation of reactive oxygen species (ROS). The most common ROS include singlet oxygen (1O2), the superoxide anion radical (O2−•), hydrogen peroxide (H2O2), and the hydroxyl radical (HO•). Uncontrolled ROS production can result in oxidation of various vulnerable molecules, disturbance in cellular homeostasis, and ultimately cell death (Benzie, 2000; Ślesak et al., 2007). However, at early stages of Earth evolution, before the appearance of life ca. 3.8–3.5 billion years ago (Holland, 2006; Miller and Cleaves, 2007), the atmosphere was anoxic; thus it is widely accepted that the first living cells were strict anaerobes (Halliwell and Gutteridge, 1999). With this in mind, a number of paradigms concerning antioxidants and antioxidant metabolism have been accepted, as follows: (1) aerobic metabolism may not have occurred until after oxygen release into Earth's atmosphere ca. 2.7–2.2 billion years ago by cyanobacterial oxygenic photosynthesis; (2) the antioxidant cellular machinery evolved at the same time as aerobic metabolism and oxygenic photosynthesis (Halliwell and Gutteridge, 1999; Alberts et al., 2002; De Las Rivas et al., 2004; Shaw, 2008). However, recently several lines of evidence suggest that the appearance of oxygenic photosynthesis preceded the Great Oxidation Event of Earth's atmosphere, and as a consequence, oxygen oases, possibly even with micromolar O2 concentrations, were present in the Archean ocean (Olson et al., 2013; Kaufman, 2014).
Aerobes are equipped with a sophisticated antioxidant defense system that involves ROS detoxification. It constitutes low molecular antioxidants (i.e., glutathione, ascorbate, tocopherols, etc.) and antioxidant enzymes that belong to the enzymatic antioxidant system (EAS), which regulates the cellular ROS homeostasis (Benzie, 2000; Ślesak et al., 2007; Foyer and Noctor, 2009; Schippers et al., 2012; Karpiński et al., 2013). The primary antioxidant enzyme that converts O2−• to H2O2 and O2 is the superoxide dismutase (SOD; EC 1.15.1.1):
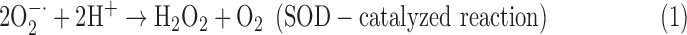 |
Four main classes of SOD, as indicated by metal ions present at the active site, have been identified: copper/zinc (Cu/ZnSOD), iron (FeSOD), manganese (MnSOD), and nickel (NiSOD) (Alscher et al., 2002; Miller, 2012). The MnSOD and FeSOD are very similar in their primary and tertiary structures, whereas Cu/ZnSOD features structural differences (Fink and Scandalios, 2002; Wolfe-Simon et al., 2005).
Another key antioxidant enzyme involved in H2O2 scavenging is catalase (CAT; EC 1.11.1.6), which decomposes H2O2 to water and oxygen:
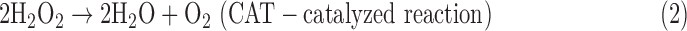 |
There are three classes of CATs: the first contains heme (typical monofunctional CATs, KatEs) in its active site; the second, heme-containing CATs with catalase-peroxidase activity (KatGs); and the non-heme CAT with Mn at the active site (MnCAT) (Zámocký et al., 2012). Under certain conditions (low pH, low H2O2 concentration), KatGs may decompose H2O2 via peroxidation (Singh et al., 2008; Ndontsa et al., 2012; Eq. 3). Recently, peroxiredoxins (PXs) were recognized for their antioxidant properties. PXs are peroxidases with low substrate specificity, which are capable of reducing diverse peroxides, such as H2O2, alkyl hydrogen peroxides, and peroxynitrite, to water and the corresponding alcohol, water, and nitrite, respectively. The general scheme of reactions catalyzed by PX with H2O2 as a substrate is
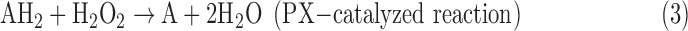 |
where AH2 and A denote the reduced and oxidized acceptor, respectively. PXs are present in organisms from all three domains of life: Archaea, Bacteria, and Eukarya. They contain a conserved cysteine residue in the N-terminal region that is the primary site of oxidation by H2O2 (Horling et al., 2002; Rhee et al., 2005; Dietz, 2011). It was found that detoxification of O2−•, unlike in the case of SOD, occurs via its reduction, a reaction catalyzed by a small non-heme Fe-containing enzyme superoxide reductase (SOR), where H2O2 is the only product (Niviére and Fontecave, 2004):
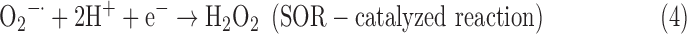 |
The presence of SOR has been mainly limited to strict anaerobes and microaerophiles that belong to Bacteria and Archaea (Imlay, 2002; Lucchetti-Miganeh et al., 2011).
The growing number of gene and protein sequences from organisms that belong to the three domains of life (Woese et al., 1990) facilitate the reconstruction of many cellular processes of the hypothetical last universal common ancestor (LUCA) of all modern organisms. The presence of SODs, the Fe and Mn forms in particular, CATs, and PXs in organisms from all three domains of life (Kornaś et al., 2010) and ROS-removing reactions in strict anaerobes have been reported previously (Ślesak et al., 2012). Thus, we made the simplified assumption that every enzyme that has archaeal, bacterial, and eukaryotic homologues was most likely present in LUCA. This postulation is allowed in the context of a general methodology used for the reconstruction of the LUCA genome (Ouzounis and Kyrpides, 1996; Mushegian, 2008). If present-day (modern) anaerobes have evolved as descendants of ancient forms, the following questions need to be raised: (1) Could LUCA be an O2/ROS-tolerant organism possessing any primordial EAS? (2) What were the potential abiotic sources of ROS and oxygen on young Earth? Our results constitute additional biological support for the unorthodox hypothesis that EAS or a rudimentary equivalent may have been present in primordial organisms on early Earth, even before the appearance of oxygenic photosynthesis. Moreover, the theoretical considerations presented here paradoxically indicate that ROS-scavenging reactions could themselves be an intracellular net source of O2/ROS inside hypothetical LUCA protocells. Additionally, we hypothesize that the occurrence of iron ion binding sites at the active site of most analyzed antioxidant enzymes is an additional piece of evidence that suggests the incorporation of iron from the ancient oceans into key antioxidant enzymes, before oxygen accumulation in the atmosphere, presumably due to oxygenic photosynthesis.
2. Materials and Methods
2.1. The selection of obligate anaerobes
To verify oxygen tolerance of various microorganisms, a query was performed of the literature (e.g., Vieira-Silva and Rocha, 2008) and available online resources, for example, the Genome List (www.ncbi.nlm.nih.gov/genomes/lproks.cgi), the European Bioinformatics Institute (www.ebi.ac.uk), and the Doe Joint Genome Institute (www.jgi.doe.gov).
2.2. Searching for components of EAS
Data indicating the presence of antioxidant enzymes, such as SOD, SOR, PX, and CAT in obligate anaerobic bacteria and archaea, were acquired from publicly available databases: STITCH 1.0 and later versions (Search Tool for Interactions of Chemicals; available at http://stitch.embl.de) and BioCyc (www.biocyc.org) (Caspi et al., 2008; Kuhn et al., 2008). Only complete genome representations were included in the present study. The completeness of each genome was verified by information from the following Web sites: www.ncbi.nlm.nih.gov/genome/browse/# and http://www.ebi.ac.uk, where full genome representation was shown at the complete genome (chromosomes and plasmids) assembly level. For Pyrococcus abyssi, full genome representation was presented at the chromosome level.
CAT denotes sequences identified as monofunctional (KatE), bifunctional (KatG), and Mn-containing catalases (MnCAT). The enzymes' amino acid sequences and their accession numbers were obtained from GenBank (www.ncbi.nlm.nih.gov; Supplementary Table S2; Supplementary Data are available online at www.liebertonline.com/ast). We also made an arbitrary typology of enzymatic antioxidant systems (EASs). Typical “minimal” functional EASs should consist of at least SOD and/or SOR, converting O2−• to H2O2, and enzymes, such as PX and/or CAT that decompose H2O2 to water and other compounds. For more clarity, we assumed that the majority of CATs decompose H2O2 to water and O2 (Eq. 2), and the peroxidase-type reaction (Eq. 3) is negligible. In fact, H2O2 degradation, according to Eq. 2, is dominant in biological systems, but as was mentioned above, it depends inter alia on pH and H2O2 concentration. The functionality of selected combinations of EASs is based on the fact that reactive O2−• is reduced to the less reactive H2O2. Subsequently, H2O2 must be decomposed to neutral or less harmful compounds, that is, H2O and O2, because it may react with O2−• and produce extremely reactive and dangerous ROS, that is, hydroxyl radical (HO•) (Ślesak et al., 2007). Moreover, the deleterious effects of H2O2 (HO• generation) depend on the presence of transition metals such as iron and copper in the environment (Halliwell and Gutteridge, 1999; Imlay, 2008a). In general, the functional EAS proposed above is typical for all aerobes (Benzie, 2000; Ślesak et al., 2007; Schippers et al., 2012). The lack of enzymes that remove O2−• (SOD, SOR) or H2O2 (PX, CAT) in an anaerobe was defined as “nonfunctional” EAS.
We selected eight functional EAS configurations: (1) SOD + SOR + PX + CAT, (2) SOD + PX + CAT, (3) SOD + PX, (4) SOD + CAT, (5) SOD + SOR + PX, (6) SOR + PX + CAT, (7) SOR + PX, (8) SOR + CAT. The nonfunctional EAS consists of (1) SOD, (2) SOR, (3) SOD + SOR, (4) PX, (5) PX + CAT, (6) CAT. Previous reports indicate that all antioxidant genes/enzymes in anaerobes are transcribed/active at about the same time, and the lack of compartmentalization of bacterial cells ensures that generation/detoxification of ROS occurs in the same intracellular area (e.g., Brioukhanov et al., 2006; Dolla et al., 2006; Imlay, 2008b). Moreover, the analyzed antioxidant enzymes more or less differ in kinetic parameters, for example, the turnover number (kcat [s−1]), but it does not affect the stoichiometry and type of the net products (www.brenda-enzymes.org; Pratt and Cornely, 2014).
This allowed us to determine the number of obligate anaerobes that possess at least one of the above-mentioned enzymes and which of them have a functional antioxidant system.
2.3. Prediction of an ion metal at the active site of antioxidant enzymes
The SODa Web tool (http://babylone.ulb.ac.be/SODa) (Kwasigroch et al., 2008) was used for prediction of a specific metal cofactor in the active site of the analyzed SODs. The prediction was shown as a score expressed in percent (Supplementary Table S3). The predictions for the occurrence of a metal in the active site of CATs were based on literature and GenBank (www.ncbi.nlm.nih.gov).
2.4. Determination of protein homology
Homology was inferred using standard protein BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) analysis. All sequences (of the same type of enzymes) were aligned with each other. The homology was estimated based on several parameters, describing the similarity of analyzed sequences: sequence amino acid identity (%) and expectation value (E-value) (Table 1). If pairwise sequence identity, for sequences longer than 100 amino acid residues, were higher than 20% (>20%), and E-values were ≤10−5 (E ≤ 1e-05), the sequences could be interpreted as homologues (Xiong, 2006; Pearson, 2013).
Table 1.
Parameters Estimated by Protein BLAST Similarity Search (See also Materials and Methods)
| Parameters | |||
|---|---|---|---|
| Enzyme | Range of amino acid sequence length | Range of amino acid identity (%) | Expectation value (E-value) |
| SOD | 188–239 | 23–100 | E ≤ 2e-08 |
| SOR | 109–161 | 26–100 | E ≤ 4e-06 |
| PX | 158–235 | 22–100 | E ≤ 4e-05a |
| KatE | 478–801 | 38–100 | E ≤ 1e-102 |
| KatG | 712–741 | 52–87 | E = 0.000 |
| MnCATb | 190–228 | 38 | E = 1e-34 |
Accession numbers below are listed according to www.ncbi.nlm.nih.gov.
For aligned sequences: NP_349898, length = 164 amino acids (C. acetobutylicum ATCC 824) vs. WP_012882691, length = 213 (D. mccartyi VS): identity = 22%, E = 0.003 (3e-03).
2.5. Phylogenetic analysis
The molecular phylogenetic analysis was based on 16S rRNA sequences of anaerobes that contain at least SOD or SOR (Supplementary Table S2) and on 16 rRNA sequences of anaerobes that lack an EAS (Supplementary Table S1). All sequences were derived from SILVA (http://www.arb-silva.de), StrainInfo (http://www.straininfo.net), and GenBank (www.ncbi.nlm.nih.gov).The sequences were aligned with the default parameters of ClustalW implemented in MEGA6 (Tamura et al., 2013). The evolutionary history was inferred by using the maximum likelihood method based on Kimura's 2-parameter model (Kimura, 1980). The bootstrap consensus tree was inferred from 100 replicates (Felsenstein, 1985). Initial tree(s) for the heuristic search were obtained by applying the neighbor-joining method to a matrix of pairwise distances estimated with the maximum composite likelihood approach. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.5461)). The rate variation model allowed for some sites to be evolutionary invariable ([+I], ∼22% sites). The analysis involved 89 nucleotide sequences. All positions that contained gaps and were missing data were eliminated. There were a total of 479 positions in the final data set. Evolutionary analyses were conducted in MEGA6 (Tamura et al., 2013).
3. Results
3.1. EAS is quite common in strict anaerobes and determination of protein homology
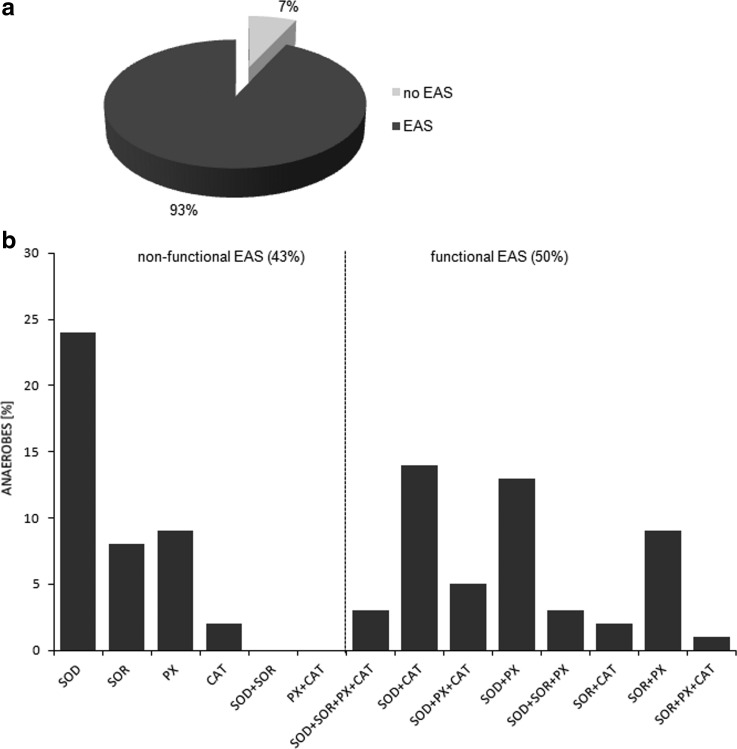
We selected 100 obligate anaerobes, representative of the domains Archaea and Bacteria, and at least one antioxidant enzyme was found in 93% of species (Supplementary Table S2, Fig. 1a). According to the proposed typology (see Materials and Methods), both nonfunctional (43%) and functional (50%) EAS was present in species, with various configurations of enzymes detoxifying O2−• (SOD and/or SOR) and enzymes responsible for H2O2 elimination (PX and/or CAT) (Fig. 1b). In Methanosarcina mazei two sequences for heme CAT (KatG) were found, but in Desulfitobacterium hafniense two different types of CATs—KatE and KatG—were noted (Supplementary Table S2). In Clostridium beijerinckii and Clostridium novyi two SODs were identified (Supplementary Table S2). It is worth pointing out that a single antioxidant—SOD or SOR—was present in a large number of the anaerobes examined. In the case of Archaea, SORs were the predominant superoxide anion radical scavengers (Supplementary Table S2).
FIG. 1.
Enzymatic antioxidant system (EAS) in analyzed obligate anaerobes. (a) The percent of organisms encoding an EAS. (b) The percent of organisms encoding functional and nonfunctional EAS.
The existence of genes encoding related proteins does not provide final proof as to whether the EAS is really active. However, it gives strong support for bona fide EAS occurrence in an organism. In fact, the majority of available protein sequences have not been experimentally characterized; most sequences are annotated by computational analysis (Schnoes et al., 2009). We hypothesized that every enzyme that has archaeal, bacterial, and eukaryotic homologues was likely present in LUCA. To test this hypothesis, it was necessary to estimate whether the analyzed sequences are homologues. Sequence homology allows one to conclude that there is a common ancestral relationship for all compared sequences drawn from comparisons when two sequences share a high-enough degree of similarity (Xiong, 2006). Therefore, annotations of all selected proteins were verified by the UniProt database (www.uniprot.org). The results indicate that the majority of the analyzed proteins: SODs, PXs, and various types of CATs were inferred from homology analysis. A small number was additionally verified experimentally (see www.uniprot.org). Moreover, BLAST analysis indicated that SOD, SOR, PX, KatE, KatG, and MnCAT sequences showed that similarity indicators—sequence identity level (22–100%) in relation to the protein sequence length and E-values (E ≤ 4e-05) (Table 1)—were in the range that allows one to conclude that compared sequences are homologues (Xiong, 2006; Pearson, 2013). Two PXs, however, were exceptions to this: (1) from C. acetobutylicum (NP_349898), predicted as atypical 2-Cys peroxiredoxin, and (2) from D. mccartyi (WP_012882691), described as 1-Cys peroxiredoxin (www.ncbi.nlm.nih.gov). After BLAST alignment, a 22% value of identity between these sequences and E = 0.003 (Table 1) was near (% of identity) or below (E-value) the threshold values accepted as indicators of sequence homology (see Materials and Methods). Such results, mainly E-value in the range of 10−1 to 10−5, may indicate that the compared sequences are distant homologues (Xiong, 2006). It has been shown, however, with active site structure and amino acid residue analysis, that these PXs belong to one family of peroxiredoxins (Nelson et al., 2011). In other words, all the studied sequences share homology; thus it can be assumed that it is most likely the ancestral form of the analyzed antioxidant enzyme that was present in LUCA.
3.2. The functional and nonfunctional EASs are net producers of O2/ROS
The following reactions describe the conversion of ROS in hypothetical EAS.
3.2.1. Functional EASs
-
(1) SOD + SOR + PX + CAT:
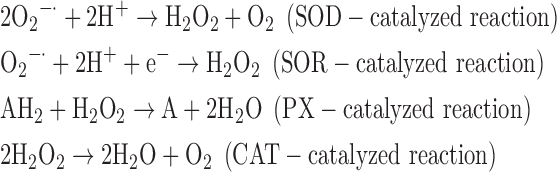
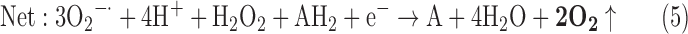
where AH2 and A denote a reduced and an oxidized acceptor, respectively.
-
(2) SOD + PX + CAT:
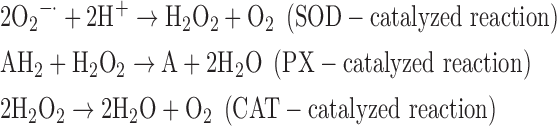
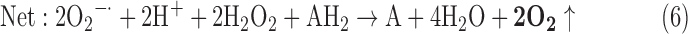
-
(3) SOD + PX:
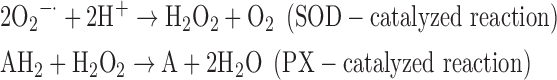
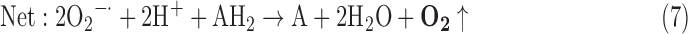
-
(4) SOD + CAT:
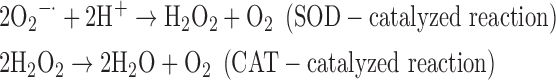
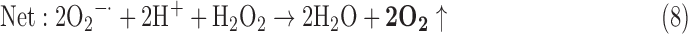
-
(5) SOD + SOR + PX:
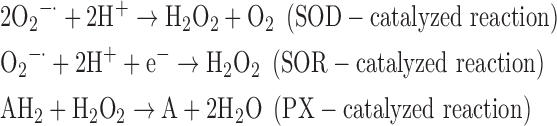
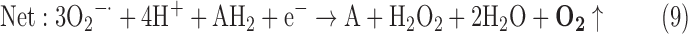
-
(6) SOR + PX + CAT:
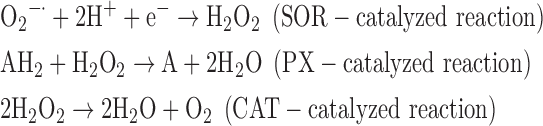
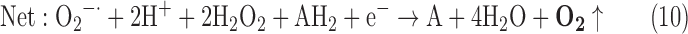
-
(7) SOR + PX:
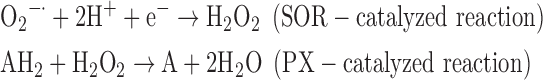
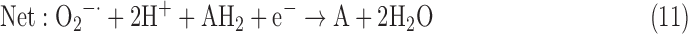
-
(8) SOR + CAT:
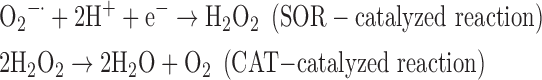
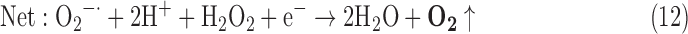
3.2.2. The nonfunctional EASs
- (1) SOD:
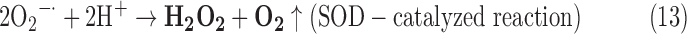
- (2) SOR:
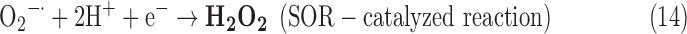
-
(3) SOD + SOR:
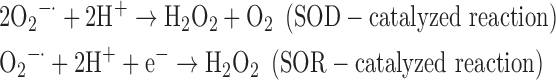
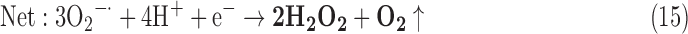
-
(4) PX:
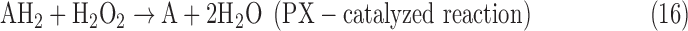
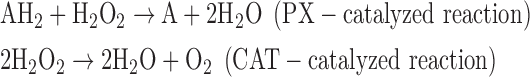
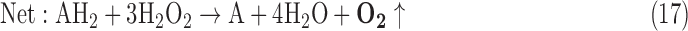
- (6) CAT:
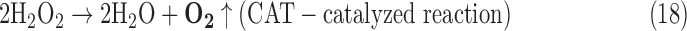
As a result, we were able to identify the net products of all the reactions. O2/ROS are marked in bold. No net O2/ROS products were found for the functional combination of two enzymes SOR and PX (Eq. 11) and the PX-containing variant for a nonfunctional system (Eq. 16). EAS: SOR + PX and the single PX were present in only 9% of the analyzed anaerobes (Fig. 1b). In fact, the results showed the net generation of O2 (Eqs. 5, 6, 7, 8, 9, 10, 12, 17, 18), O2 and H2O2 (Eqs. 13, 15), or H2O2 only (Eq. 14) in both functional and nonfunctional EAS combinations, which were identified in analyzed anaerobes. It should be emphasized that, in most EASs, O2 was the only net product, and O2−• and H2O2, as unstable and highly reactive intermediates, were not the net final products in most cases. These results suggest that the activity of antioxidant enzymes, annotated for each anaerobe (Supplementary Table S2), could be an intracellular source of molecular oxygen.
3.3. Molecular phylogenetic analysis
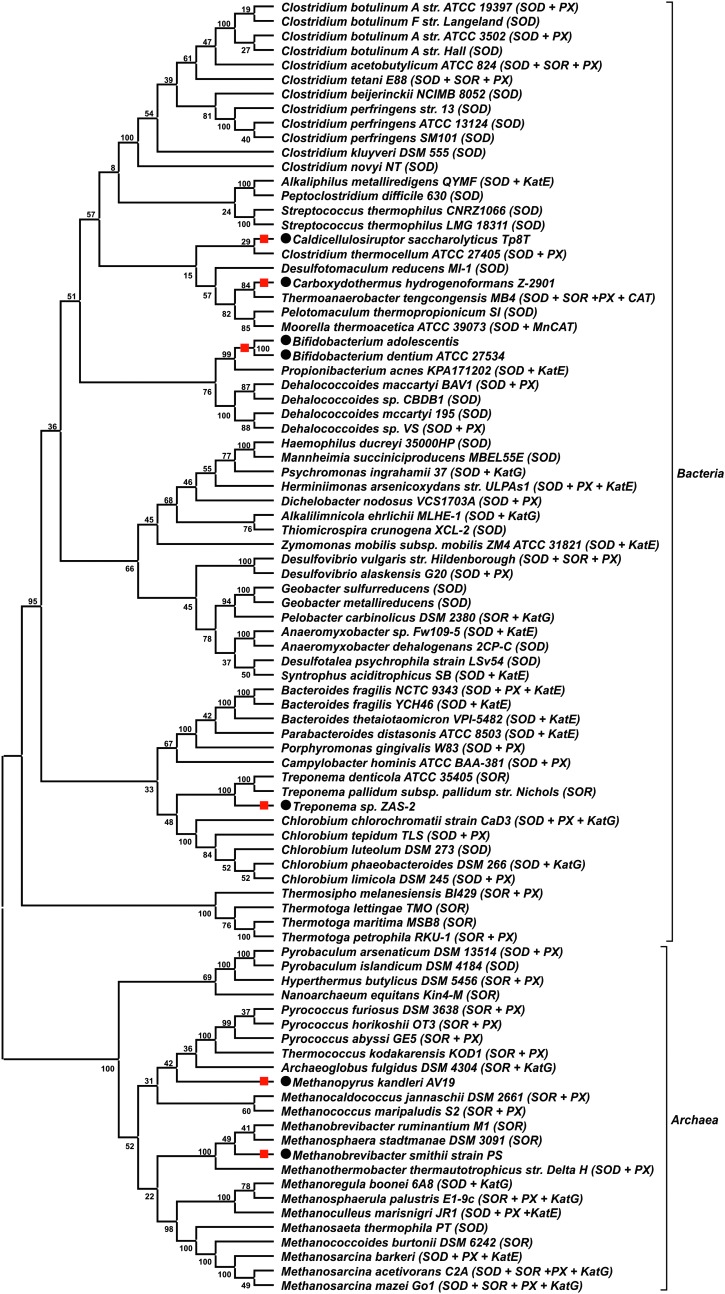
To simplify a test with regard to the hypothesis concerning the loss of EAS (functional or nonfunctional), we selected anaerobes that contained at least one of the most commonly annotated antioxidant enzymes, that is, SOD or SOR (Supplementary Table S2, Fig. 1b), and reconstructed a phylogenetic tree based on the 16S rRNA sequences from these anaerobes and those without EAS (Supplementary Table S1). According to the tree (Fig. 2), non-EAS anaerobes, which include Methanobrevibacter smithii, Methanopyrus kandleri, Treponema sp. ZAS-2 (alias T. primitia ZAS-2), Bifidobacterium adolescentis, Bifidobacterium dentium, Carboxydothermus hydrogenoformans, and Caldicellulosiruptor saccharolyticus, are, in general, scattered among various groups with EAS. It seems that most analyzed anaerobes, in relation to their ancestors, lost two antioxidant enzymes that constitute functional EAS. Moreover, the results might suggest that an occurrence of EAS was a primordial attribute of so-called obligate anaerobes, which was accidentally lost in progeny lineages (Fig. 2).
FIG. 2.
Unrooted 16S rRNA tree (cladogram) for anaerobes possessing at least SOD or SOR. Species labeled with black circles have no antioxidant response system EAS (Supplementary Table S1). Red squares indicate lineages that possibly lost at least SOD and/or SOR genes. All antioxidant enzymes encoded in genome of each organism are presented in parentheses. Numbers at nodes represent bootstrap values (based on 100 replications).
3.4. Prediction of the specificity of the ion metal present in the active site of antioxidant enzymes
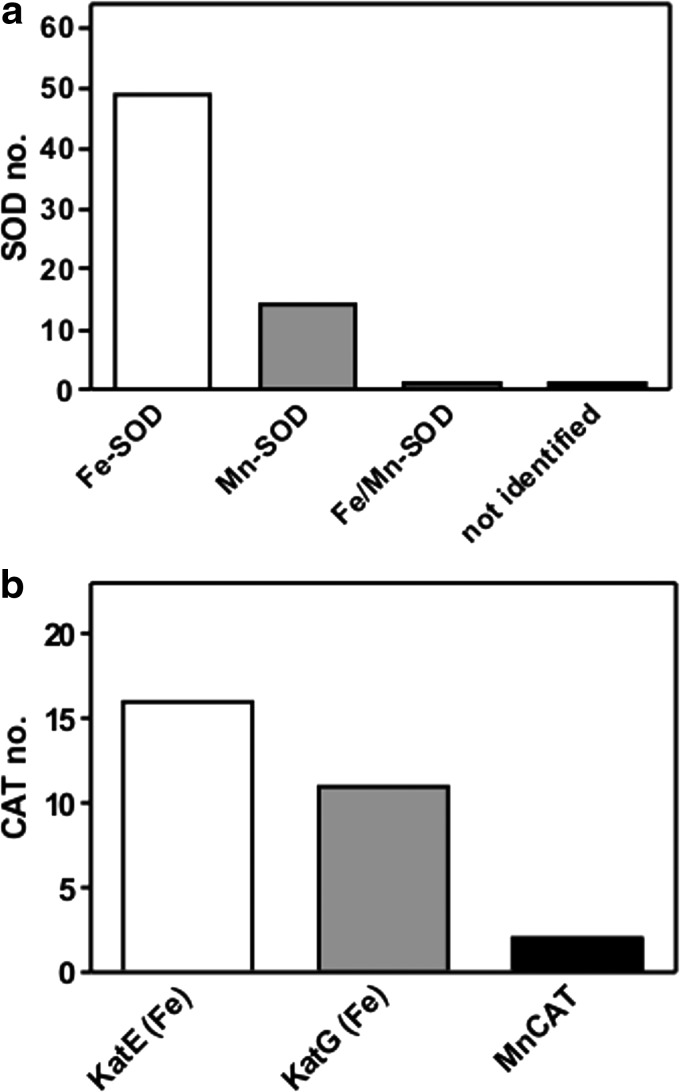
We predicted the presence of iron only in SOD and CAT active sites, because all SORs belong to non-heme proteins and PXs are not metalloenzymes (see Introduction). Among all SODs, 49 were predicted as Fe-SODs, 14 putatively contained Mn, one was probably Fe/Mn-SOD, and for one enzyme (accession number NP_349176, Supplementary Table S3) the SODa bioinformatic tool did not identify a metal cofactor (Fig. 3a), though GenBank classified it as a protein belonging to the Fe/Mn-SOD family (Supplementary Table S3). The detailed values concerning metal prediction are shown as score expressed in percent (Supplementary Table S3). Among 29 CATs (Supplementary Table S2), 16 were putatively identified as monofunctional (typical) heme-containing CATs (KatEs), 11 as catalase-peroxidases (KatGs), and 2 presumably are Mn-containing enzymes (MnCAT) (Fig. 3b).
FIG. 3.
The number of various classes of SODs (a) and CATs (b) predicted by SODa Web tool (http://babylone.ulb.ac.be/SODa) in analyzed anaerobes. For more details see Results. Fe-SOD, iron-containing SOD; Mn-SOD, manganese-containing SOD; Fe/Mn-SOD, manganese/iron-containing SOD; KatE (Fe), iron-containing monofunctional CATs; KatG (Fe), iron-containing catalase-peroxidases; MnCAT, manganese CAT.
4. Discussion
Most likely, life on Earth evolved under anoxic conditions, with the hypothetical LUCA being an anaerobe. In general, prebiotic chemistry for synthesis of many biomolecules is impossible in the presence of molecular oxygen (e.g., Lazcano and Miller, 1996; Miller and Cleaves, 2007). However, reanalysis of samples from Miller's spark discharge experiment has shown the occurrence of hydroxylated compounds in samples (Johnson et al., 2008), suggesting the generation of ROS, such as hydroxyl radicals (HO•), which may have reacted with amino acid precursors or amino acids themselves (Ring and Miller, 1984; Johnson et al., 2008). Nevertheless, the concentration of molecular oxygen in the atmosphere of early Earth was estimated to be very low, ca. 10−5 of the present atmospheric level, only with traces of O2 in shallow oceans (Holland, 2006). However, the timing of the rise of O2 in the ancient atmosphere is still under vigorous debate (Yamaguchi, 2005; Kump, 2008; Shaw, 2008; Lyons et al., 2014). Numerous reports suggest that local areas with increased O2 concentration appeared several hundred million year before the Great Oxidation Event (Crowe et al., 2013; Olson et al., 2013; Lyons et al., 2014; Planavsky et al., 2014); therefore, many ancient anaerobes had already been adapted to O2/ROS.
4.1. Modern anaerobes
Anaerobes, mainly prokaryotes, are defined as organisms that do not require oxygen to obtain metabolic energy for growth. Among them, obligate or strict anaerobes never use O2 in their metabolism (Sanz, 2011). It is most likely that such a broad criterion was used for classification of most modern obligate anaerobes described in databases and literature. Nevertheless, no habitat is free even from an occasional increase of O2, and so-called obligate anaerobes share similar microbiota as facultative anaerobes. Moreover, the adaptability of obligate anaerobes to O2 might have been overlooked because laboratory conditions failed to mimic the dynamic of aeration in natural environments (Imlay, 2008b). In fact, in modern anaerobes, components of aerobic respiration, and EAS, such as various cytochrome oxidases (Baughn and Malamy, 2004; Das et al., 2005; Dolla et al., 2006), NADH oxidases (Yang and Ma, 2007), SODs (Gregory et al., 1978; Dos Santos et al., 2000; Brioukhanov and Netrusov, 2004; Dolla et al., 2006; Horne and Lessner, 2013), SORs (Niviére and Fontecave, 2004; Dolla et al., 2006), and CATs (Brioukhanov and Netrusov, 2004; Dolla et al., 2006; Mishra and Imlay, 2013) have been identified previously. According to other reports, enzymes and metabolic pathways that are directly or indirectly oxygen-dependent are present in many anaerobes (Peregrin-Alvarez et al., 2003; Freitas et al., 2005; Raymond and Segrè, 2006; Passardi et al., 2007; Ślesak et al., 2012). Moreover, according to theoretical studies, Fe/MnSOD and CAT would have been required for basic metabolism of the hypothetical LUCA (Ouzounis et al., 2006).
The minority of the analyzed anaerobes have a double set of antioxidant enzymes that perform the same functions: SOD + SOR and CAT + PX (Supplementary Table S2, Fig. 2). It cannot be excluded that such redundancy is, in part, a result of horizontal gene transfer. The presence of EAS in contemporary obligate anaerobes from the domains Archaea and Bacteria raises questions concerning the evolution of antioxidant cellular pathways. Only 7% of the analyzed anaerobes do not have antioxidant enzymes (Fig. 1a). The simplest explanation for the variable absence of SOD and SOR genes on the 16S rRNA tree is that these genes were present in their common ancestor but were later lost in some lineages (Fig. 2). Based on this result, the reductive genome evolution could be considered to be a possible explanation of the selective loss of SOD/SOR genes in some analyzed anaerobes. Reductive evolution is related to genome reduction, which occurs via two modes: (i) the neutral gene loss ratchet and (ii) adaptive genome streamlining, which dominates in parasitic and symbiotic organisms. Reductive evolution has also been observed in several organisms that evolved a commensal lifestyle (Wolf and Koonin, 2013). The examples in our study are bifidobacteria (B. dentium, B. adolescentis) and M. smithii, which inhabit human gut and intestines (Samuel et al., 2002; Klijn et al., 2005). It is interesting to note that no genes for SOD have been identified in bifidobacteria, but divalent metal ions (Fe2+ and Mn2+) may play the role of SOD (Chang and So, 1998). Moreover, a loss of various genes was previously suggested for the catalase (katG) gene even in oxygen-producing cyanobacteria (Morris et al., 2012). If the modern anaerobic metabolism of obligate anaerobes is representative for their ancestors, our analyses strongly suggest that EAS appeared at the very early period of Earth evolution, yet before the evolution of oxygen-producing cyanobacteria. Subsequently, components of EAS were lost in selected microbial lines. According to our typology (based on aerobes' antioxidant machinery), 43% of EAS found were nonfunctional (Fig. 1b); however, we have scarce data confirming the nonfunctionality of such enzyme combinations in specific anaerobic species.
It should be emphasized that, in the case of the evolution of oxygenic photosynthesis, we have the “chicken-and-the-egg problem”. Photosystem II (PSII)–mediated O2 evolution requires antioxidant enzymes that limit O2 derivatives, that is, ROS toxicity, while antioxidant enzymes cannot evolve without O2/ROS (McKay and Hartman, 1991; Kirschvink and Kopp, 2008). Therefore, if the unorthodox respiration-early hypothesis (Castresana and Saraste, 1995) is true, SOD, SOR, PX, and CAT were key enzymes of EAS, which evolved before the oxygenic photosynthesis–dependent rise of atmospheric O2. Nevertheless, the verification of such a hypothesis requires the identification of early nonphotosynthetic (abiotic) O2 sources.
4.2. Formation of oxygen and related species on early Earth
Here, we have identified potential intracellular sources of O2/ROS as by-products of net EAS activity in a hypothetical ancestor of anaerobes. Nevertheless, we are unable to expand upon our current understanding of the mechanisms of abiotic O2/ROS production in early Earth's atmosphere; a reminder of the possible nonbiological sources of O2/ROS is necessary for the clarity of our argumentation. In ancient Earth's atmosphere, ca. 3.85–2.45 O2/ROS could have been produced abiotically by water photolysis (UV radiation), radiolysis, and CO2 photolysis (Halliwell and Gutteridge, 1999; Baumstark-Khan and Facius, 2002; Azrague et al., 2005; Draganić, 2005; Haqq-Misra et al., 2011). All these processes were present before oxygenic photosynthesis. Moreover, some of the O2/H2O2 molecules would have been produced in cosmic space and then imported to Earth's atmosphere by comets and other icy interstellar bodies (Zheng et al., 2006; Cooper et al., 2008). Recently, ROS generation at the oxide/water interface has also been reported (Xu et al., 2013). All these hypothetical scenarios strongly indicate that prebiotic ROS could have been, at least to some extent, a primordial source of O2. This assumption has been supported by experimental evidence, which has shown that pyrite/aqueous suspensions generate H2O2, via hydroxyl radicals (HO•), in the absence of O2 (Borda et al., 2001; Cohn et al., 2006). Moreover, this fact corresponds to the theory that the early events in the evolution of life took place in iron-rich environments containing iron-sulfide minerals (Martin and Russel, 2003; Wächtershäuser, 2007) and that Fe was abundant mainly in the reduced soluble form Fe2+ (Saito et al., 2003). According to this, it would seem that Fe2+ was initially the most probable transition metal in the active site of the first SOD (Bannister et al., 1991; Asada, 2000). In a similar way, the evolution of catalases, from free Fe2+ through iron-heme complexes to the formation of the active site of the CAT protein, was proposed by Calvin (1969). Iron is still present in many enzymes of contemporary organisms (Dupont et al., 2006; Nitschke et al., 2013) and can be treated as a vestige of ancient Fe-containing proteins. The increase of oxygen in the atmosphere resulted in a number of immediate geochemical effects. O2 reacted with soluble iron (Fe2+) in the ancient water reservoirs to form layers of insoluble iron oxides (Fe3+), now known as banded iron formations (Bekker et al., 2010); therefore, the incorporation of Fe2+ into the active sites' enzymes was hindered after the release of major amounts of O2 into the atmosphere. The presence of iron in active sites of analyzed antioxidant enzymes may suggest that they appeared at very early stages of life evolution yet before the release of O2 into the atmosphere. The results obtained from the ion metal prediction in SOD and CAT active sites clearly indicate that putative iron-metalloenzymes dominate among analyzed CATs and SODs (Fig. 2). Only a minority of them have been predicted to be manganese-containing enzymes, which suggests that Mn ions were less available in ancient oceans, as has been suggested in previous geochemical studies (Saito et al., 2003).
According to available reports and our in silico analyses presented here, a common strategy for dealing with oxidative stress exists not only in aerobes but also in anaerobes. Obligate anaerobes also neutralize harmful ROS by a series of reactions where the product is always less toxic then the substrate, but inevitably the net by-product of such a series of events is molecular oxygen or rarely H2O2. Molecular oxygen is less toxic than its derivatives (ROS), but O2 itself is an intracellular source of ROS. Recently, metagenomic studies revealed another intracellular O2-generating pathway in the anaerobic denitrifying bacterium Candidatus Methylomirabilis oxyfera (Ettwig et al., 2010). The results presented here clearly indicate that abiotically derived O2 was not the only source of O2 available for the first microorganisms before oxygenic photosynthesis evolved. These organisms probably account for the availability of endogenous, metabolically derived O2.
4.3. Conclusions
Given the outcome of this study, we draw the following conclusions. The presence of iron-containing antioxidant enzymes in many modern strict anaerobes may be an evolutionary vestige inherited from early ancestors of anaerobic prokaryotes, which had to have had the ability to cope with ROS. Nevertheless, our data strongly suggest that the occurrence of EAS in obligate anaerobes could have appeared at an early stage of life evolution. Besides environmental abiogenic sources of O2/ROS, rudimentary EASs could also have been endogenous net producers of O2/ROS in LUCA. This hypothesis may explain why harmful effects of cyanobacterial-produced O2 were restricted for their producers. Ancient nonphotosynthesizing cyanobacterial ancestors may have already been equipped with some key antioxidant protoenzymes inherited from O2/ROS-tolerant ancestors.
Supplementary Material
Abbreviations Used
- CAT
catalase
- EAS
enzymatic antioxidant system
- LUCA
last universal common ancestor
- PX
peroxiredoxin
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SOR
superoxide reductase
Acknowledgments
This work was supported by the Polish National Science Centre project 2011/03/B/NZ9/01619. We also thank anonymous reviewers for several suggestions that helped strengthen this paper.
Author Disclosure Statement
No competing financial interests exist.
References
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K., and Walter P. (2002) Energy conversion: mitochondria and chloroplasts. In Molecular Biology of the Cell, 4th ed., Garland Science, New York, pp 767–829 [Google Scholar]
- Alscher R.G., Erturk N., and Heath L.S. (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341 [PubMed] [Google Scholar]
- Asada K. (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond B Biol Sci 355:1419–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azrague K., Bonnefille E., Pradines V., Pimienta V., Oliveros E., Maurette M.-T., and Benoit-Marquité F. (2005) Hydrogen peroxide evolution during V-UV photolysis of water. Photochem Photobiol Sci 4:406–408 [DOI] [PubMed] [Google Scholar]
- Bannister W.H., Bannister J.V., Barra D., Bond J., and Bossa F. (1991) Evolutionary aspects of superoxide dismutase: the copper/zinc enzyme. Free Radic Res Commun 12–13:349–361 [DOI] [PubMed] [Google Scholar]
- Baughn A.D. and Malamy M.H. (2004) The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 427:441–444 [DOI] [PubMed] [Google Scholar]
- Baumstark-Khan C. and Facius R. (2002) Life under conditions of ionizing radiation. In Astrobiology: The Quest for the Conditions of Life, edited by Horneck G. and Baumstark-Khan C., Springer, Berlin, pp 260–283 [Google Scholar]
- Bekker A., Slack J., Planavsky N., Krapež B., Hofmann A., Konhauser K., and Rouxel O. (2010) Iron formation: the sedimentary product of a complex interplay among mantle, tectonic, oceanic, and biospheric processes. Econ Geol 105:467–508 [Google Scholar]
- Benzie I.F.F. (2000) Evolution of antioxidant defence mechanisms. Eur J Nutr 39:53–61 [DOI] [PubMed] [Google Scholar]
- Borda M.J., Elsetinow A.R., Schooen A.R., and Strongin D.R. (2001) Pyrite-induced hydrogen peroxide formation as a driving force in the evolution of photosynthetic organisms on an early Earth. Astrobiology 1:283–288 [DOI] [PubMed] [Google Scholar]
- Brioukhanov A.L. and Netrusov A.I. (2004) Catalase and superoxide dismutase: distribution, properties, and physiological role in cells of strict anaerobes. Biochemistry (Moscow) 69:949–962 [DOI] [PubMed] [Google Scholar]
- Brioukhanov A.L., Netrusov A.I., and Eggen R.I.L. (2006) The catalase and superoxide dismutase genes are transcriptionally up-regulated upon oxidative stress in the strictly anaerobic archaeon Methanosarcina barkeri. Microbiology 152:1671–1677 [DOI] [PubMed] [Google Scholar]
- Calvin M. (1969) Chemical Evolution: Molecular Evolution Towards the Origin of Living Systems on the Earth and Elsewhere, Oxford University Press, New York, pp 144–161 [Google Scholar]
- Caspi R., Foerster H., Fulcher C.A., Kaipa P., Krummenacker M., Latendresse M., Paley S., Rhee S.Y., Shearer A.G., Tissier C., Walk T.C., Zhang P., and Karp P.D. (2008) The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res 36:D623–D631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J. and Saraste M. (1995) Evolution of energetic metabolism: the respiration-early hypothesis. Trends Biochem Sci 20:443–448 [DOI] [PubMed] [Google Scholar]
- Chang W.S. and So J.S. (1998) False positive SOD activity of Bifidobacterium spp. grown in MRS medium. J Microbiol Biotechnol 8:305–309 [Google Scholar]
- Cohn C.A., Mueller S., Wimmer E., Leifer N., Greenbaum S., Strongin D.R., and Schoonen M.A.A. (2006) Pyrite-induced hydroxyl radical formation and its effect on nucleic acids. Geochem Trans 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P.D., Moore M.H., and Hudson R.L. (2008) Radiation chemistry of H2O + O2 ices. Icarus 194:379–388 [Google Scholar]
- Crowe S.A., Døssing L.N, Beukes N.J., Bau M., Kruger S.J., Frei R., and Canfielf D.E. (2013) Atmospheric oxygenation three billion years ago. Nature 501:535–539 [DOI] [PubMed] [Google Scholar]
- Das A., Silaghi-Dumitrescu R., Ljungdahl L.G., and Kurtz D.M. (2005) Cytochrome bd oxidase, oxidative stress, and dioxygen tolerance of the strictly anaerobic bacterium Moorella thermoacetica. J Bacteriol 187:2020–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Las Rivas J., Balsera M., and Barber J. (2004) Evolution of oxygenic photosynthesis: genome-wide analysis of the OEC extrinsic proteins. Trends Plant Sci 9:18–24 [DOI] [PubMed] [Google Scholar]
- Dietz K.-J. (2011) Peroxiredoxins in plant and cyanobacteria. Antioxid Redox Signal 15:1129–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolla A., Fournier M., and Dermoun Z. (2006) Oxygen defense in sulfate-reducing bacteria. J Biotechnol 126:87–100 [DOI] [PubMed] [Google Scholar]
- Dos Santos W.G., Pacheco I., Liu M.-Y., Teixeira M., Xavier A.V., and LeGall J. (2000) Purification and characterization of an iron superoxide dismutase and a catalase from the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol 182:796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganić I.G. (2005) Radiolysis of water: a look at its origin and occurrence in the nature. Radiat Phys Chem 72:181–186 [Google Scholar]
- Dupont C.L., Yang S., Palenik B., and Bourne P.E. (2006) Modern proteomes contain putative imprints of ancient shifts in trace metal geochemistry. Proc Natl Acad Sci USA 103:17822–17827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettwig K.F., Butler M.K., Le Paslier D., Pelletier E., Mangenot S., Kuypers M.M.M., Schroeiber F., Dutilh B.E., Zedelius J., De Beer D., Gloerich J., Wessels H.J.C.T., Van Alen T., Luesken F., Wu M.L., Van de Pas-Schoonen K.T., Op den Camp H.J.M., Janssen-Megens E.M., Francoijs K.J., Stunnenberg H., Weissenbach J., Jetten M.S.M., and Strous M. (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548 [DOI] [PubMed] [Google Scholar]
- Felsenstein J. (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791 [DOI] [PubMed] [Google Scholar]
- Fink R.C. and Scandalios J.G. (2002) Molecular evolution and structure-function relationships of the superoxide dismutase gene families in angiosperms and their relationship to other eukaryotic and prokaryotic superoxide dismutases. Arch Biochem Biophys 399:19–36 [DOI] [PubMed] [Google Scholar]
- Foyer C. and Noctor G. (2009) Redox regulation in photosynthetic organisms: signalling, acclimation, and practical implications. Antioxid Redox Signal 11:861–905 [DOI] [PubMed] [Google Scholar]
- Freitas T.A., Saito J.A., Hou S., and Alam M. (2005) Globin-coupled sensors, protoglobins, and the last universal common ancestor. J Inorg Biochem 99:23–33 [DOI] [PubMed] [Google Scholar]
- Gregory E.M., Moore W.E., and Holdeman L.V. (1978) Superoxide dismutase in anaerobes: survey. Appl Environ Microbiol 35:988–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. and Gutteridge J.M.C. (1999) Oxygen is a toxic gas—an introduction to oxygen toxicity and reactive oxygen species. In Free Radicals in Biology and Medicine, Oxford University Press, Oxford, pp 1–35 [Google Scholar]
- Haqq-Misra J., Kasting J.F., and Lee S. (2011) Availability of O2 and H2O2 on pre-photosynthetic Earth. Astrobiology 11:293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland H.D. (2006) The oxygenation of the atmosphere and oceans. Philos Trans R Soc Lond B Biol Sci 361:903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horling F., König J., and Dietz K.-J. (2002) Type II peroxiredoxin C, a member of the peroxiredoxin family of Arabidopsis thaliana: its expression and activity in comparison with other peroxiredoxins. Plant Physiol Biochem 40:491–499 [Google Scholar]
- Horne A.J. and Lessner D.J. (2013) Assessment of the oxidant tolerance of Methanosarcina acetivorans. FEMS Microbiol Lett 343:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J.A. (2002) What biological purpose is served by superoxide reductase? J Biol Inorg Chem 7:659–663 [DOI] [PubMed] [Google Scholar]
- Imlay J.A. (2008a) Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J.A. (2008b) How obligatory is anaerobiosis? Mol Microbiol 68:801–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.P., Cleaves H.J., Dworkin J.P., Glavin D.P., Lazcano A., and Bada J.L. (2008) The Miller volcanic spark discharge experiment. Science 322:404. [DOI] [PubMed] [Google Scholar]
- Karpiński S., Szechyńska-Hebda M., Wituszyńska W., and Burdiak P. (2013) Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant Cell Environ 36:736–744 [DOI] [PubMed] [Google Scholar]
- Kaufman A.J. (2014) Early Earth: cyanobacteria at work. Nat Geosci 7:253–254 [Google Scholar]
- Kimura M. (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120 [DOI] [PubMed] [Google Scholar]
- Kirschvink J.L. and Kopp R.E. (2008) Paleoproterozoic ice houses and the evolution of oxygen-mediating enzymes: the case for late origin of photosystem II. Philos Trans R Soc Lond B Biol Sci 363:2755–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klijn A., Mercenier A., and Arigoni F. (2005) Lessons from the genomes of bifidobacteria. FEMS Microbiol Rev 29:491–509 [DOI] [PubMed] [Google Scholar]
- Kornaś A., Kuźniak E., Ślesak I., and Miszalski Z. (2010) The key role of the redox status in regulation of metabolism in photosynthesizing organisms. Acta Biochim Pol 57:143–151 [PubMed] [Google Scholar]
- Kuhn M., von Mering C., Campillos M., Jensen L.J., and Bork P. (2008) STITCH: interaction networks of chemicals and proteins. Nucleic Acids Res 36:D684–D688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kump L.R. (2008) The rise of atmospheric oxygen. Nature 451:277–278 [DOI] [PubMed] [Google Scholar]
- Kwasigroch J.M., Wintjens R., Gilis D., and Rooman M. (2008) SODa: an Mn/Fe superoxide dismutase prediction and design server. BMC Bioinformatics 9:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazcano A. and Miller S.L. (1996) The origin and early evolution of life: prebiotic chemistry, the pre-RNA world, and time. Cell 85:793–798 [DOI] [PubMed] [Google Scholar]
- Lucchetti-Miganeh C., Goudenège D., Thybert D., Salbert G., and Barloy-Hubler F. (2011) SORGOdb: superoxide reductase gene ontology curated database. BMC Microbiol 11:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons T.W., Reinhard C.T., and Planavsky N.J. (2014) The rise of oxygen in Earth's early ocean and atmosphere. Nature 506:307–315 [DOI] [PubMed] [Google Scholar]
- Martin W. and Russel M.J. (2003) On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos Trans R Soc Lond B Biol Sci 358:59–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay C.P. and Hartman H. (1991) Hydrogen peroxide and the evolution of oxygenic photosynthesis. Orig Life Evol Biosph 21:157–163 [DOI] [PubMed] [Google Scholar]
- Miller A.F. (2012) Superoxide dismutases: ancient enzymes and new insights. FEBS Lett 586:585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.L. and Cleaves H.J. (2007) Prebiotic chemistry on the primitive Earth. Systems Biology. Vol. I In Genomics, edited by Rigoutsos I. and Stephanopoulos G., Oxford University Press, Oxford, pp 3–56 [Google Scholar]
- Mishra S. and Imlay J.A. (2013) An anaerobic bacterium, Bacteroides thetaiomicron, uses a consortium of enzymes to scavenge hydrogen peroxide. Mol Microbiol 90:1356–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.J., Lenski R.E., and Zinser E.R. (2012) The Black Queen Hypothesis: evolution of dependencies through adaptive gene loss. MBio 3:e00036–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushegian A. (2008) Gene content of LUCA, the last universal common ancestor. Front Biosci 13:4657–4666 [DOI] [PubMed] [Google Scholar]
- Ndontsa E.N., Moor R.L., and Goodwin D.C. (2012) Stimulation of KatG catalase activity by peroxidatic electron donors. Arch Biochem Biophys 525:215–222 [DOI] [PubMed] [Google Scholar]
- Nelson K.J., Knutson S.T., Soito L., Klomsiri C., Poole L.B., and Fetrow J.S. (2011) Analysis of the peroxiredoxin family: using active site structure and sequence information for global classification and residue analysis. Proteins 79:947–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke W., McGlynn S.E., Milner-White E.J., and Russell M.J. (2013) On the antiquity of metalloenzymes and their substrates in bioenergetics. Biochim Biophys Acta 1827:871–881 [DOI] [PubMed] [Google Scholar]
- Niviére V. and Fontecave M. (2004) Discovery of superoxide reductase: an historical perspective. J Biol Inorg Chem 9:119–123 [DOI] [PubMed] [Google Scholar]
- Olson S.L., Kump L.R., and Kasting J.F. (2013) Quantifying the areal extent and dissolved oxygen concentrations of Archean oxygen oases. Chem Geol 362:35–43 [Google Scholar]
- Ouzounis C. and Kyrpides N. (1996) The emergence of major cellular processes in evolution. FEBS Lett 390:119–123 [DOI] [PubMed] [Google Scholar]
- Ouzounis C.A., Kunin V., Darzentas N., and Goldovsky L. (2006) A minimal estimate for the gene content of the last universal common ancestor—exobiology from terrestrial perspective. Res Microbiol 157:57–68 [DOI] [PubMed] [Google Scholar]
- Passardi F., Zámocký M., Favet J., Jakopitsch C., Penel C., Obinger C., and Dunand C. (2007) Phylogenetic distribution of catalase-peoxidases: are there patches of order in chaos? Gene 397:101–113 [DOI] [PubMed] [Google Scholar]
- Pearson W.R. (2013) An introduction to sequence similarity (“homology”) searching. Curr Protoc Bioinform 42:3.1.1–3.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peregrin-Alvarez J.M., Tsoka S., and Ouzounis C.A. (2003) The phylogenetic extent of metabolic enzymes and pathways. Genome Res 13:422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planavsky N.J., Asaei D., Hofmann A., Reinhard C.T., Lalonde S.V., Knudsen A., Wang X., Ossa Ossa F., Pecoits E., Smith A.J.B., Beukes N.J., Bekker A., Johnson T.M., Konhauser K.O., Lyons T.W., and Rouxel O.J. (2014) Evidence for oxygenic photosynthesis half a billion years before the Great Oxidation Event. Nat Geosci 7:283–286 [Google Scholar]
- Pratt C.W. and Cornely K. (2014) Enzyme kinetics and inhibition. In Essential Biochemistry, 3rd ed., John Wiley and Sons, Hoboken, NJ, pp 188–219 [Google Scholar]
- Raymond J. and Segrè D. (2006) The effect of oxygen on biochemical networks and the evolution of complex life. Science 311:1764–1767 [DOI] [PubMed] [Google Scholar]
- Rhee S.G., Chae H.Z., and Kim K. (2005) Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 38:1543–1552 [DOI] [PubMed] [Google Scholar]
- Ring D. and Miller S.L. (1984) The spark discharge synthesis of amino acids from various hydrocarbons. Orig Life Evol Biosph 15:7–15 [DOI] [PubMed] [Google Scholar]
- Saito M.A., Sigman D.M., and Morel F.M.M. (2003) The bioinorganic chemistry of the ancient ocean: the co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean-Proterozoic boundary? Inorganica Chim Acta 356:308–318 [Google Scholar]
- Samuel B.S., Hansen E.E., Manchester J.K., Coutinho P.M., Henrissat B., Fulton R., Latreille P., Kim K., Wilson R.K., and Gordon J.I. (2002) Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc Natl Acad Sci USA 104:10643–10648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz J.L. (2011) Anaerobe. In Encyclopedia of Astrobiology, edited by Gargaud M., Amils R., Cernicharo R., Cleaves H.J., II, Irvine W.M., Pinti D.L., and Viso M., Springer-Verlag, Berlin, p 44 [Google Scholar]
- Schippers J.H.M., Nguyen H.M., Lu D., Schmidt R., and Mueller-Roeber B. (2012) ROS homeostasis during development: an evolutionary conserved strategy. Cell Mol Life Sci 69:3245–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoes A.M., Brown S.D., Dodevski I., and Babbitt P.C. (2009) Annotation error in public databases: misannotation of molecular function in enzyme superfamilies. PLoS Comput Biol 5:e1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G.H. (2008) Earth's atmosphere—Hadean to early Proterozoic. Chem Erde 68:235–264 [Google Scholar]
- Singh R., Wiseman B., Deemagrn T., Jha V., Świtała J., and Loewen P.C. (2008) Comparative study of catalase-peroxidases (KatGs). Arch Biochem Biophys 471:207–214 [DOI] [PubMed] [Google Scholar]
- Ślesak I., Libik M., Karpinska B., Karpiński S., and Miszalski Z. (2007) The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim Pol 54:39–50 [PubMed] [Google Scholar]
- Ślesak I., Ślesak H., and Kruk J. (2012) Oxygen and hydrogen peroxide in the early evolution of life on Earth. In silico analysis of biochemical pathways. Astrobiology 12:775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., and Kumar S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira-Silva S. and Rocha E.P.C. (2008) An assessment of the impacts of molecular oxygen on the evolution of proteomes. Mol Biol Evol 25:1931–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächtershäuser G. (2007) On the chemistry and evolution of pioneer organism. Chem Biodivers 4:584–602 [DOI] [PubMed] [Google Scholar]
- Woese C.R., Kandler O., and Wheelis M.L. (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 87:4576–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y.I. and Koonin E.V. (2013) Genome reduction as the dominant mode of evolution. Bioessays 35:829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe-Simon F., Grzebyk D., Schofield O., and Falkowski P.G. (2005) The role and evolution of superoxide dismutases in algae. J Phycol 41:453–465 [Google Scholar]
- Xiong J. (2006) Pairwise sequence alignment. In Essential Bioinformatics, Cambridge University Press, New York, pp 31–50 [Google Scholar]
- Xu J., Sahai N., Eggleston C.M., and Schoonen M.A.A. (2013) Reactive oxygen species at the oxide/water interface: formation mechanisms and implications for prebiotic chemistry and the origin of life. Earth Planet Sci Lett 363:156–167 [Google Scholar]
- Yamaguchi K.E. (2005) Evolution of the atmospheric oxygen in the early Precambrian: an updated review of geological “evidence.” In Frontier Research on Earth Evolution, Vol. 2, edited by Fukao Y., Institute of Frontier Research for Earth Evolution (IFREE), Yokosuka, Japan, pp 4–23 [Google Scholar]
- Yang X. and Ma K. (2007) Characterization of an exceedingly active NADH oxidase from the anaerobic hyperthermophilic bacterium Thermotoga maritima. J Bacteriol 189:3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zámocký M., Gasselhuber B., Furtmüller P.G., and Obinger C. (2012) Molecular evolution of hydrogen peroxide degrading enzymes. Arch Biochem Biophys 525:131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Jewitt D., and Kaiser R.I. (2006) Formation of hydrogen, oxygen, and hydrogen peroxide in electron-irradiated crystalline water ice. Astrophys J 639:534–548 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.