Abstract
Objective: We sought to evaluate the neurophysiology of mindfulness-based cognitive therapy for children (MBCT-C) in youth with generalized, social, and/or separation anxiety disorder who were at risk for developing bipolar disorder.
Methods: Nine youth (mean age: 13 ± 2 years) with a generalized, social, and/or separation anxiety disorder and a parent with bipolar disorder completed functional magnetic resonance imaging (fMRI) while performing a continuous processing task with emotional and neutral distractors (CPT-END) prior to and following 12 weeks of MBCT-C.
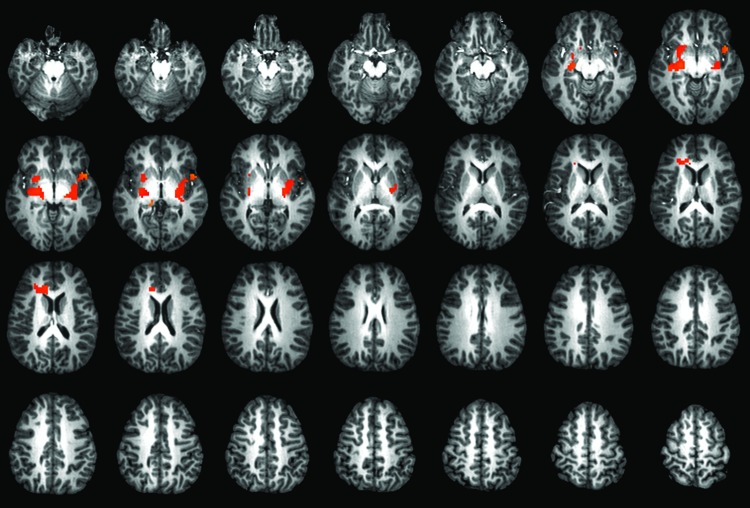
Results: MBCT-C was associated with increases in activation of the bilateral insula, lentiform nucleus, and thalamus, as well as the left anterior cingulate while viewing emotional stimuli during the CPT-END, and decreases in anxiety were correlated with change in activation in the bilateral insula and anterior cingulate during the viewing of emotional stimuli (p < 0.05, uncorrected; p < 0.005 corrected; cluster size, 37 voxels).
Conclusions: MBCT-C treatment in anxious youth with a familial history of bipolar disorder is associated with increased activation of brain structures that subserve interoception and the processing of internal stimuli—functions that are ostensibly improved by this treatment.
Introduction
Anxiety disorders are among the most common psychiatric conditions affecting children and adolescents (Kessler et al. 2012) and increase the risk of suicide attempts (Husky et al. 2012; Nock et al. 2013) as well as of secondary mood and other anxiety disorders (Pine et al. 1998; Beesdo et al. 2007; Beesdo-Baum and Knappe 2012). Additionally, anxiety disorders are common in youth at risk for developing bipolar disorder (Henin et al. 2005; Hirshfeld-Becker et al. 2006; Duffy et al. 2007) and anxious youth with a bipolar parent have three times the normal risk of developing mania, the onset of which occurs typically during adolescence (Singh et al. 2007). In youth with anxiety disorders, psychotherapeutic interventions are recommended in treatment guidelines, although when pharmacotherapy is considered, the first-line psychopharmacologic interventions for youth with anxiety disorders –antidepressants – are the mainstay of treatment (Connolly and Bernstein 2007; Strawn et al. 2012b). However, antidepressants may worsen the outcome and accelerate the onset of mania or hypomania in some youth with a familial risk of bipolar disorder (Reichart and Nolen 2004). Nonetheless, antidepressants are commonly used to treat anxiety disorders in youth at high risk for developing bipolar disorder (Strawn et al. 2014a). Additionally, the emergence of an earlier age of onset of manic episodes may be associated with increased use of antidepressant medications in patients who may already be at risk of developing bipolar disorder (Reichart and Nolen 2004). However, not all data support this association (Chang et al. 2010; Goldsmith et al. 2011).
Although there are few data that directly or prospectively address the safety and tolerability of antidepressants in pediatric patients who are at risk for developing bipolar disorder, one prospective study of nine patients (7–16 years of age) with a family history of bipolar disorder treated with the selective serotonin reuptake inhibitor (SSRI), paroxetine, or with paroxetine and divalproex, found that >50% of youth experienced manic or hypomanic symptoms or developed suicidality (Findling et al. 2008). Additionally, we observed, in patients 9–20 years of age, that 50% of patients experienced an antidepressant-related adverse event leading to discontinuation (Strawn et al. 2014a). Therefore, given the poor tolerability of antidepressants—the first-line psychopharmacologic interventions for anxiety disorders (Connolly and Bernstein 2007; Strawn et al. 2012b)—in youth at high risk for developing bipolar disorder, innovative nonpharmacologic treatments are urgently needed.
Mindfulness is a dynamic process involving the self-regulation of attention toward present moment experiences with an attitude of openness, curiosity, and non-judgment (Kabat-Zinn 2003, 2011). Mindfulness-based cognitive therapy (MBCT) is an evidence-based, manualized treatment for emotional disorders in adults that combines features of mindfulness training and cognitive-behavior therapy (CBT) (Segal and Williams 2001; Hofmann et al. 2010). This protocol has recently been adapted specifically for use in children and adolescents with anxiety disorders (MBCT-Child version [MBCT-C]), and shows promising efficacy data (Semple et al. 2005; Lee et al. 2008; Semple and Lee 2008; Paul et al. 2012). Involving weekly group sessions, regular home practice, and the core curriculum of formal mindfulness practices (e.g., body scan, sitting, movement, and walking meditations), MBCT-C guides patients through mindfulness meditation practices, facilitator-led discussions of experiences, and psychoeducation. Most importantly, and in contrast to antidepressants in youth with a familial risk for bipolar disorder, MBCT-C is associated with minimal adverse effects.
In pediatric patients with the most prevalent anxiety disorders, generalized anxiety disorder (GAD), social anxiety disorder, and separation anxiety disorder (SAD) (i.e., the pediatric anxiety triad, [Strawn et al., 2015]), functional and structural abnormalities are present in prefrontal emotional processing circuits. These circuits, which modulate emotional and social behavior and, ultimately, maintain emotional homeostasis (Strakowski et al. 2011), include the amygdala, medial prefrontal cortex (Brodmann area [BA] 10/11), rostral insula, subgenual/rostral anterior cingulate cortex (ACC) (BA 25, BA 24/32), ventrolateral prefrontal cortex (VLPFC) (BA 10/47), and dorsolateral prefrontal cortex, structures that are frequently implicated in pediatric anxiety disorders (Blackford and Pine 2012; Strawn et al. 2012c, 2013, 2014b).
Additionally, recent neuroimaging data implicate a variety of these structures in both the practice of mindfulness and MBCT in adults. Specifically, during the practice of mindfulness, task-specific activation occurs in a constellation of medial structures that subserve reflective function and interoception (e.g., the anterior insula, ACC, medial prefrontal cortex, and bilateral precuneus) (Ives-Deliperi et al. 2011). Functional magnetic resonance imaging (fMRI) studies in subjects with affective and anxiety disorders, as well as in healthy subjects, reveal treatment-related effects of MBCT. In this regard, Allen and colleagues (2012) noted that, in the context of a longitudinal trial of MBCT in adults, employing a visual Stroop task, treatment increased dorsolateral prefrontal cortex activation. Also, in this sample, individuals with large improvements in mindfulness demonstrated increased use of the dorsal ACC, medial prefrontal cortex, and right anterior insula while processing negative stimuli.
To date, one study examined a related mindfulness-based therapy (i.e., mindfulness-based stress reduction [MBSR]) and found that in adults with social phobia (n = 16) – in whom amygdala activation is increased compared with healthy subjects −10 weeks of MBSR was associated with decreased amygdala activation (Goldin and Gross 2010). Finally, a study of adults with bipolar disorder (n = 23), observed that improvements in anxiety and depressive symptoms were associated with increased, posttreatment activity in the medial prefrontal cortex and posterior parietal cortex, and found a relationship between activation in the medial prefrontal cortex and increases in mindfulness (Ives-Deliperi et al. 2013).
Given 1) the poor tolerability of antidepressants in anxious youth who are at risk for developing bipolar disorder (Findling et al. 2008; Strawn et al. 2013) and 2) the heterogeneity in response to cognitive behavioral therapy, which although a first-line psychotherapeutic intervention in youth with anxiety disorders, may not be effective in all patients (Connolly and Bernstein 2007), we conducted a pilot of trial of MBCT-C with 10 anxious youth at familial risk for bipolar disorder, and have previously reported the effects on mood and anxiety (Cotton et al. 2015). Clinician-rated anxiety (p < 0.01) and youth-rated trait anxiety (p = 0.03) were significantly reduced following treatment. In addition, parent-rated emotion regulation significantly increased after the MBCT-C intervention (p = 0.05) and increases in mindfulness were associated with decreases in anxiety (p = 0.03) (Cotton et al. 2015). A second aim of this pilot trial was to examine the neurofunctional changes in emotional processing associated with mindfulness training for anxious youth who are at high risk for developing bipolar disorder. Specifically, we hypothesized that MBCT-C would be associated with increases in activation in prefrontal structures, which have been implicated in the pathophysiology of anxiety disorders (Strawn et al. 2014), including the VLPFC and insula. These two structures were examined given their central role in the pathophysiology of anxiety. In this regard, most fMRI studies of youth with anxiety disorders suggest increased activation of the VLPFC (Monk et al. 2006; McClure et al. 2007; Guyer et al. 2008; Monk et al. 2008; Beesdo et al. 2009; Strawn et al. 2012a) and also suggest structural deficits in this region in children and adolescents with anxiety disorders (Strawn et al. 2015). The VLPFC subserves a number of regulatory functions, including modulation of amygdala activity (Monk et al. 2008) and is responsible for conscious regulation of affect (Phillips et al. 2008). The insula is implicated in interoception, a process that is directly affected by MCBT-C, and that is neurofunctionally implicated in the neurobiology of pediatric anxiety disorders (Roy et al. 2013).
Methods
Study design
Children and adolescents with a family history of bipolar disorder who met Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) (American Psychiatric Association, 1994) criteria for at least one of the pediatric triad anxiety disorders (i.e., GAD, social anxiety disorder, and/or separation anxiety disorder) were recruited from an ongoing naturalistic, longitudinal study of youth with a familial history of bipolar disorder. Participants underwent fMRI at the Center for Imaging Research at the University of Cincinnati prior to and following 12 sessions of MBCT-C, as previously described (American Psychiatric Association 1994; Cotton et al. 2015).
Assessments and analyses
Clinical diagnoses were confirmed with the Kiddie Schedule for Affective Disorders and Schizophrenia, and all subjects were evaluated using the Crovitz Handedness Questionnaire, the Duke Tanner stage self-assessment, the Pediatric Anxiety Rating Scale (PARS) (2002), and the Child and Adolescent Mindfulness Measure (CAMM) as previously described, obtained at baseline and end-point (week 12, or early termination) (Cotton et al. 2015). The PARS, a clinician-administered scale, includes a 50 item symptom checklist, as well as a second section consisting of 7 severity and impairment items that are rated on a six point scale, with higher scores representing more severe symptoms and impairment. The CAMM, a patient-reported scale, assess the use of mindfulness skills, particularly one's ability to observe internal experiences, act with awareness of the present moment, and accept internal experiences without judgment. Scores on the CAMM range from 0 to 40 (higher scores indicate greater levels of mindfulness).
Neuroimaging
All subjects were scanned on a 4.0 Tesla Varian Unity INOVA Whole Body MRI system (Varian Inc., Palo Alto, CA). To provide anatomical localization for activation maps, a high-resolution, T1-weighted, three-dimensional (3-D) brain scan was obtained using a modified driven equilibrium Fourier transform (MDEFT) sequence after which a multiecho reference scan was obtained. Subjects completed the fMRI session in which whole-brain images (volumes) were acquired every 2 seconds while they were performing the continuous processing task with emotional and neutral distracters (CPT-END) (Yamasaki et al. 2002) using a T2*-weighted gradient-echo echoplanar imaging (EPI) pulse sequence. The CPT-END, which has been utilized in pediatric patients with anxiety disorders (Strawn et al. 2012a), utilizes a visual oddball paradigm in which 70% of the cues are simple, colored squares, 10% are simple colored circles (targets), 10% are emotionally neutral pictures, and 10% are emotionally unpleasant pictures, with the neutral and emotional distractors (fear and disgust-evoking images) that originated from the International Affective Picture System (University of Florida, Gainesville, Florida). In response to each visual cue, participants provided a unique response: Button 2 when presented with a colored circle (target) and button 1 when presented with the other stimuli. Each session consisted of two runs, each consisting of 158 visual cues (at 3 ond intervals, for 2 seconds' duration of presentation). Blood oxygen level-dependent (BOLD) signal was acquired by means of an echo planar single-shot gradient echo pulse sequence (matrix, 64 × 64; repetition time, 3000 ms; echo time, 30 ms; field of view, 240 mm; 256 × 256 voxels, 4 × 4 × 4 mm).
fMRI analysis
The CPT-END allows the ventral (emotional) and dorsal (cognitive) prefrontal networks—which are putatively affected by MBCT-C and subserve processes that are affected by MBCT-C (e.g., self-regulation, attention)—to be evaluated. In this task, subjects distinguished targets from frequent background shapes and interspersed novel images varied for emotional valence. Consequently, the CPT-END is an ideally suited cognitive-emotional probe for studying treatment-associated changes in pediatric patients with anxiety disorders (Strawn et al. 2012a). Anatomic and fMRI data were co-registered and fMRI data were motion corrected. The fMRI data were analyzed using analysis of functional neuroimages, and magnetic resonance images were reconstructed to convert raw scanner data into AFNI format. Individual voxel-wise event-related activation maps were created following standard AFNI procedures using an algorithm that compared the actual hemodynamic response to a canonical hemodynamic response function. Event-related response functions were calculated for the emotional pictures, neutral pictures, and circles. Squares provided the baseline against which hemodynamic responses were assessed. A voxelwise statistical analysis was performed to identify regions that exhibited significant differences (group-by-cue [groups, baseline or posttreatment; cue, circle, emotional, or neutral image]) to identify treatment-associated differences in response patterns. Based on a Monte Carlo simulation using 10,000 iterations, we performed individual group-by-cue voxelwise contrasts at a significance level of p < 0.005 with a cluster of 37 voxels, which resulted in a corrected threshold of p < 0.05.
A post-hoc examination of the relationship between baseline functional activity and improvement in anxiety was also undertaken. Specifically, regression analyses were performed for PARS score and BOLD signal (in response to emotional images) in regions of interest (ROIs) based on regions in which significant treatment-related differences were observed in the more conservative voxelwise analysis. The ROI activation in response to emotional images was extracted from measurements obtained from the neuroanatomic scans using semiautomatic segmentation (gray and white matter) and volumetric analyses performed as previously described (Strakowski et al, 2011; Strawn et al. 2012a). Findings were considered statistically significant at the p < 0.05 level.
Results
Subject characteristics
As previously described, the participants were 10 children and adolescents 9–16 years (mean age: 13.2 ± 1.8 years, eight female) (Cotton et al. 2015); however, in this analysis, only nine patients completed pre- and posttreatment fMRI (mean age: 12.9 ± 2). Additional characteristics of these patients are described in Table 1.
Table 1.
Demographic Characteristics of Patients
| n (%) | Mean | |
|---|---|---|
| Age | 12.9 ± 2 | |
| Sex (male) | 2 (22) | |
| Race | ||
| Caucasian | 3 (33) | |
| African American | 4 (44) | |
| Mixed | 2 (22) | |
| Baseline Pediatric Anxiety Rating Scale (PARS) score | 11.3 ± 2.9 | |
| Baseline Child and Adolescent Mindfulness Measure (CAMM) Score | 21.7 ± 8.47 | |
| Co-occurring conditions | ||
| ADHD | 6 (66) | |
| Depressive disorder | 3 (33) | |
Means are expressed ± standard deviations, and these demographics relate to the patients who completed baseline and end-point fMRI (n = 9).
ADHD, attention-deficit/hyperactivity disorder.
Changes in emotional processing during the CPT-END
Treatment with MBCT-C was associated with increases in activation of the bilateral insula, lentiform nucleus, and thalamus, as well as the left anterior cingulate, while viewing emotional stimuli (compared with baseline [squares], Fig. 1) during the CPT-END (p < 0.05, uncrorrected; p < 0.005 corrected; cluster size, 37 voxels).
FIG. 1.
Treatment-related changes in activation following mindfulness-based cognitive therapy (MBCT). Increased activation was observed in the bilateral insula and left thalamus as well as in the left anterior cingulate cortex following treatment with MBCT (p < 0.05, uncrorrected; p < 0.005 corrected; cluster size, 37 voxels). A color version of this figure is available in the online article at www.liebertpub.com/cap.
Attentional processing during the CPT-END
No statistically significant differences in accuracy were noted between the baseline and posttreatment CPT-END (baseline percent correct, 92.7 ± 12.9; posttreatment percent correct, 90.7 ± 16). Similarly, reaction times did not differ between the baseline and posttreatment scans (0.7 ± 0.2 sec vs. 0.7 ± 0.2 sec). Finally, no differences in activation in any brain region were observed for circles (compared with squares [baseline]) or for the neutral images (compared with squares [baseline]).
Relationship among anxiety, mindfulness, and activation during emotional processing
In the voxelwise analysis, decreases in PARS score were associated with decreased functional activity in the anterior insula bilaterally (right, p = 0.012; left, p = 0.009), change in left ACC activation (p = 0.009), and a trend toward significance in the left ACC (p = 0.052). No significant associations were observed between changes in CAMM scores activation in these structures.
Baseline neurofunctional predictors of change in anxiety symptom severity
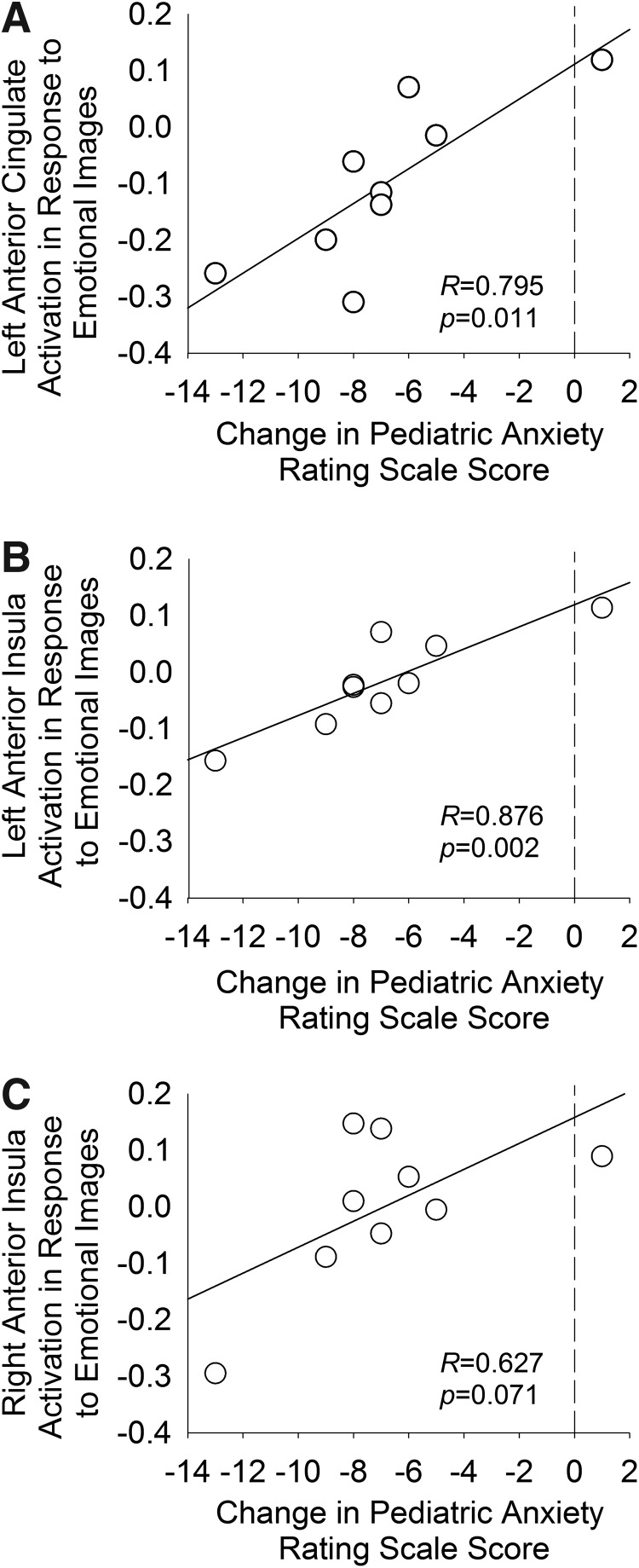
A post-hoc ROI-based analysis of activation in prefrontal structures in which treatment-related changes were observed in the voxelwise analysis described, revealed a statistically significant relationship among baseline activation (in response to emotional images), change in anxiety symptom severity in the left anterior cingulate (r = 0.795, p = 0.011), and right anterior insula (r = 0.876, p = 0.002); however, this relationship only trended toward significance in the left anterior insula (r = 0.63, p = 0.071) (Fig. 2).
FIG. 2.
Relationship between region of interest (ROI) activation in response to emotional images and anxiety symptom severity. Correlations between baseline activation in each ROI identified in the voxelwise analysis, and changes in symptom severity are shown for the left anterior cingulate (A) and for the insula, bilaterally (B, C).
Discussion
This is the first pilot trial in children and adolescents to demonstrate that MBCT-C treatment in anxious youth with a familial history of bipolar disorder may be associated with increased activation of structures (e.g., insula and anterior cingulate) that subserve the interoceptive representation of one's affective state and the processing of internal and extrapersonal stimuli: functions that are ostensibly improved by MBCT-C.
Our preliminary observation that MBCT-C increased activation in the cingulate cortex is noteworthy, given that this structure subserves the contemporaneous processing of cognitive and emotional information, and integrates these two streams (Yamasaki et al. 2002). fMRI studies of this region in youth with GAD have revealed increased activation during the viewing of fearful faces (McClure et al. 2007) and ACC activation correlates with amygdala and VLPFC activation in youth with GAD. Further, in the present sample, baseline activation of this structure predicted treatment-associated changes in anxiety symptoms. Taken together, these findings raise the possibility that a treatment-related increase in ACC activity – during emotional processing – may represent increased functioning of this region and, possibility, improved integrative capacity with regard to emotional processing in anxious youth at risk for developing bipolar disorder.
Our findings of treatment-associated increases in functional insula activity during the processing of emotional images are of significant interest for several reasons. First, the insula is involved in cognitive (i.e., attentional), affective, and regulatory (e.g., interoceptive awareness, emotional responsivity) processes (Menon and Uddin 2010) that are putatively disrupted in patients with anxiety disorders. Second, and likely of central relevance to mindfulness-based treatments, accumulating data implicate the role of the insula in interoception and representation of bodily states. Insula activation in adults correlates with the perception of somatic states of arousal (Straube and Miltner 2011). Third, anticipatory anxiety in response to threat-related stimuli is associated with activation of the insula in adult patients with a myriad anxiety disorders ranging from social anxiety disorder to GAD to specific phobia (Boshuisen et al. 2002; Simmons et al. 2004; Nitschke et al. 2006; Simmons et al. 2006; Stein et al. 2007; Straube et al. 2007; Boehme et al. 2014). Moreover, in anxious adolescents, 11–17 years of age, using the same task as in the present study (i.e., CPT-END), insula activity inversely correlates with the severity of anxiety symptoms, suggesting a compensatory role for this structure in anxiety (Strawn et al. 2012a). This finding is consistent with one prior study of mindfulness training in marines who demonstrated training-associated changes in anterior insula and ACC activation during loaded breathing, a paradigm that assesses responsiveness to aversive stimuli, suggesting modification of a neural substrate for the processing of aversive and interoceptive stimuli (Haase et al. 2014), at least in young adults. Additionally, it has recently been hypothesized that the insula (and in particular the anterior insula) may orchestrate an “integral hub” (p. 655) function coordinating interactions among attentional networks and those that subserve self-oriented cognition (Menon and Uddin 2010) which – if the increased activity in this region is related to increased functioning – would be consistent with the notion that MBCT enhances the internally oriented processing of fear or anxiety. Last, the increases in bilateral insula activation observed in the present study highlight a complex relationship between the insula and anxiety symptoms in youth (Strawn et al. 2015). Specifically, activation of the insula during the CPT-END in prior studies of anxious youth (Strawn et al, 2012a) supports the possibility that the insula and VLPFC operate as a coordinated system to compensate for the increased amygdala activity seen in other studies of anxious youth. Consistent with this notion, several studies reveal that both insula and VLPFC activation – although increased in anxious youth – inversely correlate with the severity of anxiety symptoms (Monk et al. 2008; Strawn et al. 2012a).
In the present study, we also observed differences between pretreatment and posttreatment activation in the thalamus/lentiform nucleus (in response to emotional images). The thalamus and lentiform nuclei, which subtend diverse functions, including information transfer to the prefrontal cortex, reward processing (Singh et al. 2013), and visuospatial processing, have been implicated in neurofunctional and neuroanatomic studies of pediatric patients with bipolar disorder (Chang et al. 2004). Additionally, in adults with fear-based anxiety disorders, a multitude of thalamic nuclei appear to exhibit disorder-specific connectivity compared with healthy subjects (Etkin et al. 2009). In this regard, in adults with GAD, the basolateral amygdala exhibits increased connectivity with targets typically associated with the centromedial amygdala, including the thalamus (Etkin et al. 2009). Importantly, however, the posttreatment neurofunctional changes observed herein in the thalamus/lentiform nucleus may relate to either “at risk” or anxiety neurocircuitry, or could be an effect of treatment. Given the diversity of thalamic functions and the central role of this structure within the prefrontal amygdala circuitry, additional studies will be critical.
The path from an initial understanding of the neurobiological effects of psychotherapeutic treatments to the identification of neurophysiologic markers of treatment response (as well as moderators or predictors of treatment response) is fraught with challenges, and will almost certainly represent a highly iterative process (i.e., relying on successive studies, each necessarily probing specific aspects of the emotional processing circuits).
Limitations
There are several limitations of this investigation. First, in the present pilot study, we lacked a control group; therefore, future studies will require comparison samples so that the specificity of neurofunctional changes with regard to either treatment or fluctuation in anxiety symptoms can be examined. Additionally, we cannot exclude the possibility that practice effects may have contributed to the baseline–end-point differences observed herein. Third, we examined a small number of patients, which limits statistical power, and, as such, null results may result from a lack of power. Additionally, the small sample size precludes correction for multiple comparisons. Fourth, there was no blinding with regard to treatment; all subjects knew that they were receiving an active psychotherapeutic treatment, which may bias the results toward positive outcomes by introducing expectancy effects. Fifth, common factor components (e.g., alliance, therapeutic expectation) (Plakun et al. 2009), which are increasingly acknowledged to be important determinants of psychotherapy-related change, were not measured in the present study and, therefore, the degree to which the neurofunctional changes are specific to the mindfulness, cognitive, or common components of the psychotherapy are unclear. Sixth, it is of interest that the CAMM – a measure of mindfulness – did not correlate with changes in brain activation, particularly given that mindfulness correlated with the reduction in anxiety symptoms (Cotton et al. 2015). This lack of a direct relationship may be attributable to several factors, including 1) an indirect or moderating relationship between mindfulness and the brain activation, 2) nonspecific effects of psychotherapeutic treatment on brain activation, or 3) type II error, particularly in this small, pilot study. However, despite these limitations, this study is a novel contribution to the extant research, given that the current data represent a rare, within-subject fMRI study of pediatric anxiety disorders and the effects of mindfulness training.
Conclusions
These preliminary data suggest that MBCT-C alters brain activation in anxious youth who are at risk for bipolar disorder. Additionally, the treatment-related neurofunctional changes observed herein occur in a constellation of structures that subserve fear processing and emotional regulation. The findings described herein, despite their preliminary nature, guide us toward important areas for future research.
Clinical Significance
This study suggests that MBCT-C in anxious youth who are at risk for developing bipolar disorder is associated with increased activation of structures (e.g., insula and anterior cingulate) that subserve the interoceptive representation of one's affective state and the processing of internal and extrapersonal stimuli functions. Importantly, these processes are ostensibly improved by MBCT-C. Moreover, this study provides initial evidence putatively linking treatment-related changes in specific structures with treatment-related changes in anxiety and mindfulness.
Disclosures
Dr. Strawn has received research support from Edgemont Pharmaceuticals, Eli Lilly, Forest Research Institute, Lundbeck, the National Institute of Mental Health, and Shire. Dr. Cotton has received research support from the National Institute of Child Health and Human Development, Pfizer, and the University of Cincinnati Neuroscience Institute. Dr. Patino has received funding from American Academy of Child and Adolescent Psychiatry (AACAP). Dr. Sears receives royalties from the publication of books on mindfulness. Dr. DelBello has received research support from Amylin, Eli Lilly, GlaxoSmithKline, Lundbeck, Martek, Merck, Otsuka, Pfizer, Purdue, Shire, and Sunovion. She is on the lecture bureau for Otsuka and has provided consultation or advisory board services for Actavis, Dey, Lundbeck, Otsuka, Pfizer, Sunovian, Supernus, and Takeda. Drs. Stahl and Eliassen, Mr. Weber, and Ms. Luberto report no biomedical conflicts of interest.
References
- Allen M, Dietz M, Blair KS, van Beek M, Rees G, Vestergaard-Poulsen P, Lutz A, Roepstorff A: Cognitive-affective neural plasticity following active-controlled mindfulness intervention. J Neurosci 32:15601–15610, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV). Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Höfler M, Lieb R, Wittchen H-U: Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry 64:903–912, 2007 [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS: Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. Psychiatr Clin North Am 32:483–524, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo-Baum K, Knappe S: Developmental epidemiology of anxiety disorders. Child Adolesc Psychiatr Clin N Am 21:457–478, 2012 [DOI] [PubMed] [Google Scholar]
- Blackford JU, Pine DS: Neural substrates of childhood anxiety disorders: A review of neuroimaging findings. Child Adolesc Psychiatr Clin N Am 21:501–525, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S, Ritter V, Tefikow S, Stangier U, Strauss B, Miltner WHR, Straube T: Brain activation during anticipatory anxiety in social anxiety disorder. Soc Cogn Affect Neurosci 9:1413–1418, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshuisen ML, Ter Horst GJ, Paans AMJ, Reinders AATS, Den Boer JA: rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biol Psychiatry 52:126–135, 2002 [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A: Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: A functional magnetic resonance imaging investigation. Arch Gen Psychiatry 61:781–792, 2004 [DOI] [PubMed] [Google Scholar]
- Chang KD, Saxena K, Howe M, Simeonova D: Psychotropic medication exposure and age at onset of bipolar disorder in offspring of parents with bipolar disorder. J Child Adolesc Psychopharmacol 20:25–32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SD, Bernstein GA: Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 46:267–283, 2007 [DOI] [PubMed] [Google Scholar]
- Cotton S, Luberto CM, Sears RW, Strawn JR, Stahl L, Wasson RS, Blom TJ, Delbello MP: Mindfulness-based cognitive therapy for youth with anxiety disorders at risk for bipolar disorder: a pilot trial. Early Interv Psychiatry, 2015. [epub ahead of print] [DOI] [PubMed]
- Duffy A, Alda M, Crawford L, Milin R, Grof P: The early manifestations of bipolar disorder: A longitudinal prospective study of the offspring of bipolar parents. Bipolar Disord 9:828–838, 2007 [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD: Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 66:1361–1372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Lingler J, Rowles BM, McNamara NK, Calabrese JR: A pilot pharmacotherapy trial for depressed youths at high genetic risk for bipolarity. J Child Adolesc Psychopharmacol 18:615–621, 2008 [DOI] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ: Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion 10:83–91, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M, Singh M, Chang K: Antidepressants and psychostimulants in pediatric populations: Is there an association with mania? Pediatr Drugs 13:225–243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JYF, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJR, Leibenluft E, Fox NA, Ernst M, Pine DS, Nelson EE: Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry 65:1303–1312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase L, Thom NJ, Shukla A, Davenport PW, Simmons AN, Paulus MP, Johnson DC: Mindfulness-based training attenuates insula response to an aversive interoceptive challenge. Soc Cogn Affect Neurosci, 2014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henin A, Biederman J, Mick E, Sachs GS, Hirshfeld-Becker DR, Siegel RS, McMurrich S, Grandin L, Nierenberg AA: Psychopathology in the offspring of parents with bipolar disorder: A controlled study. Biol Psychiatry 58:554–561, 2005 [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Henin A, Faraone S V., Dowd ST, De Petrillo LA, Markowitz SM, Rosenbaum JF: Psychopathology in the young offspring of parents with bipolar disorder: A controlled pilot study. Psychiatry Res 145:155–167, 2006 [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D: The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol 78:169–183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husky MM, Olfson M, He J, Nock MK, Swanson SA, Merikangas KR: Twelve-month suicidal symptoms and use of services among adolescents: Results from the National Comorbidity Survey. Psychiatr Serv 63:989–996, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives-Deliperi VL, Howells F, Stein DJ, Meintjes EM, Horn N: The effects of mindfulness-based cognitive therapy in patients with bipolar disorder: A controlled functional MRI investigation. J Affect Disord 150:1152–1157, 2013 [DOI] [PubMed] [Google Scholar]
- Ives-Deliperi VL, Solms M, Meintjes EM: The neural substrates of mindfulness: An fMRI investigation. Soc Neurosci 6:231–242, 2011 [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J: Mindfulness-based interventions in context: Past, present, and future. Clin Psychol Sci Pract 10:144–156, 2003 [Google Scholar]
- Kabat-Zinn J: Why mindfulness matters. In: Boyce Barry, Ed., The mindfulness revolution/ Leading psychologists scientists artists and meditation teachers on the power of mindfulness in daily life. Shambhala Publications, Inc, Boston, MA, 2011, pp 57–62 [Google Scholar]
- Kessler RC, Avenevoli S, Costello J, Green JG, Gruber MJ, McLaughlin KA, Petukhova M, Sampson NA, Zaslavsky AM, Merikangas KR: Severity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry 69:381–389, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Semple RJ, Rosa D, Miller L: Mindfulness-based cognitive therapy for children: Results of a pilot study. J Cogn Psychother 22:15–28, 2008 [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS: Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 64:97–106, 2007 [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ: Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 1–13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJR, Chen G, Charney DS, Ernst M, Pine DS: Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry 163:1091–1097, 2006 [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, Chen G, McClure-Tone EB, Ernst M, Pine DS: Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 65:568–576, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, MacKiewicz KL, Schaefer HS, Davidson RJ: Functional neuroanatomy of aversion and its anticipation. Neuroimage 29:106–116, 2006 [DOI] [PubMed] [Google Scholar]
- Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, Kessler RC: Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: Results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry 70:300–310, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul HA, Semple RJ, Lee J: Mindfulness-based cognitive therapy for anxious children: A manual for treating childhood anxiety. Child Fam Behav Ther 34:167–172, 2012 [Google Scholar]
- Pediatric Anxiety Rating Scale (PARS): Development and psychometric properties. J Am Acad Child Adolesc Psychiatry 41:1061–1069, 2002 [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC: A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13:829, 833–857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y: The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry 55:56–64, 1998 [DOI] [PubMed] [Google Scholar]
- Plakun EM, Sudak DM, Goldberg D: The Y model: An integrated, evidence-based approach to teaching psychotherapy competencies. J Psychiatr Pract 15:5–11, 2009 [DOI] [PubMed] [Google Scholar]
- Reichart CG, Nolen WA: Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord 78:81–84, 2004 [DOI] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS a, Daniele T, Carlisi C, Benson B, Castellanos FX, Milham MP, Pine DS, Ernst M: Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 52:290–299.e2, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple RJ, Lee J: Treating anxiety with mindfulness: Mindfulness-based cognitive therapy for children. In: Acceptance and Mindfulness Treatments for Children and Adolescents. A Practitioners Guide. New Harbinger Publications, Oakland, CA, 2008, pp. 63–87 [Google Scholar]
- Semple RJ, Reid EFG, Miller L: Treating anxiety with mindfulness: An open trial of mindfulness training for anxious children. J Cogn Psychother 19:379–392, 2005 [Google Scholar]
- Simmons A, Matthews SC, Stein MB, Paulus MP: Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport 15:2261–2265, 2004 [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB: Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry 60:402–409, 2006 [DOI] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Kelley RG, Cui X, Sherdell L, Howe ME, Gotlib IH, Reiss AL: Reward processing in adolescents with bipolar I disorder. J Am Acad Child Adolesc Psychiatry 52:68–83, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, DelBello MP, Stanford KE, Soutullo C, McDonough-Ryan P, McElroy SL, Strakowski SM: Psychopathology in children of bipolar parents. J Affect Disord 102:131–136, 2007 [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP: Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 164:318–327, 2007 [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, Lee J-H, Welge JA, DelBello MP, Fleck DE, Adler CM: Functional magnetic resonance imaging brain activation in bipolar mania: Evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry 69:381–388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WHR: Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. Neuroimage 37:1427–1436, 2007 [DOI] [PubMed] [Google Scholar]
- Straube T, Miltner WHR: Attention to aversive emotion and specific activation of the right insula and right somatosensory cortex. Neuroimage 54:2534–2538, 2011 [DOI] [PubMed] [Google Scholar]
- Strawn JR, McReynolds DJ: An evidence-based approach to treating pediatric anxiety disorders. 16–21, 2012 [Google Scholar]
- Strawn JR, Adler CM, McNamara RK, Welge JA, Bitter SM, Mills NP, Barzman DH, Cerullo MA, Chang KD, Strakowski SM, DelBello MP: Antidepressant tolerability in anxious and depressed youth at high risk for bipolar disorder: A prospective naturalistic treatment study. Bipolar Disord 16:523–30, 2014a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Bitter SM, Weber WA, Chu WJ, Whitsel RM, Adler C, Cerullo MA, Eliassen J, Strakowski SM, Delbello MP: Neurocircuitry of generalized anxiety disorder in adolescents: A pilot functional neuroimaging and functional connectivity study. Depress Anxiety 29:939–947, 2012a [DOI] [PubMed] [Google Scholar]
- Strawn JR, Dominick KC, Patino LR, Doyle CD, Picard LS, Phan KL: Neurobiology of pediatric anxiety disorders. Curr Behav Neurosci Rep 1:154–160, 2014b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Hamm L, Fitzgerald DA, Fitzgerald KD, Monk CS, Phan KL: Neurostructural abnormalities in pediatric anxiety disorders. J Anxiety Disord 32:81–88, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Sakolsky DJ, Rynn MA: Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am 21:527–539, 2012b [DOI] [PubMed] [Google Scholar]
- Strawn JR, Wehry AM, Chu WJ, Adler CM, Eliassen JC, Cerullo MA, Strakowski SM, Delbello MP: Neuroanatomic abnormalities in adolescents with generalized anxiety disorder: A voxel-based morphometry study. Depress Anxiety 30:842–848, 2013 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Wehry AM, Delbello MP, Rynn MA, Strakowski S: Establishing the neurobiologic basis of treatment in children and adolescents with generalized anxiety disorder. Depress Anxiety 29:328–339, 2012c [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G: Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A 99:11447–11451, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindel V. Segal J. Mark G. Williams JDT: Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse: Medicine & Health Science Books. New York: The Guilford Press; 2001 [Google Scholar]