Abstract
Background: Iron deficiency anemia (IDA) affects 2%–5% of reproductive-age women. Screening is based on risk factors, such as a low-iron diet and menstruation. However, published IDA risk factors fail to consider age-related risks specific to adolescent women, potentially limiting identification of high-risk adolescents for objective testing. The goal of the study was to examine IDA risk factors in a nationally representative sample of younger (12–21 years) and older (22–49 years) reproductive-age women.
Materials and Methods: Data were obtained from the National Health and Nutrition Examination Survey (NHANES) 2003–2010. IDA was defined using hemoglobin, ferritin, soluble transferrin receptor, standard NHANES laboratory measures. Sex-, age-, and race-specific hemoglobin values defined anemia. Iron deficiency was calculated using ferritin and soluble transferrin receptor in the body iron formula. Logistic regression assessed the association of potential risk factors (race, body mass index, poverty, iron intake, tobacco/nicotine exposure, physical activity, menses, and contraceptive use) with IDA in younger and older women.
Results: The prevalence of IDA was 2.4% and 5.5% among younger and older women, respectively. Among younger women, contraceptive use was marginally protective from IDA (risk ratio 0.50, 95% confidence interval [CI] 0.25–1.00). Among older women, significant variables included Black race (risk ratio 2.31, 95% CI 1.33–4.02) and increased years menstruating (≥25 years vs. <25 years; risk ratio 1.93, 95% CI 0.99–3.76).
Conclusions: Risk factors for IDA among older reproductive-age women do not apply to adolescent women. To better inform the timing and frequency of screening recommendations, further research must identify adolescent-specific IDA risk factors.
Introduction
Anemia may have multiple etiologies, but the most common cause among reproductive-age women is iron deficiency.1–3 Iron deficiency anemia (IDA) affects 2%–5% of reproductive-age women in the United States with clearly documented morbidity.1,2,4–9 Adolescent women with IDA may experience negative effects on cognitive function, audiovisual reaction time, and physical performance.10–14 Symptoms improve rapidly with iron supplementation.10,13 Although anemia is a late-stage indicator of iron deficiency, laboratory testing is commonly based on a hemoglobin level to screen for anemia due to its widespread availability and ease of interpretation.2,15
The Centers for Disease Control and Prevention (CDC) recommends testing “all nonpregnant women for anemia every 5–10 years throughout their childbearing years during routine health examinations.” Women with risk factors (extensive menstrual or other blood loss, low iron intake, and previous diagnosis of IDA) are recommended for annual objective testing, although risk assessment tools for blood loss and low iron intake are either not available or not widely used.15,16
Published risk factors for IDA, including race, poverty, education, low iron intake, heavy menses, parity, and a previous diagnosis of IDA, are based on all women of reproductive age, variably defined between 12 and 49 years.9,15–19 Adolescent women differ in many ways from older reproductive-age women, including nutritional requirements, duration of menses, and contraceptive use.20–23 For adolescent IDA, the guidelines do not advise how many years after menarche to screen.15,16,18 Adolescents may struggle to accurately quantify menstrual blood loss or iron intake.23 If a 13- or 14-year-old girl has a normal hemoglobin result, healthcare providers strictly following the guidelines may not repeat testing during adolescence.15,16,18
The objective of the study was to examine variables associated with IDA in younger (12–21 years) and older (22–49 years), nonpregnant, reproductive-age women using data from the National Health and Nutrition Examination Survey (NHANES) 2003–2010. Understanding of IDA risk factors is needed to inform evidence-based IDA risk assessment in women 12–21 years of age.
Materials and Methods
Study participants
NHANES is a program of studies to assess the health and nutritional status of the US population.24 The nationally representative surveys combine household interviews and physical examinations conducted in mobile examination centers by the National Center for Health Statistics, CDC. The NHANES interview includes demographic, socioeconomic, dietary, and health-related questions. Participants are selected via a stratified multistage probability with oversampling of certain groups (e.g., African Americans, Hispanics) to produce reliable statistics.24

We included all women 12–49 years of age with hemoglobin, ferritin, and soluble transferrin receptor values recorded in NHANES 2003–2010 to have an adequate sample size to consider IDA risk factors. Participants were excluded for a history of blood transfusion as this suggests their IDA may have been due to causes (e.g., trauma, malignancy) other than the IDA most commonly seen in routine primary care.2 Pregnant or breastfeeding participants also were excluded. Those with cancer, malignancy, or chronic kidney or liver disease were excluded, but these questions were only asked for participants older than 20 years. As acute and chronic infection or inflammation may influence the iron indices, consistent with Cogswell et al., we also excluded participants with a white blood cell count >10.0 × 103/μL or a C-reactive protein >0.6 mg/dL.25 This project was given a not human research determination by the Penn State College of Medicine Institutional Review Board.
Conceptual framework and measures
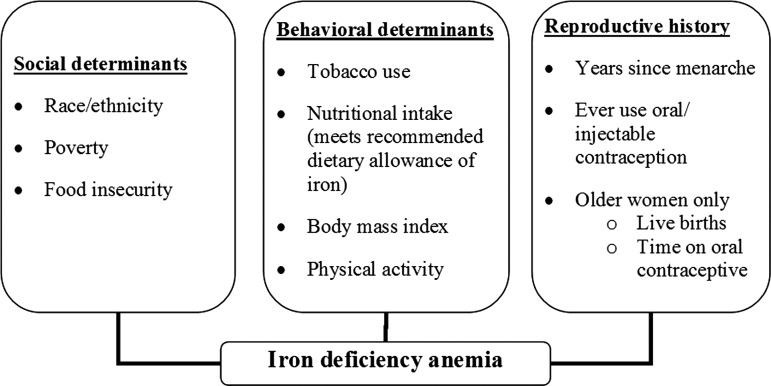
Based on a literature review, clinical expertise, and the consistency of selected variables measured in NHANES across the sample period, a conceptual framework for the possible predictors of IDA in reproductive-age women was constructed (Fig. 1).
FIG. 1.
A conceptual framework for predictors of iron deficiency anemia among nonpregnant reproductive-age women 12–49 years of age.
Dependent variable
The dependent variable, IDA, was defined based on measures of hemoglobin (to determine anemia) and body iron (to determine iron deficiency), which was calculated from ferritin and soluble transferrin receptor. Hemoglobin is a measure of the concentration of iron-containing protein in circulating red blood cells. In the clinical setting, hemoglobin is used as a surrogate for the amount of functional iron in the body.15 Anemia was defined by the CDC standard hemoglobin cutoffs based on sex and age. For women aged 12 through 14 years, anemia is defined by a hemoglobin concentration <11.8 g/dL. For women aged 15 years or older, anemia is defined by a hemoglobin concentration <12.0 g/dL.15 For self-identified Black women, threshold for anemia diagnosis is 1 g/dL lower as recommended by the International Nutritional Anemia Consultative Group and the World Health Organization.26 Parameters for the complete blood count, which includes hemoglobin level, are based on the Beckman Coulter method of counting and sizing. This is performed in combination with an automatic diluting and mixing device for sample processing and a single-beam photometer for hemoglobin measurement.27
Iron deficiency is calculated using the following body iron formula developed by Cook et al.25,28
The formula requires laboratory values for ferritin and soluble transferrin receptor, which were included in the NHANES data set from 2003 to 2010. In this case, a negative value (<0 mg/kg) is indicative of iron deficiency.25
Two methods were used to measure ferritin in 2003–2004. The National Center for Environmental Health analyzed all 2003 samples with a Bio-Rad assay and all 2004 samples with a Roche/Hitachi assay.29 Before the release of the 2003 data, piecewise linear regression equations were applied to adjust the 2003 ferritin data to be comparable to the 2004 ferritin data.29 The Roche/Hitachi 912 nephelometric immunoassay was used in 2005–2008.30,31 In 2009–2010, the Roche Elecsys sandwich immunoassay was used.32 The Roche/Hitachi ferritin assay was previously compared to the Cook ferritin assay with no difference found between the two methods.25 Thus, the 2009–2010 ferritin measurements obtained using the Roche Elecsys 170 method were converted to the Roche/Hitachi 912 scale using the following equation:
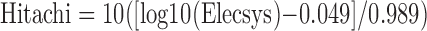 |
Soluble transferrin receptor was measured by immunoturbidimetry using Roche kits on the Hitachi 912 clinical analyzer from 2003 to 2008.33–35 In 2009–2010, the Roche Hitachi Mod P immunoturbidimetric method was used. A crossover study was performed between the two methods, but no adjustment of the data was needed.36 As per Cogswell et al., the Roche soluble transferrin receptor values were converted to the equivalent in the Flowers assay used in developing the body iron model.25
Social determinants
Race/ethnicity was measured in four categories: non-Hispanic White, non-Hispanic Black, Mexican/Hispanic, and other. Poverty was defined using the family poverty-to-income ratio and dichotomized into <1 (indicates household income below the poverty level) versus ≥1 (above the poverty level).37 Persons 16 years and older and emancipated minors were interviewed directly regarding family income, which was used to calculate the family poverty-to-income ratio. A proxy provided information for participants younger than 16 years or those who could not answer the question.38 Household food insecurity was measured by the US Food Security Survey Module (FSSM).39 There are 18 items for households with children younger than 18 years and 10 items for households without children.39 The survey is completed by an adult in the household.40 Responses were dichotomized into full household food security versus marginal, low, or very low household food security.
Behavioral determinants
Tobacco use was assessed for both younger and older women via a yes/no response to tobacco or nicotine use within the last 5 days. Smoking is known to artificially elevate hemoglobin levels, potentially masking anemia.26
Iron intake was examined using the two NHANES dietary recalls. The first dietary recall was an in-person interview querying food intake in the preceding 24 hours. Children 12 years and older answered for themselves.41 The second 24-hour dietary recall was conducted by telephone 3–10 days later.41 Participants were categorized based on meeting the recommended dietary allowance (RDA) of iron based on standard cutoffs for sex and age. For adolescent females 12–13 years of age, the RDA is 8 mg/day of iron.16,20 For those 14–18 years of age, the RDA of iron is 15 mg/day, and for those 19–49 years of age, this increases to 18 mg/day.16,20 Participants fell into one of the three categories: met the RDA of iron on both days, met the RDA of iron on 1 day, or did not meet the RDA of iron on either day.
Elevated body mass index (BMI) and physical activity were included as proxies for poor health and nutrition/low iron intake.42 BMI-for-age percentile was examined up to age 20 and for those older than 20 years using the CDC BMI classifications for adults. Participants were initially classified into four categories: underweight, normal weight, overweight, and obese.43 However, the final subset of older women with complete data for the multivariable analysis did not include any underweight participants. Thus, BMI was collapsed into underweight/normal weight, overweight, and obese. Physical activity was assessed via a yes/no question asked directly to participants.44,45 The question changed slightly over the study period, retaining similar response categories. For NHANES 2003–2006, the question was phrased, “Over the past 30 days, did you do any vigorous activities for at least 10 minutes that caused heavy sweating or large increases in breathing or heart rate? Some examples are running, lap swimming, aerobics classes, or fast bicycling.”44 For NHANES 2007–2010, the question was phrased, “Do you do any vigorous-intensity sports, fitness, or recreational activities that cause large increases in breathing or heart rate like running or basketball for at least 10 minutes continuously?”45
Reproductive history
A variable defining the years since menarche was created by subtracting age at menarche from the participant's age at the time of the survey. Years since menarche was then dichotomized for both younger and older women at the median value, which was <3 years versus ≥3 years and <25 years versus ≥25 years, respectively. Ever using either oral contraceptive pills (OCPs) or injectables (e.g., medroxyprogesterone acetate) was included to account for the protective effect against IDA seen with many contraceptive products.23,46 For older women only, reproductive questions related to the time on OCPs (categorized as <1 year, 1–5 years, and >5 years) and number of live births (categorized as 0–1, 2, and >2) were included. These data were not available for the younger women.
Statistical analyses
Statistical analyses were performed using SUDAAN software, version 11.0.1, and in particular the RLOGIST procedure to fit logistic regression models (Research Triangle Institute, Research Triangle Park, NC).47 SUDAAN is a commercial software package designed specifically for complex survey analysis by taking into account the sampling weights and complex study design to calculate proper variances of estimates.
Predictors were assessed for collinearity. For items that were strongly correlated (e.g., age and years of menstruation or race/ethnicity and immigrant status), only one of the predictors was retained in the final model based on clinical applicability. Initially, bivariate analysis was used to examine the association of predictors with IDA. For ease of interpretation, potential predictors were converted to binary variables where possible.
Potential predictors of IDA were used to build a multivariable model. All predictors included in the conceptual framework were retained in the model regardless of significance level to gain a clear understanding of the impact of the various IDA risk factors in both younger and older women. Descriptive data are reported as raw frequencies and percentages. From the multivariable logistic regression analyses, model-adjusted risk ratios and corresponding 95% confidence intervals (CIs) are reported.48 Two models were built for older women, one with and one without the inclusion of measures unavailable for the younger women.
Results
Characteristics of the sample population
A sample of 13,015 women aged 12–49 years participating in NHANES 2003–2010 was initially identified. This represented 18.7% of the NHANES 2003–2010 participants. After applying the exclusion criteria, the sample included 7,658 women, of which 6,602 had hemoglobin, ferritin, and transferrin receptor values in the data set.
Of the 6,602 women with the necessary laboratory values, 2,985 (45.2%) were 12–21 years of age and 3,617 (54.8%) were 22–49 years of age. Table 1 presents the characteristics of the sample population based on the predictors in the conceptual model. Among younger women, 70 (2.4%) had IDA compared to 198 (5.5%) older women.
Table 1.
Characteristics of Younger (12–21 Years) and Older (22–49 Years) Women
| Younger, N = 2,985a | Older, N = 3,617a | |
|---|---|---|
| Primary outcome | n (%) | n (%) |
| Anemia and body iron deficiency | ||
| Anemic and body iron deficient | 70 (2.4) | 198 (5.5) |
| Normal | 2,915 (97.7) | 3,419 (94.5) |
| Sample characteristics | ||
| Race/ethnicity | ||
| White | 962 (32.2) | 1,777 (49.1) |
| Mexican/Hispanic | 1,010 (33.8) | 942 (26.0) |
| Black | 856 (28.7) | 722 (20.0) |
| Other | 157 (5.3) | 176 (4.9) |
| Poverty-to-income ratio | ||
| <1.00 | 909 (32.4) | 706 (20.8) |
| ≥1.00 | 1,893 (67.6) | 2,692 (79.2) |
| Food security | ||
| Full | 1,854 (63.3) | 2,501 (69.7) |
| Not full | 1,077 (36.8) | 1,085 (30.3) |
| Tobacco/nicotine | ||
| Yes | 301 (11.0) | 788 (24.5) |
| No | 2,442 (89.0) | 2,433 (75.5) |
| RDA of iron | ||
| 0 days compliant | 1,145 (43.0) | 2,049 (65.5) |
| 1 day compliant | 794 (29.8) | 805 (25.7) |
| 2 days compliant | 723 (27.2) | 274 (8.8) |
| BMI | ||
| Underweight | 82 (2.8) | 88 (2.4) |
| Normal | 1,830 (62.2) | 1,393 (38.7) |
| Overweight | 541 (18.4) | 1,070 (29.7) |
| Obese | 489 (16.6) | 1,051 (29.2) |
| Physical activity | ||
| Yes | 1,683 (58.1) | 1,118 (30.9) |
| No | 1,212 (41.9) | 2,499 (69.1) |
| Menstrual years | ||
| <3 | 877 (33.6) | |
| ≥3 | 1,734 (66.4) | |
| <25 | 1,513 (47.4) | |
| ≥25 | 1,676 (52.6) | |
| OCPs/Depo-Provera/injectables | ||
| Yes | 702 (26.8) | 2,669 (83.1) |
| No | 1,921 (73.2) | 543 (16.9) |
| Duration of OCP use | ||
| 0 years | 660 (20.6) | |
| <12 months | 511 (16.0) | |
| 1–5 years | 1,016 (31.8) | |
| >5 years | 1,011 (31.6) | |
| Live births | ||
| Never pregnant | 638 (20.5) | |
| 0 or 1 | 618 (19.9) | |
| 2 | 906 (29.2) | |
| >2 | 946 (30.4) | |
Sample sizes listed are based on having a hemoglobin level recorded and information to determine body iron deficiency status. Sample sizes of the predictor variables may be smaller due to missing data.
BMI, body mass index; OCP, oral contraceptive pill; RDA, recommended dietary allowance.
Multivariable logistic regression: 12- to 21-year-old women
The final model of IDA predictors in young women is depicted in Table 2. Contraceptive use was marginally significant as protective for IDA, with a risk ratio of 0.50 (95% CI 0.25–1.00), p = 0.05. Menstruation for ≥3 years approached significance (risk ratio 2.95, 95% CI 0.91–8.94). None of the other variables were significantly predictive of IDA. In younger women, analyzing underweight as a separate BMI category did not change the results.
Table 2.
Multivariable Model of Anemia and Body Iron Deficiency Predictors for Younger Women (12–21 Years), N = 2,174
| Predictor | Comparison | Risk ratio (95% CI) | Overall p-value |
|---|---|---|---|
| Race | 0.76 | ||
| Mexican/Hispanic versus White | 0.71 (0.32–1.58) | ||
| Black versus White | 0.58 (0.19–1.82) | ||
| Other versus White | 0.63 (0.16–2.41) | ||
| BMI | 0.36 | ||
| Overweight versus underweight/normal | 1.44 (0.63–3.29) | ||
| Obese versus underweight/normal | 0.50 (0.09–2.93) | ||
| Household food security | 0.24 | ||
| Marginal/low/very low food security versus full food security | 1.67 (0.71–3.95) | ||
| Family poverty-to-income ratio | 0.41 | ||
| <1.00 versus ≥1.00 | 1.40 (0.62–3.17) | ||
| RDA of iron | 0.84 | ||
| Compliant with US RDA on both days versus not compliant with US RDA on either day | 0.82 (0.29–2.34) | ||
| Compliant with US RDA on 1 day versus not compliant with US RDA on either day | 0.76 (0.27–2.14) | ||
| Tobacco/nicotine in last 5 days | 0.74 | ||
| Yes versus no | 0.82 (0.24–2.82) | ||
| Vigorous physical activity | 0.27 | ||
| Yes versus no | 0.67 (0.33–1.36) | ||
| Ever used OCPs/Depo-Provera/injectables | 0.05 | ||
| Yes versus no | 0.50 (0.25–1.00) | ||
| Menstrual years | 0.07 | ||
| ≥3 versus <3 | 2.85 (0.91–8.94) |
CI, confidence interval.
Multivariable logistic regression: 22- to 49-year-old women
The final model of IDA predictors in older reproductive-age women is depicted in Table 3. Black race was a significant predictor, with a risk ratio of 2.31 (95% CI 1.33–4.02). Menstruation for ≥25 years was a marginally significant predictor, with a risk ratio of 1.93 (95% CI 0.99–3.76), p = 0.05. Meeting the RDA of iron and the family poverty-to-income ratio also approached significance.
Table 3.
Multivariable Model of Anemia and Body Iron Deficiency Predictors for Older Women (22–49 Years), N = 2,707
| Predictor | Comparison | Risk ratio (95% CI) | Overall p-value |
|---|---|---|---|
| Race | 0.03 | ||
| Mexican/Hispanic versus White | 1.73 (0.97–3.08) | ||
| Black versus White | 2.31 (1.33–4.02) | ||
| Other versus White | 1.37 (0.39–4.85) | ||
| BMI | 0.22 | ||
| Overweight versus underweight/normal | 0.61 (0.33–1.14) | ||
| Obese versus underweight/normal | 0.99 (0.49–2.00) | ||
| Household food security | 0.41 | ||
| Marginal/low/very low food security versus full food security | 0.77 (0.41–1.44) | ||
| Family poverty-to-income ratio | 0.08 | ||
| <1.00 versus ≥1.00 | 1.88 (0.93–3.79) | ||
| RDA of iron | 0.08 | ||
| Compliant with US RDA on both days versus not compliant with US RDA on either day | 0.31 (0.06–1.60) | ||
| Compliant with US RDA on 1 day versus not compliant with US RDA on either day | 1.48 (0.86–2.55) | ||
| Tobacco/nicotine in last 5 days | 0.22 | ||
| Yes versus no | 0.69 (0.38–1.25) | ||
| Vigorous physical activity | 0.45 | ||
| Yes versus no | 1.27 (0.68–2.36) | ||
| Ever used OCPs/Depo-Provera/injectables | 0.86 | ||
| Yes versus no | 1.06 (0.57–1.98) | ||
| Menstrual years | 0.05 | ||
| ≥25 versus <25 | 1.93 (0.99–3.76) |
The addition of live births and years of OCP use to the model for older reproductive-age women resulted in significant changes to the final model (Table 4). Black race, family poverty-to-income ratio, meeting the RDA of iron, tobacco/nicotine exposure, and number of live births (>2 vs. 2) were significantly associated with IDA. While BMI and food security were also significant, the direction of the association was the opposite from what would have been expected. Overweight status and less than full food security demonstrated a protective effect for IDA.
Table 4.
Multivariable Model of Anemia and Body Iron Deficiency Predictors for Older Women (22–49 Years), Including Years of OCP Use and Live Births, N = 2,038
| Predictor | Comparison | Risk ratio (95% CI) | Overall p-value |
|---|---|---|---|
| Race | 0.05 | ||
| Mexican/Hispanic versus White | 1.60 (0.89–2.87) | ||
| Black versus White | 2.06 (1.22–3.49) | ||
| Other versus White | 1.12 (0.30–4.23) | ||
| BMI | 0.05 | ||
| Overweight versus underweight/normal | 0.47 (0.25–0.86) | ||
| Obese versus underweight/normal | 0.75 (0.38–1.51) | ||
| Household food security | 0.03 | ||
| Marginal/low/very low food security versus full food security | 0.52 (0.28–0.94) | ||
| Family poverty-to-income ratio | 0.05 | ||
| <1.00 versus ≥1.00 | 1.98 (1.04–3.80) | ||
| RDA of iron | <0.001 | ||
| Compliant with US RDA on both days versus not compliant with US RDA on either day | 0.04 (0.01–0.27) | ||
| Compliant with US RDA on 1 day versus not compliant with US RDA on either day | 1.52 (0.86–2.67) | ||
| Tobacco/nicotine in last 5 days | 0.02 | ||
| Yes versus no | 0.49 (0.27–0.89) | ||
| Vigorous physical activity | 0.17 | ||
| Yes versus no | 1.57 (0.83–2.98) | ||
| Duration of OCP use, years | 0.46 | ||
| >5 versus never | 0.48 (0.17–1.33) | ||
| >5 versus <1 | 0.64 (0.19–2.12) | ||
| >5 versus 1–5 | 0.54 (0.20–1.44) | ||
| Menstrual years | 0.35 | ||
| ≥25 versus <25 | 1.40 (0.69–2.82) | ||
| Number of live births | 0.06 | ||
| >2 versus 0 or 1 | 1.77 (0.80–3.91) | ||
| >2 versus 2 | 2.27 (1.12–4.60) |
Discussion
Adolescent IDA risk factors were not identified in this nationally representative survey of the general population. This analysis demonstrates that adolescent IDA risk factors are not the same as those for older reproductive-age women. Specifically, Black race and years menstruating were found to be risk factors for IDA for older reproductive-age women, but these variables were not significant predictors for adolescent women. Also among older women, the number of live births, family poverty-to-income ratio, meeting the RDA of iron, and tobacco/nicotine exposure predicted IDA. None of the measured variables significantly predicted IDA in women 12–21 years of age.
The above-mentioned risk factors have demonstrated an association with IDA in previous studies using NHANES data. Looker et al., using NHANES 1988–1994, found an association with IDA and race, poverty, education, and parity among women 20–49 years of age.9 Frith-Terhune et al. used NHANES 1988–1994 to demonstrate a higher prevalence of IDA among Mexican American women 12–39 years of age compared to non-Hispanic White women.19 Eicher-Miller et al. demonstrated an association between food security and IDA among 12- to 15-year-old children of both sexes.49 All these studies used the ferritin model (requires 2 out of 3 iron deficiency laboratory studies out of range along with a low hemoglobin level) to define IDA.9,19,49
Use of the newer body iron formula in defining IDA in this study may explain why the model for older women excluding live births and years of oral contraceptive use found few significant IDA risk factors. Additionally, all risk factors were retained in the model regardless of significance to gain a clear understanding of the impact of the various IDA risk factors in both younger and older women. Use of forward selection or backward elimination may have changed these results. Including live births and years of oral contraceptive use significantly changed the model for older women. Despite careful consideration of the variables in the collinearity analysis, it is possible that these variables introduced additional interactions, which were not considered.
Less information is available specific to IDA among adolescent females and using the newer body iron formula. Our findings suggest that current risk-based IDA screening recommendations are inadequate for adolescent women. The CDC guidelines to test nonpregnant reproductive-age women at low risk for IDA every 5–10 years versus annual testing for high-risk women assume that risk factors are applicable to the population under examination.15,16 Overall, the prevalence of IDA among adolescent women in this sample is lower than that seen among older reproductive-age women (2.4% vs. 5.5%). Thus, tailoring IDA screening guidelines to best identify high-risk adolescents by addressing screening timing, frequency, and high-risk factors would fill a gap in current preventive healthcare for women. The lack of association for risk factors measured in this study with adolescent IDA may also be due to the comparatively shorter exposure time for adolescents. For example, tobacco use and menses may have to occur over a more prolonged time span to demonstrate a significant association with IDA. Other risk factors, for example, live births, are less applicable to the adolescent population in general.
The current guidelines provided by the CDC (1) do not reflect the difference in prevalence of IDA for younger and older women, (2) provide a wide margin for clinicians regarding who, when, and how often to conduct IDA screening, and (3) do not tailor risk factors for young women. Inconsistency in screening is supported by prior work.50 For example, by the guidelines, a young woman with a normal hemoglobin result at age 13 years who is determined to be at low-risk for IDA might not be checked again until 18 or even 23 years old.15 The decision to repeat screening is based on an individual clinician's interpretation of what corresponds to high-risk.
As the study did not detect statistically significant predictors of IDA in adolescents using data from the general population, a key research question is whether such brief survey-based questions are sufficient to identify adolescents at risk of IDA. The negative results for adolescents may be due to inadequate measures. For example, adolescents may have difficulty quantifying their iron intake due to a poor understanding of which foods are iron rich, what constitutes an adequate serving size, and more erratic eating schedules compared to older women.22 Physical activity questions may need to specifically focus on the types of activities in which adolescent women participate. The study of IDA and food insecurity by Eicher-Miller in 12- to 15-year-old adolescents used only the 8 child-specific questions, which are part of the larger 18-item FSSM used in our analysis. Thus, adolescent-specific questions related to food security may have yielded different results.49
Reframing IDA risk assessment tools to target risks specifically applicable to adolescent women could more accurately identify high-risk adolescents. As NHANES is not designed specifically for adolescents, it may not focus on risks or behaviors specific to adolescent women. From a clinical perspective, focusing on common adolescent symptoms (e.g., fatigue, depression, poor concentration) linked to IDA could be an important step in the development of an adolescent-specific IDA screening tool.10–14 In addition, identifying a reliable set of questions to delineate heavy menstrual blood flood in adolescence is needed. The development of validated measures of these factors would be a useful focus for researchers.
Limitations
The current study was limited by the available NHANES questions to consider as candidate risk factors for IDA. The NHANES survey questions were not designed specifically for IDA but cover a broad range of health topics. In particular, the analysis would have been enhanced by the availability of questions related to menstrual blood flow that have been validated in both adolescents and adults. In addition, some measures available for older reproductive-age women (e.g., live births) were not available in NHANES for adolescents.
Conclusions
IDA risk factors differ between younger and older reproductive-age women. To best inform guidelines on the timing and frequency of adolescent IDA screening, research is needed to better identify IDA risk factors specific to the adolescent population.
Acknowledgments
Dr. Sekhar's research is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under BIRCWH Award No. K12HD055882, “Career Development Program in Women's Health Research at Penn State.” The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cusick SE, Mei Z, Freedman DS, et al. Unexplained decline in the prevalence of anemia among US children and women between 1988–1994 and 1999–2002. Am J Clin Nutr 2008;88:1611–1617 [DOI] [PubMed] [Google Scholar]
- 2.Friedman AJ, Chen Z, Ford P, et al. Iron deficiency anemia in women across the life span. J Womens Health 2012;21:1282–1289 [DOI] [PubMed] [Google Scholar]
- 3.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011. (WHO/NMH/NHD/MNM/11.1). Available at: www.who.int/vmnis/indicators/haemoglobin.pdf Accessed June1, 2015 [Google Scholar]
- 4.Carter RC, Jacobson JL, Burden MJ, et al. Iron deficiency anemia and cognitive function in infancy. Pediatrics 2010;126:e427–e434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beard JL, Hendricks MK, Perez EM, et al. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr 2005;135:267–272 [DOI] [PubMed] [Google Scholar]
- 6.Perez EM, Hendricks MK, Beard JL, et al. Mother-infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr 2005;135:850–855 [DOI] [PubMed] [Google Scholar]
- 7.Deal JA, Carlson MC, Xue QL, Fried LP, Chaves PH. Anemia and 9-year domain-specific cognitive decline in community-dwelling older women: The Women's Health and Aging Study II. J Am Geriatr Soc 2009;57:1604–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ando K, Morita S, Higashi T, et al. Health-related quality of life among Japanese women with iron-deficiency anemia. Qual Life Res 2006;15:1559–1563 [DOI] [PubMed] [Google Scholar]
- 9.Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA 1997;277:973–976 [DOI] [PubMed] [Google Scholar]
- 10.Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr 2007;85:778–787 [DOI] [PubMed] [Google Scholar]
- 11.Halterman JS, Kaczorowski JM, Aligne CA, Auinger P, Szilagyi PG. Iron deficiency and cognitive achievement among school-aged children and adolescents in the United States. Pediatrics 2001;107:1381–1386 [DOI] [PubMed] [Google Scholar]
- 12.Falkingham M, Abdelhamid A, Curtis P, Fairweather-Tait S, Dye L, Hooper L. The effects of oral iron supplementation on cognition in older children and adults: A systematic review and meta-analysis. Nutr J 2010;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaki PB, Chandra RK, Geisser P. Effects of oral iron(III) hydroxide polymaltose complex supplementation on hemoglobin increase, cognitive function, affective behavior and scholastic performance of adolescents with varying iron status: A single centre prospective placebo controlled study. Arzneimittelforschung 2009;59:303–310 [DOI] [PubMed] [Google Scholar]
- 14.Nelson M, Bakaliou F, Trivedi A. Iron-deficiency anaemia and physical performance in adolescent girls from different ethnic backgrounds. Br J Nutr 1994;72:427–433 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep 1998;47:1–29 [PubMed] [Google Scholar]
- 16.Holt K, Wooldridge N, Story M, Sofka D, eds. Iron-deficiency anemia. Bright Futures Nutrition, 3rd ed. Elk Grove Village, IL: American Academy of Pediatrics, 2011 [Google Scholar]
- 17.Centers for Disease Control and Prevention. Iron deficiency—United States, 1999–2000. MMWR Morb Mortal Wkly Rep 2002;51:897–899 [PubMed] [Google Scholar]
- 18.Hagan JF, Shaw JS, Duncan PM, eds. Bright futures: Guidelines for health supervision for infants, children, and adolescents, 3rd ed. Elk Grove Village, IL: American Academy of Pediatrics, 2008 [Google Scholar]
- 19.Frith-Terhune AL, Cogswell ME, Khan LK, Will JC, Ramakrishnan U. Iron deficiency anemia: Higher prevalence in Mexican American than in non-Hispanic white females in the third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 2000;72:963–968 [DOI] [PubMed] [Google Scholar]
- 20.US National Library of Medicine. Iron in diet. Recommendations. [Updated February 18, 2013]. Available at: www.nlm.nih.gov/medlineplus/ency/article/002422.htm Accessed June23, 2015
- 21.Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. Natl Health Stat Report 2012;60:1–25 [PubMed] [Google Scholar]
- 22.Grooms LP, Walsh M, Monnat LE. Treatment of anemia in the adolescent female. Pediatr Ann 2013;42:36–39 [DOI] [PubMed] [Google Scholar]
- 23.Holland-Hall C. Heavy menstrual bleeding in adolescents: Normal variant or a bleeding disorder? Contemp Pediatr 2012;29:24–40 [Google Scholar]
- 24.NCHS. National Health and Nutrition Examination Survey. About the National Health and Nutrition Examination Survey. [Updated February 3, 2014]. Available at: www.cdc.gov/nchs/nhanes/about_nhanes.htm Accessed June23, 2015
- 25.Cogswell ME, Looker AC, Pfeiffer CM, et al. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am J Clin Nutr 2009;89:1334–1342 [DOI] [PubMed] [Google Scholar]
- 26.Nestel P. Adjusting hemoglobin values in program surveys, 2002. Available at: http://pdf.usaid.gov/pdf_docs/PNACQ927.pdf Accessed June23, 2015
- 27.NHANES. 2009–2010 Data documentation, codebook, and frequencies. Complete blood count with 5-part differential in whole blood (CBC_F). Description of Laboratory Methodology. [Updated January 2012]. Available at: wwwn.cdc.gov/Nchs/Nhanes/2009-2010/CBC_F.htm#Description_of_Laboratory_Methodology Accessed June23, 2015
- 28.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–3364 [DOI] [PubMed] [Google Scholar]
- 29.NHANES. 2003–2004 Data documentation, codebook, and frequencies. Laboratory component. Ferritin and transferrin receptor (L06TFR_C). Analytic notes. [Updated December 2007]. Available at: www.cdc.gov/nchs/nhanes/nhanes2003-2004/L06TFR_C.htm#Analytic_Notes Accessed October5, 2015
- 30.NHANES. 2005–2006 Data documentation, codebook, and frequencies. Ferritin (FERTIN_D). Description of laboratory methodology. [Published April 2008]. Available at: wwwn.cdc.gov/Nchs/Nhanes/2005-2006/FERTIN_D.htm#Description_of_Laboratory_Methodology Accessed October5, 2015
- 31.NHANES. 2007–2008 Data documentation, codebook, and frequencies. Ferritin (FERTIN_E). Description of laboratory methodology. [Published September 2009]. Available at: wwwn.cdc.gov/Nchs/Nhanes/2007-2008/FERTIN_E.htm#Description_of_Laboratory_Methodology Accessed October5, 2015
- 32.NHANES. 2009–2010 Data documentation, codebook, and frequencies. Ferritin (FERTIN_F). Analytic notes. [Published February 2012]. Available at: wwwn.cdc.gov/Nchs/Nhanes/2009-2010/FERTIN_F.htm#Analytic_Notes Accessed October5, 2015
- 33.NHANES. 2003–2004 Data documentation, codebook, and frequencies. Ferritin and transferrin receptor (L06TFR_C). Description of laboratory methdology. [Updated December 2007]. Available at: wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L06TFR_C.htm#Description_of_Laboratory_Methodology Accessed October5, 2015
- 34.NHANES. 2005–2006 Data documentation, codebook, and frequencies. Transferrin receptor (TFR_D). Description of laboratory methodology. [Published April 2008]. Available at: wwwn.cdc.gov/Nchs/Nhanes/2005-2006/TFR_D.htm#Description_of_Laboratory_Methodology Accessed October5, 2015
- 35.NHANES. 2007–2008 Data documentation, codebook, and frequencies. Transferrin receptor (TFR_E). Description of laboratory methdology. [Published September 2009]. Available at: wwwn.cdc.gov/Nchs/Nhanes/2007-2008/TFR_E.htm#Description_of_Laboratory_Methodology Accessed October5, 2015
- 36.NHANES. 2009–2010 Data documentation, codebook, and frequencies. Transferrin receptor (TFR_F). [Published February 2012]. Available at: wwwn.cdc.gov/Nchs/Nhanes/2009-2010/TFR_F.htm#Analytic_Notes Accessed October5, 2015
- 37.US Census Bureau. How the Census Bureau measures poverty. [Updated September 16, 2015]. Available at: www.census.gov/hhes/www/poverty/about/overview/measure.html Accessed October5, 2015
- 38.NHANES. 2009–2010 Data documentation, codebook, and frequencies. Demographic variables and sample weights (DEMO_F). [Published September 2011]. Available at: wwwn.cdc.gov/Nchs/Nhanes/2009-2010/DEMO_F.htm#Interview_Setting_and_Mode_of_Administration Accessed June17, 2015
- 39.Carlson SJ, Andrews MS, Bickel GW. Measuring food insecurity and hunger in the United States: Development of a national benchmark measure and prevalence estimates. J Nutr 1999;129(2S Suppl):510S–516S [DOI] [PubMed] [Google Scholar]
- 40.NHANES. 2009–2010 Data documentation, codebook, and frequencies. Food security (FSQ_F). [Published March 2013]. Available at: wwwn.cdc.gov/Nchs/Nhanes/2009-2010/FSQ_F.htm Accessed June23, 2015
- 41.NHANES. Data documentation, codebook, and frequencies. Dietary interview—Individual foods, first day (DR1IFF_F). [Updated May 2013]. Available at: wwwn.cdc.gov/Nchs/Nhanes/2009-2010/DR1IFF_F.htm Accessed June17, 2015
- 42.Nead KG, Halterman JS, Kaczorowski JM, Auinger P, Weitzman M. Overweight children and adolescents: A risk group for iron deficiency. Pediatrics 2004;114:104–108 [DOI] [PubMed] [Google Scholar]
- 43.CDC. Body mass index. [Updated May 15, 2015]. Available at: www.cdc.gov/healthyweight/assessing/bmi/ Accessed June23, 2015
- 44.NHANES. 2005–2006 Data documentation, codebook and frequencies. Physical activity (PAQ_D), 2007. Available at: wwwn.cdc.gov/Nchs/Nhanes/2005-2006/PAQ_D.htm#PAD200 Accessed June23, 2015
- 45.NHANES. 2009–2010 Data documentation, codebook, and frequencies. Physical activity (PAQ_F), 2011. Available at: wwwn.cdc.gov/Nchs/Nhanes/2009-2010/PAQ_F.htm#PAQ650 Accessed June23, 2015
- 46.Gray SH, Emans SJ. Abnormal vaginal bleeding in adolescents. Pediatr Rev 2007;28:175–182 [DOI] [PubMed] [Google Scholar]
- 47.Hosmer D, Lemeshow S. Applied logistic regression, 2nd ed. New York: Wiley, 2000 [Google Scholar]
- 48.Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. Am J Epidemiol 2010;171:618–623 [DOI] [PubMed] [Google Scholar]
- 49.Eicher-Miller HA, Mason AC, Weaver CM, McCabe GP, Boushey CJ. Food insecurity is associated with iron deficiency anemia in US adolescents. Am J Clin Nutr 2009;90:1358–1371 [DOI] [PubMed] [Google Scholar]
- 50.Sekhar DL, Murray-Kolb LE, Wang L, Kunselman AR, Paul IM. Adolescent anemia screening during ambulatory pediatric visits in the United States. J Commun Health 2015;40:331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]