Abstract
Nerve conduits prefilled with hydrogels are frequently explored in an attempt to promote nerve regeneration. This study examines the interplay in vivo between the porosity of the conduit wall and the level of bioactivity of the hydrogel used to fill the conduit. Nerve regeneration in porous (P) or nonporous (NP) conduits that were filled with either collagen only or collagen enhanced with a covalently attached neurite-promoting peptide mimic of the glycan human natural killer cell antigen-1 (m-HNK) were compared in a 5 mm critical size defect in the mouse femoral nerve repair model. Although collagen is a cell-friendly matrix that does not differentiate between neural and nonneural cells, the m-HNK-enhanced collagen specifically promotes axon growth and appropriate motor neuron targeting. In this study, animals treated with NP conduits filled with collagen grafted with m-HNK (CollagenHNK) had the best overall functional recovery, based on a range of histomorphometric observations and parameters of functional recovery. Our data indicate that under some conditions, the use of generally cell friendly fillers such as collagen may limit nerve regeneration. This finding is significant, considering the frequent use of collagen-based hydrogels as fillers of nerve conduits.
Introduction
Functional recovery after peripheral nerve repair is often poor because of the slow rate of axonal extension and the limited ability of neurons to navigate long gaps and reconnect with their proper distal targets.1–3 To improve upon the clinical outcome, conduits, or tubes, are commonly used to enclose a nerve injury site and physically guide regenerating axons from the proximal stump to their distal targets.3,4 However, even in the presence of a conduit, regeneration cannot successfully occur without the formation of a fibrin matrix, a physical bridge that forms across the nerve gap after injury and that provides a structure for cells to migrate across.
In an attempt to expedite regeneration and synthetically mimic the natural fibrin matrix, many nerve regeneration studies use prefilled nerve conduits with a three-dimensional inner lumen hydrogel matrix derived from biopolymers such as laminin, alginate, or collagen.5–14 The chemical attachment of a cell-signaling or neurite-promoting moiety to the filler matrix can enhance regeneration further. Among many others, brain-derived neurotrophic factor,15 platelet-derived growth factor,16 and glial growth factor17,18 have been explored. The presence of such molecules in the filler generally improves nerve regeneration to varying degrees over nerve conduits filled with a ligand-free version of the matrix.19
The wall of the conduits can be fabricated to be either porous (P) or nonporous (NP). When the wall pores are larger than 10 μm, porous conduits allow for the infiltration of non-neural cells into the conduit lumen, whereas nonporous conduits provide a cell-impermeable conduit wall. Nonporous conduits work well when bridging gaps are <1.0 cm, when nutrient and waste exchange from the ends of the conduits is sufficient. It is likely that longer nerve gaps will require porous conduits that allow for nutrient and waste exchange along the length of the conduit and even allow for infiltration of blood vessels as observed with autografts.20 Effects of conduit pore size on the outcome of nerve regeneration have been investigated and, although results and interpretations of this critical aspect vary widely, the optimal pore size for conduits was reported to be in the 5–30 μm range to enable nutrient and waste diffusion.21–24 Considering that non-neural cells can easily penetrate through pores of these sizes, it is of interest to determine how infiltration of non-neural cells into porous conduits affects nerve regeneration when combined with a cell-friendly filler matrix.
In this study, we investigated the interplay between nerve regeneration and non-neural cell infiltration into the conduit lumen. More specifically, we compared functional nerve recovery across four different conduit conditions in a 5 mm clinical size gap in the mouse femoral nerve.25–27 This nerve injury model, first introduced by Brushart et al.,25 allows for both morphological and functional measures of recovery after treatment. Specifically, the femoral nerve bifurcates into a motor branch and a sensory branch, making it possible to assess the level of correct motor targeting of the regenerating motor neurons. This is a significant feature of the femoral nerve model that is absent in the more commonly used rat sciatic nerve model. Since one of the biological activities of glycan human natural killer cell antigen-1 (m-HNK) is to support preferential motor neuron targeting, we have used the mouse femoral nerve model in this and other studies.28 Four specific conduit conditions were evaluated as shown in Table 1. Conduits had either a porous or nonporous outer wall and were filled with either a general cell friendly matrix (collagen), shown to effectively improve nerve regeneration after peripheral nerve injury,10,11 or a neurite-specific promoting matrix (CollagenHNK) containing a glycomimetic of the m-HNK. This peptide mimic, m-HNK, was selected as it has been used successfully in soluble form to treat peripheral nerve injuries of the femoral nerve in both mouse29,30 and nonhuman primate31 models. The m-HNK-1 peptide mimic is neurostimulatory and enhances regeneration of peripheral motor neurons in the mouse femoral model when administered either in soluble form or when grafted to collagen.31,32 In addition, m-HNK also enhanced nerve regeneration in a nonhuman primate.33 More recently, m-HNK covalently attached to a collagen hydrogel, identical to the neurite-promoting matrix used in this study, has been used as a conduit filler by Shreiber and colleagues to effectively enhance functional recovery in the mouse femoral nerve model.32
Table 1.
The Four Specific Conduit Conditions Evaluated in This Study
| Porosity | |||
|---|---|---|---|
| Yes | No | ||
| Neurite promoting | Yes | P-CollagenHNK | NP-CollagenHNK |
| Matrix | No | P-Collagen | NP-Collagen |
Conduits either had a porous outer wall denoted by “P” or a nonporous outer wall denoted by “NP.” In addition, conduits either had a neurite-specific promoting matrix containing m-HNK denoted by “CollagenHNK” or had only a generally cell-friendly matrix denoted by “Collagen.”
The conduits employed in this study were composed of a tyrosine-derived polycarbonate terpolymer composed of 89.5 mol% desaminotyrosyl tyrosine ethyl ester, 10 mol% desaminotyrosyl tyrosine, and 0.5 mol% poly(ethylene glycol) (Mw = 1 kDa) [designated as E10-0.5(1K)]. We reported on the use of this terpolymer to facilitate nerve regeneration previously.28 We are now reporting on the continuation of our studies, exploring the complex in vivo relationship between the porosity of the conduit wall and the material used to fill the inner lumen of the conduit.
Materials and Methods
Conduit fabrication
E10-0.5(1K) was synthesized using published procedures.34 Porous and nonporous conduits 5 mm in length were fabricated using a dip-coating method as described.28 In brief, the nonporous conduits were made by repeatedly dip coating a mandrel in a solution of 900 mg of E10-0.5(1K) in 3 mL of methylene chloride, followed by drying. The porous conduits were made in the same way by dip coating the mandrel in a solution of 450 mg of E10-0.5(1K) in 3 mL of methylene chloride that also contained 450 mg of crystals sieved to a size of 25–45 μm, followed by drying and then by leaching in water to remove the sugar crystals. The only difference between the nonporous scaffolds and porous scaffolds is the presence of pores created when the sugar crystals were dissolved by exposure to water. Porosity was evaluated by using ImageJ software to analyze scanning electron micrograph (SEM) images. In brief, SEM images of the nerve conduit surfaces were imported into ImageJ software and converted into binary images. Each converted image was then inverted and analyzed for regions of interest, representing open pores. Total pore area was measured as well as individual pore diameters. Ten representative images of each conduit type were analyzed and the averages and standard deviation were reported.
Preparation of collagen and functionalized collagen
Type-I calf skin collagen (Elastin Products Company, Inc.) was prepared into hydrogels using a published procedure.35 In brief, 2.0 mg/mL collagen hydrogels were prepared using solutions in the following ratios: 2% of 1 M Hepes (Fluka), 14% of 0.1 N NaOH, 10% of 10× minimum essential medium (Sigma), 5.2% M199 (Sigma), 0.1% penicillin/streptomycin (Sigma), 1% l-glutamine (Sigma), and 67.7% native collagen (3 mg/mL).32 For the functionalized collagen, the peptide mimetic of the m-HNK33 (FLHTRLFV, MW: 1032.24, synthesized by GenScript) was grafted onto the collagen using EDC chemistry, as described.32,36 This peptide has been shown to encourage nerve regeneration and axonal targeting.30,31 Peptide grafting efficiency was measured indirectly by grafting FITC-tagged m-HNK (GenScript) to collagen and comparing the fluorescence intensity of grafted collagen after reconstitution to a standard curve created by admixing fluorescent peptide into collagen solution. Using these comparisons, the peptide grafting efficiency was found to range between 50% and 60% of the original mass of added peptide. This percentage equates to 130–160 μg of coupled peptide per milliliter collagen matrix, based on the molar ratio of peptide to monomeric type-I bovine collagen fibers.36 These values are similar to the effective doses administered in solution in previous in vivo nerve regeneration studies.29,30 Collagen only and functionalized collagen solutions were injected into conduits using a 22-gauge syringe, allowing excess to completely fill all voids. Before implantation, the conduits were incubated at 37°C to allow self-assembly of the hydrogel.
Surgical methods and animal groups
All experiments were conducted in accordance with the Rutgers Animal Care and Facilities Committee and the Institutional Animal Care and Use Committee (IACUC) using published protocols with the following modifications: conduits were prefilled with collagen or collagen grafted with m-HNK rather than filled with saline at the time of transplantation. In brief, 3-month-old female C57BL/6J mice were anesthetized by intraperitoneal injections of a mixture of ketamine (80 mg/kg) and xylazine (12 mg/mg). The left femoral nerve was surgically exposed, and a nerve transection was performed at a distance ∼3 mm proximal to the bifurcation of the nerve. The cut ends of the nerve were inserted into the conduits prefilled with the collagen gels and fixed on each end with a 10-0 nylon suture (Ethicon) so that a 5 mm gap was present between the proximal and distal stump. The incised skin was closed with wound clips, which were removed 2 weeks postsurgery. Four animal groups (eight animals each) were compared over a 15 week time period, including porous conduits filled with collagen (P-Collagen), porous conduits filled with collagen grafted with m-HNK (P-CollagenHNK), nonporous conduits filled with collagen (NP-Collagen), and nonporous conduits filled with collagen grafted with m-HNK (NP-CollagenHNK).
Motor function recovery
After implantation of the nerve conduits, functional recovery was assessed using a single-frame motion analysis (SFMA) approach.28,32,37 Animals were trained to perform a classical beam test before implantation of the conduit. Postsurgery, this test was performed weekly until the endpoint of the experiment. Videos of the mice walking from the rear were collected using a high-speed camera (A602fc; Basler). SimiMotion (SIMI Reality Motion Systems) was used to analyze the movements of the hind legs during the normal gait cycle from individual video frames. The foot base angle (FBA)37 was measured to evaluate the function of the quadriceps muscle. This parameter is measured during the walking cycle at the time of the toe-off position when the sole of the foot is parallel to the transverse plane. The FBA is formed by the line dividing the sole into two symmetric halves and the medial horizontal plane.
To assess supraspinal control, used during voluntary movements, and proprioceptive control, used during movements that require precision, the pencil grip test was performed. During this test, the mouse is held by its tail and allowed to grasp a pencil with its forelimb paws. The hind limbs, which are away from the pencil, alternate between limb flexion to maximum extension attempting to grasp the pencil tip. In intact animals, these protractions are symmetric. After injury, the limb is not able to completely extend. To quantify this deficit, the limb protraction limb ratio (PLR) is measured. The PLR is a ratio of the relative length of the intact limb to that of the injured limb using the distance between the most distal midpoint of the extremity to a fixed point in the sagittal plane on the animal to measure limb length.37
For each parameter, the FBA and the PLR, a recovery index (RI) was calculated for each animal to provide a relative measure of functional recovery. This RI value was calculated as a percentage using the following formula:
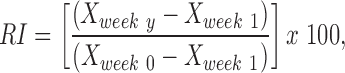 |
where 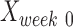 ,
, 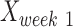 and
and 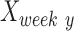 are intact values at week 0 (either FBA or PLR), values measured at week 1 after injury, and at week y (where y is the endpoint of the study, week 15), respectively.37 An RI value of 100 indicates complete recovery of the femoral nerve.
are intact values at week 0 (either FBA or PLR), values measured at week 1 after injury, and at week y (where y is the endpoint of the study, week 15), respectively.37 An RI value of 100 indicates complete recovery of the femoral nerve.
Histomorphometric analysis of explanted nerve
After perfusion with 4% formaldehyde solution at 16 weeks, femoral nerves were dissected from animals and morphometric analysis was performed according to standard protocol.28,31 Total number of myelinated axons per nerve cross section, raw tissue area, cross-sectional area of the regenerating cable, and the percentage of nerve regeneration were measured with ImageJ.
Statistical analysis
The study was designed to allow comparison of the effects fillers made of collagen or collagen grafted with m-HNK (CollagenHNK) in both porous and nonporous conduits on nerve regeneration. Variance analysis using a one-way ANOVA was used followed by post hoc planned comparisons with Tukey's test. Differences were considered significant at p < 0.05.
Results
Fabrication and in vitro characterization of conduits
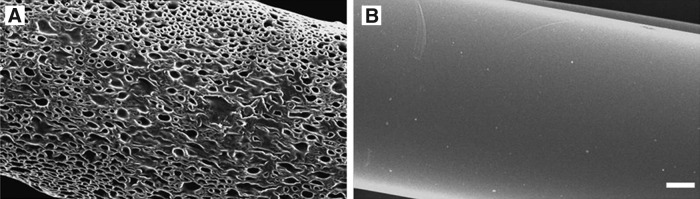
Porous conduits composed of E10-0.5(1K) showed an interconnected porous structure within the outer wall, whereas the corresponding nonporous conduits had an apparently impermeable, smooth outer wall structure28 (Fig. 1). The degree of porosity was calculated by analyzing SEM images of the conduit surfaces. An average pore size of 35.7 ± 9.0 μm and an overall porosity of 55.2% ± 1.2% were measured for porous conduits (Fig. 1A). Nonporous conduits showed no discernable pore structure and correspondingly have an overall porosity of 0% as calculated based on SEM images using the same technique (Fig. 1B). The conduits have an internal diameter of 580 μm and an external diameter of 680 μm.
FIG. 1.
Scanning electron microscope images of conduits evaluated in the mouse femoral nerve repair model. (A) Outer wall of a porous E10-0.5(1K) conduit and (B) outer wall of a nonporous E10-0.5(1K) conduit. All scale bars: 100 μm.
In vivo evaluation
Porous and nonporous E10-0.5(1K) conduits were prefilled with collagen or collagen grafted with m-HNK (CollagenHNK). The two types of fillers were allowed to gel within the conduits before implantation within the mouse femoral defect. Four groups were tested: (1) porous conduits filled with collagen (P-Collagen), (2) nonporous conduits filled with collagen (NP-Collagen), (3) porous conduits filled with collagen (P-CollagenHNK), and (4) nonporous conduits filled with CollagenHNK (NP-CollagenHNK).
Motor function recovery
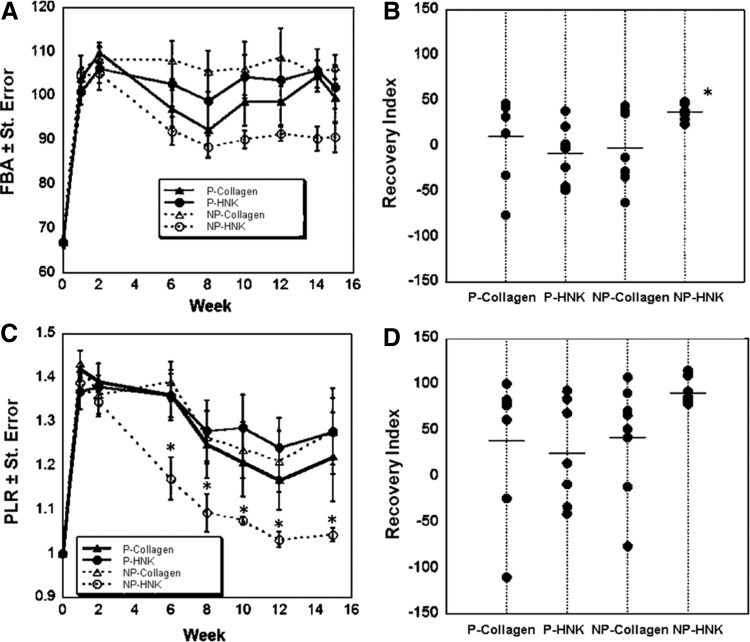
SFMA of the FBA and PLR was used to quantify functional recovery (Fig. 2).37 Animals that received P-Collagen, NP-Collagen, and P-CollagenHNK conduits showed little to no functional improvement in the FBA parameter over the full 15 weeks of the study (Fig. 2A). The endpoint FBA did not differ from the FBAs measured for these groups the week after the injury, as evidenced by the percentage of RI for each animal (Fig. 2B). NP-CollagenHNK conduits were the only group that showed significant improvement in the FBA over the course of the study, showing a tight grouping of RIs of the animals within this treatment group. The final measured FBA for the NP-CollagenHNK compares favorably to the maximal FBA achieved in other studies using this animal model, even for much smaller noncritical size gaps.28,32,38
FIG. 2.
Metrics of functional recovery for porous and nonporous conduits in the mouse femoral nerve repair model. Conduits are either P or NP, and are filled with either a generally cell-friendly matrix (Collagen) or a matrix consisting of m-HNK grafted to collagen (CollagenHNK). (A) FBA for a 15-week period after surgical insertion of the four different conduit types. (B) RI for FBA at week 15. Each dot represents one animal in the group. The line indicates the average RI for the group (*p < 0.05, one-way ANOVA with Fisher's LSD post hoc test). (C) PLR for all conduit types (*p < 0.0001, one-way ANOVA with Tukey post hoc test). (D) RI for PLR at week 15. Each dot represents one animal in the group. The line indicates the average RI for the group. FBA, foot base angle; NP, nonporous; P, porous; PLR, protraction limb ratio; RI, recovery index.
This same trend was observed for the PLR, which, in comparison with the reflexive movements measured by the FBA, is a voluntary movement.37 Significant improvement of the PLR for animals that received NP-CollagenHNK conduits was evident at 6 weeks postinjury (Fig. 2C). P-CollagenHNK, P-Collagen, and NP-Collagen groups showed lesser degrees of PLR recovery and the RI values were scattered as compared with those for the NP-CollagenHNK group (Fig. 2D). Taken together, results collected up to 15 weeks demonstrate that functional recovery was highest in the animals treated with nonporous conduits and a neurite-promoting cell matrix grafted with m-HNK (NP-CollagenHNK). For FBA, the difference in RI for these animals was statistically significant as compared with the other treatment groups.
Histomorphometric analysis
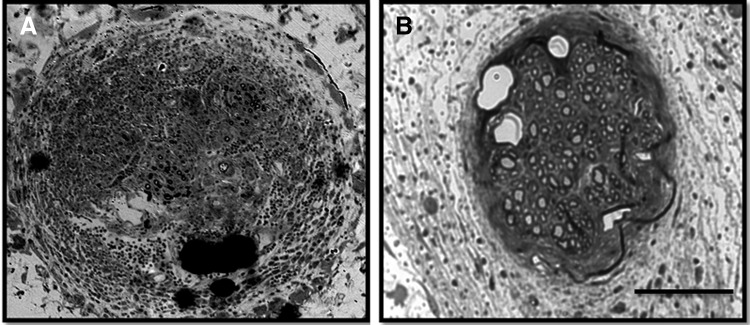
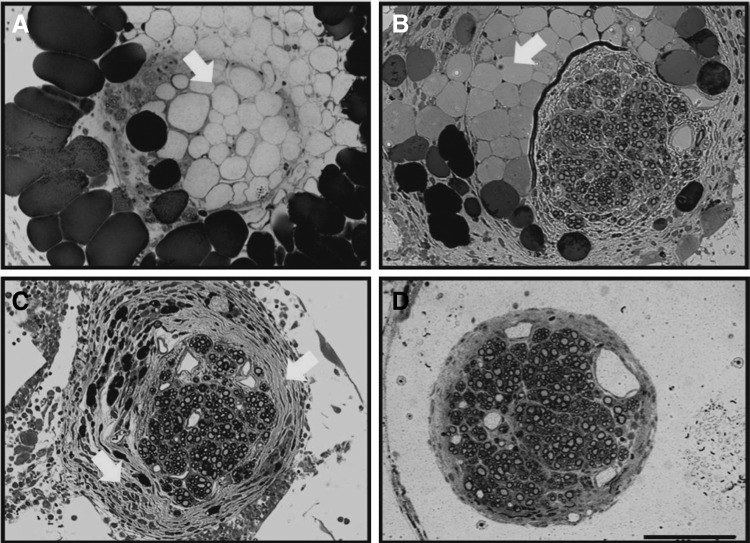
After the final time point of the study, implant sites were excised and nerves were collected and analyzed for histomorphometric features. Histological differences between treatment options were evident from sections of the mid-conduit regenerative nerve cable (Fig. 3). In the P-Collagen conduits (Fig. 3A), distinct regenerative cables did not always form. Rather, the inner lumen was often filled with dense fibrous tissue (Fig. 4A) or large acellular regions (Fig. 5A). In the NP-Collagen conduits, dense fibrous tissue was present, but in a more organized manner (Figs. 3C and 5C). The tissue commonly displayed a circular encasement of the regenerative cable. Animals treated with P-CollagenHNK conduits demonstrated distinct regenerative nerve cables with a perineurial layer. This layer entirely separated the neural tissue from the surrounding non-neural tissue and acellular regions (Figs. 3B, 4B, and 5B). All animals treated with NP-CollagenHNK conduits exhibited distinct regenerative cables with little to no fibrous tissue surrounding them (Figs. 3D and 5D).
FIG. 3.
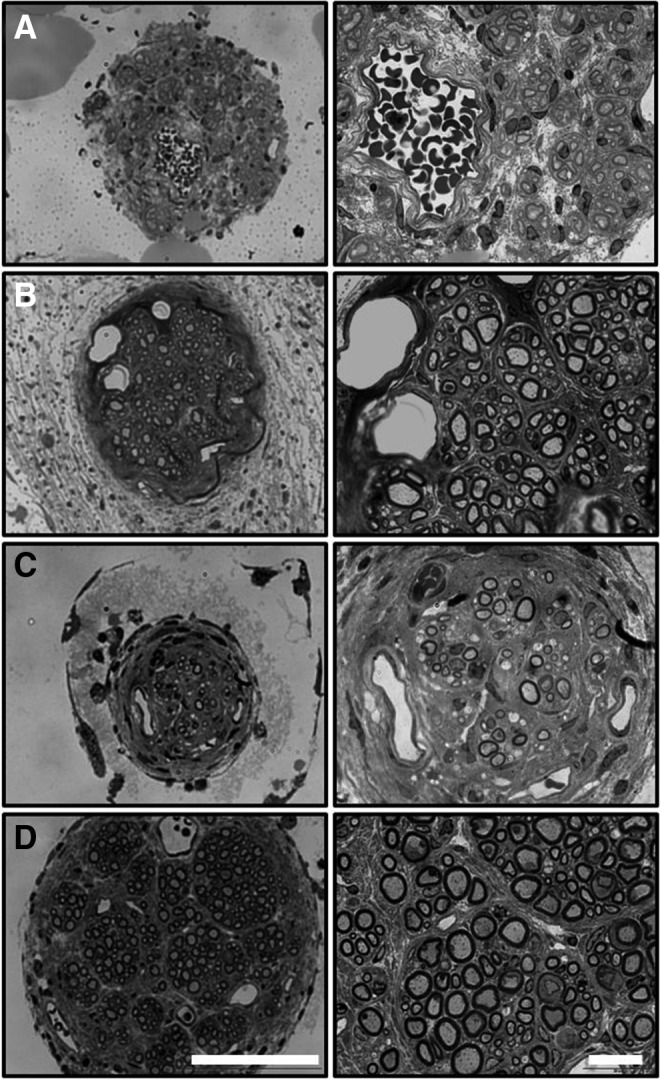
Representative cross-sectional images (40× and 100×) of nerve sections stained with toluidine blue from the midpoint of the regenerated femoral nerve after tubulization with four different types of conduits. (A) P-Collagen (B) P-CollagenHNK (C) NP-Collagen (D) NP-CollagenHNK. Scale bars: 100 and 20 μm, respectively.
FIG. 4.
Representative cross-sectional images of nerve sections exhibiting a high degree of non-neural fibrous tissue infiltration. Sections are taken from the midpoint of the regenerated femoral nerve after tubulization with either a (A) porous conduit filled with collagen (P-Collagen) or (B) a porous conduit filled with m-HNK grafted to collagen (P-CollagenHNK). Scale bar: 100 μm.
FIG. 5.
Representative cross-sectional images of nerve sections stained with toluidine blue from the midpoint of regenerated femoral nerve after tubulization. Images show various histological appearances including examples of acellular regions, dense fibrotic tissue, and distinct regenerative cables. Animals were treated with the following types of conduits: (A) P-Collagen (B) P-CollagenHNK (C) NP-Collagen, or (D) NP-CollagenHNK. Large black circles are adipocytes common to nerve repair, acting as a soft “cushioning” around the nerve to protect it from mechanical trauma. Images show examples of obstruction to axonal regeneration because of remnants of the collagen gel filler (producing acellular regions within the regenerating nerve cable indicated by white arrows in A, B) and over deposition of fibrous tissue (indicated by white arrows in C), leading to dense areas through which axons cannot navigate. Image (D) illustrates the distinct regenerative cable found in an animal treated with NP-CollagenHNK. Little to no fibrous tissue deposition occurs in these conduits. Scale bar: 100 μm.
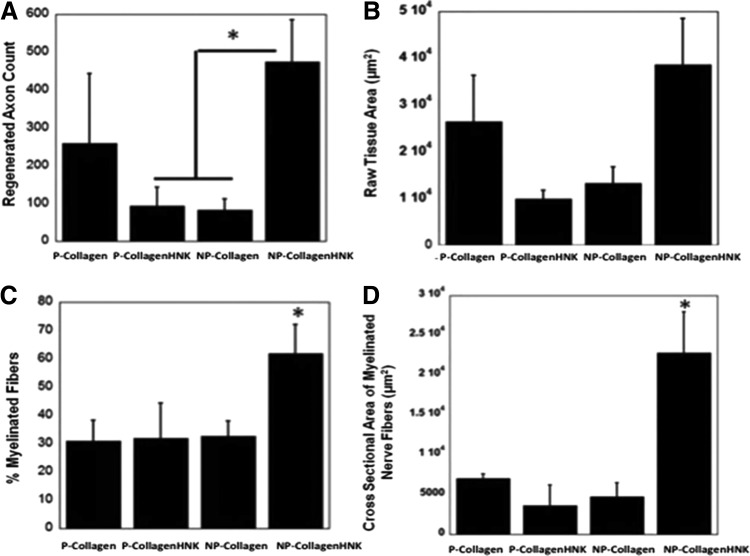
Quantitative analysis revealed no statistical differences between the number of axons, raw tissue area, percentage of myelinated nerve fibers, or cross-sectional area of myelinated nerve fibers in animals treated with P-Collagen and NP-Collagen conduits. There were a greater number of axons within regenerating nerve cables formed within NP-CollagenHNK conduits than other treatment conditions (Fig. 6A). The cross-sectional area of the myelinated nerve fibers was greater in NP-CollagenHNK conduits than in the other conduits, and a greater percentage of this area was occupied by myelinated nerve fibers (Fig. 6B, C). There was no statistical difference between the raw tissue areas measured from the nerve samples regenerated in each of the conduit types (Fig. 6B).
FIG. 6.
Histomorphometric analysis of regenerative nerve cable after conduit implantation. (A) Axon count of myelinated axons in the regenerative cable in the mid-conduit nerve section for each conduit type (*p < 0.05, one-way ANOVA with Fisher's LSD post hoc test). (B) Raw tissue area. (C) Percentage of myelinated nerve fibers in regenerating nerve cable (*p < 0.1, one-way ANOVA with Fisher's LSD post hoc test). (D) Cross-sectional area of regenerated nerve fibers (*p < 0.05, one-way ANOVA with Fisher's LSD post hoc test).
Discussion
This study presents evidence that the use of cell-friendly fillers in nerve conduits can be detrimental to nerve regeneration and examines the relationship between cell-friendly fillers and the porosity of the conduit wall. Specifically, we interpret the experimental results of this study as indicating that the use of a cell-friendly filler can negatively affect nerve regeneration when the porosity of the conduit wall allows for the uncontrolled infiltration of fibrous tissue into the conduit. In this study, the addition of a nerve-specific stimulatory factor (m-HNK) to the cell-friendly collagen filler enhanced nerve regeneration even when the conduit wall was porous.
Nerve regeneration was measured by functional recovery as well as histomorphometric analysis of the regenerative cables. Although not all parameters ranked conduit designs equally, when evaluated across all measured parameters, functional nerve regeneration in animals treated with NP-CollagenHNK conduits was superior to regeneration in all other experimental groups (P-Collagen, NP-Collagen, and P-CollagenHNK). The differences between P-Collagen, NP-Collagen, and P-CollagenHNK were difficult to assess, but based on several outcome measures, NP-Collagen was superior over P-Collagen and P-CollagenHNK was superior over NP-CollagenHNK.
Histology of the regenerated nerves within the conduits strongly suggested that the degree of fibrous tissue infiltration was the likely cause of the difference in the degree of nerve regeneration between porous and nonporous conduits. Porous conduits correlated with an extensive amount of fibrous tissue within the conduit lumen. In the most severe cases, the fibrous tissue filled the luminal space to the exclusion of nerve tissue. In less severe cases, histology showed fibrous tissue heavily interspersed within axons, preventing the formation of an organized nerve cable.
Although many nerve repair studies have used a conduit filled with a cell-friendly matrix,5–14 our study suggests that the effects of using such a filler may only be beneficial if conduits are impermeable to infiltrating fibrous tissue. When porous conduits are filled with a hydrogel, a balance must be achieved between the conduit porosity and the bioactivity of the filler matrix to encourage faster axonal regeneration than fibrotic tissue infiltration.10,39–42 As longer conduits are required in more severe nerve injuries, a greater porosity will be necessary to overcome limitations of nutrient and waste exchange. In these cases, the cell-friendly filler may become more detrimental to nerve regeneration by increasing the risk of fibrous tissue occluding the conduit lumen.
With the addition of a neurite-promoting factor, such as m-HNK, to the generally cell-friendly filler, the inner lumen may become more attractive to neurite outgrowth. We observed that for porous conduits (P-CollagenHNK), some functional recovery was observed even if the inner lumen of the porous conduit contained a substantial amount of non-neural cells. For the corresponding nonporous conduits (NP-CollagenHNK), the absence of fibrous tissue ingrowth provided an optimal environment for functional recovery (Figs. 2, 3D, and 4D).
Conclusions
Our results show that a cell-friendly filler added to the conduit inner lumen has different effects depending upon the conduit porosity. In the case of porous conduits, whether or not m-HNK is present, pores appear to facilitate the infiltration of non-neural cells along the entire conduit length, allowing them to deposit tissue and obstruct neurite outgrowth.43 In the case of nonporous conduits, tissue infiltration is only possible at the anastomosis with the nerve stumps, resulting in less infiltration of non-neural tissue and an environment that favors nerve regeneration. However, the addition of a neurite-promoting factor such as m-HNK to the filler can partially overcome the negative impact of non-neural cell infiltration. The functional and morphological results of this study show that the use of cell-friendly fillers in nerve conduits can be detrimental to nerve regeneration unless the infiltration of non-neural tissue into the conduit is carefully controlled.
Acknowledgments
This research was sponsored by the Armed Forces Institute of Regenerative Medicine award number W81XWH-08-2-0034. The US Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, MD 21702–5014, is the awarding and administering acquisition office. The content of the article does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred. Research was conducted in compliance with the Animal Welfare Act Regulations and other Federal statutes relating to animals and experiments involving animals and adheres to the principles set forth in the Guide for Care and Use of Laboratory Animals, National Research Council, 1996. This work was also supported by the Center for Military Biomaterials Research (CeMBR) award number W81XWH-04-2-0031, RESBIO—The National Resource for Polymeric Biomaterials supported by the National Institutes of Health (NIH grant EB001046), and the New Jersey Center for Biomaterials at Rutgers University. The authors would like to thank Dr. Jian Chen for performing the surgeries, Dr. Ijaz Ahmed for preparation of the tissue samples, and Dr. Shirley Masand for her assistance with these studies.
Disclosure Statement
No competing financial interests exist
References
- 1.Jiang X., Lim S.H., Mao H.-Q., and Chew S.Y. Current applications and future perspectives of artificial nerve conduits. Exp Neurol 223, 86, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Seddon H.J., Medawar P.B., and Smith H. Rate of regeneration of peripheral nerves in man. J Physiol 102, 191, 1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valentini R. The Biomedical Engineering Handbook, 2nd ed., 2 Volume Set. Boca Raton: CRC Press, 1999 [Google Scholar]
- 4.Heijke G.C.M., Klopper P.J., Van Doorn I.B.M., and Baljet B. Processed porcine collagen tubulization versus conventional suturing in peripheral nerve reconstruction: an experimental study in rabbits. Microsurgery 21, 84, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Ceballos D., Navarro X., Dubey N., Wendelschafer-Crabb G., Kennedy W.R., and Tranquillo R.T. Magnetically aligned collagen gel filling a collagen nerve guide improves peripheral nerve regeneration. Exp Neurol 158, 290, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Dubey N., Letourneau P.C., and Tranquillo R.T. Engineered alignment in media equivalents: magnetic prealignment and mandrel compaction. Exp Neurol 158, 338, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Labrador R.O., Butí M., and Navarro X. Influence of collagen and laminin gels concentration on nerve regeneration after resection and tube repair. Exp Neurol 149, 243, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Madison R., da Silva C.F., Dikkes P., Chiu T.-H., and Sidman R.L. Increased rate of peripheral nerve regeneration using bioresorbable nerve guides and a laminin-containing gel. Exp Neurol 88, 767, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Madison R.D., Da Silva C.F., and Dikkes P. Entubulation repair with protein additives increases the maximum nerve gap distance successfully bridged with tubular prostheses. Brain Res 447, 325, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Rosen J.M., Padilla J.A., Nguyen K.D., Siedman J., and Pham H.N. Artificial nerve graft using collagen as an extracellular matrix for nerve repair compared with sutured autograft in a rat model. Ann Plast Surg 25, 375, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Satou T., Nishida S., Hiruma S., Tanji K., Takahashi M., et al. A morphological study on the effects of collagen gel matrix on regeneration of severed rat sciatic nerve in silicone tubes. Pathol Int 36, 199, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Verdú E., Labrador R.O., Rodríguez F.J., Ceballos D., Forés J., and Navarro X. Alignment of collagen and laminin-containing gels improve nerve regeneration within silicone tubes. Restor Neurol Neurosci 20, 169, 2002 [PubMed] [Google Scholar]
- 13.Rosen J.M., Padilla J.A., Nguyen K.D., Siedman J., and Pham H.N. Artificial nerve graft using glycolide trimethylene carbonate as a nerve conduit filled with collagen compared to sutured autograft in a rat model. J Rehabil Res Dev 29, 1, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Rosen J.M., Pham H.N., Abraham G., Harold L., and Hentz V.R. Artificial nerve graft compared to autograft in a rat model. J Rehabil Res Dev 26, 1, 1989 [PubMed] [Google Scholar]
- 15.Midha R., Munro C.A., Dalton P.D., Tator C.H., and Shoichet M.S. Growth factor enhancement of peripheral nerve regeneration through a novel synthetic hydrogel tube. J Neurosurg 99, 555, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Wells M.R., Kraus K., Batter D.K., Blunt D.G., Weremowitz J., et al. Gel matrix vehicles for growth factor application in nerve gap injuries repaired with tubes: a comparison of biomatrix, collagen, and methylcellulose. Exp Neurol 146, 395, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Mohanna P.N., Young R.C., Wiberg M., and Terenghi G. A composite poly-hydroxybutyrate-glial growth factor conduit for long nerve gap repairs. J Anat 203, 553, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohanna P.-N., Terenghi G., and Wiberg M. Composite PHB-GGF conduit for long nerve gap repair: a long-term evaluation. Scand J Plast Reconstr Surg Hand Surg 39, 129, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Pfister L.A., Papaloïzos M., Merkle H.P., and Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst 12, 65, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Griffith L.G. Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann N Y Acad Sci 961, 83, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain L.J., Yannas I.V., Arrizabalaga A., Hsu H.P., Norregaard T.V., and Spector M. Early peripheral nerve healing in collagen and silicone tube implants: myofibroblasts and the cellular response. Biomaterials 19, 1393, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Jenq C.-B., and Coggeshall R.E. Nerve regeneration through holey silicone tubes. Brain Res 361, 233, 1985 [DOI] [PubMed] [Google Scholar]
- 23.Jenq C.-B., and Coggeshall R.E. Permeable tubes increase the length of the gap that regenerating axons can span. Brain Res 408, 239, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Jenq C.-B., Jenq L.L., and Coggeshall R.E. Nerve regeneration changes with filters of different pore size. Exp Neurol 97, 662, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Brushart T. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci 8, 1026, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brushart T. Motor axons preferentially reinnervate motor pathways. J Neurosci 13, 2730, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brushart T.M.E., and Seiler Iv W.A. Selective reinnervation of distal motor stumps by peripheral motor axons. Exp Neurol 97, 289, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Ezra M., Bushman J., Shreiber D., Schachner M., and Kohn J. Enhanced femoral nerve regeneration after tubulization with a tyrosine-derived polycarbonate terpolymer: effects of protein adsorption and independence of conduit porosity. Tissue Eng Part A 20, 518, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehanna A., Mishra B., Kurschat N., Schulze C., Bian S., et al. Polysialic acid glycomimetics promote myelination and functional recovery after peripheral nerve injury in mice. Brain 132, 1449, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Simova O., Irintchev A., Mehanna A., Liu J., Dihné M., et al. Carbohydrate mimics promote functional recovery after peripheral nerve repair. Ann Neurol 60, 430, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Irintchev A., Wu M.M., Lee H.J., Zhu H., Feng Y.P., et al. Glycomimetic improves recovery after femoral injury in a non-human primate. J Neurotrauma 28, 1295, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Masand S.N., Chen J., Perron I.J., Hammerling B.C., Loers G., et al. The effect of glycomimetic functionalized collagen on peripheral nerve repair. Biomaterials 33, 8353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon-Haldi M., Mantei N., Franke J., Voshol H., and Schachner M. Identification of a peptide mimic of the L2/HNK-1 carbohydrate epitope. J Neurochem 83, 1380, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Magno M.H.R., Kim J., Srinivasan A., McBride S., Bolikal D., et al. Synthesis, degradation and biocompatibility of tyrosine-derived polycarbonate scaffolds. J Mater Chem 20, 8885, 2010 [Google Scholar]
- 35.Shreiber D.I., Barocas V.H., and Tranquillo R.T. Temporal variations in cell migration and traction during fibroblast-mediated gel compaction. Biophys J 84, 4102, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monteiro G.A., Fernandes A.V., Sundararaghavan H.G., and Shreiber D.I. Positively and negatively modulating cell adhesion to type I collagen via peptide grafting. Tissue Eng Part A 17, 1663, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irintchev A., Simova O., Eberhardt K.A., Morellini F., and Schachner M. Impacts of lesion severity and tyrosine kinase receptor B deficiency on functional outcome of femoral nerve injury assessed by a novel single-frame motion analysis in mice. Eur J Neurosci 22, 802, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Bushman J., Mishra B., Ezra M., Gul S., Schulze C., et al. Tegaserod mimics the neurostimulatory glycan polysialic acid and promotes nervous system repair. Neuropharmacology 79, 456, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams L.R. Exogenous fibrin matrix precursors stimulate the temporal progress of nerve regeneration within a silicone chamber. Neurochem Res 12, 851, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Tona A., and Perides G. Effect of Highly Concentrated Gels of Sodium Hyaluronate on Early Phases of Regeneration in the Transected and Tubulized Rat Sciatic Nerve. Amsterdam, Pays-Bas: IOS Press, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Navarro X., Kennedy W.R., Stewart N.J., and Furcht L.T. Effects of laminin on functional reinnervation of target organs by regenerating axons. Neuroreport 2, 37, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Valentini R.F., Aebischer P., Winn S.R., and Galletti P.M. Collagen- and laminin-containing gels impede peripheral nerve regeneration through semipermeable nerve guidance channels. Exp Neurol 98, 350, 1987 [DOI] [PubMed] [Google Scholar]
- 43.Meek M.F., Dunnen W.F.A.D. Porosity of the wall of a Neurolac nerve conduit hampers nerve regeneration. Microsurgery 29, 473, 2009 [DOI] [PubMed] [Google Scholar]