Abstract
Alzheimer's disease (AD) is the most common form of dementia in elder people, characterised by a progressive decline in memory as a result of an impairment of cholinergic neurotransmission. To date acetylcholinesterase inhibitors (AChEIs) have become the most prescribed drugs for the symptomatic treatment of mild to moderate AD. However, the traditional “one molecule-one target” paradigm is not sufficient and appropriate to yield the desired therapeutic efficacy since multiple factors, such as amyloid-β (Aβ) deposits, neuroinflammation, oxidative stress, and decreased levels of acetylcholine (ACh) have been thought to play significant roles in the AD pathogenesis. New generation of multi-target drugs is earnestly demanded not only for ameliorating symptoms but also for modifying the disease. Herein, we delineated the catalytic and non-catalytic functions of AChE, and summarized the works of our group and others in research and development of novel AChEI-based multi-target-directed ligands (MTDLs), such as dual binding site AChEIs and multi-target AChEIs inhibiting Aβ aggregation, regulating Aβ procession, antagonizing platelet-activating factor (PAF) receptor, scavenging oxygen radical, chelating metal ions, inhibiting monoamine oxidase B (MAO-B), blocking N-methyl-D-aspartic acid (NMDA) receptor and others.
Keywords: Acetylcholinesterase inhibitor, alzheimer's disease, multi-target-directed ligand.
1. INTRODUCTION
Alzheimer's disease (AD) is the most common form of dementia affecting the elderly population and contributes to 60-70% cases. AD is characterised by a progressive decline in memory and general cognitive abilities. The cause for most AD cases is still unknown except for 1% to 5% of cases where genetic mutations have been identified. Several existed hypotheses try to explain the cause of the disease, such as genetics, cholinergic hypothesis, amyloid hypothesis, and Tau hypothesis. But it has been widely accepted that the cognitive deficit of AD patients is a result of the loss of cholinergic neurons and synapses and the resulting impairment of cholinergic neurotransmission in the cerebral cortex and certain subcortical regions [1]. However, none of the ongoing treatments developed on the basis of the “cholinergic hypothesis” i.e. acetylcholinesterase inhibitors (AChEIs), and even the more recently approved N-methyl-D-aspartic acid (NMDA) receptor antagonist, memantine, has proven to be effective to stop the progression of AD [2, 3]. Given the relative ineffectiveness of AChEIs and the current understanding of AD molecular biology, it is now recognized that modulating multiple targets simultaneously, instead of the “one molecule-one target” paradigm, appears to be the best pharmacological instrument for tackling the disease.
2. STRUCTURAL AND FUNCTIONAL DETERMINANTS OF ACETYLCHOLINESTERASE (ACHE) IN AD ETIOLOGY
2.1. Catalytic Function of AChE and Cholinergic Deficit Hypothesis of AD
AChE is a hydrolase belonging to carboxylesterase family of enzymes. Its classic function is hydrolyzing neuronal signal messenger acetylcholine (ACh) in the synaptic cleft to terminate the cholinergic neurotransmission. The “cholinergic-deficit hypothesis” arising in the mid-1970s asserts that loss of the cholinergic neurons (nucleus basalis of meynert) of the basal forebrain that project to the cortex and hippocampus, also marked by reduced choline acetyltransferase (ChAT) and ACh, is accountable for the memory loss and cognitive disturbances in AD [4]. Accordingly, AChE inhibition could increase the amount of ACh and agonize M1 muscarinic acetylcholine receptor in hippocampus, and consequently leads to the downstream regulation related to amyloid precursor protein (APP) processing [5, 6], neuroprotection [7, 8], learning and memory [9] (Fig. 1). Thus, AChE is conferred as one of the rare available drug targets for AD intervention.
Fig. (1).
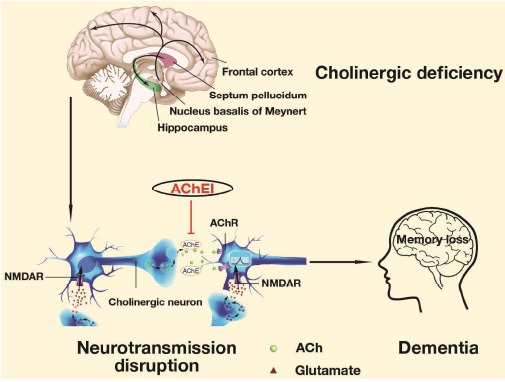
Mechanism of action of the enzyme AChE in cholinergic-deficit hypothesis. Revised from Francis PT et al. (2012). Expert Review of Neurotherapeutics, 12, 1351-1365 and Serge G et al. CMAJ, (2002), 166(5), 616-23.
Owing to the efforts made by Sussman JL group, the first X-ray crystal structure of AChE from torpedo californica was unveiled in 1993 [10]. AChE possesses the core catalytic triad: Ser 200, His 440 and Glu 327, which is deep inside the narrow gorge responsible for ACh hydrolysing. The elucidation of key catalytic region in AChE facilitates scientific research in AChE catalytic mechanism and molecular binding modes. Since then a lot of AChEIs have been developed taking advantage of the precise binding pockets information provided by structural biologists. There are currently four FDA-proved AChEIs including donepezil (Aricept®), rivastigmine (Exelon®), galantamine (Reminyl®, Razadyne®), and tacrine (Cognex®) commercially available [11, 12]. Huperzine A, a potent reversible and selective AChEI, is proved to be used for AD in China. Nowadays new AChE structure of homo sapiens exhibits subtle but significant difference from that of torpedo californica or other species, which provides more accurate evidence for rational AChEI design [13].
2.2. Non-catalytic Function of AChE and Amyloid Hypothesis of AD
According to the alternative splicing of AChE mRNA, there are three main post-transcriptional AChEs (AChE-T, AChE-R, and AChE-H). Different splicing variants present distinctive tissue distributions and consequently diverse functions, such as neurogenesis, cell adhesion, synaptotoxicity, apoptosis, etc. The non-classic function of AChE is defined as all non-catalytic activities on account of polymorphism and has gained more and more attention from researchers worldwide [14, 15]. Distributing in central neuronal system, AChE-T is the main isoform discussed in AD.
As we know, amyloid hypothesis suggests that Aβ deposition is an important pathogenic marker of the onset and progressive AD. Excluding hydrolyzing ACh, AChE is also found to colocalize with Aβ in senile plaques. Study from Inestrosa et al. [16] revealed AChE as a molecular chaperone, which accelerates Aβ assembling into oligomers and fibrils in amyloidosis via peripheral anionic sites (PAS). PAS, located around the external region of AChE narrow gorge, serve as the secondary binding sites of ACh and quaternary ligands without enzymatic activity and are accountable for some of non-catalytic activities. PAS inhibitors could sterically block ACh entrance into catalytic core in an uncompetitive mode as well as PAS-induced Aβ oligomerization. Therefore, PAS has emerged as a hotspot for novel AChEI development.
In line with the traditional approach “one molecule-one target”, the discovery of AChEIs has been perceived as a major breakthrough in the field. However, this class of drugs has not met with the expected success since they can only cause a modest improvement in memory and cognitive function but can not exert real disease-altering effects in terms of reversing or slowing the progression of the neurodegeneration [17]. Thus, dual AChEIs simultaneously blocking both the catalytic and peripheral sites might not only alleviate the cognitive deficit of AD patients by elevating ACh levels in synaptic cleft but also act as disease-modifying agents delaying amyloid plaque formation.
Over the past few years, our laboratory has been committed to the dual AChEIs with successful achievements. Our attention focused particularly on meptazinol (MEP) known as a racemic opioid analgesic exhibiting moderate AChE inhibition potency. We synthesized a series of bis-(-)-nor-MEP derivatives (Fig. 2a) by binding two (-)-nor-MEP monomers with alkylene linkers to interact simultaneously with both catalytic and peripheral sites of AChE [18]. These derivatives displayed AChE inhibitory activities closely related to the length of the alkylene chain, in contrast to butyrocholinesterase (BuChE) inhibition. The most active AChE inhibitor within the series possessed an alkylene chain of nine methylene groups between both (-)-nor-MEP units (Fig. 2b,c), exhibiting AChE inhibitive activity 10000-fold higher than that of (-)-MEP (IC50 = 3.9 nM vs 41 μM). Moreover, this compound prevented AChE-induced Aβ aggregation as expected, with an IC50 value of 16.6 µM comparable with that of propidium (12.8 µM) and could significantly reverse the scopolamine-induced memory deficits in mice.
Fig. (2).
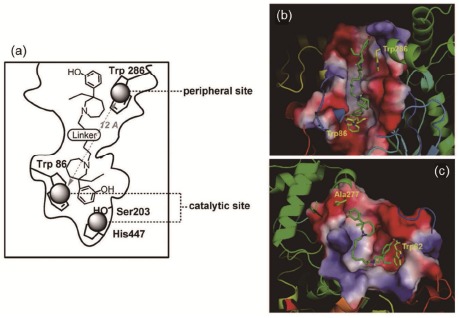
The catalytic and peripheral sites of AChE (a) and the binding model of bis(9)-(-)-nor-MEP to AChE (b) and BuChE (c). Xie, Q., Wang, H. et al. Journal of medicinal chemistry 2008, 51(7), 2027-36.
Based on the fact that PAS ligand propidium could partially inhibit AChE induced Aβ aggregation [16], additional binding sites on AChE were suggested participating in AChE-Aβ interaction. Hou et al. from our group have discovered a motif located in the N-terminal of AChE (N7-20) showed highly structural similarity with Aβ16-40, and induced Aβ aggregation evidently in vitro [19]. Studies from Vaux et al. have found another aggregation-prone motif in the C-terminal of AChE, which is homologous to Aβ [20]. Aβ oligomers play a crucial role in AD pathology through α7 nicotine acetylcholine receptor (α7 nAChR) interaction. Greenfield group has confirmed the regulatory effect of this C-terminal motif on α7 nAChR [14]. Compared with cell membrane-binding C-terminal, N-terminal of AChE faces intercellular space and may possess more freedom to interact with Aβ, even α7 nAChR. Therefore, the potential interaction between the N-terminal of AChE and α7 nAChR, which may contribute to understanding the pathogenesis of AD, should be unveiled urgently.
3. TOWARDS A FURTHER UNDERSTANDING OF THE MULTIFACTORIAL NATURE OF AD
In the light of new compelling evidence, neuroscientists have come to the conclusion that AD has been handled in an inappropriate way and agreed to acknowledge that no sustained therapeutic solution can be expected from AChEIs alone. In fact, AD is multifactorial and heterogeneous in nature and extends well beyond the cholinergic system (Fig. 3) [21]. Its classical neuropathological features comprise fibrillar Aβ deposits, neurofibrillary tangles, neuronal cell death and synaptic loss; but it is just the tip of the iceberg. Ingenuity® Pathway Analysis (IPA®) indicates that dozens of targets and hundreds of endogenous biomolecules have been proved to participate in AD pathophysiology. More recently, the amyloid cascade hypothesis [22] that links the cause of AD solely to β-sheet-rich amyloid fibrils deposits has been revised. Low molecular-weight oligomeric assemblies of Aβ peptide have been confirmed as the key pathogenetic effectors in the earlier stage of AD.
Fig. (3).
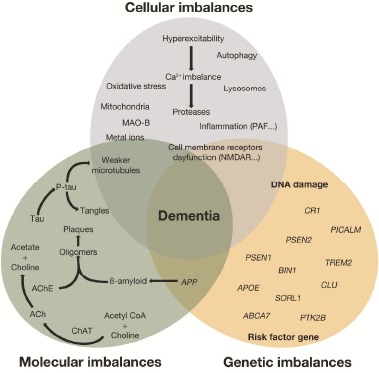
Diagram for multifactorial nature of Alzheimer’s disease. Revised from Herrup K, et al. Nature Neuroscience, 2015, 794-799.
Recent advances provide a fascinating insight into the complexities of AD physiopathology. Behind the scene, it’s an integrated network with genetic risks, molecular interactions and cellular homeostasis regulation. By virtue of genome-wide association study (GWAS) and whole-exome/genome sequencing techniques, genetic risk factor genes or loci such as APOE, PSEN1, TREM2, and SORL1 are constantly discovered to provide us more underlying pathogenic drivers. The expression of familial AD (fAD) and sporadic AD (sAD) related risk genes trigger the downstream molecules chaos. Excessive Aβ-initiated pathological cascade can give rise to chronic inflammation and oxidative stress, two hallmarks reported to play a key role in AD pathogenesis and progression.
It is now well documented that all signs of inflammatory microglial and astroglial activation are evident around Aβ deposits and along the axons of neurons with intracellular neurofibrillary tangles. Aβ-activated microglia sparks the release of several neurotoxic inflammatory factors such as inducible nitric oxide synthase (iNOS), interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α) which in turn lead to neuronal apoptosis [23]. In addition, the platelet-activating factor (PAF), a potent pro-inflammatory mediator, has been recognized as an essential component underlying the devastating effects of Aβ that lead to neuronal death and dementing disorder [24-26].
There is also a great deal of evidence demonstrating that mitochondria damage, cell membrane receptors dysfunction and autophagy accompany with the appearance of senile plaques and neurofibrillary tangles. Moreover, monoamine oxidase B (MAO-B) activity is also increased in the AD brain, reflecting gliosis which results in oxidative stress [23, 27]. Another relevant finding is that amyloid peptide induces an excessive release of glutamate that promotes Ca2+ influx into neuronal cells through glutamate receptor-coupled channels such as NMDA receptor [12, 28]. This process ends in a substantial increase in [Ca2+]i responsible for the hyper-activation of NO synthase, the production of reactive oxygen species (ROS) and the up-regulation of a variety of kinases involved in tau protein phosphorylation.
All of these pathogenic events are potential targets and can be viewed as conclusive evidence supporting the fact that targeting AChE alone, or the “one molecule-one target” concept in general, appears clinically irrelevant and inadequate to handle effectively a complex syndrome like AD. Thus, multifunctional compounds may be beneficial in AD therapy and any drug design strategy should take into consideration this compelling hypothesis.
4. THE MULTI-TARGET DIRECTED LIGANDS (MTDLS), A NEW PARADIGM FOR AD THERAPY
Due to the complexities of AD physiopathology, multi-target approaches develop fast in the recent years. Among them AChE inhibition is usually taken in consideration for its symptomatic amelioration. As we summarised in the third section, many factors involved in AD pathology might be the potential targets for the disease therapy, such as PAF, beta-site amyloid precursor protein cleavage enzyme 1 (BACE1), ROS, MAO-B, metal ions, and so on. The further understanding of cellular and molecular mechanisms underlying AD neurodegeneration helps reshape drug design strategies to counter particular step of the neurotoxic cascade. Today, there is a growing recognition that modulating multiple targets including AChE simultaneously may considerably enhance efficacy with highly satisfactory outcomes [29-32]. Fortunately, medicinal chemists are capable to rationally design specific compounds exhibiting various pharmacological actions. Using universal chemical approaches, this challenging task can be achieved by carefully combining two or several structural features with specific single-target activity into one structure. Another way of achieving success is simply to perform appropriate modifications on the basic structure of existing molecules to yield multiple ligands able to span several targets. The challenges lying behind these rational approaches are enormous; reaching the desired dual or multiple profiles may turn out to be a tough process that ends up in failure. Actually, the constraints related to the structure-activity relationships (SAR) of the interested chemical motif make it sometimes difficult to link together several distinct pharmacophores without losing their associated functionalities.
In clinic, AChEIs are sometimes prescribed with other drugs to achieve more clinical efficacy. The memantine-donepezil or mematine-rivastigmine combination therapy leads to significant benefit over donepezil or rivastigmine alone [33-36]. In contrast to “drug cocktails”, MTDLs may present, along with therapeutic benefits, some attractive assets in terms of satisfactory pharmacoeconomy profile, predictable pharmacokinetic and pharmacodynamic relationship coupled with enhanced patient compliance. However it should be noted that, in some cases, designing multifunctional drugs by binding several pharmacophores together could be a limiting factor, as it could lead to high-molecular-weight molecules exhibiting poor "drug-likeness" [37]. The paradigm of AD drug development mainly focuses on AChEI-based MTDLs including 1) dual binding site AChEIs inhibiting Aβ aggregation, 2) novel AChEIs regulating Aβ procession, 3) novel AChEIs antagonizing PAF receptor, 4) novel AChEIs scavenging oxygen radical, 5) novel AChEIs inhibiting MAO-B, 6) novel AChEIs chelating metal ions, 7) novel AChEIs blocking NMDA receptor and others.
4.1. Dual Binding Site AChEIs Inhibiting Aβ Aggregation
Represented by bis-(-)-nor-MEP derivatives mentioned above, dual binding site AChEIs targeting both CAS and PAS of AChE opened the prelude of AChEI-based MTDLs. As tacrine was the first FDA-approved drug for AD therapy, now rarely used owing to its hepatotoxicity, it was chosen to be a preferred backbone for novel AChEIs. Gemma et al. reported the development of a series of novel tacrine-huperzine A (THA-HA) hybrids synthesized on 3-methylbicyclo-[3.3.1]non-3-ene scaffold [38]. These THA-HA hybrids were
able to simultaneously interact with multiple interaction sites (catalytic, mid-gorge, and peripheral) of either AChE or BuChE. The introduction of the 3-methylbicyclo-[3.3.1]non-3-ene moiety conferred on THA-HA hybrids a markedly improved inhibitory potency against both hAChE and hBuChE (in the nanomolar range) compared with tacrine and huperzine A. Similar studies were undertaken by Muñoz-Ruiz et al. who reported the design, synthesis and in vitro pharmacology of a series of tacrine-indole heterodimers as dual binding site AChEIs able to interfere with Aβ aggregation [39]. These heterodimeric derivatives contain a 1,2,3,4-tetrahydroacridine (tacrine) ring as catalytic anionic site binding unit and an indole ring as peripheral site binding unit, connected by an appropriate linker; the synthesis methodology was using either carbamate or amide functionalities within the linker. Later, Kwon et al. reported the synthesis and pharmacological evaluation of new piperidine derivatives having dual inhibition of AChE and Aβ aggregation [40]. To bind to the AChE catalytic site, they designed an ester with aromatic entity. As for the peripheral site, another aromatic group was associated. The blockage of Aβ oligomerization was brought about by a long and linear molecular structure containing a hydrophobic group.
4.2. Novel AChEIs Regulating Aβ Procession
On the basis of the involvement of PAS in Aβ aggregation process, Piazzy et al. reported two coumarin derivatives AP2238 and AP2243 as AChEIs with anti-Aβ aggregating activities [41]. As they were also interested in another attractive target, BACE1, a transmembrane aspartyl protease responsible for N-terminal cleavage of APP and Aβ peptide production, they performed some modifications on AP2243 structure [42] and obtained potent BACE1 inhibitors AP2238 with IC50 ranging up to 99 nM. After further modification of AP2238, a new derivative AP2469 was able to inhibit Aβ42 self-aggregation, Aβ42 oligomer neurotoxicity and ROS formation in neuronal and microglial cells [43]. That makes AP2469 a potential multifunctional AD therapeutic candidate together with its prototypical AChE and BACE1 inhibitory and antioxidant performance.
There were also AChEIs regulating amyloidogenesis by directly interfering with APP production. One of (-)-MEP phenylcarbamate derivatives reported by our group, Meserine, executed dual actions against cholinergic deficiency and amyloidogenesis by inhibiting AChE and APP translation. In vivo study showed that Meserine could ameliorate scopolamine-induced dementia and alleviate amyloidogenesis in mice [44].
Recent update from Cedric et al. reported donecopride based on combination of AChEI donepezil and a partial serotonin subtype 4 receptor (5-HT4R) agonist RS67333 [45]. Apart from being AChEI (IC50 = 16 nM) and 5-HT4R partial agonist (Ki = 10.4 nM), it also promoted soluble amyloid precursor protein α (sAPPα) release (EC50 = 11.3 nM) as APP non-amyloidogenic cleavage promotor. In vivo test showed donecopride could improve memory performances. All these results suggested donecopride as a promising AD treatment candidate.
4.3. Novel AChEIs Antagonizing PAF Receptor
It’s well known that neuroinflammation is an obvious sign in microglial and astroglial activation accompany with Aβ deposition and neurofibrillary tangles. Severe neuro-inflammatory reaction may exacerbate neurodegenerative progression. As a potent pro-inflammatory mediator, PAF shows elevated level in AD brain. Thus, MTDLs against PAF and AChE are expected with more desired treatment potency.
In the last decade, our collaborators Prof. Godfroid et al. synthesized a series of tetrahydrofuran derivatives presenting a dual inhibition of PAF receptor and AChE [46, 47]. PMS777 (Fig. 4a), one of the promising compounds within the series, has proven to exert multiple functions in vitro and even in vivo. PMS777 (1-100 µM) could dose-dependently inhibit PAF-induced rabbit platelet aggregation and markedly inhibit brain AChE activity in mice with a modest selectivity for AChE [48]. It was also able to fight oxidative injury [49, 50], modulate the release of pro-inflammatory mediators [51], attenuate PAF-induced neurocytotoxicity and neuroinflammation [52, 53], and regulate APP processing in vitro [54]. Additionally, in vivo study found that PMS777 could reverse spatial memory deficits induced by scopolamine in mouse model [48]. Thereafter, the group synthesized another series of piperazine derivatives, among which PMS1339 (Fig. 4b) was the most effective one with an additional inhibition of AChE-induced Aβ aggregation compared with PMS777. Enzymatic analysis showed PMS1339 inhibited AChE in a mixed-mode competitive way. Molecular docking results showed PMS1339 fit well into the active site gorge of AChE, simultaneously binding the catalytic and peripheral site residues (Fig. 4c) [55].
Fig. (4).
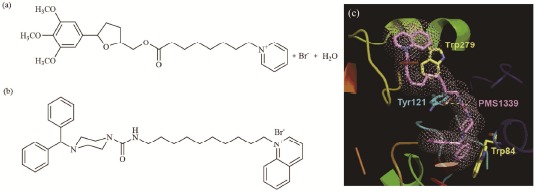
Structural formula of PMS777 (a) and PMS1339 (b) and the binding model of PMS1339 to AChE (c)
4.4. Novel AChEIs Scavenging Oxygen Radical
The latest advances in AD research highlighted the crucial role of free radical formation, oxidative cell damage in the pathogenesis and progression of AD. Drugs that specifically scavenge oxygen radical may have a particular therapeutic efficacy. For this reason, Rodríguez-Franco et al. focused their research on dual acting drugs that combine AChE inhibitory and antioxidant properties in a single molecule [56]. They successfully synthesized new hybrids of tacrine and melatonin. It is worth noting that melatonin is a neurohormone whose secretion decreases during aging, especially in AD patients. It has been reported that melatonin possesses strong antioxidant properties with a direct scavenging function on a variety of ROS, exhibits protective effects against Aβ-induced apoptosis in microglia and improves learning and memory in AD model rats [57-59]. In addition to antioxidant properties, the new tacrine-melatonin derivatives exhibited in vitro AChE inhibitory activity (IC50 values ranging from sub-nanomolar to picomolar) with selectivity for AChE over BuChE. Recently updated research about melatonin scaffold came from Cheng SB et al. who designed a series of novel (-)-meptazinol-melatonin hybrids [60]. Compared with parental drugs, one of these derivatives 7c displayed dual inhibitory potency toward AChE and Aβ self-aggregation and AChE-induced A( aggregation, in addition to oxygen radical absorbance capacity (ORAC). Rosini et al. also proposed the design of molecules that can simultaneously exhibit several pharmacological properties, such as the enhancement of cholinergic transmission, inhibition of Aβ accumulation and oxidative stress [61]. They thereby reported the synthesis of hybrids yielded by connecting together the key structural features of both tacrine (THA) and lipoic acid (LA). Particular attention has been focused on LA in consistence with its pharmacological properties including antioxidant activity and neuroprotection against A(-induced neurotoxicity [62-64]. As expected, lipocrine, one of the THA-LA derivatives emerged from subsequent in vitro assessments as an effective compound endowed with multiple biological properties including the inhibition of both AChE and BuChE, inhibition of AChE-induced Aβ aggregation and protection of neurons against ROS.
Bolognesi et al. used a series of polyamine derivatives possessing dual actions (i.e. AChE inhibition and muscarinic M2 receptor antagonism) as starting scaffolds, and converted them into MTDLs by incorporating into their backbones a chemical entity with antioxidant function [65]. Still maintaining the AChE inhibitory activity, the replacement of the inner polymethylene chain of the polyamine derivatives by a benzoquinone moiety conferred on the resulting molecules, 2,5-bis(diamino)-1,4-benzoquinone derivatives, various additional functions including ROS scavenging function, inhibition of BACE1 and AChE-mediated Aβ aggregation. Another interesting observation in their study was that memoquin, one of these derivatives, caused an effective recovery from the cholinergic deficit, tau hyperphosphorylation, Aβ deposition, and behavioral abnormalities in AD11 anti-NGF mice, a transgenic model of AD. Interestingly, Rizzo et al. designed a series of 2-arylbenzofuran derivatives capable of inhibiting AChE and ROS [66]. Moreover, one compound showed good selectivity and moderate affinity to cannabinoid receptor (CB1) receptor, which is beneficial to neuroprotection. Indanone and ebselen were also considered as functional groups in AChEIs design with additional antioxidant property. Colleagues from Li’s lab developed new AD drug candidates such as a fusion of donepezil and ebselen [67] acquired AChE inhibition and peroxide scavenging activity. Furthermore, a series of indanone derivatives [68] were designed with nanomolar inhibition of AChE, some of which showed significant anti-Aβ aggregation and antioxidant activities.
4.5. Novel AChEIs Inhibiting MAO-B
Recent studies have shown that MAO-B activity is increased in the cortex of AD patients and consequently produces an elevation of brain levels of hydroxyl radicals which is connected to the deposition of Aβ plaques. Thus, MAO-B inhibitors have been proposed for the treatment of AD. Sterling et al. describes the preparation and preliminary in vitro screening of two series of dual MAO and AChE inhibitors. They used rasagiline and selegiline, MAO-B inhibitors with neuroprotective functions related to their propargyl groups, and introduced a carbamate moiety to confer on either rasagiline (Series I, N-propargylaminoindans) or selegiline (Series II, N-propargylphenethylamines) the AChE inhibitory activity. In an exploration of coumarin derivatives as MAO inhibitors, Bruhlmann et al. surprisingly discovered that some of them were also endowed with inhibitory activity towards AChE [69]. They thereby undertook some investigations relating to AChE inhibition of several analogues of 7-hydroxycoumarin that strongly inhibit MAO-A and MAO-B with marked MAO-B selectivity. After many attempts, they obtained 7-[(chlorobenzyl) oxy]-3,4-dimethylcoumarin which was particularly interesting because it was the best AChE inhibitor ranking among the best MAO-B inhibitors as well. The next step for these authors is to set up a better assessment and optimization of anti-AChE activity without the loss of MAO-B inhibition. What’s more, based on ASS234, an antioxidant and AChE and Aβ aggregation inhibitor, MBA236 was identified as a promising new cholinesterase and MAO dual inhibitor by Bautista-Aguilera et al. [70].
4.6. Novel AChEIs Chelating Metal Ions
Metal ion is an indispensable part in AD pathology. Two hallmarks of AD, both Aβ and tau, have enrolled metal ions in respective proteopathy. High-level accumulation of zinc, copper and iron ions has been observed in the amyloid plagues of AD patient, and the function of these metal ions in amyloid formation has been well documented [71]. Metal ions cause metal-specific changes in the kinetics of Aβ aggregation and also contribute to higher ROS production and toxicity since metal ions such as copper can catalyse ROS itself. Therefore, based on metal ion clearance strategy, metal chelators have become one of the most employed function groups for AChEI-based MTDLs.
More recently, Bolognesi et al. proposed a design strategy to convert a dual-binding site AChEI into triple functional compounds [65]. Their starting scaffold was a bivalent ligand that encompassed two tacrine units bound together by a heptylene linker of optimized length in order to contact simultaneously with both catalytic and peripheral sites of AChE (bis-tacrine: Y= (CH2)5, Fig. 5a). To rationally convert this bivalent ligand into a triple functional compound, the authors focused on the linker as the carrier of a third biological activity defined by metal chelation. Moreover, bis-tacrine derivatives have been yielded by successively replacing the heptylene chain of bis-tacrine with carbonyl, oxalamide and polyethylene glycol chains of similar length. Subsequent in vitro assays demonstrated that the bis-tacrine derivatives maintain a potent inhibiting activity against AChE and AChE-induced Aβ aggregation, and at the same time exhibit an additional property as metal chelators.
Fig. (5).
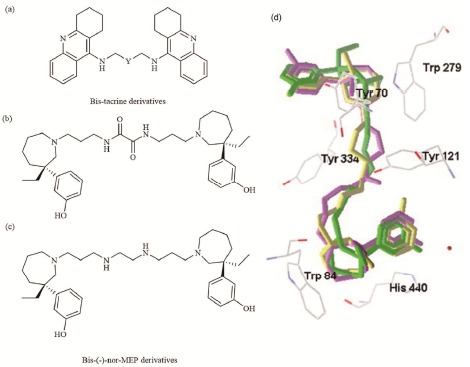
Structural formula of a) bis-tacrine derivatives, b), c) bis-MEP derivatives and d) the binding modes of bis-MEP, bis-MEP derivatives (green, purple and yellow, respectively) at the TcAChE gorge.
In order to augment metal chelating function, our group designed two novel bis-(-)-nor-MEP derivatives (Fig. 5b, c) by inserting oxalamide or ethylenediamine group to the linker [72]. This modification retained the original potency of bis-(-)-nor-MEP and endowed these derivatives with metal ion chelation competence. Docking studies suggested that they were able to interact with both the catalytic and peripheral anionic sites of AChE (Fig. 5d).
Akiko Kochi et al. combined an AChEI and an Aβ-targeted metal chelator into a single molecule to contribute a novel hybrid of 6-chlorotacrine and metal-Aβ modulator [73]. The hybrid showed potent inhibition of AChE, interaction with Cu2+, Zn2+, control of metal-free or metal-associated Aβ aggregation and disaggregation.
4.7. Novel AChEIs Blocking NMDA Receptor and Others
AD pathology presents neuronal lesion on multi-neuro-transmission systems, including cholinergic, glutamatergic, dopaminergic, serotoninergic and etc. Synapse receptors enriched in hippocampus are important hubs for neuronal regulation. Compounds targeting on corresponding receptors will ameliorate synaptotoxicity and be beneficial to AD treatment. Among these receptors, NMDA receptor, associated with synapse plasticity, has been proved to be involved in glutamatergic dysfunction in AD pathology (Fig. 1) and become a promising AD therapeutic target [74]. Yvonne Rook et al. reported some bivalent β-carbolines as potent NMDA receptor blockers with AChE/BuChE inhibition. The most promising compound was N9-homobivalent β-carboline with a nonylene spacer, which displayed IC50 values of 0.5 nM for AChE, 5.7 nM for BuChE, and 1.4 µM for NMDA receptor, respectively [75]. Comparable success was achieved by hybrids, carbacrine (linking tacrine and carvedilol) and memagal (bridging galantamine and memantine) [76, 77], which were multifunctional inhibitors inhibiting Aβ aggregation and ROS generation as well as AChE and NMDA receptor.
For the growing knowledge of AD pathophysiology, more and more new targets are under consideration in multi-target strategies of AD therapy, such as serotonin transporter [78], cannabinoid CB1 receptor [79], Ca2+ channel [80], histamine receptor [81], etc. These studies provide us fresh angles to dig out solutions to the disease.
4.8. Others
Recently, natural drugs have gained great attention due to their excellent efficacy and low side effects. Traditional Chinese herb medicine, which is one of precious treasures in China, has become a rich source of innovative drugs [82]. In China, herb medicine has been used to treat dementia for a very long time [83]. A diversity of bioactive compounds with different chemical scaffolds has been derived from the medicinal herbs and proved to be effective in preclinical and clinical studies, some of which even possess multi-target properties. Huperzine A is an alkaloid isolated from the Chinese herb Huperzia serrata. It is a potent, highly selective, reversible AChEI with neuroprotective and antioxidant activities. Huperzine A has been a licensed anti-AD drug in China since 1994 and now is commercially available as a food supplement in US for its ability to improve memory and mental function [84]. Moreover, well-studied natural compounds such as (-)-Epigallocatechin-3-gallate (EGCG) and resveratrol have been reported as AChE inhibitors [85]. And natural medicine gingko biloba, curcumin and quinoline exhibit neuroprotective, antioxidant and antinflammatory properties beneficial for the treatment of AD.
Based on the template of nature compounds, new AChEI-based MTDLs were designed by scaffold hopping, fragment assembly, or structure optimization. Several derivatives of huperzine A have been prepared to achieve multi-target strategies. ZT-1 is a Schiff derivative of Huperzine A and hydrolyzed nonenzymatically into the active compound Huperzine A in the body. ZT-1 reversed the memory deficits in AD rat and monkey models and showed safe and well tolerated in phase I clinical study [86]. Phase II clinical trial for efficacy assessment in mild and moderate AD patients has been completed in Europe. Bis-huperzine A is a dual binding AChEI targeting both active and peripheral anionic sites of AChE by linking two huperzine A compounds with a nonamethylene spacer. Both in vitro and in vivo studies have proved it as a potential dual inhibitor of AChE and Aβ aggregation [87]. Notably, informatics technique such as computer aided drug design (CADD) and bioinformatics have been widely used in drug discovery and biomedical research to seek more disease related targets and regulators. Bansode et al. applied FDA-approved CNS drugs in docking studies for drug repositioning. The tricyclic antidepressant, representative protriptyline, was suggested potential inhibitors against AChE, BACE1 and Aβ aggregation [88]. Similar docking protocol suggested silibinin, a hepatoprotective agent, as an inhibitor of AChE and Aβ peptide aggregation for the treatment of AD [89].
Recently, by collecting and mining the compound structural data of Jun, Chen, Zuo, and Shi herbs in Buzhongyiqi decoction prescription, a Traditional Chinese Medicine (TCM) recipe used to treat dementia and myasthenia gravis, and the AChE inhibitor structural data, we proved that the active components of Buzhongyiqi decoction are mainly existing in the Jun and Shi herbs. These active components are flavonoid derivatives as AChE inhibitors. Our work provided a precise insight into TCM efficacy and a solid method of drug discovery by identifying active components from Chinese herb medicine.
5. CONCLUSION AND PERSPECTIVES
Given the recent achievements relating to the field of AD, there is no reason we can still expect a sustained therapeutic solution from “one molecule-one target” AChEIs. It is apparent from observations above-mentioned that any drug design strategy should comply with the emerging “one molecule-multiple targets” approach to effectively address the multifactorial nature of AD. In contrast to traditional AChEIs, the new generation of multi-target AChEIs may be more relevant than ever. The present investigations showed that, in addition to AChE inhibiting activity, the effects of novel anti-AChE agents can be strengthened by various other functions including inhibition of AChE-induced Aβ aggregation and/or Aβ self-aggregation, metal chelation, free radical-scavenging activity, MAO inhibition, etc. Hence, their capacity to modulate several molecular targets simultaneously makes them as real disease-modifying agents with improved clinical outcome in AD. Following the rise of more powerful diagnostic tools such as molecular probes for neuroimaging or whole genome sequencing for precision medicine, AD pathology may be dissected into specific progressive periods according to the evolvement of related pathologic targets. Then specifically drug or gene interventions will be expected for AD individuals' recovery. As the mainstream treatment for AD, AChEI-based MTDLs will be persisted as the cornerstone for the development of versatile anti-AD drugs.
ACKNOWLEDGEMENTS
We sincerely thank the National Natural Science Foundation of China (NO. 30772553, 30801393, 30801435 , 30973509, 81373395 and 81573415), National Basic Research Program of China (2007CB935800 and 2010CB529806), and National Innovative Drug Development Project (2009ZX09103-077 and 2009ZX09301-011) and the Shanghai Municipal Science and Technology Commission (No. 10431902700 and 14431905600) for financial support. We are grateful to Prof. F. Massicot (Faculté des Sciences Pharmaceutiques et Biologiques, Universite Paris Descartes), F. Heymans (Unite de Pharmacochimie Moleculaire et Systemes Membranaires (EA2381), Université Paris 7-Denis Diderot,) and Z. Qiu (School of Pharmacy, Fudan University) and Prof. J. Xu (School of Pharmaceutical Sciences, Sun Yat-Sen University) for our long-lasting collaboration on the development of multi-target AChEIs.
LIST OF ABBREVIATIONS
- ACh =
acetylcholine
- AChE =
acetylcholinesterase
- AChEI =
acetylcholinesterase inhibitor
- AD =
alzheimer disease
- Aβ =
amyloid beta
- BACE 1 =
beta-site amyloid precursor protein cleavage enzyme 1
- BuChE =
butyrocholinesterase
- CADD =
computer aided drug design
- CB1 =
cannabinoid receptor
- ChAT =
choline acetyltransferase
- GWAS =
genome-wide association study
- IL-1β =
interleukin-1β
- IL-6 =
interleukin-6
- iNOS =
nitric oxide synthase
- MAO =
monoamine oxidase B
- MEP =
meptazinol
- MTDL =
multi-target directed ligand
- NMDA =
N-methyl-D-aspartic acid
- PAF =
platelet-activating factor
- PAS =
peripheral anionic site
- ROS =
reactive oxygen species
- SAPPα =
soluble amyloid precursor protein α
- SAR =
structure-activity relationship
- THA-HA =
tacrine-huperzine A
- TNF-α =
tumour necrosis factor-α
- 5-HT4 =
serotonin subtype 4
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanatkar H.R., Grossberg G.T. Transdermal rivastigmine in the treatment of Alzheimer’s disease: current and future directions. Expert Rev. Neurother. 2014;14(10):1119–1125. doi: 10.1586/14737175.2014.955852. [DOI] [PubMed] [Google Scholar]
- 3.Tracey I., Flower R. The warrior in the machine: neuroscience goes to war. Nat. Rev. Neurosci. 2014;15(12):825–834. doi: 10.1038/nrn3835. [DOI] [PubMed] [Google Scholar]
- 4.Contestabile A. The history of the cholinergic hypothesis. Behav. Brain Res. 2011;221(2):334–340. doi: 10.1016/j.bbr.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 5.Qiu Y., Wu X. J., Chen H. Z. Simultaneous changes in secretory amyloid precursor protein and beta-amyloid peptide release from rat hippocampus by activation of muscarinic receptors. Neurosci. Lett. 2014;352(1):41–44. doi: 10.1016/j.neulet.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Zhu X., Zhou W., Cui Y., Zhu L., Li J., Xia Z., Shao B., Wang H., Chen H. Muscarinic activation attenuates abnormal processing of beta-amyloid precursor protein induced by cobalt chloride-mimetic hypoxia in retinal ganglion cells. Biochem. Biophys. Res. Commun. 2009;384(1):110–113. doi: 10.1016/j.bbrc.2009.04.080. [DOI] [PubMed] [Google Scholar]
- 7.Zhou W., Zhu X., Zhu L., Cui Y.Y., Wang H., Qi H., Ren Q.S., Chen H.Z. Neuroprotection of muscarinic receptor agonist pilocarpine against glutamate-induced apoptosis in retinal neurons. Cell. Mol. Neurobiol. 2008;28(2):263–275. doi: 10.1007/s10571-007-9251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan P.P., Yuan H.H., Zhu X., Cui Y.Y., Li H., Feng X.M., Qiu Y., Chen H.Z., Zhou W. Activation of muscarinic receptors protects against retinal neurons damage and optic nerve degeneration in vitro and in vivo models. CNS Neurosci. Ther. 2014;20(3):227–236. doi: 10.1111/cns.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H., Lu Y., Chen H.Z. Differentiating effects of anisodamine on cognitive amelioration and peripheral muscarinic side effects induced by pilocarpine in mice. Neurosci. Lett. 2003;344(3):173–176. doi: 10.1016/S0304-3940(03)00444-0. [DOI] [PubMed] [Google Scholar]
- 10.Harel M., Schalk I., Ehret-Sabatier L., Bouet F., Goeldner M., Hirth C., Axelsen P.H., Silman I., Sussman J.L. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc. Natl. Acad. Sci. USA. 1993;90(19):9031–9035. doi: 10.1073/pnas.90.19.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachovchin D.A., Cravatt B.F. The pharmacological landscape and therapeutic potential of serine hydrolases. Nat. Rev. Drug Discov. 2012;11(1):52–68. doi: 10.1038/nrd3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paoletti P., Bellone C., Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 13.Cheung J., Rudolph M.J., Burshteyn F., Cassidy M.S., Gary E.N., Love J., Franklin M.C., Height J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012;55(22):10282–10286. doi: 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]
- 14.Greenfield S. Discovering and targeting the basic mechanism of neurodegeneration: the role of peptides from the C-terminus of acetylcholinesterase: non-hydrolytic effects of ache: the actions of peptides derived from the C-terminal and their relevance to neurodegeneration. Chem. Biol. Interact. 2013;203(3):543–546. doi: 10.1016/j.cbi.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann M. Neuronal AChE splice variants and their non-hydrolytic functions: redefining a target of AChE inhibitors? Br. J. Pharmacol. 2013;170(5):953–967. doi: 10.1111/bph.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inestrosa N.C., Alvarez A., Pérez C.A., Moreno R.D., Vicente M., Linker C., Casanueva O.I., Soto C., Garrido J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer’s fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16(4):881–891. doi: 10.1016/S0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 17.Koch M.W., Korngut L., Patry D.G., Agha-Khani Y., White C., Sarna J.R., Yeung M., Yong V.W., Heng D.Y., Cutter G., Metz L. The promise of futility trials in neurological diseases. Nat. Rev. Neurol. 2015;11(5):300–305. doi: 10.1038/nrneurol.2015.34. [DOI] [PubMed] [Google Scholar]
- 18.Xie Q., Wang H., Xia Z., Lu M., Zhang W., Wang X., Fu W., Tang Y., Sheng W., Li W., Zhou W., Zhu X., Qiu Z., Chen H. Bis-(-)-nor-meptazinols as novel nanomolar cholinesterase inhibitors with high inhibitory potency on amyloid-beta aggregation. J. Med. Chem. 2008;51(7):2027–2036. doi: 10.1021/jm070154q. [DOI] [PubMed] [Google Scholar]
- 19.Hou L.N., Xu J.R., Zhao Q.N., Gao X.L., Cui Y.Y., Xu J., Wang H., Chen H.Z. A new motif in the N-terminal of acetylcholinesterase triggers amyloid-β aggregation and deposition. CNS Neurosci. Ther. 2014;20(1):59–66. doi: 10.1111/cns.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cottingham M.G., Hollinshead M.S., Vaux D.J. Amyloid fibril formation by a synthetic peptide from a region of human acetylcholinesterase that is homologous to the Alzheimer’s amyloid-beta peptide. Biochemistry. 2002;41(46):13539–13547. doi: 10.1021/bi0260334. [DOI] [PubMed] [Google Scholar]
- 21.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat. Neurosci. 2015;18(6):794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 22.Benilova I., Karran E., De Strooper B. The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat. Neurosci. 2012;15(3):349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 23.Heppner F.L., Ransohoff R.M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16(6):358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 24.Ryan S.D., Whitehead S.N., Swayne L.A., Moffat T.C., Hou W., Ethier M., Bourgeois A.J., Rashidian J., Blanchard A.P., Fraser P.E., Park D.S., Figeys D., Bennett S.A. Amyloid-beta42 signals tau hyperphosphorylation and compromises neuronal viability by disrupting alkylacylglycerophosphocholine metabolism. Proc. Natl. Acad. Sci. USA. 2009;106(49):20936–20941. doi: 10.1073/pnas.0905654106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bate C., Salmona M., Williams A. The role of platelet activating factor in prion and amyloid-beta neurotoxicity. Neuroreport. 2004;15(3):509–513. doi: 10.1097/00001756-200403010-00025. [DOI] [PubMed] [Google Scholar]
- 26.Bate C., Kempster S., Williams A. Platelet-activating factor antagonists protect amyloid-beta damaged neurons from microglia-mediated death. Neuropharmacology. 2006;51(2):173–181. doi: 10.1016/j.neuropharm.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Trillo L., Das D., Hsieh W., Medina B., Moghadam S., Lin B., Dang V., Sanchez M.M., De Miguel Z., Ashford J.W., Salehi A. Ascending monoaminergic systems alterations in Alzheimer’s disease. translating basic science into clinical care. Neurosci. Biobehav. Rev. 2013;37(8):1363–1379. doi: 10.1016/j.neubiorev.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Parameshwaran K., Dhanasekaran M., Suppiramaniam V. Amyloid beta peptides and glutamatergic synaptic dysregulation. Exp. Neurol. 2008;210(1):7–13. doi: 10.1016/j.expneurol.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 29.León R., Garcia A.G., Marco-Contelles J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013;33(1):139–189. doi: 10.1002/med.20248. [DOI] [PubMed] [Google Scholar]
- 30.Dias K.S., Viegas C., Jr Multi-Target Directed Drugs: A Modern Approach for Design of New Drugs for the treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2014;12(3):239–255. doi: 10.2174/1570159X1203140511153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agis-Torres A., Sölhuber M., Fernandez M., Sanchez-Montero J.M. Multi-Target-Directed Ligands and other Therapeutic Strategies in the Search of a Real Solution for Alzheimer’s Disease. Curr. Neuropharmacol. 2014;12(1):2–36. doi: 10.2174/1570159X113116660047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rochais C., Lecoutey C., Gaven F., Giannoni P., Hamidouche K., Hedou D., Dubost E., Genest D., Yahiaoui S., Freret T., Bouet V., Dauphin F., Sopkova de Oliveira Santos J., Ballandonne C., Corvaisier S., Malzert-Fréon A., Legay R., Boulouard M., Claeysen S., Dallemagne P. Novel multitarget-directed ligands (MTDLs) with acetylcholinesterase (AChE) inhibitory and serotonergic subtype 4 receptor (5-HT4R) agonist activities as potential agents against Alzheimer’s disease: the design of donecopride. J. Med. Chem. 2015;58(7):3172–3187. doi: 10.1021/acs.jmedchem.5b00115. [DOI] [PubMed] [Google Scholar]
- 33.Xiong G., Doraiswamy P.M. Combination drug therapy for Alzheimer’s disease: what is evidence-based, and what is not? Geriatrics. 2005;60(6):22–26. [PubMed] [Google Scholar]
- 34.Dantoine T., Auriacombe S., Sarazin M., Becker H., Pere J.J., Bourdeix I. Rivastigmine monotherapy and combination therapy with memantine in patients with moderately severe Alzheimer’s disease who failed to benefit from previous cholinesterase inhibitor treatment. Int. J. Clin. Pract. 2006;60(1):110–118. doi: 10.1111/j.1368-5031.2005.00769.x. [DOI] [PubMed] [Google Scholar]
- 35.Klatte E.T., Scharre D.W., Nagaraja H.N., Davis R.A., Beversdorf D.Q. Combination therapy of donepezil and vitamin E in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2003;17(2):113–116. doi: 10.1097/00002093-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Weycker D., Taneja C., Edelsberg J., Erder M.H., Schmitt F.A., Setyawan J., Oster G. Cost-effectiveness of memantine in moderate-to-severe Alzheimer’s disease patients receiving donepezil. Curr. Med. Res. Opin. 2007;23(5):1187–1197. doi: 10.1185/030079907X188071. [DOI] [PubMed] [Google Scholar]
- 37.Morphy R., Rankovic Z. The physicochemical challenges of designing multiple ligands. J. Med. Chem. 2006;49(16):4961–4970. doi: 10.1021/jm0603015. [DOI] [PubMed] [Google Scholar]
- 38.Gemma S., Gabellieri E., Huleatt P., Fattorusso C., Borriello M., Catalanotti B., Butini S., De Angelis M., Novellino E., Nacci V., Belinskaya T., Saxena A., Campiani G. Discovery of huperzine A-tacrine hybrids as potent inhibitors of human cholinesterases targeting their midgorge recognition sites. J. Med. Chem. 2006;49(11):3421–3425. doi: 10.1021/jm060257t. [DOI] [PubMed] [Google Scholar]
- 39.Muñoz-Ruiz P., Rubio L., García-Palomero E., Dorronsoro I., del Monte-Millán M., Valenzuela R., Usán P., de Austria C., Bartolini M., Andrisano V., Bidon-Chanal A., Orozco M., Luque F.J., Medina M., Martínez A. Design, synthesis, and biological evaluation of dual binding site acetylcholinesterase inhibitors: new disease-modifying agents for Alzheimer’s disease. J. Med. Chem. 2005;48(23):7223–7233. doi: 10.1021/jm0503289. [DOI] [PubMed] [Google Scholar]
- 40.Kwon Y.E., Park J.Y., No K.T., Shin J.H., Lee S.K., Eun J.S., Yang J.H., Shin T.Y., Kim D.K., Chae B.S., Leem J.Y., Kim K.H. Synthesis, in vitro assay, and molecular modeling of new piperidine derivatives having dual inhibitory potency against acetylcholinesterase and Abeta1-42 aggregation for Alzheimer’s disease therapeutics. Bioorg. Med. Chem. 2007;15(20):6596–6607. doi: 10.1016/j.bmc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Piazzi L., Rampa A., Bisi A., Gobbi S., Belluti F., Cavalli A., Bartolini M., Andrisano V., Valenti P., Recanatini M. 3-(4-[[Benzyl(methyl)amino]methyl]phenyl)-6,7-dimethoxy-2H-2-chromenone (AP2238) inhibits both acetylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation: a dual function lead for Alzheimer’s disease therapy. J. Med. Chem. 2003;46(12):2279–2282. doi: 10.1021/jm0340602. [DOI] [PubMed] [Google Scholar]
- 42.Guo T., Hobbs D.W. Development of BACE1 inhibitors for Alzheimer’s disease. Curr. Med. Chem. 2006;13(15):1811–1829. doi: 10.2174/092986706777452489. [DOI] [PubMed] [Google Scholar]
- 43.Tarozzi A., Bartolini M., Piazzi L., Valgimigli L., Amorati R., Bolondi C., Djemil A., Mancini F., Andrisano V., Rampa A. From the dual function lead AP2238 to AP2469, a multi-target-directed ligand for the treatment of Alzheimer’s disease. Pharmacol. Res. Perspect. 2014;2(2):e00023. doi: 10.1002/prp2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao B.Y., Xia Z., Xie Q., Ge X.X., Zhang W.W., Sun J., Jiang P., Wang H., Le W.D., Qiu Z.B., Lu Y., Chen H.Z. Meserine, a novel carbamate AChE inhibitor, ameliorates scopolamine-induced dementia and alleviates amyloidogenesis of APP/PS1 transgenic mice. CNS Neurosci. Ther. 2014;20(2):165–171. doi: 10.1111/cns.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lecoutey C., Hedou D., Freret T., Giannoni P., Gaven F., Since M., Bouet V., Ballandonne C., Corvaisier S., Malzert Fréon A., Mignani S., Cresteil T., Boulouard M., Claeysen S., Rochais C., Dallemagne P. Design of donecopride, a dual serotonin subtype 4 receptor agonist/acetylcholinesterase inhibitor with potential interest for Alzheimer’s disease treatment. Proc. Natl. Acad. Sci. USA. 2014;111(36):E3825–E3830. doi: 10.1073/pnas.1410315111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Texier L., Favre E., Redeuilh C., Blavet N., Bellahsene T., Dive G., Pirotzky E., Godfroid J.J. Structure-activity relationships in platelet-activating factor (PAF). 7. Tetrahydrofuran derivatives as dual PAF antagonists and acetylcholinesterase inhibitors. Synthesis and PAF-antagonistic activity. J. Lipid Mediat. Cell Signal. 1996;13(3):189–205. doi: 10.1016/0929-7855(95)00046-1. [DOI] [PubMed] [Google Scholar]
- 47.Le Texier L., Favre E., Ronzani N., Massicot F., Blavet N., Pirotzky E., Godfroid J.J. Structure-activity relationships in platelet-activating factor (PAF). 8. Tetrahydrofuran derivatives as dual PAF antagonists and acetylcholinesterase inhibitors: anti-acetylcholinesterase activity and comparative SAR. J. Lipid Mediat. Cell Signal. 1996;13(3):207–222. doi: 10.1016/0929-7855(95)00053-4. [DOI] [PubMed] [Google Scholar]
- 48.Li J., Huang H., Miezan Ezoulin J.M., Gao X.L., Massicot F., Dong C.Z., Heymans F., Chen H.Z. Pharmacological profile of PMS777, a new AChE inhibitor with PAF antagonistic activity. Intl. J. Neuropsychopharmacol. / Oficial Sci. J Collegium Internationale Neuropsychopharmacologicum. 2007;10(1):21–29. doi: 10.1017/S1461145705006425. [CINP]. [DOI] [PubMed] [Google Scholar]
- 49.Ezoulin M.J., Dong C.Z., Liu Z., Li J., Chen H.Z., Heymans F., Lelièvre L., Ombetta J.E., Massicot F. Study of PMS777, a new type of acetylcholinesterase inhibitor, in human HepG2 cells. Comparison with tacrine and galanthamine on oxidative stress and mitochondrial impairment. Toxicol. In Vitro. 2006;20(6):824–831. doi: 10.1016/j.tiv.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Ezoulin M.J., Li J., Wu G., Dong C.Z., Ombetta J.E., Chen H.Z., Massicot F., Heymans F. Differential effect of PMS777, a new type of acetylcholinesterase inhibitor, and galanthamine on oxidative injury induced in human neuroblastoma SK-N-SH cells. Neurosci. Lett. 2005;389(2):61–65. doi: 10.1016/j.neulet.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 51.Ezoulin M.J., Liu Z., Dutertre-Catella H., Wu G., Dong C.Z., Heymans F., Ombetta J.E., Rat P., Massicot F. A new acetylcholinesterase inhibitor with anti-PAF activity modulates oxidative stress and pro-inflammatory mediators release in stimulated RAW 264.7 macrophage cells. Comparison with tacrine. Int. Immunopharmacol. 2007;7(13):1685–1694. doi: 10.1016/j.intimp.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 52.Li J., Hu J., Shao B., Zhou W., Cui Y., Dong C., Ezoulin J.M., Zhu X., Ding W., Heymans F., Chen H. Protection of PMS777, a new AChE inhibitor with PAF antagonism, against amyloid-beta-induced neuronal apoptosis and neuroinflammation. Cell. Mol. Neurobiol. 2009;29(4):589–595. doi: 10.1007/s10571-009-9351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Shao B., Zhu L., Cui Y., Dong C., Miezan Ezoulin J.M., Gao X., Ren Q., Heymans F., Chen H. PMS777, a bis-interacting ligand for PAF receptor antagonism and AChE inhibition, attenuates PAF-induced neurocytotoxicity in SH-SY5Y cells. Cell. Mol. Neurobiol. 2008;28(1):125–136. doi: 10.1007/s10571-007-9190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang H.Q., Sun Z.K., Zhao Y.X., Pan J., Ba M.W., Lu G.Q., Ding J.Q., Chen H.Z., Chen S.D. PMS777, a new cholinesterase inhibitor with anti-platelet activated factor activity, regulates amyloid precursor protein processing in vitro. Neurochem. Res. 2009;34(3):528–535. doi: 10.1007/s11064-008-9816-4. [DOI] [PubMed] [Google Scholar]
- 55.Miezan E.J., Shao B.Y., Xia Z., Xie Q., Li J., Cui Y.Y., Wang H., Dong C.Z., Zhao Y.X., Massicot F., Qiu Z.B., Heymans F., Chen H.Z. Novel piperazine derivative PMS1339 exhibits tri-functional properties and cognitive improvement in mice. Intl. J. Neuropsychopharmacol / Official Sci. J. Collegium Internationale Neuropsychopharmacologicum. 2009;12(10):1409–1419. doi: 10.1017/S1461145709000455. [CINP]. [DOI] [PubMed] [Google Scholar]
- 56.Rodríguez-Franco M.I., Fernández-Bachiller M.I., Pérez C., Hernández-Ledesma B., Bartolomé B. Novel tacrine-melatonin hybrids as dual-acting drugs for Alzheimer disease, with improved acetylcholinesterase inhibitory and antioxidant properties. J. Med. Chem. 2006;49(2):459–462. doi: 10.1021/jm050746d. [DOI] [PubMed] [Google Scholar]
- 57.Pappolla M.A., Sos M., Omar R.A., Bick R.J., Hickson-Bick D.L., Reiter R.J., Efthimiopoulos S., Robakis N.K. Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J. Neurosci. 1997;17(5):1683–1690. doi: 10.1523/JNEUROSCI.17-05-01683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baldwin W.S., Barrett J.C. Melatonin attenuates hydrogen peroxide toxicity in MCF7 cells only at pharmacological concentrations. Biochem. Biophys. Res. Commun. 1998;250(3):602–605. doi: 10.1006/bbrc.1998.9370. [DOI] [PubMed] [Google Scholar]
- 59.Lahiri D.K., Chen D.M., Lahiri P., Bondy S., Greig N.H. Amyloid, cholinesterase, melatonin, and metals and their roles in aging and neurodegenerative diseases. Ann. N. Y. Acad. Sci. 2005;1056:430–449. doi: 10.1196/annals.1352.008. [DOI] [PubMed] [Google Scholar]
- 60.Cheng S., Zheng W., Gong P., Zhou Q., Xie Q., Yu L., Zhang P., Chen L., Li J., Chen J., Chen H., Chen H. (-)-Meptazinol-melatonin hybrids as novel dual inhibitors of cholinesterases and amyloid-β aggregation with high antioxidant potency for Alzheimer’s therapy. Bioorg. Med. Chem. 2015;23(13):3110–3118. doi: 10.1016/j.bmc.2015.04.084. [DOI] [PubMed] [Google Scholar]
- 61.Rosini M., Andrisano V., Bartolini M., Bolognesi M.L., Hrelia P., Minarini A., Tarozzi A., Melchiorre C. Rational approach to discover multipotent anti-Alzheimer drugs. J. Med. Chem. 2005;48(2):360–363. doi: 10.1021/jm049112h. [DOI] [PubMed] [Google Scholar]
- 62.Ono K., Hirohata M., Yamada M. Alpha-lipoic acid exhibits anti-amyloidogenicity for beta-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2006;341(4):1046–1052. doi: 10.1016/j.bbrc.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 63.Jesudason E.P., Masilamoni J.G., Jesudoss K.S., Jayakumar R. The protective role of DL-alpha-lipoic acid in the oxidative vulnerability triggered by Abeta-amyloid vaccination in mice. Mol. Cell. Biochem. 2005;270(1-2):29–37. doi: 10.1007/s11010-005-3301-z. [DOI] [PubMed] [Google Scholar]
- 64.Jesudason E.P., Masilamoni J.G., Ashok B.S., Baben B., Arul V., Jesudoss K.S., Jebaraj W.C., Dhandayuthapani S., Vignesh S., Jayakumar R. Inhibitory effects of short-term administration of DL-alpha-lipoic acid on oxidative vulnerability induced by Abeta amyloid fibrils (25-35) in mice. Mol. Cell. Biochem. 2008;311(1-2):145–156. doi: 10.1007/s11010-008-9705-9. [DOI] [PubMed] [Google Scholar]
- 65.Bolognesi M.L., Cavalli A., Valgimigli L., Bartolini M., Rosini M., Andrisano V., Recanatini M., Melchiorre C. Multi-target-directed drug design strategy: from a dual binding site acetylcholinesterase inhibitor to a trifunctional compound against Alzheimer’s disease. J. Med. Chem. 2007;50(26):6446–6449. doi: 10.1021/jm701225u. [DOI] [PubMed] [Google Scholar]
- 66.Rizzo S., Tarozzi A., Bartolini M., Da Costa G., Bisi A., Gobbi S., Belluti F., Ligresti A., Allarà M., Monti J.P., Andrisano V., Di Marzo V., Hrelia P., Rampa A. 2-Arylbenzofuran-based molecules as multipotent Alzheimer’s disease modifying agents. Eur. J. Med. Chem. 2012;58:519–532. doi: 10.1016/j.ejmech.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 67.Luo Z., Sheng J., Sun Y., Lu C., Yan J., Liu A., Luo H.B., Huang L., Li X. Synthesis and evaluation of multi-target-directed ligands against Alzheimer’s disease based on the fusion of donepezil and ebselen. J. Med. Chem. 2013;56(22):9089–9099. doi: 10.1021/jm401047q. [DOI] [PubMed] [Google Scholar]
- 68.Huang L., Miao H., Sun Y., Meng F., Li X. Discovery of indanone derivatives as multi-target-directed ligands against Alzheimer’s disease. Eur. J. Med. Chem. 2014;87:429–439. doi: 10.1016/j.ejmech.2014.09.081. [DOI] [PubMed] [Google Scholar]
- 69.Brühlmann C., Ooms F., Carrupt P.A., Testa B., Catto M., Leonetti F., Altomare C., Carotti A. Coumarins derivatives as dual inhibitors of acetylcholinesterase and monoamine oxidase. J. Med. Chem. 2001;44(19):3195–3198. doi: 10.1021/jm010894d. [DOI] [PubMed] [Google Scholar]
- 70.Bautista-Aguilera O.M., Samadi A., Chioua M., Nikolic K., Filipic S., Agbaba D., Soriano E., de Andrés L., Rodríguez-Franco M.I., Alcaro S., Ramsay R.R., Ortuso F., Yañez M., Marco-Contelles J. N-Methyl-N-((1-methyl-5-(3-(1-(2-methylbenzyl)piperidin-4-yl)propoxy)-1H-indol-2-yl)methyl)prop-2-yn-1-amine, a new cholinesterase and monoamine oxidase dual inhibitor. J. Med. Chem. 2014;57(24):10455–10463. doi: 10.1021/jm501501a. [DOI] [PubMed] [Google Scholar]
- 71.Nasica-Labouze J., Nguyen P.H., Sterpone F., Berthoumieu O., Buchete N.V., Coté S., De Simone A., Doig A.J., Faller P., Garcia A., Laio A., Li M.S., Melchionna S., Mousseau N., Mu Y., Paravastu A., Pasquali S., Rosenman D.J., Strodel B., Tarus B., Viles J.H., Zhang T., Wang C., Derreumaux P. Amyloid β Protein and Alzheimer’s Disease: When Computer Simulations Complement Experimental Studies. Chem. Rev. 2015;115(9):3518–3563. doi: 10.1021/cr500638n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng W., Li J., Qiu Z., Xia Z., Li W., Yu L., Chen H., Chen J., Chen Y., Hu Z., Zhou W., Shao B., Cui Y., Xie Q., Chen H. Novel bis-(-)-nor-meptazinol derivatives act as dual binding site AChE inhibitors with metal-complexing property. Toxicol. Appl. Pharmacol. 2012;264(1):65–72. doi: 10.1016/j.taap.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 73.Kochi A., Eckroat T.J., Green K.D., Mayhoub A.S., Lim M.H., Garneau-Tsodikova S. A novel hybrid of 6-chlorotacrine and metal-amyloid-beta modulator for inhibition of acetylcholinesterase and metal-induced amyloid-beta aggregation. Chem. Sci. (Camb.) 2013;4(11):4137–4145. doi: 10.1039/c3sc51902c. [DOI] [Google Scholar]
- 74.Danysz W., Parsons C.G. Alzheimer’s disease, β-amyloid, glutamate, NMDA receptors and memantine--searching for the connections. Br. J. Pharmacol. 2012;167(2):324–352. doi: 10.1111/j.1476-5381.2012.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rook Y., Schmidtke K.U., Gaube F., Schepmann D., Wünsch B., Heilmann J., Lehmann J., Winckler T. Bivalent beta-carbolines as potential multitarget anti-Alzheimer agents. J. Med. Chem. 2010;53(9):3611–3617. doi: 10.1021/jm1000024. [DOI] [PubMed] [Google Scholar]
- 76.Rosini M., Simoni E., Minarini A., Melchiorre C. Multi-target design strategies in the context of Alzheimer's disease: acetylcholinesterase inhibition and NMDA receptor antagonism as the driving forces. Neurochem. Res. 2014;39(10):1914–1923. doi: 10.1007/s11064-014-1250-1. [DOI] [PubMed] [Google Scholar]
- 77.Simoni E., Daniele S., Bottegoni G., Pizzirani D., Trincavelli M.L., Goldoni L., Tarozzo G., Reggiani A., Martini C., Piomelli D., Melchiorre C., Rosini M., Cavalli A. Combining galantamine and memantine in multitargeted, new chemical entities potentially useful in Alzheimer’s disease. J. Med. Chem. 2012;55(22):9708–9721. doi: 10.1021/jm3009458. [DOI] [PubMed] [Google Scholar]
- 78.Toda N., Kaneko T., Kogen H. Development of an efficient therapeutic agent for Alzheimer's disease: design and synthesis of dual inhibitors of acetylcholinesterase and serotonin transporter. Chem. Pharm. Bull. 2010;58(3):273–287. doi: 10.1002/chin.201031237. [DOI] [PubMed] [Google Scholar]
- 79.Lange J.H., Coolen H.K., van der Neut M.A., Borst A.J., Stork B., Verveer P.C., Kruse C.G. Design, synthesis, biological properties, and molecular modeling investigations of novel tacrine derivatives with a combination of acetylcholinesterase inhibition and cannabinoid CB1 receptor antagonism. J. Med. Chem. 2010;53(3):1338–1346. doi: 10.1021/jm901614b. [DOI] [PubMed] [Google Scholar]
- 80.Marco-Contelles J., León R., de los Ríos C., Samadi A., Bartolini M., Andrisano V., Huertas O., Barril X., Luque F.J., Rodríguez-Franco M.I., López B., López M.G., García A.G., Carreiras Mdo.C., Villarroya M. Tacripyrines, the first tacrine-dihydropyridine hybrids, as multitarget-directed ligands for the treatment of Alzheimer’s disease. J. Med. Chem. 2009;52(9):2724–2732. doi: 10.1021/jm801292b. [DOI] [PubMed] [Google Scholar]
- 81.Fang J., Li Y., Liu R., Pang X., Li C., Yang R., He Y., Lian W., Liu A.L., Du G.H. Discovery of multitarget-directed ligands against Alzheimer’s disease through systematic prediction of chemical-protein interactions. J. Chem. Inf. Model. 2015;55(1):149–164. doi: 10.1021/ci500574n. [DOI] [PubMed] [Google Scholar]
- 82.Harvey A.L., Edrada-Ebel R., Quinn R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015;14(2):111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 83.Sun Z.K., Yang H.Q., Chen S.D. Traditional Chinese medicine: a promising candidate for the treatment of Alzheimer's disease. Transl. Neurodegeneration. 2013;2(1):6. doi: 10.1186/2047-9158-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheung T.S., Song T.H., Ng T.B., Wu F.H., Lao L.X., Tang S.C., Ho J.C., Zhang K.Y., Sze S.C. Therapeutic Effects of Herbal Chemicals in Traditional Chinese Medicine on Alzheimer’s Disease. Curr. Med. Chem. 2015;22(19):2392–2403. doi: 10.2174/0929867322666150520095509. [DOI] [PubMed] [Google Scholar]
- 85.Murray A.P., Faraoni M.B., Castro M.J., Alza N.P., Cavallaro V. Natural AChE Inhibitors from Plants and their Contribution to Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2013;11(4):388–413. doi: 10.2174/1570159X11311040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jia J.Y., Zhao Q.H., Liu Y., Gui Y.Z., Liu G.Y., Zhu D.Y., Yu C., Hong Z. Phase I study on the pharmacokinetics and tolerance of ZT-1, a prodrug of huperzine A, for the treatment of Alzheimer’s disease. Acta Pharmacol. Sin. 2013;34(7):976–982. doi: 10.1038/aps.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei G., Xiao S., Lu R., Liu C. Simultaneous determination of ZT-1 and its metabolite Huperzine A in plasma by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006;830(1):120–125. doi: 10.1016/j.jchromb.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 88.Bansode S. B., Jana A. K., Batkulwar K. B., Warkad S. D., Joshi R. S., Sengupta N., Kulkarni M. J. Molecular Investigations of Protriptyline as a Multi-Target Directed Ligand in Alzheimer's Disease. Plos One. 2014;9(8) doi: 10.1371/journal.pone.0105196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duan S., Guan X., Lin R., Liu X., Yan Y., Lin R., Zhang T., Chen X., Huang J., Sun X., Li Q., Fang S., Xu J., Yao Z., Gu H. Silibinin inhibits acetylcholinesterase activity and amyloid β peptide aggregation: a dual-target drug for the treatment of Alzheimer’s disease. Neurobiol. Aging. 2015;36(5):1792–1807. doi: 10.1016/j.neurobiolaging.2015.02.002. [DOI] [PubMed] [Google Scholar]


