Abstract
Alemtuzumab is a monoclonal antibody that depletes T and B cells and is used as induction therapy for renal transplant recipients. Without long-term calcineurin inhibitor (CNI) therapy, alemtuzumab-treated patients have a propensity to develop alloantibody and may undergo antibody-mediated rejection (AMR). In pursuit of a mechanistic explanation, we analyzed peripheral B cells and serum of these patients for BAFF (Blys) and BAFF-R, factors known to be integral for B-cell activation, survival, and homeostasis. Serum BAFF levels of 22/24 alemtuzumab-treated patients were above normal range, with average levels of 1967 pg/mL compared to 775 pg/mL in healthy controls (p = 0.006). BAFF remained elevated 2 years posttransplant in 78% of these patients. BAFF-R on CD19+ B cells was significantly downregulated, suggesting ligand/receptor engagement. BAFF mRNA expression was increased 2–7-fold in CD14+ cells of depleted patients, possibly linking monocytes to the BAFF dysregulation. Addition of recombinant BAFF to mixed lymphocyte cultures increased B-cell activation to alloantigen, as measured by CD25 and CD69 coexpression on CD19+ cells. Of note, addition of sirolimus (SRL) augmented BAFF-enhanced B-cell activation whereas CNIs blocked it. These data suggest associations between BAFF/BAFF-R and AMR in alemtuzumab-treated patients.
Keywords: Alemtuzumab, Campath-1H, BAFF, sirolimus, renal transplant
Introduction
Therapy with the CD52-specific monoclonal antibody alemtuzumab (Campath-1H) results in profound depletion of T and B cells in the peripheral blood. T cells are slow to replete, with CD4+ lymphocytes taking up to 3 years to achieve 50% baseline levels (1). In contrast, CD20+ B lymphocyte levels usually recover by 1 year often exceeding baseline (1,2). Monocytes are depleted less efficiently and repopulate in 2–3 months (1–3).
Alemtuzumab-treated renal transplant patients receiving maintenance sirolimus (SRL) therapy have an increased frequency of antibody-mediated rejection (AMR) (4–7), have a propensity to develop donor-specific alloantibody (4,5), and may be at increased risk for chronic allograft injury (8). The incidence of AMR can be reduced by the use of calcineurin inhibitors (CNIs) in combination with or in place of SRL (5,6,9,10). However, patients withdrawn from CNI maintenance after alemtuzumab induction tend to have a humoral component to their rejection episodes (6).
The increased incidence of AMR in alemtuzumab-depleted patients suggests a dysregulation of B-cell activation. The concurrent depletion of regulatory cells of either the T- or B-cell lineage may very well play a role in aberrant activation. In addition, abnormal expression of factors affecting B-cell survival and homeostasis are logical suspects. Two such factors are B-cell activating factor BAFF/Blys and APRIL (a proliferation-induced ligand), both members of the TNF-ligand family (11–15). Increases in BAFF expression can enhance B-cell survival, heighten B-cell proliferation and lower BCR activation thresholds to autologous and foreign antigens (11–16). As such, BAFF transgenic mice develop pathology similar to SLE and Sjogren’s syndrome (SS) (17–20). In accordance with this, increases in BAFF have been identified in patients with SLE and SS and found to be associated with autoantibody production (21–23).
BAFF is expressed in both membrane-bound and soluble form (11,13). The primary sources of BAFF in the periphery are monocytes and neutrophils (11,13). Nonmyeloid cells such as bone marrow stromal cells, astrocytes, and activated lymphocytes also produce BAFF (11,13,16,24–26). BAFF is the sole known ligand for the BAFF receptor (BAFF-R), which is preferentially expressed on B cells (13). T cells also express BAFF-R that upon engagement acts to costimulate T-cell activation (16,27–29). TACI and BCMA are receptors for APRIL and BAFF. TACI is expressed on many cell types including activated monocytes, T cells and B cells (30). Recent studies have shown that BCMA expression is mainly limited to plasma cells and appears to control their survival (12,13).
With the aim of understanding alloantibody responses in alemtuzumab-depleted patients, we analyzed their sera for expression of APRIL and BAFF. Interestingly, we found abnormally high levels of BAFF but not APRIL in these patients, as well as changes in expression of BAFF and BAFF-R on peripheral B cells. In vitro experiments demonstrate the power of BAFF in allogeneic B-cell responses. How these findings might impact the allo- and autoimmune B-cell responses in vivo are discussed.
Materials and Methods
Patients
Forty patients and 7 healthy controls were enrolled under IRB-approved protocols at the University of Wisconsin-Madison after informed consent regarding the nature of the study.
All patients were recipients of primary kidney allografts. Male or female patients aged 18–75 years who had received induction with alemtuzumab, and at least 2 months of CNI therapy, MMF/EC-MPS, and prednisone were enrolled. All 40 patients were enrolled posttransplant. Twenty-six of the 40 patients were enrolled between 2 and 4 months posttransplant/postdepletion.
Thirty-six of the 40 patients enrolled in the study received one 30-mg dose of alemtuzumab on the day 0 posttransplant. Four patients received two 30-mg doses of alemtuzumab but were not included in our analysis because their enrollment was a year or more posttransplant and we wished to focus on those that were enrolled early posttransplant. Steroid treatment consisted of 500 mg of methylprednisolone on day 0, 250 mg IV on day 1 and 10 mg of prednisone orally on day 3 and thereafter. Corticosteroid dosing was reduced to 5–7.5 mg/day if the patient in either treatment group remained rejection-free at 6 months poststudy enrollment.
Patients were receiving maintenance MMF (i.e., 500 mg BID) or EC-MPS (i.e., 360 mg BID) at the time of study enrollment. Postenrollment, patients were randomized for CNI withdrawal by 1 month, but continued MMF/EC-MPS dosing, as tolerated, up to a maximum of 1000/720 mg BID, respectively.
The six nondepleted patients in this study received anti-CD25 (Basiliximab, Novartis, East Hanover, NJ) induction therapy, along with conventional long-term immunosuppression (CsA, steroids and MMF).
ELISAs
Blood was collected in tubes without anticoagulant, spun at 2000 rpm for 10 min. Serum was collected and frozen in liquid nitrogen long term. BAFF serum levels were detected using a BAFF Quantikine Immunoassay (R&D Systems, Minneapolis, MN). APRIL ELISA kits were purchased by Bender MedSystems, Inc. (Burlingame, CA). Standards and sera were assayed in duplicate wells. Sera from normal individuals were always run alongside patient sera.
QPCR
CD14+ cells were purified from frozen PBMCs by using CD14+ microbeads for positive selection via AutoMacs separation (Miltenyi Biotec, Auburn, CA). Total mRNA was purified using the SV Total RNA Isolation Kit (Promega Corporation, Madison, WI). PCR primers for BAFF and BAFF-R were purchased from Qiagen (Valencia, CA) as were Quantitect SYBR Green RT-PCR kits for one step RT-QPCR. We chose to use a Roche Lightcycler for RT-QPCR.
Flow cytometry
PBMCs were isolated via Ficoll and stored in LN2 until time of flow analysis. All time points were run together for each patient, and normal PBMCs were run together with patient samples for consistency. Cell surface proteins were detected by flow cytometry by using standard (BD Bioscience) protocols. Labeled antibodies used are as follows: FITC anti-BAFF-R, clone 8A7 (eBioscience, San Diego, CA); PE-anti-TACI, clone 11H3, eBioscience; FITC-anti-BCMA, pAb, R&D Systems; FITC-anti-BAFF, clone 1D6, eBioscience; PE-anti-CD25, clone M-A251 (BD Biosciences, San Jose, CA); APC-anti-CD69, clone FN50, BD Biosciences; PerCP-anti-CD19, clone 4G7, BD Biosciences; APC-anti-CD14, clone M5E2, BD Biosciences; PE-anti-CD3, clone HIT3a, BD Biosciences.
Luminex analysis for cytokines and HLA-specific antibodies
Fluorescent bead technology was utilized to assay 50 μL of cell culture supernatants for cytokine levels by using the TH1/TH2 human cytokine 9-plex kit (BioRad, Inc., Hercules, CA). Fluorescence was detected using the Bio-Plex 200 (BioRad, Inc.). In addition, Luminex technology was used to detect single antigen specific anti-HLA antibody for both class I and class II (LabScreen; One Lambda, Inc., Canoga Park, CA) for the patients with rejection episodes.
In vitro BAFF/MLR assay
Mixed lymphocyte reactions by using PBMCs from normal healthy individuals were set up as follows. In 48-well plates, 200 μL of responder PBMCs (1×106 cells) were added to 200 μL of stimulator PBMCs (1 × 106 cells), which had been irradiated with 3000 rads. Increasing concentrations of recombinant human (rh)BAFF (R&D Systems) was immediately added in 100 μL volume for a total of 500 μL. In immunosuppression experiments, 50 μL of each drug (tacrolimus, Fujisawa, Deerfield, IL; rapamycin/sirolimus, BioMol, Plymouth Meeting, PA) was added along with 50 μL of rhBAFF to maintain the 500-μL volume. Cells were cultured for 4 days, after which supernatants were collected and cells prepared for flow cytometry analysis. Four different responder–donor pairs were analyzed in four separate experiments, all with similar results.
Statistical methods
Statistical differences in serum BAFF levels between groups were determined via an unpaired two-tailed t-test. Pearson correlation coefficients were generated to test whether an association exists between BAFF levels versus CD20+ B-cell levels, and BAFF levels versus monocyte BAFF mRNA levels. An unpaired two-tailed t-test was also used to determine the significance for differences in BAFF and BAFF-R levels between BAFFmed and BAFFhigh groups. An unpaired two-tailed t-test was also used to determine the significance of activated B-cell percentages versus addition of exogenous BAFF.
To correlate patient demographics and BAFF levels, Pearson correlation coefficients (r) were calculated for continuous variables age, PRA and number of HLA-mismatches. R2 coefficients were calculated for discrete/categorical variables gender, cause of ESRD, donor source and maintenance immunosuppression. Mean BAFF concentrations along with standard deviations were calculated for all categorical variables (Table 1). Patients samples used for this analysis were taken at the month 6 postenrollment time point.
Table 1.
Correlation of patient demographics with BAFF levels
| Demographic variables | Correlation to BAFF (r) | Correlation to BAFF (R2) | Mean BAFF [pg/mL] (+/−SD) |
|---|---|---|---|
| Age | 0.072 | N/A | N/A |
| Gender (M/F) | N/A | 0.0016 | |
| • M (n = 16) | 1688 (715) | ||
| • F (n = 5) | 1760 (1077) | ||
| Cause of ESRD | N/A | 0.177 | |
| • DM (n = 6) | 1674 (558) | ||
| • Chronic GN (n = 7) | 1413 (540) | ||
| • HTN (n = 1) | 1487 | ||
| • PCK (n = 4) | 2325 (1268) | ||
| • Others (n = 3) | 1694 (1003) | ||
| PRA (peak/historical) | −0.21 | N/A | N/A |
| HLA mismatches | N/A | N/A | |
| • HLA-A | 0.15 | ||
| • HLA-B | 0.20 | ||
| • HLA-DR | 0.16 | ||
| • Total | 0.20 | ||
| Donor source | N/A | 0.125 | |
| • DD (n = 13) | 1857 (907) | ||
| • LRD (n = 6) | 1280 (395) | ||
| • LURD (n = 2) | 1992 (372) | ||
| Maintenance | N/A | 0.074 | |
| Immunosuppression | |||
| • CNI/MMF/STR (n = 9) | 1945 (731) | ||
| • MMF/STR (n = 12) | 1524 (806) | ||
N/A = not applicable.
Results
Serum BAFF levels are increased in alemtuzumab-treated patients
The patients enrolled in this trial were treated with a single 30-mg dose of alemtuzumab, and until enrollment were maintained on CNI and MMF/EC-MPS. Enrollment varied between 2 and 16 months after transplant. The vast majority of depleted patients were enrolled 2–4 months post-transplant/postdepletion. At the time of enrollment, half were randomized for CNI withdrawal but all patients were maintained on MMF/EC-MPS. Serum samples were taken at the time of enrollment and at 6, 12 and 24 months thereafter.
Serum BAFF was analyzed for three control groups: healthy individuals, renal transplant patients’ day 1 pretransplant and nondepleted transplant patients. The latter cohort was analyzed 6–12 months after transplant and had been treated on day 0 with anti-CD25 (basiliximab) therapy and maintenance IS of CNI, MMF and prednisone. Finally, six alemtuzumab-treated patients were chosen because they had been enrolled at 6–12 months posttransplant and had been maintained on CNI and MMF/EC-MPS up to the time of enrollment. In this way, the maintenance immunosuppression between the nondepleted and depleted patients were comparable.
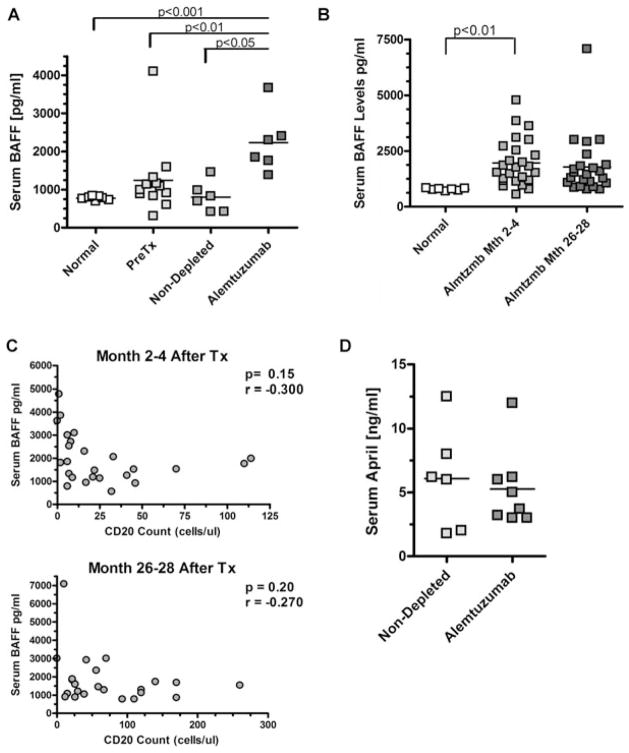
As shown in Figure 1A, levels of serum BAFF in seven healthy individuals ranged between 700 and 800 pg/mL with little variation (SD = 49.8 pg/mL). For 12 patients’ pretransplant, mean values were 1245 pg/mL (SD = 226 pg/mL). Mean BAFF levels of six nondepleted control patients were 801 pg/mL (SD = 160 pg/mL). In contrast, serum BAFF levels in six alemtuzumab-depleted patients were elevated to 2228 pg/mL (SD = 325 pg/mL), differing significantly from healthy individuals (p = 0.0005), patients pretransplant (p = 0.003) and control patients (p = 0.046).
Figure 1. BAFF but not APRIL levels are elevated in alemtuzumab-treated transplant patients.
(A) Serum BAFF levels measured by ELISA for the following groups: healthy control individuals, renal transplant patients pretransplant, nondepleted transplant patients treated with anti-CD25 induction therapy (measured 6 months posttransplant), depleted transplant patients treated with alemtuzumab (measured at 6 months posttransplant). Statistics performed via two-tailed t-test. (B) Long-term BAFF levels of alemtuzumab treated renal transplant cohorts 2–4 months posttransplant and 24 months later. Absolute CD20 counts (X-axis) at the 2–4 month time point and 24 months later. Pearson correlation coefficients were generated to test whether an association exists between BAFF levels and CD20+ B-cell levels. (D) Serum APRIL levels measured by ELISA for nondepleted transplant patients treated with anti-CD25 induction therapy (measured 6 months posttransplant) and depleted transplant patients treated with alemtuzumab (measured at 6 months posttransplant). Statistics performed via unpaired two-tailed t-test.
We next analyzed serum BAFF levels to gauge whether the increases were transient or maintained long term. The majority of patients in this study (26/40) were enrolled 2–4 months posttransplant. Thus, to try and maximize the number of patients for analysis while imparting some uniformity for the posttransplant time frame, we analyzed sera that were collected at this enrollment time point and then 24 months later (or 26–28 months posttransplant).
At 2–4 months, serum BAFF levels were increased with a mean of 1967 pg/mL (SD = 1054 pg/mL) compared to a mean of 775 pg/mL for healthy controls (p = 0.006, Figure 1B). Two years later (26–28 months posttransplant) BAFF levels in alemtuzumab-depleted patients were relatively unchanged, with a mean of 1792 pg/mL (SD = 1342 pg/mL, p = 0.35). Importantly, at the latter time point, one-half of these patients had been withdrawn from their CNI for almost 2 years. However, there was no significant difference in BAFF levels between the two cohorts of patients (Table 1), suggesting that serum BAFF concentrations were not influenced by CNIs. Furthermore, BAFF levels did not correlate significantly with patient demographics such as ESRD, age, gender, donor source, PRA, individual number of HLA-A, -B, -DR mismatches or total number of HLA mismatches (Table 1). All patients received primary allografts and therefore the number of previous transplants was not a factor in degree of BAFF increase. Together, these data strongly suggest that alemtuzumab-mediated depletion induces the BAFF increase in these patients and that factors other than demographics determine the extent of this dysregulation.
A principal receptor for BAFF in the peripheral blood is BAFF-R, expressed primarily on B cells (13). To examine whether increased soluble BAFF in immune-depleted patients was simply a result of the lack of available BAFF-R because of B-cell depletion, we looked for an inverse correlation between BAFF levels and absolute peripheral B-cell numbers. Figure 1C shows BAFF levels at 2–4 and 26–28 months postdepletion and the absolute CD20 counts in the peripheral blood. At both time points, extent of B-cell depletion did not appear to correlate with BAFF levels (correlation coefficients r = 0.026 for 2–4 months, r = 0.060 for 26–28 months).
We tested if APRIL, a ligand closely related to BAFF (13), was also increased in patients treated with alemtuzumab. Notably, serum levels of APRIL were not significantly different between depleted and nondepleted patients 6 months posttransplant (Figure 1D, p = 0.66). Both were within range of previously reported levels in healthy individuals (31,32).
BAFF expression is increased in CD14+ monocytes
BAFF can be expressed as a membrane-bound molecule on monocytes, which is cleaved by a furin-like protease to produce soluble BAFF (11). In an attempt to find a possible source for increased BAFF production, we isolated mRNA from the CD14+ monocytes of alemtuzumab patients with serum BAFF levels ranging from 1000 pg/mL to greater than 5000 pg/mL, at months 2–4 and 26–28 posttransplant. Monocyte mRNA was also isolated from four normal controls. RT-QPCR was subsequently performed with BAFF primers. As shown in Figure 2A, BAFF mRNA was expressed at 2–7-fold higher levels than normal controls. Interestingly, at the early time point, BAFF mRNA levels in CD14+ cells correlated with serum BAFF levels (Figure 2B), suggesting that the dysregulation of BAFF may at least in part is due to monocytes. At the 2-year time point, BAFF mRNA levels did not appear to correlate with serum BAFF since patients with low serum BAFF levels had an increase in monocyte BAFF mRNA (data not shown).
Figure 2. BAFF mRNA is increased in monocytes of alemtuzumab patients.
(A) RT-QPCR performed for eight patients at 2–4 months posttransplant and four patients 26–28 months post-transplant. (B) Correlation between BAFF mRNA levels and serum BAFF levels in the eight patients measured at the 2–4 month time point. Pearson correlation coefficients were generated to test whether an association exists between BAFF levels and monocyte BAFF mRNA levels. (C) MCF of BAFF on the monocytes of normal, BAFFmed and BAFFhigh alemtuzumab patients at early and late posttransplant time points. Statistics performed via unpaired two-tailed t-test.
To determine whether increased BAFF mRNA translated to cell surface BAFF, we performed flow cytometry on CD14+ monocytes of a subset of patients with a range of serum BAFF levels. Although we saw a shift in cell surface BAFF expression in a subset of patients at the later time point (Figure 2C), this increase was not significant overall. This suggests that the increases in BAFF protein may result mostly in the soluble and not the membrane-bound form.
BAFF, BAFF-R, TACI, and BCMA expression on lymphocytes
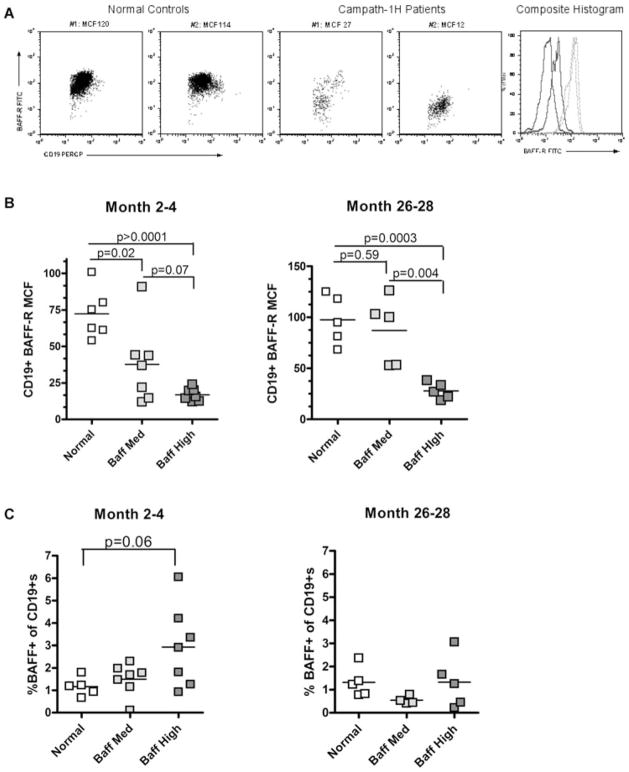
We further examined whether expression of BAFF, BAFF-R, TACI, and BCMA were altered on the cell surface of repopulating lymphocytes of alemtuzumab-treated patients. To that end, and with the use of flow cytometry, we analyzed the expression of these molecules on CD19+ and CD3+ cell subsets from patients who had a wide range of BAFF levels at 2–4 months and 26–28 months posttransplant. PBMCs from normal individuals (BAFF levels below 800 pg/mL) served as controls.
For CD3+ cells, the expression of BAFF, BAFF-R and TACI were not significantly different from that of healthy controls (data not shown). For CD19+ B cells, TACI and BCMA expression was comparable to normal controls (data not shown). However, the expression of BAFF-R was dramatically altered. Figure 3A depicts representative dot plots and histograms of lymphocyte-gated CD19+ cells for their expression of BAFF-R for two healthy controls and two alemtuzumab-treated patients at 2–4 months posttransplant. The expression of BAFF-R of the healthy controls shown had a mean channel fluorescence (MCF) of 114 and 120. In the depleted patients, the MCF of BAFF-R was 12 and 27. When separated between BAFFmed (900–1000 pg/mL) versus BAFFhigh (>2500 pg/mL) groups, BAFF-R was significantly downregulated in the BAFFhigh patients compared to healthy controls at both the months 2–4 and 26–28 time points (p = 0.0003 and p < 0.0001 respectively; Figure 3B). Importantly, there was a significant difference in BAFF-R downregulation at month 26–28 between BAFFmed and BAFFhigh patients (p = 0.004). BAFF-R QPCR revealed no differences between normal controls and alemtuzumab-treated patients (data not shown), suggesting that downregulation is controlled at the posttranscriptional level.
Figure 3. BAFF-R is downregulated on peripheral CD19± lymphocytes.
(A) Representative dot plots of BAFF-R expression (Y-axis) in two healthy control individuals and two alemtuzumab (campath-1H)-treated patients at M2–4 posttransplant. CD19+ cells (X-axis) are derived from the lymphocyte gate. Composite histograms are also shown: gray lines are healthy controls and black lines are depleted patients. (B) BAFF-R expression on CD19+ cells of patients that are BAFFmed (900–1000 pg/mL) versus BAFFhigh (>2500 pg/mL) at the M2–4 and M26–28 posttransplant time points. (C) The percent of CD19+ cells in the lymphocyte gate which is BAFF+, at both the early and late time points posttransplant. All statistics performed via an unpaired two-tailed t-test.
Increases in surface BAFF on B cells can be an indication of B-cell activation (33). Analysis of BAFF expression on B cells of alemtuzumab-depleted patients revealed that at the early 2–4 months posttransplant time point, the percent of CD19+BAFF+ cells increased only in the BAFFhigh patients, approaching statistical significance (p = 0.06, Figure 3C). However, BAFF expression on B cells returned to normal levels 2 years thereafter (Figure 3C).
BAFF increases allo-B-cell activation in vitro and may act synergistically with SRL
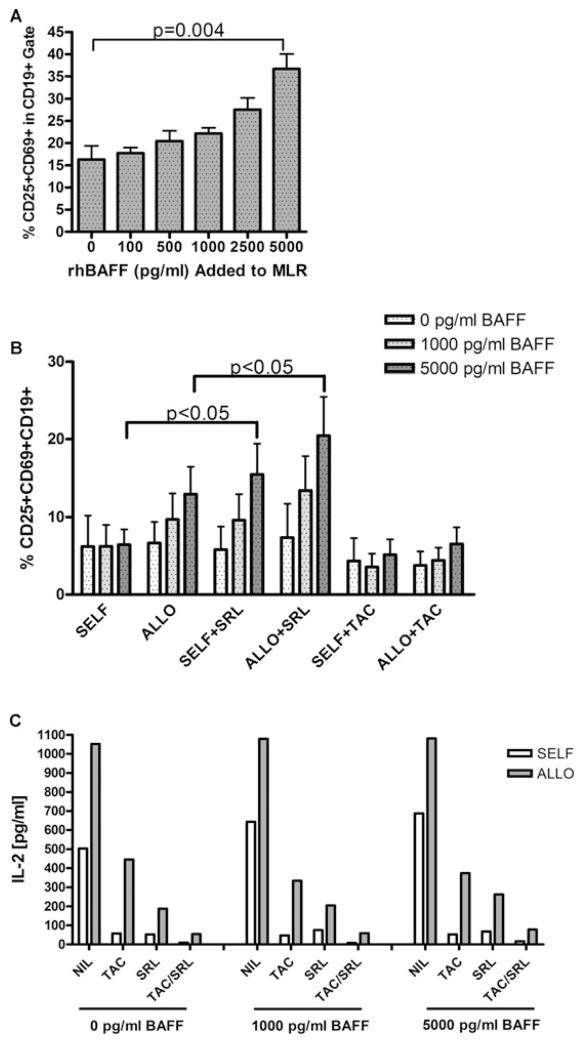
Since the effects of high BAFF levels on allo-specific B cells were not possible to thoroughly address in vivo, we asked what the consequences of increased BAFF concentrations would be in the context of alloantigen in vitro. We performed one-way mixed lymphocyte reactions in the presence of increased exogenous rhBAFF (0–5000 pg/mL) using HLA-disparate responder/stimulator PBMCs from four different pairs of healthy individuals. On day 4, activation markers CD25 and CD69 were analyzed on B cells by flow cytometry. As shown in Figure 4A, the percentage of CD25+CD69+ double positive CD19+ B cells increased significantly by adding rhBAFF, more than doubling at the 5000 pg/mL rhBAFF concentration (p = 0.004).
Figure 4. Exogenous rhBAFF increases the percentage activated CD19± lymphocytes in MLR; SRL augments this effect.
(A) Percent of activated lymphocytes after 4-day one-way MLR with increasing concentrations of exogenous recombinant human BAFF. Activation is measured as the percentage CD25+CD69+ double positives within the CD19+ cells of the lymphocyte gate. These data are the combination of four responder/stimulator pairs. Statistics were performed via a two-tailed t-test. (B) Comparison of self (autologous) versus allogeneic B-cell stimulation with exogenous rhBAFF and immunosuppressive agents SRL or TAC. Activation is measured by percentage CD25+CD69+ double positives within the CD19+ cells of the lymphocyte gate. Statistics were performed via an unpaired two-tailed t-test. (C) IL-2 T-cell cytokine analysis of MLR supernatants of experiments from (B). Both cytokines, as well as a host of others, are effectively downregulated by both immunosuppressants.
We further examined the effects of tacrolimus (TAC) and SRL on BAFF-mediated B-cell activation. We performed four separate MLR experiments using different responder/stimulator pairs. At concentrations of 10 ng/mL, TAC was effective at suppressing B-cell activation even at high BAFF levels of 5000 pg/mL (Figure 4B). However, SRL (10 ng/mL) was ineffective at halting BAFF-mediated increases in alloactivation of B cells and tended to augment BAFF-enhanced B-cell activation (p = 0.048). The activation of autologous B cells was likewise augmented in the presence of SRL (p = 0.031). As for the combination of TAC and SRL, B-cell activation did not significantly increase, suggesting that CNIs override the SRL-induced effects of BAFF (data not shown). Thus, BAFF increased allostimulated B-cell activation, and SRL may augment BAFF-mediated increases in allo-specific B-cell activation.
Cytokine multiplex analysis of tissue culture supernatants on day 4 of the MLR revealed that the addition of exogenous BAFF did not increase T-cell cytokines. Importantly, however, both TAC and SRL reduced IL-2, IFN-γ, GM-CSF, IL-13, and IL-10 (shown is IL-2, Figure 4C). This demonstrates that both immunosuppressants effectively dampened T-cell activation in culture and that SRL specifically effected BAFF-enhanced B lymphocyte activation.
Finally, in this cohort of alemtuzumab-treated patients, CNI was withdrawn for half of the patients. There was a higher incidence of biopsy-proven rejection in those patients withdrawn from their CNI than for those maintained on CNI (4/20 versus 0/20, respectively). Table 2 displays the levels of BAFF at enrollment for whom we had serum samples (three of the four patients). All had higher than normal levels of BAFF at enrollment, ranging from 1160 pg/mL to 1533 pg/mL. One patient (Pt. C) had a relatively early rejection episode at day 46 after enrollment. We had obtained sera at day 30 after enrollment for this patient, at which time BAFF levels were very high (3800 pg/mL). Three of the four biopsies were positive for C4d suggestive of AMR. Furthermore, using single antigen detection for alloantibody, the three patients tested were all positive for both Class I and Class II donor-specific antibody (DSA) at the time of rejection (Table 2).
Table 2.
Alemtuzumab-induced patients presenting with biopsy-proven rejection
| Patient | CNI1 withdrawal | Time from CNI withdrawal to rejection (days) | BAFF levels at withdrawal (pg/mL)2 | DSA class I | DSA class II | C4d+ |
|---|---|---|---|---|---|---|
| A | Yes | 97 | ND | ND | ND | Yes |
| B | Yes | 433 | 1160 | Yes | Yes | Yes |
| C | Yes | 46 | 1257 | Yes | Yes | No |
| D | Yes | 272 | 1533 | Yes | Yes | Yes |
ND = not done.
Patients were withdrawn from CNI on the day of enrollment.
Normal BAFF levels (775 SD+/−50 pg/mL).
Discussion
Alemtuzumab (Campath-1H) is being increasingly utilized for renal transplantation (34) yet little is understood about its effects on humoral alloreactivity. In this study, we have attempted to find anomalies in the environment in which B cells are repopulating after alemtuzumab-mediated immune depletion. We found that serum concentrations of the B-cell activating factor BAFF (Blys) are significantly increased in most alemtuzumab-treated patients and that BAFF levels can remain elevated long term.
Unlike the tight regulation in BAFF levels in our normal control individuals, levels in depleted patients are unusually high and wide range. Although alemtuzumab-induction therapy appears to mediate the increase in serum BAFF levels, it is apparent that the increase is not solely due to decreased numbers of B cells. In our study, BAFF levels did not fully correlate with peripheral B-cell levels, and alemtuzumab-treated patients with very similar peripheral B-cell levels could have dramatically different levels of BAFF. This suggests that BAFF production is dysregulated in these patients.
BAFF levels in alemtuzumab-treated patients did not correlate significantly with patient demographics. Importantly, serum BAFF levels appear to be independent of the presence of CNIs, as those patients on CNI’s did not have significantly different levels of BAFF than those withdrawn from CNI’s. Likewise, MMF/EC-MPS and steroids are unlikely to have caused an increase in BAFF, as the nondepleted control patients had been maintained on these drugs as well, and did not demonstrate increases in BAFF levels. Whether or not SRL alters BAFF levels has yet to be determined. However, the conclusion supported by our data is that alemtuzumab-mediated depletion can increase serum BAFF concentrations.
We hypothesize that monocytes are one possible source of increased BAFF production in these patients as BAFF mRNA expression was increased in CD14+ cells. Activation of monocytes via interferons or Fcγ R cross-linking has been shown to induce BAFF production, inducing its release from intracellular stores as well as the release from the cell surface (35,36). We speculate that alemtuzumab may play a role in activating monocytes, perhaps via the cross-linking of Fc receptors and/or via cytokine release. In addition, B-cell depletion may also perturb the balance between BAFF production and BAFF utilization, and perhaps dysregulate an autocrine-like negative feedback loop. As such, once BAFF production is triggered by such overwhelming factors, it may not be able to be turned off efficiently. This may explain why in many alemtuzumab-treated patients’ BAFF levels remain elevated long term.
We did not definitively prove that BAFF is a key factor in allo-specific B-cell activation in our patients. However, the correlation of our in vitro data with what is witnessed clinically is intriguing. For example, alemtuzumab-treated patients maintained on SRL monotherapy had a high incidence of AMR (5,8) while those maintained on CNIs did not (6,8,10,34). Our in vitro data support these findings, as BAFF in the presence of CNI had little effect on B-cell activation, whereas BAFF was able to act synergistically with SRL to augment B-cell activation. Furthermore, alemtuzumab-treated patients withdrawn from CNI maintenance tend to have a humoral component to their rejection episode (6). We show that the patients with rejection episodes had higher than normal levels of BAFF before withdrawal and had DSA in their serum at the time of rejection. Future studies will attempt to correlate long-term BAFF levels with donor-specific alloantibody production and whether or not CNI’s can regulate allo-specific B cells in the presence of high BAFF levels.
A key question remains as to whether BAFF-augmented activation is a mediator of alloantibody production in alemtuzumab-treated patients maintained on SRL. This would require that B cells are allowed to proliferate and differentiate in the presence of SRL, which should be prevented by this drug under normal circumstances (37). However, SRL-induced immunosuppression may be different in a lymphopenic environment in which homeostatic proliferation is increased. Furthermore, SRL may not be able to effectively control memory cells that are spared by alemtuzumab.
Interestingly, average BAFF levels in alemtuzumab patients were in range of levels found in the serum of patients with SLE and Sjogren’s syndrome (11,15,17,19,38). Alemtuzumab mediated depletion has been associated with autoimmune syndromes such as Grave’s disease, autoimmune hemolytic anemia and ITP (39–42). Increased BAFF levels may offer an explanation for these observations.
Flow analyses of the receptors for BAFF were informative as to the actual implications of high BAFF levels. On B cells, the BAFF-R was significantly more downregulated in BAFFhigh patients than BAFFmed, suggesting that the BAFF-R is engaged by ligand. This may have physiological consequences. For example, BAFF-R downregulation has likewise been shown in patients with SLE and primary Sjogren’s syndrome (44,45) and interestingly, it was receptor downregulation more so than BAFF levels that were associated with disease activity.
In conclusion, serum BAFF levels are increased significantly in alemtuzumab-treated kidney allograft patients. These data suggest a mechanism for allo-specific B-cell activity if proper maintenance immunosuppression is not used. It will be of interest to see whether currently available anti-BAFF therapies may assist in curtailing alloantibody production in these patients.
Acknowledgments
The authors wish to thank clinical coordinators Christine Lillesand, Chris Janus, Nancy Radke, and Aimee Sundberg for their assistance and expertise. We also wish to extend our gratitude to Dr. Allan Kirk for critical review of the manuscript. This work was funded by NIH Grant #R01 AI050938-03.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest.
References
- 1.Bloom DD, Hu H, Fechner JH, Knechtle SJ. T-lymphocyte alloresponses of Campath-1H-treated kidney transplant patients. Transplantation. 2006;81:81–87. doi: 10.1097/01.tp.0000191940.13473.59. [DOI] [PubMed] [Google Scholar]
- 2.Noris M, Casiraghi F, Todeschini M, et al. Regulatory T cells and T cell depletion: Role of immunosuppressive drugs. J Am Soc Nephrol. 2007;18:1007–1018. doi: 10.1681/ASN.2006101143. [DOI] [PubMed] [Google Scholar]
- 3.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120–129. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 4.Cai J, Terasaki PI, Bloom DD, et al. Correlation between human leukocyte antigen antibody production and serum creatinine in patients receiving sirolimus monotherapy after Campath-1H induction. Transplantation. 2004;78:919–924. doi: 10.1097/01.tp.0000134398.86243.81. [DOI] [PubMed] [Google Scholar]
- 5.Knechtle SJ, Pirsch JD, Fechner H, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: Results of a pilot study. Am J Transplant. 2003;3:722–730. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 6.Pascual J, Bloom D, Torrealba J, et al. Calcineurin inhibitor withdrawal after renal transplantation with alemtuzumab: Clinical outcomes and effect on T-regulatory cells. Am J Transplant. 2008;8:1529–1536. doi: 10.1111/j.1600-6143.2008.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascual J, Pirsch J, Torrealba J, et al. Antibody-mediated rejection (AMR) in renal allograft recipients treated with alemtuzumab induction. Am J Transplant. 2008;8:237. [Google Scholar]
- 8.Ruggenenti P, Perico N, Gotti E, et al. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation. 2007;84:956–964. doi: 10.1097/01.tp.0000284808.28353.2c. [DOI] [PubMed] [Google Scholar]
- 9.Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351:1701–1702. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 10.Matsushita T, Fujimoto M, Hasegawa M, et al. Elevated serum APRIL levels in patients with systemic sclerosis: Distinct profiles of systemic sclerosis categorized by APRIL and BAFF. J Rheumatol. 2007;34:2056–2062. [PubMed] [Google Scholar]
- 11.Daridon C, Youinou P, Pers JO. BAFF, APRIL, TWE-PRIL: Who’s who? Autoimmun Rev. 2008;7:267–271. doi: 10.1016/j.autrev.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: A tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 13.Bossen C, Schneider P. BAFF, APRIL and their receptors: Structure, function and signaling. Semin Immunol. 2006;18:263–275. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Woodland RT, Schmidt MR, Thompson CB. BLyS and B cell homeostasis. Semin Immunol. 2006;18:318–326. doi: 10.1016/j.smim.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: Fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–336. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–289. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pers JO, Daridon C, Devauchelle V, et al. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann N Y Acad Sci. 2005;1050:34–39. doi: 10.1196/annals.1313.004. [DOI] [PubMed] [Google Scholar]
- 20.Stohl W, Xu D, Kim KS, et al. BAFF overexpression and accelerated glomerular disease in mice with an incomplete genetic predisposition to systemic lupus erythematosus. Arthritis Rheum. 2005;52:2080–2091. doi: 10.1002/art.21138. [DOI] [PubMed] [Google Scholar]
- 21.Mariette X, Roux S, Zhang J, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren’s syndrome. Ann Rheum Dis. 2003;62:168–171. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stohl W, Metyas S, Tan SM, et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: Longitudinal observations. Arthritis Rheum. 2003;48:3475–3486. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Roschke V, Baker KP, et al. Cutting edge: A role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 24.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198:937–945. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krumbholz M, Theil D, Derfuss T, et al. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med. 2005;201:195–200. doi: 10.1084/jem.20041674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaumann DH, Tuischer J, Ebell W, Manz RA, Lauster R. VCAM-1-positive stromal cells from human bone marrow producing cytokines for B lineage progenitors and for plasma cells: SDF-1, flt3L, and BAFF. Mol Immunol. 2007;44:1606–1612. doi: 10.1016/j.molimm.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Huard B, Schneider P, Mauri D, Tschopp J, French LE. T cell costimulation by the TNF ligand BAFF. J Immunol. 2001;167:6225–6231. doi: 10.4049/jimmunol.167.11.6225. [DOI] [PubMed] [Google Scholar]
- 28.Huard B, Arlettaz L, Ambrose C, et al. BAFF production by antigen-presenting cells provides T cell co-stimulation. Int Immunol. 2004;16:467–475. doi: 10.1093/intimm/dxh043. [DOI] [PubMed] [Google Scholar]
- 29.Mackay F, Mackay CR. The role of BAFF in B-cell maturation, T-cell activation and autoimmunity. Trends Immunol. 2002;23:113–115. doi: 10.1016/s1471-4906(01)02159-7. [DOI] [PubMed] [Google Scholar]
- 30.Mackay F, Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008;19:263–276. doi: 10.1016/j.cytogfr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Jonsson MV, Szodoray P, Jellestad S, Jonsson R, Skarstein K. Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjogren’s syndrome. J Clin Immunol. 2005;25:189–201. doi: 10.1007/s10875-005-4091-5. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe R, Fujimoto M, Yazawa N, et al. Increased serum levels of a proliferation-inducing ligand in patients with bullous pemphigoid. J Dermatol Sci. 2007;46:53–60. doi: 10.1016/j.jdermsci.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Chu VT, Enghard P, Riemekasten G, Berek C. In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J Immunol. 2007;179:5947–5957. doi: 10.4049/jimmunol.179.9.5947. [DOI] [PubMed] [Google Scholar]
- 34.Morris PJ, Russell NK. Alemtuzumab (Campath-1H): A systematic review in organ transplantation. Transplantation. 2006;81:1361–1367. doi: 10.1097/01.tp.0000219235.97036.9c. [DOI] [PubMed] [Google Scholar]
- 35.Lavie F, Miceli-Richard C, Ittah M, Sellam J, Gottenberg JE, Mariette X. B-cell activating factor of the tumour necrosis factor family expression in blood monocytes and T cells from patients with primary Sjogren’s syndrome. Scand J Immunol. 2008;67:185–192. doi: 10.1111/j.1365-3083.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Su K, Ji C, et al. Immune opsonins modulate BLyS/BAFF release in a receptor-specific fashion. J Immunol. 2008;181:1012–1018. doi: 10.4049/jimmunol.181.2.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heidt S, Roelen DL, Eijsink C, van Kooten C, Claas FH, Mulder A. Effects of immunosuppressive drugs on purified human B cells: Evidence supporting the use of MMF and rapamycin. Transplantation. 2008;86:1292–1300. doi: 10.1097/TP.0b013e3181874a36. [DOI] [PubMed] [Google Scholar]
- 38.Groom J, Mackay F. B cells flying solo. Immunol Cell Biol. 2008;86:40–46. doi: 10.1038/sj.icb.7100142. [DOI] [PubMed] [Google Scholar]
- 39.Elimelakh M, Dayton V, Park KS, et al. Red cell aplasia and autoimmune hemolytic anemia following immunosuppression with alemtuzumab, mycophenolate, and daclizumab in pancreas transplant recipients. Haematologica. 2007;92:1029–1036. doi: 10.3324/haematol.10733. [DOI] [PubMed] [Google Scholar]
- 40.Emmerich F, Bal G, Barakat A, et al. High-level serum B-cell activating factor and promoter polymorphisms in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2007;136:309–314. doi: 10.1111/j.1365-2141.2006.06431.x. [DOI] [PubMed] [Google Scholar]
- 41.Haider I, Cahill M. Fatal thrombocytopaenia temporally related to the administration of alemtuzumab (MabCampath) for refractory CLL despite early discontinuation of therapy. Hematology. 2004;9:409–411. doi: 10.1080/10245330400001942. [DOI] [PubMed] [Google Scholar]
- 42.Kirk AD, Hale DA, Swanson SJ, Mannon RB. Autoimmune thyroid disease after renal transplantation using depletional induction with alemtuzumab. Am J Transplant. 2006;6(5 Pt 1):1084–1085. doi: 10.1111/j.1600-6143.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 43.Otton SH, Turner DL, Frewin R, Davies SV, Johnson SA. Autoimmune thrombocytopenia after treatment with Campath 1H in a patient with chronic lymphocytic leukaemia. Br J Haematol. 1999;106:261–262. doi: 10.1046/j.1365-2141.1999.01576.x. [DOI] [PubMed] [Google Scholar]
- 44.Sellam J, Miceli-Richard C, Gottenberg JE, et al. Decreased B cell activating factor receptor expression on peripheral lymphocytes associated with increased disease activity in primary Sjogren’s syndrome and systemic lupus erythematosus. Ann Rheum Dis. 2007;66:790–797. doi: 10.1136/ard.2006.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carter RH, Zhao H, Liu X, et al. Expression and occupancy of BAFF-R on B cells in systemic lupus erythematosus. Arthritis Rheum. 2005;52:3943–3954. doi: 10.1002/art.21489. [DOI] [PubMed] [Google Scholar]