Abstract
Protein misfolding, followed by aggregation and amyloid formation is an underlying pathological hallmark in a number of prevalent diseases, including Parkinson's (PD), Alzheimer's (AD) and Type 2 diabetes (T2D). In the case of PD, the aggregation of α-synuclein protein (α-syn) has been shown to be highly cytotoxic and to play a key role in the death of dopaminergic cells. Thus, inhibition of the aggregation process may be considered as an attractive avenue for therapeutic intervention. In this respect, molecular chaperones, known to promote proper folding of proteins, are able to inhibit protein aggregation thus preventing amyloid formation. In this work, the effect of the constitutively expressed chaperone Hsc70 and its various domains on α-syn aggregation have been investigated using different approaches. The results show that the C-terminal domain alone (residues 386-646) is as efficient in inhibiting α-syn aggregation as the entire Hsc70 protein, by increasing the lag phase for α-syn oligomeric nucleus formation, suggesting that the chaperone interacts with and stabilizes α-syn monomers and/or small aggregates. Deletion of the C-terminal helices (residues 510-646), which are known to play the role of a lid locking target peptide ligands in the peptide-binding site of the chaperone, strongly reduced the efficiency of inhibition of α-syn aggregation indicating that these helices play an essential in stabilizing the interaction between Hsc70 and α-syn. Furthermore, the effects of Hsc70 and its structural domains on aggregation appear to correlate with those on cytotoxicity, by reducing the fraction of α-syn toxic species to various degrees. Together these results suggest a mechanism in which inhibition of synuclein aggregation is the result of monomeric synuclein binding to the chaperone as any monomeric target unfolded protein or peptide binding to the chaperone.
Keywords: Alpha synuclein, Hsc70 molecular chaperone, aggregation, amyloid fibril
1. Introduction
A common theme shared by Parkinson's disease (PD) and many other disorders is the abnormal folding or clearance of potentially cytotoxic protein species. In the case of PD, the property of aggregation is attributed to the α-synuclein protein (α-syn) [1,2]. The cascade of α-syn is believed to progress from the protein misfolding, to the formation of oligomers, the maturation of protofibrils and finally the formation of mature fibrils [2]. This pathological conversion of misfolded proteins to amyloid aggregates can form cytotoxic species [2].
It is well established that molecular chaperones play an important role in in preventing protein misfolding and aggregation [3,4,5]. Chaperones and particularly the 70 kDa Heat Shock Protein Family (Hsp70s) have thus emerged as key modulators of protein amyloidogenesis [6,7], and several studies demonstrated their role in inhibiting the fibrillogenesis of different amyloidogenic proteins such as α-syn [8,9,10] and β-amyloid [11]. Other studies indicated that Hsp70 protect against α-syn toxicity in vitro [8, 11] and reduce the amount of α-syn aggregates in vivo [8].
At the mechanistic level, Hsp70s exhibits two activities, an ATP-independent “holding” activity that consists in a mere, high affinity, binding to an unfolded protein thus preventing it from aggregation, and an ATP- and co-chaperones-dependent “folding” activity that results in the efficient folding, by coupling repeated cycles of ATP binding and release to cycles of association and dissociation to the misfolded protein [12,13,14]. The constitutive member of the Hsp70s family is made of two structural and functional domains [7,15,16,17]: an N-terminal ATPase domain, known also as nucleotide binding domain, NBD (residues 1-385) [18] and a C-terminal peptide-binding domain (PBD: residues 386-646) involved in unfolded protein binding [19,20] (Figure 1). The C-terminal domain is in turn composed of 2 subdomains: the peptide-binding subdomain (SBSD) per se (residues 386-509) and the C-terminal α-helical subdomain (residues 510-646) that plays the role of a lid (composed of 5 helices) that regulates substrate binding and release under the control of ATP binding and hydrolysis [19,20].
Figure 1. Representation of Hsc70 and its respective structural domains.
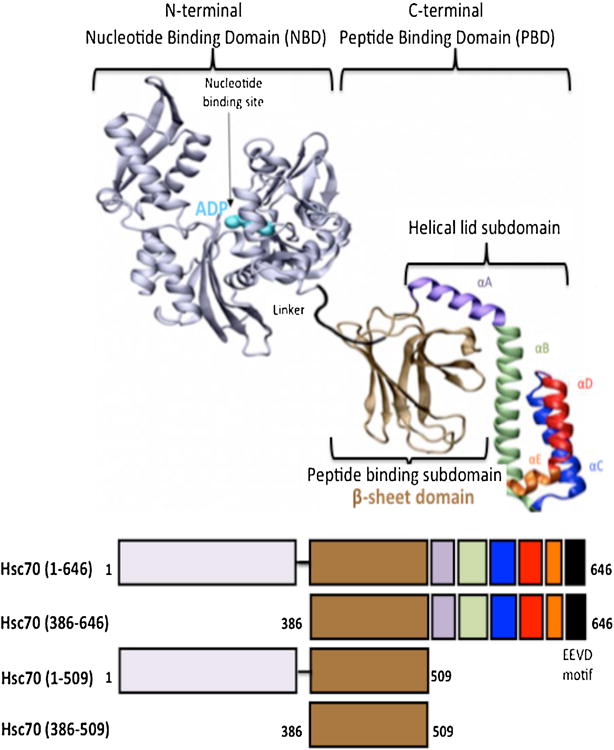
Top: Structure of DnaK based on the PDB file 2KHO [37] 19. Bottom: Schematic representation of Hsc70 and its structural domains represented by different colors.
In this work, the effect of the constitutively expressed chaperone Hsc70 and its various domains on α-syn aggregation have been investigated using different approaches.
2. Material and methods
Expression and purification of α-syn
Recombinant wild type α-syn was overexpressed in E.coli BL21 using a pET-28a vector coded with a T7 promoter and was purified as described in [21]. α-syn concentration was determined using an extinction coefficient of 5960 M-1cm-1 at 280 nm. Before using, pure lyophilized α-syn was resuspended in 50 mM Tris-HCl (pH 7.4), 100 mM KCl buffer and was filtered through 0.22 μM pore size filter.
Aliquot of the final purification step were analysed by SDS-Polyacrylamide Gel Electrophoresis (PAGE) to confirm purity (Fig. 2). To determine the molecular mass of the native protein and to check if the purified protein is in an aggregated form, we analysed an aliquot of the final purification step with Size Exclusion Chromatography (SEC) using a superdex 70 column previously equilibrated with 5 mM phosphate buffer, pH 7.4 with a flow of 0.5ml/min (Fig. 2).
Figure 2. Analysis of purified α-syn, Hsc70 and its structural domains by size exclusion chromatography and SDS-PAGE (inset).
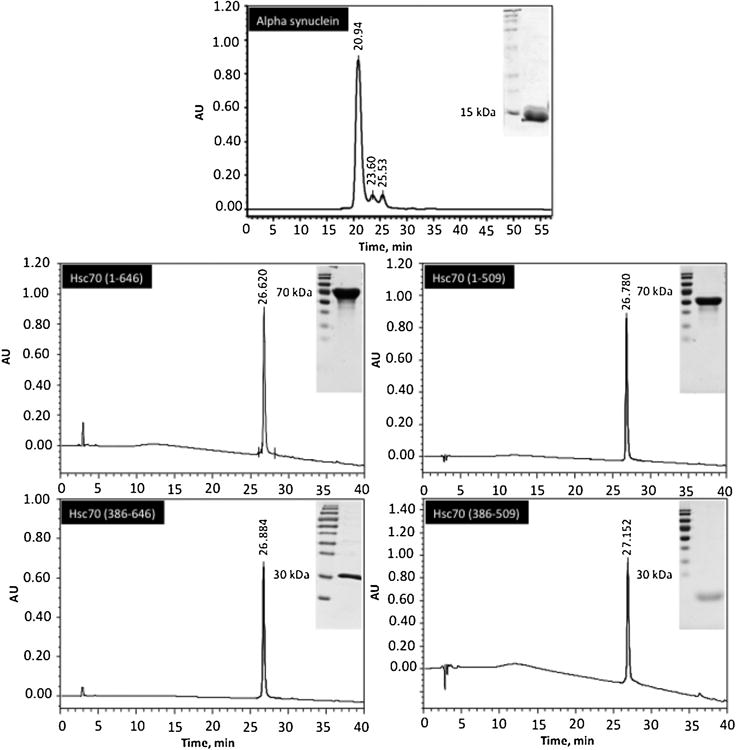
5 mM of these proteins were loaded on a superdex 75 column using high performance liquid chromatography (HPLC) and eluted as described under « Materials and methods ». Inset: 15 mg of each purified protein were analyzed by 12% SDS-PAGE as described under « Materials and methods ».
Expression and purification of Hsc70 and its structural domains
These proteins were overexpressed in BL21 pLysS E.coli using Pet14 vector and were purified as described previously [22,23,24]. After purification, Hsc70 and its respective structural domains are in a PBS buffer (1.5 mM Kh2PO4, 150 mM NaCl, 3 mM KCl and 8 mM Na2HPO4). The purified Hsc70 proteins were concentrated by Milipore Centricon, dialyzed against 50 mM Tris-HCl (pH 7.4), 150 mM KCl, filtered through a sterile 0.22 μM pore size filter and stored at -20°C. Aliquots of each purification step were analyzed by SDS-PAGE to confirm purity (Fig. 2). To determine the molecular mass of the native protein and to check if the purified protein is in an aggregate form, we have analyzed an aliquot of the final purification step with high performance liquid chromatography (HPLC) using a superdex 70 column previously equilibrated with 5 mM phosphate buffer (pH 7.4) with a flow rate of 0.5 ml/min (Fig. 2. Protein concentrations were determined routinely using the Lowry procedure.
The activity of the purified hsc70 alone was done using a luciferase refolding assay as described [25].
Assembly of α-syn into fibrils
To induce fibril structures, lyophilized α-syn (1mg/ml) dissolved in 50 mM Tris-HCl (pH 7.4), 100 mM KCl was incubated for a few days at 37°C in a thermomixer in presence or absence of various concentrations of Hsc70 and respective structural domains. Samples for analysis were taken after regular time intervals and stored at 4°C.
Thioflavine T (ThT) binding assay
ThT is a dye widely used as an indicator of the protein aggregation state and that fluoresces upon binding to fibrillar species [26]. Samples co-incubated without or with Hsc70 and its deleted mutants were diluted 25-fold with ThT solution (20 μM). ThT fluorescence measurements were performed using Perkin Elmer model LS 55. An excitation of 440 nm and an emission of 480 nm were used [21]. The emission and excitation slits were set at 5 nm and 1 cm cuvette was used for all experiments. Nonspecific background fluorescence was subtracted from the samples by using appropriate blanks in absence of proteins. The time-dependence of ThT fluorescence will be fitted to a sigmoidal growth model using:
Where Y is the intensity of fluorescence at different times, x is the incubation time, yi and yf are the maximal and minimal fluorescence intensity, x0 is the incubation time when we have 50% of the maximal of fluorescence, τ is the inverse of the apparent constant of growth Kapp and mi, mf the adjustment parameters. The lag time tlag is described by tlag= x0 - 2τ. The effect of chaperones on α-syn aggregation was analyzed by comparison of the time courses in presence or absence of Hsc70 and its domains.
Dynamic Light Scattering (DLS) assay
DLS (Zetasizer, Malvern) was used to measure the average diffusion coefficient distribution of α-syn particles. Each particle in the sample is characterized by its hydrodynamic radius (RH). Samples co-incubated without or with Hsc70 and its respective structural domains were diluted 10-fold and 50 μl was placed into 96 well plate and the total light scattering intensity was collected using a 10s averaging acquisition time. Particle translational diffusion coefficients were calculated from auto correlated light intensity data (usually 30–40 points) and converted to RH with the Stokes–Einstein equation.
Atomic Force Microscopy (AFM)
An aliquot of 5 μl from the monomeric and the aggregate forms were deposited onto freshly cleaved mica sheets for AFM inspection. The samples were prepared and the AFM pictures were analyzed as described in (20).
Cell culture
SH-SY5Y neuronal cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS) and antibiotics in a 5% CO2 humidified atmosphere at 37°C. Cells were plated at a density of 104 cells/well in 96-well plates (for the cell viability assays), incubated for 24h, and then the medium was changed before incubation with α-syn amyloid. Samples of α-syn solutions with and without chaperones were initially diluted in the culture medium and then added to the cells to achieve the required final concentrations. Treated cells with amyloid incubation buffer were used as control.
Cell viability assay
The toxicity of soluble and fibrillar α-syn in presence or absence of Hsc70 and its domains was assessed by the 3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction inhibition assay, as described by Pieiri et al. [27]. In our experiments, MTT (Sigma) was added to cells to have a final concentration of 1mg/ml. After that, cells were incubated for 2 h before removing cell culture medium containing MTT. Finally, a buffer containing 25 μl 0.1 M glycine, 0.1 M NaCl (pH 10.5) and 200 μl of DMSO (Sigma) will be added per well and absorbance will be measured at 550 nm [27]. This experiment was repeated 3 times and treated cells with amyloid incubation buffer are used as control.
3. Results
3. 1. α-syn aggregation and fibril formation
In order to verify that α-syn purified in this work is able to aggregate, the aggregation process was monitored using Thioflavin T binding as described in materials and methods. As shown in figure 3A, monomeric α-syn (70 μM (1 mg/ml)) aggregation follows a sigmoidal curve, made up of an initial lag phase of about 12h (table 1), a subsequent exponential phase and a final stationary phase. This aggregation process is dependent on α-syn concentration, which reduces the lag time and the ThT fluorescence intensity. AFM microscopy shows that α-syn (sample1 at 0h, Fig 3A) is initially monomeric (Fig. 3B1). However, incubation for 52 h of 70 μM of α-syn (sample2 at 52h, Fig 3A) resulted in the formation of fibrils (Fig. 3B2). These results were confirmed by DLS experiments of the same samples, which show that α-syn used at 0h is monomeric and monodisperse with an average RH of 3.1 nm (Fig 3C1), whereas α-syn incubated for 52h is clearly oligomeric with an RH of about 176 nm (Fig 3C2). In this case, polydispersity of the sample is observed indicating that the fibrils size shown previously by AFM (Fig 3B2) is heterogeneous.
Figure 3. Aggregation of α-syn.
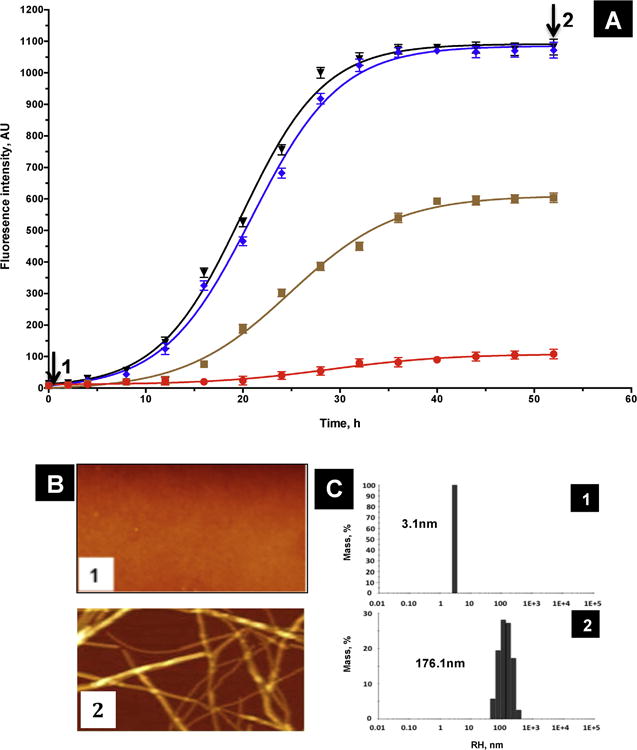
A) Time course of aggregation monitored by ThT fluorescence (10 μM, red; 35 μM, orange; 70 μM, blue and 100 μM, black). The time-dependence of ThT fluorescence was fitted to a sigmoidal growth model as described under « Materials and methods ». ThT fluorescence results are the means of three independent experiments. B) AFM images of 70 μM α-syn at 0 h (sample 1), and 52h (sample 2). C) DLS histograms of 70 μM α-syn at 0 h (sample 1) and 52h (sample 2).
Table 1.
Kinetic parameters of α-syn aggregation in presence and absence of Hsc70 and its structural domains as determined from fluorescence assays. % of inhibition at 52 h is calculated from figure 5A and the dissociation constant Kd is obtained from the hyperbolic equation Y = Bmax*X/(Kd + X).
| X0 (t50) (h) | Tlag (h) | % of inhibition at 52h | Dissociation constant Kd (μM) | |
|---|---|---|---|---|
| α-syn | 20.95 | 11.75 | 0 | |
| Hsc70 (1-646) | 32.67 | 15.95 | 88 | 0.921 |
| Hsc70 (1-509) | 29.05 | 14.35 | 59 | 1.705 |
| Hsc70 (386-646) | 32.02 | 20.24 | 92 | 0.8217 |
| Hsc70 (386-509) | 28.64 | 13.91 | 56 | 1.467 |
3. 2. The effect of Hsc70 of α-syn aggregation
The effect of the molecular chaperone Hsc70 on α-syn aggregation was monitored as described in material and methods. As shown in figure 4A increasing concentrations of Hsc70 lead to a decrease in ThT fluorescence indicating that the inhibitory effect of Hsc70 is concentration dependent. The inhibitory effect of Hsc70 is observed with concentrations as low as 1/100 relative to α-syn and the maximum of the inhibition effect (88%, table 1) is observed with a ratio of 1/10 of [Hsc70]/[α-syn] (Fig 4A inset). Figure 4A also shows that the length of the lag phase preceding α-syn aggregation is increased as a function of Hsc70 concentrations. The same results are obtained in presence of nucleotides ATP or ADP (results not shown). As shown by AFM, the presence of Hsc70 inhibits completely α-syn fibril formation, even though a few oligomers are still present (Fig 4B2, compare Fig 4B2 to Fig 3B2). This result was confirmed by DLS which indicates that the RH of the particles present in figure 4C2 is about 6.5nm which corresponds to low molecular weigh aggregates compared to fibrils which exhibit an RH of about 176.1nm (Fig 3C2).
Figure 4. Aggregation of α-syn in presence of Hsc70.
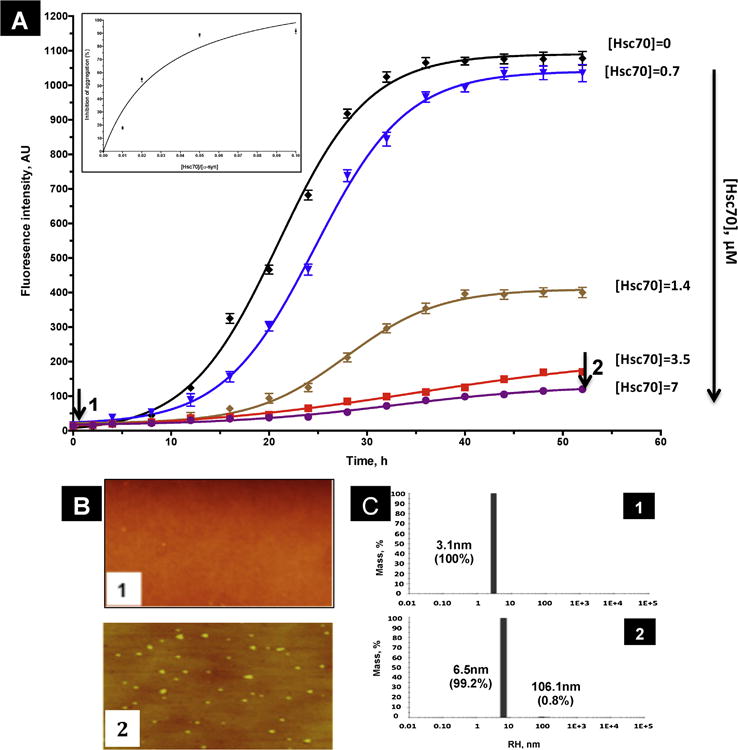
A) Time course of α-syn aggregation monitored by ThT fluorescence in the absence (black) and presence of increasing amounts of Hsc70 (0.7 μM, bleu; 1.4 μM, brown; 3.5 μM, red and 7 μM, violet). The time-dependence of ThT fluorescence was fitted to a sigmoidal growth model as described under « Materials and methods ». Inset: percentage of Inhibition of α-syn aggregation at increasing ratios of [Hsc70]/[α-syn]. This % of inhibition was calculated at 52h taking the fluorescence of -syn in absence of Hsc70 as 0% of aggregation. ThT fluorescence results are the means of three independent experiments. B) AFM images of 70 μM α-syn at 0 h (sample 1), and 52h in presence of 7μM of Hsc70 (sample 2). C) DLS histograms of 70 μM α-syn at 0 h (sample 1) and 52h in presence of 7μM of Hsc70 (sample 2).
3. 3. The effect of isolated Hsc70 structural domains on α-syn aggregation
It is well known from previous studies that the C-terminal domain of Hsc70 is responsible for binding to peptides and target proteins, since it contains the peptide-binding site [28]. The N-terminal ATPase binding domain however is not directly involved in the binding of the target proteins but plays a role in regulation of peptide binding [28,29]. Therefore, the isolated C-terminal domain was used for further studies. The C-terminal domain of Hsc70 is composed of 2 sub-domains: a peptide-binding subdomain (PBSD) (residues 386-509) entirely made of β-sheets and a lid subdomain (residues 510-646) made exclusively of alpha helices.
As shown in figure 5A, the C-terminal domain alone is able to inhibit α-syn aggregation, to an extent of 92% after 52h of incubation (table 1). This is confirmed by DLS experiments which give an RH of about 4.5 nm for the particles formed in the presence of C-terminal domain (Fig 5C5) as compared to those obtained at t=0, that exhibit an RH of about 3,1nm. Furthermore, the time at which 50% of inhibition is obtained (x0 (t50)), is identical for Hsc70 and its C-terminal domain (table 1). This inhibitory effect is observed with concentrations as low as 1/100 relative to α-syn, and almost maximum effect is observed with a ratio 1/10 [Hsc70]/[α-syn] (Fig 5B). Altogether, these results indicate that the C-terminal domain alone is as efficient in inhibiting α-syn aggregation as the entire Hsc70 (Fig 5A). Interestingly, as shown in table 1, the lag time, defined, as the time required reaching 10 % of the total fluorescence intensity, of α-syn aggregation in the presence of the isolated C-terminal domain (20.24h) (table 10) is about 25% longer than that obtained of the presence of the entire Hsc70 (15.95h). This result suggests that the isolated C-terminal domain may stabilize the interaction with monomeric and small aggregated forms of α-syn somewhat more effectively than the entire Hsc70, thereby delaying the formation of seeds and fibrils.
Figure 5. The inhibition of α-syn aggregation by structural domains of Hsc70.
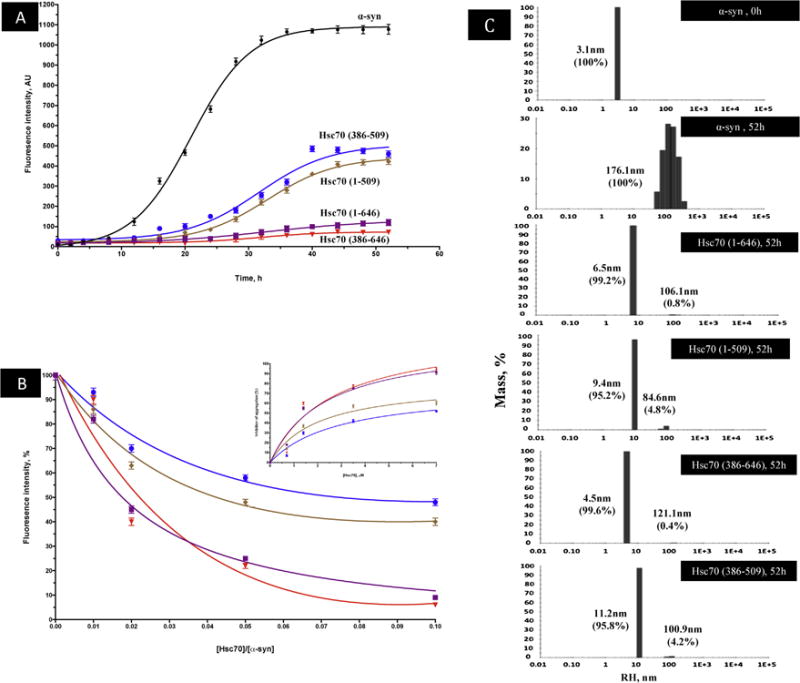
A) The time dependence of amyloid inhibition by Hsc70 and its structural domains. B) Extent of inhibition (%) of α-syn aggregation by Hsc70 and its structural domains (% calculated with respect to the ThT fluorescence of α-syn at 52h) at increasing ratios of [Hsc70]/[α-syn]. Inset: Percentage of Inhibition of α-syn aggregation at a constant α-syn concentration (70 μM) and increasing concentrations of Hsc70 and its respective structural domains at 52h of incubation. This inhibition was fitted using the hyperbolic equation Y = Bmax*X/(Kd + X). Where Bmax is the maximum specific binding in the same units as Y. It is the specific binding extrapolated to very high concentrations of [Hsc70], and so its value is almost always higher than any specific binding measured in the experiment. Kd is the equilibrium binding constant, in the same units as X. It is the chaperone concentration needed to achieve a half-maximum binding at equilibrium.
ThT fluorescence results are the means of three independent experiments. C) DLS analysis of α-syn in presence and absence of Hsc70 and its structural domains. The histograms represent the mass percentage of aggregates species.
In order to investigate further the respective roles of the PBSD and the C-terminal α-helical lid in α-syn binding, the same experiments were performed with the isolated PBSD consisting only of the β-sheets holding the peptide-binding site (residues 386-509) (see figure1). As shown in figure 5A, although the PBSD lacking the lid is able to inhibit α-syn aggregation (56% of inhibition is observed at 52h with a stoichiometry of 1/10 [Hsc70 (386-509)]/[α-syn]), it does so with much less efficiency than the entire C-terminal domain that contain the lid. This result is also confirmed by DLS data indicating that species of about 11.2 nm of RH are obtained in the presence of the PBSD, suggesting that higher sizes of aggregates are formed the presence of the C-terminal lid (Fig 5C).
Moreover, as shown in table 1, the dissociation constant Kd for PBSD binding to alpha-synuclein (1.467 μM) is about 2 fold that of the intact C-terminal domain (0.82 μM), indicating that the presence of the lid stabilizes α-syn binding to the chaperone. This is consistent with the fact that Kd for the entire protein (0.92 μM) is similar to that of the isolated C-terminal domain and that the Kd of the PBSD (1.467) is similar to that of the ATPase domain linked to the PBSD (1.705 μM). Altogether, these results indicate that α-syn binding to the PBSD is stabilized by the helical lid, but is not affected by the presence of ATPase domain.
3. 4. Hsc70 and its structural domains attenuate α-synuclein cytotoxicity
A common theme shared by PD and many other neurodegenerative disorders is the abnormal folding or clearance of potentially cytotoxic protein species [1,2]. Since Hsc70 and its respective structural domains were able to inhibit α-syn aggregation by inhibiting fibrillation and stabilizing monomers and/or small oligomers it was of interest to determine the cytotoxicity of the newly formed species.
α-syn fibril toxicity was assessed using neuronal SHSY5Y cells, previously shown to be highly affected by different protein aggregates [30]. Figure 6 shows that 1 μM of α-syn fibrils obtained after 52h of incubation (see Fig 3B2) are highly toxic to the cells, reducing cell viability to ∼44%. However, α-syn incubated in the presence of Hsc70 or its structural domains alleviates this toxicity (Fig 6B). 90% of cell viability is maintained for cells exposed to α-syn incubated with the C-terminal domain alone and 75% viability is seen for incubations with full length Hsp70 (Fig 6B). In contrast, incubation in the presence of constructs lacking the lid and containing only the PBSD show lower cell viabilities of only 55% (isolated PBSD) and 52% (ATPase domain plus PBSD) respectively (Fig 6). These results suggest that the intact C-terminal domain is necessary and sufficient for effective rescue of cells exposed to toxic α-syn fibrils.
Figure 6. Cell viability assays.
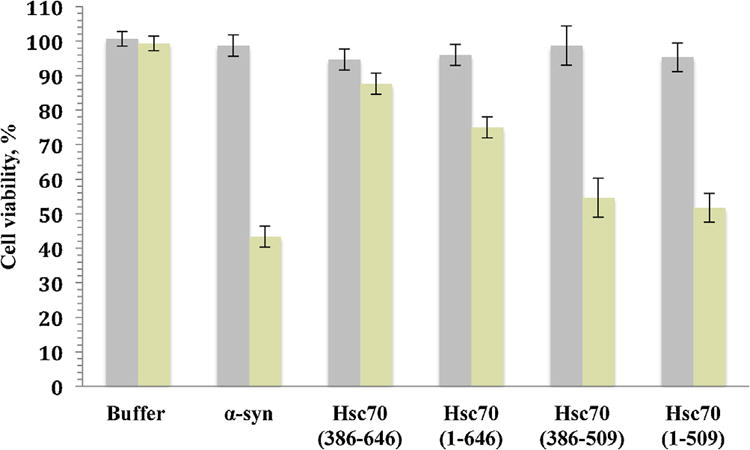
Viability of SHSY5Y neuronal cells upon exposure to soluble α-syn at 0h (grey histogram) or α-syn aggregates in the absence and presence of Hsc70 and its domains sampled at 52h (yellow histogram). The final protein concentration within the culture medium was 1 μM. The cells were incubated in the absence and presence of Hsc70 chaperone and its structural domains for 24 h. Cell viability is expressed as the percentage of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction using cells treated with the same volume of buffer as a reference (100% 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reduction). The values (averages ± S.D.) are obtained from three independent experiments.
4. Discussion
It is now well established that members of the 70 kDa Heat Shock Protein Family (Hsp70s) play an important role in dealing with protein misfolding and aggregation. By binding to hydrophobic regions of an unfolded protein, these molecular chaperones prevent incorrect intra- and intermolecular interactions, thus promoting correct folding over aggregation [7,15,16,17]. To do so, Hsp70s exhibits two activities, an ATP-independent “holding” activity that consists in a mere, high affinity, binding to an unfolded protein thus preventing it from aggregation, and an ATP- and co-chaperones-dependent “folding” activity that results in the efficient folding, by coupling repeated cycles of ATP binding and release to cycles of association and dissociation to the misfolded protein [12,13,14]. Although the interaction of Hsp70s with α-syn has been studied previously [8,9,10], the molecular details of which species of α-syn (monomers, prefibrillar or fibrillar) the chaperone binds to in order to inhibit α-syn aggregation and fiber formation remain controversial ([8,9,10]).
In an attempt to better understand this process, different mutant versions of Hsc70 have been used in this work to test for their effects on α-syn aggregation kinetics. We show here that Hsc70 is able to interact with α-syn and prevents its aggregation solely by its holding activity (Figure 4), irrespective of whether ATP is present or not (results not shown). This is confirmed by the fact that wholesale deletion of the N-terminal, ATPase domain does not alter the effect of Hsp70 on the aggregation kinetics, indicating that the peptide binding capacity of the C-terminal domain (holding activity) is sufficient to inhibit α-syn aggregation and fiber growth (Figure 5). These results fit in the Hsp70 functional cycle in which, in absence of ATP, free or ADP-Hsc70 bind to unfolded proteins with high affinity maintaining them in an off-pathway of aggregation [15,29,31]. This is also in line with results obtained in the presence of ATP where α-syn aggregation is strongly delayed by the chaperone, but not inhibited, leading to fiber formation [32]. In this case, and as expected, the ATP-chaperone having low affinity for α-syn does not form stable complexes with it and thus is unable to inhibit fiber formation. All together, these results suggest that the chaperone binds to monomeric α-syn in a way similar to that of any unfolded protein substrate by recognizing hydrophobic stretches and shielding them from aggregation.
This interpretation is supported by the observation that Hsc70 prolongs the lag phase of α-syn aggregation by about 2 fold at substoichiometric concentrations (Figure 4) indicating that the chaperone delays the nucleation phase, that is inhibits the formation and/or propagation of small soluble oligomers that act as a growing point for fiber elongation [33,34,35]. Thus, as a chaperone, Hsc70 at substoichiometric levels relative to α-syn would interact naturally with the intrinsically unfolded α-syn monomers, sequestering them off the aggregation pathway. Because any remaining monomers could continue to form nuclei and then aggregate, it is likely that the equilibrium between chaperone-bound and chaperone-free α-syn is established on a time scale that is faster than the time scale of nucleus formation. Thus a small amount of chaperone can prevent a much higher amount of protein from forming nuclei. The net effect on α-syn aggregation would be longer lag times, as observed (Figure 4), effectively as if the α-syn concentration is greatly decreased. This is also consistent with the fact that while partial inhibition of aggregation is observed at very low levels of Hsc70 relative to α-syn, full inhibition is obtained at a still sub-stoichiometric 1:10 chaperone:protein concentrations (Figure 4, inset). Indeed, DLS shows that α-syn species of 6.5 nm and 100 nm (compare to monomer size of about 3 nm) can still be formed in the presence of substoichiometric levels Hsc70, with species also visible by AFM in the form of spherical particles (Figure 4B2 and 4C2). However, it was reported previously that Hsp70s bind to small aggregates and/or fibrillar species of α-syn at a stoichiometry of 1 Hsp70 per 10 α-syn monomers [10]. We view this as unlikely since the target hydrophobic region of α-syn responsible for its aggregation would have to be inaccessible to the chaperone in the monomeric state but present in the small aggregate. Whatever the type of interaction, whether it is with the monomer, small oligomers or both, Hsc70 inhibits α-syn aggregation by delaying the nucleation phase thus inhibiting fibril formation.
From the structural point of view, the results obtained fit the known properties of substrate binding and stabilization by Hsp70s. Indeed, experiments using the entire Hsc70 and its respective N- and C-terminal structural domains show that even though the entire Hsc70 is able to inhibit α-syn aggregation, the isolated C-terminal peptide-binding domain (residues 386-646) (Figure 1) is necessary and sufficient for α-syn stabilization and inhibition of fibrils formation, the N-terminal, ATPase domain (residues 1-380) being dispensable (Figure 5A). Moreover, deletion of the C-terminal helices constituting the lid, whether from the entire protein or from the C-terminal peptide binding domain of the peptide binding domain, reduces significantly the binding of α-syn to Hsc70 and the inhibition of aggregation, suggesting that α-syn binds to the chaperone in a way similar to that of any unfolded protein: In a first step, α-syn would bind to the peptide binding site of the chaperone, then it is locked into place by the helical lid thus leading to the closed conformation that stabilizes the chaperone-unfolded protein complex [28,31,36]. This mode of interaction might explain how the chaperone inhibits α-syn aggregation by diverting α-syn monomers from the aggregation pathway. As expected, deletion of the lid (mutant Hsc70 residues 1-509, Figure 5A and B) or the effects of ATP binding and hydrolysis in the context of the intact protein ([32], Figure 1), lead to the same result, the destabilization of the chaperone-synuclein complex by either the absence or the opening of the lid thus inducing the release of the unfolded synuclein and aggregation then fibril formation.
Finally, the fact that aggregated α-syn preparation obtained, after 52 hours of incubation, is polydisperse with species ranging from 80 to 800 nm (Figure 5C) is indicative of the presence of small aggregates, prefibrils and fibrils. This might explain the partial toxicity of α-syn to the cells (cell viability reduced by 60% relative to the control, Figure 6), and is in agreement with the hypothesis that prefibrillar aggregates are the toxic species [8].
The presence of Hsc70 and those of its structural domains that are shown to inhibit nucleus formation (see above), alleviate the toxicity of α-syn (Figure 6) by reducing the fraction of toxic species. This is corroborated by the fact that there is a strong correlation between the presence of the lid and reduction of toxicity: The C-terminal domain, containing the α-syn binding site and the lid that stabilizes the synuclein-chaperone complex and delaying the formation of the nucleus, is able to almost completely abrogate synuclein toxicity. Likewise, the absence of the lid destabilizes the chaperone-synuclein complex thus allowing the formation of more synuclein aggregates and reestablishing synuclein toxicity (Figure 6).
In conclusion, it is tempting to propose that molecular chaperones inhibit aggregation and amyloidogenic proteins by mechanisms similar to those involved in binding target unfolded proteins: By binding to hydrophobic regions of monomeric species, the chaperones prevent them from aggregation.
Acknowledgments
This work was supported by the Qatar National Research fund (QNRF) under the contract grant number: NPRP 4-1371-1-223 and by NIH/NIA grant R37AG019391 (to David Eliezer).
Abbreviations
- α-syn
alpha synuclein
- Hsp70
Heat shock protein 70
- PD
Parkinson Disease
- ThT
Thioflavine T
- DLS
Dynamic Light Scattering
- RH
Hydrodynamic radius
- AFM
Atomic Force Microscopy
- PBSD
Peptide binding sub domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uversky VN, Eliezer D. Biophysics of Parkinson's disease: structure and aggregation of alpha-synuclein. Curr Protein Pept Sci. 2009;10:483–499. doi: 10.2174/138920309789351921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 3.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 4.Hayden MR, Tyagi SC, Kerklo MM, Nicolls MR. Type 2 diabetes mellitus as a conformational disease. JOP. 2005;6:287–302. [PubMed] [Google Scholar]
- 5.Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- 6.Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol. 2009;16:574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- 7.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 8.Dedmon MM, Christodoulou J, Wilson MR, Dobson CM. Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J Biol Chem. 2005;280:14733–14740. doi: 10.1074/jbc.M413024200. [DOI] [PubMed] [Google Scholar]
- 9.Huang C, Cheng H, Hao S, Zhou H, Zhang X, Gao J, Sun QH, Hu H, Wang CC. Heat shock protein 70 inhibits alpha-synuclein fibril formation via interactions with diverse intermediates. J Mol Biol. 2006;364:323–336. doi: 10.1016/j.jmb.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 10.Pemberton S, Madiona K, Pieri L, Kabani M, Bousset L, Melki R. Hsc70 protein interaction with soluble and fibrillar alpha-synuclein. J Biol Chem. 2011;286:34690–34699. doi: 10.1074/jbc.M111.261321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magrane J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci. 2004;24:1700–1706. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langer T, Lu C, Echols H, Flanagan J, Hayer MK, Hartl FU. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992;356:683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- 13.Hohfeld J, Minami Y, Hartl FU. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 14.Rudiger S, Buchberger A, Bukau B. Interaction of Hsp70 chaperones with substrates. Nat Struct Biol. 1997;4:342–349. doi: 10.1038/nsb0597-342. [DOI] [PubMed] [Google Scholar]
- 15.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 16.Ellis RJ, Hartl FU. Principles of protein folding in the cellular environment. Curr Opin Struct Biol. 1999;9:102–110. doi: 10.1016/s0959-440x(99)80013-x. [DOI] [PubMed] [Google Scholar]
- 17.Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 Reduces alpha-Synuclein Aggregation and Toxicity. J Biol Chem. 2004;279:25497–25502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- 18.Hershko A, Ciechanover A, Heller H, Haas AL, Rose IA. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 1980;77:1783–1786. doi: 10.1073/pnas.77.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciechanover A, Heller H, Elias S, Haas AL, Hershko A. ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc Natl Acad Sci U S A. 1980;77:1365–1368. doi: 10.1073/pnas.77.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaari A, Horchani H, Frikha F, Verger R, Gargouri Y, Ladjimi M. Surface behavior of alpha-Synuclein and its interaction with phospholipids using the Langmuir monolayer technique: A comparison between monomeric and fibrillar alpha-Synuclein. Int J Biol Macromol. 2013;58C:190–198. doi: 10.1016/j.ijbiomac.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 22.Amor-Mahjoub M, Gomez-Vrielyunck N, Suppini JP, Fouchaq B, Benaroudj N, Ladjimi M. Analysis of monomeric mutants of HSC70: a possible relationship between oligomerization and functional properties. Protein Pept Lett. 2007;14:761–765. doi: 10.2174/092986607781483624. [DOI] [PubMed] [Google Scholar]
- 23.Amor-Mahjoub M, Gomez-Vrielyunck N, Suppini JP, Fouchaq B, Benarouj N, Ladjimi M. Involvement of the interdomain hydrophobic linker and the C-terminal helices in self-association of the molecular chaperone HSC70. Arch Inst Pasteur Tunis. 2006;83:53–62. [PubMed] [Google Scholar]
- 24.Amor-Mahjoub M, Suppini JP, Gomez-Vrielyunck N, Ladjimi M. The effect of the hexahistidine-tag in the oligomerization of HSC70 constructs. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:328–334. doi: 10.1016/j.jchromb.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Pemberton S, Madiona K, Pieri L, Kabani M, Bousset L, Melki R. Hsc70 protein interaction with soluble and fibrillar alpha-synuclein. J Biol Chem. 286:34690–34699. doi: 10.1074/jbc.M111.261321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfe LS, Calabrese MF, Nath A, Blaho DV, Miranker AD, Xiong Y. Protein-induced photophysical changes to the amyloid indicator dye thioflavin T. Proc Natl Acad Sci U S A. 2010;107:16863–16868. doi: 10.1073/pnas.1002867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pieri L, Bucciantini M, Guasti P, Savistchenko J, Melki R, Stefani M. Synthetic lipid vesicles recruit native-like aggregates and affect the aggregation process of the prion Ure2p: insights on vesicle permeabilization and charge selectivity. Biophys J. 2009;96:3319–3330. doi: 10.1016/j.bpj.2008.12.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyle SM, Genest O, Wickner S. Protein rescue from aggregates by powerful molecular chaperone machines. Nat Rev Mol Cell Biol. 2013;14:617–629. doi: 10.1038/nrm3660. [DOI] [PubMed] [Google Scholar]
- 29.Marcinowski M, Rosam M, Seitz C, Elferich J, Behnke J, Bello C, Feige MJ, Becker CF, Antes I, Buchner J. Conformational selection in substrate recognition by Hsp70 chaperones. J Mol Biol. 2013;425:466–474. doi: 10.1016/j.jmb.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 30.Gharibyan AL, Zamotin V, Yanamandra K, Moskaleva OS, Margulis BA, Kostanyan IA, Morozova-Roche LA. Lysozyme amyloid oligomers and fibrils induce cellular death via different apoptotic/necrotic pathways. J Mol Biol. 2007;365:1337–1349. doi: 10.1016/j.jmb.2006.10.101. [DOI] [PubMed] [Google Scholar]
- 31.Schlecht R, Erbse AH, Bukau B, Mayer MP. Mechanics of Hsp70 chaperones enables differential interaction with client proteins. Nat Struct Mol Biol. 2011;18:345–351. doi: 10.1038/nsmb.2006. [DOI] [PubMed] [Google Scholar]
- 32.Roodveldt C, Bertoncini CW, Andersson A, van der Goot AT, Hsu ST, Fernandez-Montesinos R, de Jong J, van Ham TJ, Nollen EA, Pozo D, Christodoulou J, Dobson CM. Chaperone proteostasis in Parkinson's disease: stabilization of the Hsp70/alpha-synuclein complex by Hip. EMBO J. 2009;28:3758–3770. doi: 10.1038/emboj.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waxman EA, Mazzulli JR, Giasson BI. Characterization of hydrophobic residue requirements for alpha-synuclein fibrillization. Biochemistry. 2009;48:9427–9436. doi: 10.1021/bi900539p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruzafa D, Morel B, Varela L, Azuaga AI, Conejero-Lara F. Characterization of oligomers of heterogeneous size as precursors of amyloid fibril nucleation of an SH3 domain: an experimental kinetics study. PLoS One. 2012;7:e49690. doi: 10.1371/journal.pone.0049690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiti F, Taddei N, Baroni F, Capanni C, Stefani M, Ramponi G, Dobson CM. Kinetic partitioning of protein folding and aggregation. Nat Struct Biol. 2002;9:137–143. doi: 10.1038/nsb752. [DOI] [PubMed] [Google Scholar]
- 36.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Bertelsen EB, Chang L, Gestwicki JE, Zuiderweg ER. Solution conformation of wild-type E. coli Hsp70 (DnaK) chaperone complexed with ADP and substrate. Proc Natl Acad Sci U S A. 2009;106:8471–8476. doi: 10.1073/pnas.0903503106. [DOI] [PMC free article] [PubMed] [Google Scholar]


