Abstract
Purpose
To review recent progress in the field of lung xenotransplantation, including mechanisms of xenograft injury, and the influence of mechanism-directed genetic modifications and other interventions that may soon enable therapeutic use of pig lungs in humans.
Recent findings
An extensive series of lung xenotransplantation experiments demonstrates that multiple genetic modifications targeting known xenogeneic lung injury mechanisms are associated with incremental improvements in lung survival or function. Addition of human complement (hCD46, hCD55), coagulation (hEPCR, hTBM, hTFPI, hCD39), or anti-inflammatory pathway regulatory genes (HO-1, HLA-E), and GalT and Neu5Gc gene knockout has each demonstrated protective effects on lung survival or function. In addition, drug treatments targeting key inflammatory and clotting pathways have been shown to attenuate residual mechanisms of lung injury. Work with other pig organs in primate models show that regimens based on costimulatory pathway blocking antibodies prolong xenograft function for months to years, suggesting that once initial lung inflammation mechanisms are fully controlled, clinically useful application of pig lung xenografts may soon be feasible.
Summary
Genetic modification of pigs coupled with drugs targeting complement activation, coagulation, and inflammation have significantly increased duration of pig lung function in ex vivo human blood perfusion models, and life supporting lung xenograft survival in vivo .
Key phrases: lung transplantation, xenotransplantation, immune regulation, coagulation
Introduction
Each year, hundreds of people die on lung transplant waiting lists due to the shortage of human organs available for transplant. Xenotransplantation, the use of organs from other species such as pigs, could offer predictable, timely availability of physiologically normal, certifiably disease-free organs. Significant progress has been made to overcome the biologic barriers to use of pig organs in preclinical models, with sustained organ function and recipient survival reaching months to years in some heart and kidney series (1–3). However pig lung injury remains problematic in human blood perfusion and non-human primate xenogeneic models, and life-supporting lung xenograft survival has been limited to days. Here we review the progress that has been achieved, and residual barriers that have been identified that currently impede clinical lung xenograft application.
Text of Review
Xenogeneic lung injury
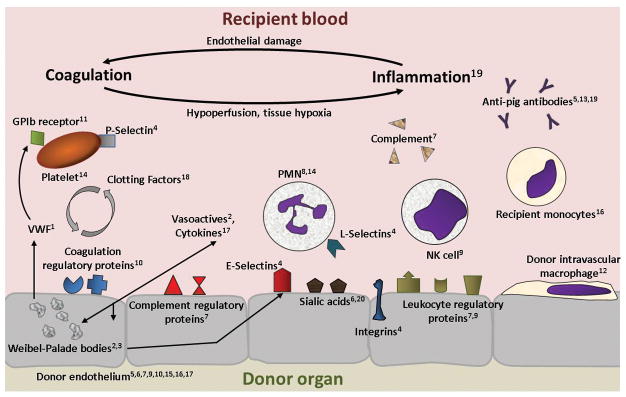
Compared with other organs, the unique anatomic structure of the lung, with a large surface area of vascular endothelium intimately associated with alveolar epithelium and a robust immune surveillance and rapid response system, are primed to trigger inflammation and are extremely susceptible to its consequences (4,5). As a result, only 30–40% of human lungs are harvested and transplanted from consented organ donors, a rate much lower than for liver (about 80%) and kidney (over 90%) (6). This increased relative vulnerability to injury is paralleled for lung xenografts in comparison to other organs. A complex interplay of inflammation, coagulation, and tissue injury leads to relatively rapid lung xenograft failure both ex vivo during perfusion with human blood and in non-human primate transplant models.
Perfusion of wild type porcine lungs with human blood causes intense coagulation and complement activation, leading to graft failure (“hyperacute rejection”) within minutes. Binding of preformed antibodies directed towards the α,1,3-galactose (Gal) epitope has been identified as one main trigger (7,8). Knockout of the galactosyltransferase enzyme (GalTKO) eliminates the carbohydrate antigen from porcine cells and was a key step to overcome hyperacute rejection of other organs (9–11). However, innate (mainly preformed antibody directed against other targets) and adaptive immune responses still persist in recipients of GalTKO organs and tissues (9, 12–15). The adaptive response to lung xenotransplants has not yet been studied since they have not yet reached a relevant duration of survival; accordingly, most lung xeno research has been focused towards early inflammation. Nonetheless, substantial progress to control adaptive anti-xeno immunity has been reported using costimulation pathway-based immunosuppressive regiments for islets (16), kidneys (3), and hearts (1,17–18), offering hope that adaptive immunity can be controlled effectively and safely for lung xenografts once initial barriers are surmounted.
Triggered at least in part by preformed anti-non-Gal antibodies, activation of human complement coupled with the absence of human complementary regulatory proteins lead to complement activation and contribute significantly to failure of GalTKO lungs within hours (9). These non-Gal antigens include carbohydrate, glycolipid, and perhaps protein structures. The most significant xenoantigen in GalTKO organs has been identified as N-Glycolylneuraminic acid (Neu5Gc).
Leukocyte and platelet sequestration occur even in experimental systems where antibody binding and complement activation are minimized, suggesting that both non-physiologic as well as physiologically appropriate adhesive mechanisms are likely to contribute to the problems observed with lung xenografts. Cytokine elaboration, cellular desialylation, and species incompatibilities between cell activation and regulatory pathways each contribute to sequestration and activation of circulating pig leukocytes and platelets by porcine endothelial cells (19–20).
Pulmonary vasculature and alveolar epithelium contain resident macrophages, including pulmonary intravascular macrophages that contribute significantly to injury of pig lungs perfused with human blood (21–22). In addition to releasing pro-inflammatory and pro-coagulant factors, pig alveolar lung and spleen macrophages and liver Kuppfer cells bind to and phagocytose human blood cells through innate cellular carbohydrate recognition by the porcine lectin sialoadhesin (23).
The signal regulatory protein alpha (SIRPα), an immune inhibitory receptor on macrophages, and CD47, a ubiquitously expressed ligand for SIRPα, serve to prevent autologous phagocytosis by providing a “don’t eat me” signal. Incompatibility in the CD47/SIRPα system across species may contribute to activation of circulating human monocyte-lineage cells and graft endothelial damage; phagocytosis of porcine cells released from the transplanted organ or infused systemically as part of a tolerance induction strategy appears to activate recipient monocytes (24,25). Species discordance of regulatory proteins similarly causes sequestration of circulating human natural killer (NK) cells: lack of negative regulatory signals such HLA-E on porcine endothelial cells, for example, leads to NK-mediated cytotoxicity through antibody-dependent and -independent mechanisms (26–28).
Physiologically inappropriate coagulation is observed in association with transplantation of pig organs or cells in multiple preclinical xeno models. Prolific coagulation pathway activation occurs at least in part as a result of inefficient inhibition or down-regulation of activated primate clotting factors due to incompatibilities between the pig and human thromboregulatory pathways. For example, porcine Tissue Factor Pathway Inhibitor (pTFPI) is a significantly less potent inhibitor of human Factor Xa than is hTFPI (29–30). In addition, although pig thrombomodulin is perfectly capable of binding human thrombin, the resulting thrombomodulin-thrombin complex is only about 10% as effective as an activator of Protein C (31); because activated Protein C (aPC) has both direct anticoagulant properties as well as potent anti-inflammatory effects on endothelial cells by activation of the endothelial protein C receptor (EPCR)/protease-activated receptor pathway, this cross-species incompatibility has both procoagulant and proinflammatory consequences. In addition, GPIb on quiescent human platelets binds weakly to human von Willebrand Factor, but undergoes conformational change to a high affinity state under flow shear stress. In contrast porcine vWF binds to and directly activates even human platelets in the absence of elevated shear stress, resulting in “non-physiologic” platelet activation and aggregation (32), thus contributing significantly to the prothrombotic milieu often observed with the vasculature of organ xenografts (33).
Experimental Models
Several models have been created to study lung xenotransplantation. In vivo lung xenotransplant experiments using non-human primates are particularly informative but extremely resource intensive and difficult to conduct. We use a pig-to-baboon model in our experiments, transplanting the left lung of a pig into the left thoracic cavity of a baboon (34). Flow probes on the pulmonary artery and ascending aorta allow for monitoring of flow through each lung. Transient occlusion of the right pulmonary artery diverts all the cardiac output through the left lung xenograft, and thus rigorously tests life-supporting capability of the xenograft.
Our ex vivo “paired” lung perfusion model has several benefits that can overcome the limitations of live animal studies (34). It allows us to compare results between the right and left lungs exposed to fresh human blood and efficiently determine the effect of a drug or other treatment added only to one side. In these experiments, blood is perfused by pump through a ventilated lung; treatments can be added to the blood or ventilation gas (35–36). In addition to being relatively cost-efficient and limiting non-human primate use to critical survival studies, perfusion with human blood is particularly valuable to study several situations where the antigenicity between humans and pigs is not accurately modeled in non-human primates; for example, humans have antibodies against Neu5Gc whereas pigs and non-human primates do not.
Several in vitro models have also been described to evaluate interactions that take place at the endothelial surface between porcine cells and human blood components. One versatile model uses microfluidic channels which can be coated with any number of ligands or cellular monolayers (37). Perfusates such as blood or blood components are flowed through the channels during microscopic observation under controlled shear stress conditions. The technique is significantly enhanced by the use of fluorescent staining to visualize adhesion, activation, or injury events involving the endothelium or blood cells.
Genetic modifications
Genomic editing is the foundation of recent progress in xenotransplantation. Numerous pathways have been successfully altered, and other targets have been proposed (38) (Figure 1). Most notably, key known porcine molecular targets of primate innate or adaptive immune mechanisms (Gal, Neu5Gc, β4Gal2NT) have been successfully “knocked out” (39). Recently we and others have shown that the GalTKO.Neu5Gc “double KO” is associated with prolonged pig lung physiology and survival during ex vivo perfusions (40–42). In addition key human regulatory genes have been inserted to overcome molecular incompatibilities in the inflammatory and coagulation cascades. For example human complementary regulatory proteins hCD46 (Membrane Cofactor Protein), hCD55 (Decay Accelerating Factor), and hCD59 (Membrane Attack Complex inhibitory protein) are associated with attenuated complement pathway amplification and prolonged lung survival. Human tissue factor pathway inhibitor (TFPI), thrombomodulin (TBM), and endothelial protein C receptor (EPCR) have been successfully introduced into pigs, generally with promising results (38, 43–51). In addition, hCD39 (an integral membrane protein expressed in endothelial and immune cell populations), and hHO-1 (Heme oxygenase-1, an anti-oxidant) have also been transfected into the pig genome; preliminary evidence with other organs and in the lung suggest that these modifications may reduce inflammation and improve function in ex vivo perfusion models (46–48). Finally, expression of the HLA-E transgene appears to limit endothelial damage by preventing NK-cell activation and resulting cytotoxicity (52–53). As deletion of vWF results in a pig with high risk of bleeding (54), current efforts seek to replace key segments of pvWF with the analogous human vWF epitope (38). We monitor platelet and neutrophil sequestration and activation, and thrombin, thromboxane, and histamine generation, as well as lung survival or pulmonary vascular resistance; we find that each of the genetic modifications discussed here is associated with some improvement in one or more of these parameters (47).
Figure 1.
- Genetic replacement of incompatible porcine vWF domains with analogous human epitopes
- Inhibition of Weibel-Palade body exocytosis and pulmonary vascular vasodilation using nitric oxide
- Pre-transplant depletion of Weibel-Palade bodies using exocytosis inducer DDAVP
- Selective or pan-selectin blockade
- Genetic knockout of porcine antigens (αGal, Neu5GC, β4GalNT2)
- Genetic knockout of porcine endogenous retroviruses, receptors involved in cellular recognition (ASGR1, sialoadhesin), or proteins necessary for expression of major histocompatibility antigens (CIITA)
- Genetic modification of donor to express human proteins, including surface complement regulatory molecules (hCD46, hCD55, hCD59) and anti-inflammatory proteins (hCTLA4-Ig, hHO-1, hTRAIL, hA20, hTNFRI-Fc);
- Inhibition of proteases using anti-inflammatory molecule alpha-1 antitrypsin
- Genetic modification of donor cells to express HLA-E. HLA-E binds the NKG2A/CD94 complex on circulating NK cells resulting in potent inhibition of NK cell cytotoxicity
- Genetic modification of donor to express coagulation regulatory proteins such as hTBM, hEPCR, hTFPI, hCD39
- GPIb, GPIIb/IIIa antagonists
- Pre-transplant depletion using liposomal clondronate
- Pre-transplant recipient antibody screening
- Inhibition of thromboxane and histamine mediated inflammation using thromboxane synthesis inhibitor 1-BIA and histamine receptor blockers, respectively
- aPC treatment of hEPCR expressing donor endothelium, activating the anti-apoptotic and anti-inflammatory EPCR/PAR1 pathway
- Genetic modification of donor to express hCD47
- Genetic modification of donor to overexpress human anti-inflammatory cytokines such as IL-10; antibody blockade of pro-inflammatory cytokines or their receptors (aIL-6R)
- Systemic anticoagulation
- Systemic immunosuppressants acting through multiple pathways, including standard agents such as corticosteroids, ATG, MMF; agents acting at other recipient sites such as novel costimulation blockers such as antiCD40 antibody 2C10.R4
- Desialylation inhibitors such as neuraminidase blockers oseltamivir, zanamivir. Alternatively, antibody mediated blockade of pig ASGR1 and sialoadhesin recognition receptors.
As each gene by itself has only limited effect, multigene targeting will be necessary for clinically meaningful lung xenograft outcomes. For this reason, perhaps the most important recent development in genetic engineering is the use of CRISP-Cas9 technology (55). In the past year this approach was used to inactivate all 62 copies of the porcine endogenous retrovirus (PERV) pol gene, which is universally integrated into the pig genome and has hypothetical risk of human transmission (56). The same group of investigators that inactivated PERV has used the same technique to simultaneously modify more than 20 other genes which regulate inflammation or coagulation pathways, although this data is not yet published (55,57).
Inducing systemic or organ-targeted expression in the mature pig is an alternative approach, allowing genetic engineering of specific donor animals as a proof-of-principle, or to apply beneficial interventions that prove deleterious when performed at the germ line level. For example, bronchoscopically delivered adenovirus containing human IL-10 during ex vivo porcine lung perfusions has promoted prolonged porcine lung allograft acceptance (58).
Targeted drug therapies
At present, recipient-directed therapeutic modalities are necessary to modulate the especially intense inflammatory response exhibited by lung xenografts which persists despite extensive genetic modifications tested to date (59). Approaches targeting innate immunity have shown some benefit in wild-type pig lungs; for example, liposomal clodronate has been used to deplete resident macrophages in the donor lung and found to aid in preventing hyperacute rejection (21, 22). In addition, thromboxane synthase inhibitors and histamine receptor blockers have also been found to decrease the inflammatory response with increased graft survival and improved function compared to untreated controls (21,60). Recently, pretreatment of hEPCR-expressing porcine endothelial cells with aPC has been shown to reduce endothelial damage as well as platelet adhesion and aggregation in vitro (43). Investigation is ongoing into the use of alpha-1-antitrypsin (AAT), a circulating anti-inflammatory glycoprotein which inhibits many proteases released by inflammatory cells, especially neutrophils. AAT has been used in other organ models and has been shown to decrease inflammatory gene expression and may inhibit xenograft rejection or even promote immunologic tolerance. Additional research is exploring blockade of selectins and integrins, cell adhesion molecules expressed on endothelial and inflammatory cells as well as platelets, to inhibit the early overwhelming platelet and neutrophil sequestration, which persists despite existing genetic and drug therapies.
Immunosuppression is another key arm of the long-term strategy to accomplish pig-to-human lung xenotransplantation. As evident from work in other organ xenograft models, immunosuppression of lung xenograft recipients will likely involve some standard clinically-used medications but also must effectively target the adaptive response to the xenograft. Antibodies directed against CD40 (anti-CD40) and CD154 (anti-CD154) appear to be particularly effective to prevent delayed xenograft rejection when used in the correct dose and with additional agents such as anti-CD20 or CTLA4-Ig (61). Anti-CD40 antibody 2C10.R4 significantly prolongs survival of xenografts and allografts in other organs, in some cases to over two years (3,17,18). An ultimate goal would be immune tolerance induction, and several approaches have been studied including induced mixed chimerism by infusion of hematopoietic stem cells, and T-cell “re-education” with donor thymic transplantation; both approaches show promise, and further research is ongoing in these areas (25).
Drug interventions aimed at coagulation dysregulation have also been associated with improved outcomes. We have used des-deoxy arginine vasopressin (DDAVP) administered to the pig prior to harvest, intending to reduce endothelial porcine vWF content by triggering exocytosis of Weibel-Palade bodies (62). This may, however, also sensitize the endothelium to injury, since P-selectin, the vasoconstrictor endothelin-1, the chemokine IL-8, and other pro-inflammatory and pro-coagulant molecules are also released. Replacement of the sites in porcine vWF that bind to human GP1b with the analogous human vWF regions may render this therapy moot, however. Meanwhile, treatment with GP1b antagonists is clinically feasible around the time of surgery, and partially attenuates platelet sequestration and activation driven by this cross-species incompatibility (63).
Conclusion
Although many challenges still abound, xenotransplantation of the lung and other organs has made great strides, and future developments are likely to further advance the field. The implications of successful clinical transplantation into humans would be transformative. Patients who are less sick at transplant would likely achieve greater benefits with less morbidity. Transplants could also be made readily available to patients with high titers of anti-human antibodies, as these rarely cross-react with pig antigens, and the pig organs will most likely be genetically engineered to modulate antibody-mediated injury. As we are better able to understand the residual mechanisms of lung xenograft injury, and alter the genome wherever possible to targeting known pathways and create more sophisticated “human-compatible” multigene swine, evaluating pig lungs’ potential for use in humans is likely to evolve from a formidable scientific puzzle to a surmountable technical challenge.
Key Points.
While functioning heart and kidney xenografts have survived for over one year after transplant in animal models, lung xenograft survival has been limited to days owing to particular organ-specific vulnerabilities arising from the lungs anatomy, perhaps confounded by additional physiologic or molecular characteristics.
Improvements in genetic engineering, including gene knock-out technology, cloning, TALENs, and especially CRISPR-Cas9, have allowed for many more and more rapid alterations to donor animal genetics than was previously possible, and greatly increased expectations for significantly accelerated advancement in this field.
Administration of targeted drugs pre- and post-transplant will almost certainly be necessary for optimal lung xenograft outcomes, and improvements in immunosuppressive strategies are showing great promise for other organ xenografts.
Acknowledgments
Agnes Azimzadeh, PhD provided valued editorial comments. The work described in this article from the Pierson/Azimzadeh lab represents many important scientific, technical, and logistical contributions over time from a large number of people, as well as essential continued support from our funders.
Financial Support and sponsorship
NIH NIAID U19 090959 and Sponsored Research Agreement with Revivicor, Inc
Footnotes
Conflicts of Interest
No conflicts of interest to declare.
References
- 1*.Mohiuddin MM, Singh KA, Corcoran PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014 Feb;14(2):488–489. doi: 10.1111/ajt.12562. This paper, although using heartmodels rather than lungs, was one of the first to demonstrate the potential for long-term xenograft survival and function using combined genetic and drug therapy including costimulation blockade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015 Jun;22(4):302–9. doi: 10.1111/xen.12174. Similar to the first reference, this paper was one of the first showing long-term survival and function in kidney xenografts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015 May;22(3):221–230. doi: 10.1111/xen.12166. Demonstrates value of antibody screening and costimulation blockade, albeit in kidney xenografts, these strategies are likely to be of benefit in lung xenotransplantation as well. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.den Hengst WA, Gielis JF, Lin JY, et al. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010 Nov;299(5):H1283–9. doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 5.Ranieri VM, Suter PM, De Tullio R, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999 Jul;282(1):54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services. Organ Transplantation: OPTN & SRTR Annual Data Report. 2012. [Google Scholar]
- 7.Pierson RN. Antibody-mediated xenograft injury: mechanisms and protective strategies. Transpl Immunol. 2009 Jun;21(2):65–9. doi: 10.1016/j.trim.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galili U, Shohet SB, Kobrin E, et al. Man, apes, and Old World monkeys differ from other mammals in the expression of alphagalactosyl epitopes on nucleated cells. J Biol Chem. 1988 Nov;263:17755–17762. [PubMed] [Google Scholar]
- 9.Nguyen BH, Azimzadeh AM, Schroeder C, et al. Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection. Xenotransplantation. 2011 Mar;18(2):94–107. doi: 10.1111/j.1399-3089.2011.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen BH, Azimzadeh AM, Zhang T, et al. Life-supporting function of genetically modified swine lungs in baboons. J Thorac Cardiovasc Surg. 2007 May;133(5):1354–63. doi: 10.1016/j.jtcvs.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 11.Phelps CJ, Koike C, Vaught TD, et al. Production of a1,3-galactosyltransferase-deficient pigs. Science. 2003 Jan 17;299(5605):411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Stawinski GV, Daggett CW, Lau CL, et al. Non-anti-Gala1-3Gal antibody mechanisms are sufficient to cause hyperacute lung dysfunction in pulmonary xenotransplantation. J Am Coll Surg. 2002 Jun;194(6):765–73. doi: 10.1016/s1072-7515(02)01162-6. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Galknockout pig kidneys. Nat Med. 2005 Dec;11(12):1295–8. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrne GW, Stalboerger PG, Davila E, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation. 2008 Jul-Aug;15(4):268–76. doi: 10.1111/j.1399-3089.2008.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne GW, Stalboerger PG, Davila E, et al. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation, Transplantation. 2011 Feb 15;91(3):287–92. doi: 10.1097/TP.0b013e318203c27d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardona K, Korbutt G, Zilas G, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006 Mar;12(3):304–6. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 17.Mohiuddin M, Singh A, Corcoran P, et al. Critical need of continuous co-stimulation blockade with anti CD40 antibody (2C10.R4) for long-term maintenance of GTKO.HCD46.hTBM pig cardiac xenograft survival in baboons [abstract] Xenotransplantation. 2015 Nov;22(S144):810. Presented at 2015 IXA conference, this abstract discusses importance of co-stimulation blockade; although in cardiac xenografts, similar strategy is likely beneficial to lungs as well. [Google Scholar]
- 18.Miller J, Singh A, Eckhaus M, et al. Histological evidence of acute rejection after terminating anti CD40 antibody (2C10R4) treatment in GTKO.hCD46.htbm pig to baboon cardiac xenografts surviving for over one year [abstract] Xenotransplantation. 2015 Nov;22(S145):811. Histological evidence demonstrating similar findings to those discussed in the preceding reference, this was presented at 2015 IXA conference. [Google Scholar]
- 19.French B, Burdorf L, Dahi S, et al. IL-8 elaboration may contribute to GalTKO.hCD46 lung xenograft inflammation [abstract] Xenotransplantation. 2015 Nov;22(S195):1055. This abstract presented at the 2015 IXA conference found that IL-8 may contribute to early neutrophil sequestration and activation in ex vivo perfusion models. [Google Scholar]
- 20.French B, Benipal P, Harris D, et al. Cellular sialylation and activation regulate xenogeneic neutrophil adhesion [abstract] Xenotransplantation. 2015 Nov;22(S67):482. This abstract presented at the 2015 IXA conference discusses the role of desialylation in neutrophil sequestration, and a possible target for intervention. [Google Scholar]
- 21.Collins BJ, Blum MG, Parker RE, et al. Thromboxane mediates pulmonary hypertension and lung inflammation during hyperacute lung rejection. J Appl Physiol. 2001 Jun;90(6):2257–68. doi: 10.1152/jappl.2001.90.6.2257. [DOI] [PubMed] [Google Scholar]
- 22.Cantu E, Gaca JG, Palestrant D, et al. Depletion of pulmonary intravascular macrophages prevents hyperacute pulmonary xenograft dysfunction. Transplantation. 2006 Apr 27;81(8):1157–64. doi: 10.1097/01.tp.0000169758.57679.2a. [DOI] [PubMed] [Google Scholar]
- 23.Brock LG, Delputte PL, Waldman JP, et al. Porcine sialoadhesin: a newly identified xenogeneic innate immune receptor. Am J Transplant. 2012 Dec;12(12):3272–82. doi: 10.1111/j.1600-6143.2012.04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ide K, Wang H, Tahara H, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007 Mar 20;104(12):5062–6. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachs D, Sykes M, Yamada K. Achieving tolerance in pig-to-primate xenotransplantation: Reality or fantasy. Transpl Immunol. 2009 Jun;21(2):101–105. doi: 10.1016/j.trim.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalfoun B, Barrat D, Watier H, et al. Development of an ex vivo model of pig kidney perfused with human lymphocytes; Analysis of xenogeneic cellular reactions. Surgery. 2000 Sep;128(3):447–57. doi: 10.1067/msy.2000.107063. [DOI] [PubMed] [Google Scholar]
- 27.Rieben R, Seebach JD. Xenograft rejection: IgG 1, complement and NK cells team up to activate and destroy the endothelium. Trends Immunol. 2005 Jan;26(1):2–5. doi: 10.1016/j.it.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Lilienfeld BG, Crew MD, Forte P, et al. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation. 2007 Mar;14(2):126–34. doi: 10.1111/j.1399-3089.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen D, Riesbeck K, McVey J, et al. Regulated inhibition of coagulation by porcine endothelial cells expressing P-selectin-tagged hirudin and tissue factor pathway inhibitor fusion proteins. Transplantation. 1999 Sep 27;68(6):832–9. doi: 10.1097/00007890-199909270-00016. [DOI] [PubMed] [Google Scholar]
- 30.Kopp CW, Siegel JB, Hancock WW, et al. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation. 1997 Mar 15;63(5):749–58. doi: 10.1097/00007890-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 31.Roussell JC, Moran CJ, Salvaris EJ, et al. Pig thrombomodulin binds human thrombin but is a poor cofactor for activation of human protein C and TAFI. Am J Transplant. 2008 Jun;8(6):1101–12. doi: 10.1111/j.1600-6143.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 32.Gaca JG, Lesher A, Aksoy O, et al. The role of the porcine von Willebrand factor: baboon platelet interactions in pulmonary xenotransplantation. Transplantation. 2002 Dec 15;74(11):1596–603. doi: 10.1097/00007890-200212150-00018. [DOI] [PubMed] [Google Scholar]
- 33.Azimzadeh AM, Kelishadi SS, Ezzelarab MB, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation. 2015 Jul-Aug;22(4):310–6. doi: 10.1111/xen.12176. This article discusses causes of early xenograft failure and progress towards made in targeting these pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burdorf L, Azimzadeh A, Pierson RN. Xenogeneic lung transplantation models. Methods Mol Biol. 2012 Apr;885:169–89. doi: 10.1007/978-1-61779-845-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pierson RN, Dorling A, Ayares D, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009 Sep-Oct;16(5):263–280. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez PG, Bittle GJ, Burdorf L, et al. State of Art: clinical ex vivo lung perfusion: rationale, current status, and future directions. J Heart Lung Transplant. 2012 Apr;31(4):339–48. doi: 10.1016/j.healun.2012.01.866. [DOI] [PubMed] [Google Scholar]
- 37.Harris BG, Benipal PK, Cheng X, et al. Four-dimensional characterization of thrombosis in a live-cell, shear-flow assay: development and application to xenotransplantation. PLoS One. 2015 Apr 1;10(4):e012301. doi: 10.1371/journal.pone.0123015. This paper describes a relatively new in vitro assay using microfluidic channels coated with porcine endothelial monolayers and perfused with human blood or serum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper DKC, Ekser B, Burlak C, et al. Clinical lung xenotransplantation - what donor genetic modifications may be necessary? Xenotransplantation. 2012 May-Jun;19(3):144–58. doi: 10.1111/j.1399-3089.2012.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation. 2015 May-Jun;22(3):194–202. doi: 10.1111/xen.12161. This paper describes properties of newly developed pigs with genetic knock-out of several non-Gal antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lutz AJ, Li P, Estrada JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and Galactose α-1,3-Galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013 Jan-Feb;20(1):27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 41*.Burdorf L, Ali F, Ramsoondar J, et al. N-Glycolylneuraminic acid (Neu5GC) knock-out in GalTKO.HCD46 pig lungs improves pulmonary function in a xenogeneic pig-to-human lung perfusion model [abstract] Xenotransplantation. 2015 Nov;22(S84):552. This study shows the additional benefit of carbohydrate antigen Neu5Gc knockout in GalTKO lungs. Other such antigens may also require knock-out. [Google Scholar]
- 42.Lutz AJ, Li P, Estrada JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and Galactose a-1,3-Galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013 Feb;20(1):27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 43.French B, Harris D, Benipal P, et al. Activated protein C modulates the thrombotic phenotype and vascular permeability of GaltKO.hCD46 porcine endothelium expressing human endothelial protein C receptor [abstract] Xenotransplantation. 2015 Nov;22(S67):483. This abstract presented at the 2015 IXA conference demonstrated the benefit of recombinant aPC when administered to EPCR expressing porcine endothelial cells in in vitro studies. [Google Scholar]
- 44.Iwase H, Miyagawa Y, Lee W, et al. Regulation of the inflammatory response by human thrombomodulin transgenic pigs in xenotransplantation [abstract] Xenotransplantation. 2015 Nov;22(S114):641. This abstract presented at the 2015 IXA conference discussed regulation of inflammatory pathways by hTBM and its benefits. [Google Scholar]
- 45.Diamond LE, Quinn CM, Martin MJ, et al. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation. 2001 Jan 15;71(1):132–42. doi: 10.1097/00007890-200101150-00021. [DOI] [PubMed] [Google Scholar]
- 46.Miyagawa S, Yamamoto A, Matsunami K, et al. Complement regulation in the GalT KO era. Xenotransplantation. 2010 Jan-Feb;17(1):11–25. doi: 10.1111/j.1399-3089.2010.00569.x. [DOI] [PubMed] [Google Scholar]
- 47**.Harris DG, Quinn KJ, French BM, et al. Metaanalysis of the independent and cumulative effects of multiple genetic modifications on pig lung xenograft performance during ex vivo perfusion with human blood. Xenotransplantation. 2015 Mar-Apr;22(2):102–11. doi: 10.1111/xen.12149. This article contains a large meta-analysis of ex-vivo lung xenoperfusions of numerous genetic modifications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daggett CW, Yeatman M, Lodge AJ, et al. Swine lungs expressing human complement-regulatory proteins are protected against acute pulmonary dysfunction in a human plasma perfusion model. J Thorac Cardiovasc Surg. 1997 Feb;113(2):390–8. doi: 10.1016/S0022-5223(97)70337-4. [DOI] [PubMed] [Google Scholar]
- 49.Westall GP, Levvey BJ, Salvaris E, et al. Sustained function of genetically modified porcine lungs in an ex vivo model of pulmonary xenotransplantation. J Heart Lung Transplant. 2013;32:1123–1130. doi: 10.1016/j.healun.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Kulick DM, Salerno CT, Dalmasso AP, et al. Transgenic swine lungs expressing human CD59 are protected from injury in a pig-to-human model of xenotransplantation. J Thorac Cardiovasc Surg. 2000;119:690–699. doi: 10.1016/S0022-5223(00)70003-1. [DOI] [PubMed] [Google Scholar]
- 51.Burdorf L, Stoddard T, Zhang T, et al. Expression of human CD46 modulates inflammation associated with GalTKO lung xenograft injury. Am J Transplant. 2014 May;14(5):1084–95. doi: 10.1111/ajt.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubicki N, Laird C, Burdorf L, et al. The effect of human leukocyte antigen-E expression on GalTKO.hCD46 lung xenograft survival and injury in an ex-vivo xenoperfusion model [abstract] Xenotransplantation. 2015 Nov;22(S193):1051. This abstract was presented at the 2015 IXA conference; it discusses benefit of added HLA-E transgenes to GalTKO.hCD46 lungs, with improved lung survival and function in an ex vivo perfusion model, and decreased NK cell cytotoxicity in in vitro assays. [Google Scholar]
- 53.Abicht J, Bongoni A, Mayr T, et al. Ex-vivo perfusion of alpha-Gal knockout, hCD46/HLA-E double transgenic pig hearts [abstract] Xenotransplantation. 2015 Nov;22(S184):933. Supporting evidence for preceding reference, with evidence of protection in HLA-E expressing cardiac xenografts. Abstract presented at the 2015 IXA conference. [Google Scholar]
- 54.Meyer C, Wolf P, Romain N, et al. Use of von Willebrand diseased kidney as donor in a pig-to-primate model of xenotransplantation. Transplantation. 1999 Jan 15;67(1):38–45. doi: 10.1097/00007890-199901150-00006. [DOI] [PubMed] [Google Scholar]
- 55.Reardon S. Gene-editing record smashed in pigs [Internet] Nature news. 2015 Oct 06; Available from http://www.nature.com/news/gene-editing-record-smashed-in-pigs-1.18525 This is a news article in Nature highlighting the significance of new gene-editing technology on the field of xenotransplantation.
- 56**.Yang L, Güell M, Niue D, et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs) Science. 2015 Nov;350(6264):1101–1104. doi: 10.1126/science.aad1191. This paper describes CRISPR-Cas9 technology as well as PERV integration into the porcine genome and the successful removal of the PERV pol genes. This same group also used the same techniques to target over 20 immune and clotting regulatory genes. [DOI] [PubMed] [Google Scholar]
- 57**.Guell M, Yang L, Shrock E, et al. Discovery and implementation of novel pig genotypes using advanced genome technologies with increased survival in primates [abstract] Xenotransplantation. 2015 Nov;22(S181):922. As in the Yang paper preceding this reference, this group used the CRISP-Cas9 technology to edit multiple different genes. Presented at 2015 IXA conference. [Google Scholar]
- 58.Cypel M, Liu M, Rubacha M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med. 2009 Oct 28;1(4):4ra9. doi: 10.1126/scitranslmed.3000266. [DOI] [PubMed] [Google Scholar]
- 59.Azimzadeh A, Cheng N, Cheng X, et al. Multiplex profiling of plasma proteins after xenogeneic lung exposure [abstract] Xenotransplantation. 2015 Nov;22(S181):1056. This abstract presented at the 2015 IXA conference demonstrates persistence of activation of inflammatory pathways despite current treatment strategies, and possible future interventions. [Google Scholar]
- 60.Schwartz E, Burdorf L, Parsell D. Effects of a thromboxane synthase inhibitor, 1-BIA, on eicosanoid metabolism in a GalTKO.hCD46 xenogeneic lung perfusion model [abstract] Xenotransplantation. 2015 Nov;22(S184):934. Presented at the 2015 IXA conference, this abstract showed that drugs targeting inflammatory pathways, in this case thromboxane, have benefit in lung xenotransplantation. [Google Scholar]
- 61.Ezzelarab MB, Ekser B, Echeverri G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012 Jul-Aug;19(4):221–32. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim YT, Lee HJ, Lee SW, et al. Pre-treatment of porcine pulmonary xenograft with desmopressin: a novel strategy to attenuate platelet activation and systemic intravascular coagulation in an ex-vivo model of swine-to-human pulmonary xenotransplantation. Xenotransplantation. 2008 Feb;15(1):27–35. doi: 10.1111/j.1399-3089.2008.00445.x. [DOI] [PubMed] [Google Scholar]
- 63.Burdorf L, Zhang T, Rybak E, et al. Combined GP1b and GP2b/3a blockade prevents sequestration of platelets in a pig-to-human lung perfusion model. J Heart Lung Transpl. 2012 Apr;31(4):S106. [Google Scholar]