Abstract
Aim of the Study:
Initial differentiation of sepsis from systemic inflammatory response syndrome (SIRS) is of prime importance for early institution of appropriate treatment. This study aimed to compare the differential diagnostic efficacy of absolute eosinophil count (AEC - a routinely available economic marker) with total leukocyte count (TLC) and procalcitonin (PCT - a costly marker available only in specialized settings).
Materials and Methods:
In this prospective observational study, 170 patients of sepsis (severe sepsis = 125; SIRS = 45) were enrolled. AEC, TLC, and PCT were measured in the blood of all patients at the time of admission and data analyzed statistically.
Results:
Median AEC was 0 cells/mm3 in both SIRS and sepsis. TLC and PCT levels were significantly higher (P < 0.001) in culture negative, culture positive, and overall sepsis groups in comparison to SIRS group. At a cutoff of < 50 cells/mm3, AEC demonstrated a sensitivity and specificity of 23% and 68%, respectively. The sensitivity, specificity, positive predictive value, and negative predictive value of TLC were 57%, 71%, 85%, 37% and of PCT were 82.4%, 82.2%, 93%, and 63%, respectively with area under curve of 0.455 for AEC, 0.640 for TLC, 0.908 for PCT.
Conclusions:
This study suggests that eosinopenia is not a reliable diagnostic tool to differentiate sepsis from SIRS. PCT and TLC are better differential diagnostic biomarkers.
Keywords: Absolute eosinophil count, negative predictive value, procalcitonin, sepsis, systemic inflammatory response syndrome
Introduction
The last decade has witnessed advances in tertiary care and management of sepsis leading to a lower fatality rate in these patients. However, owing to the significant increase in prevalence, the number of deaths due to sepsis is still on the rise.[1,2] Emerging antibiotic resistance may have contributed to this increased incidence and hence mortality.[3]
According to the American College of Chest Physicians (ACCP)/Society of Critical Care Medicine (SCCM) guidelines 2001, sepsis is accepted as a systemic inflammatory response syndrome (SIRS) in the presence of suspected or proven infection.[4] Although SIRS is inherent in the definition of sepsis, it is important to differentiate SIRS patients from sepsis, since their management is quite different. However, the clinical parameters for SIRS such as alteration in heart rate, respiratory rate, and temperature are nonspecific and are often manifested in most critically ill patients.[5,6] Microbial culture remains the gold standard to diagnose sepsis till date, but unfortunately, culture results are not immediately available. Moreover, a significant percentage of sepsis patients remain culture negative.[7,8] Diagnosis of this pool of sepsis patients is therefore based on clinical parameters and subjective clinical judgment, making these patients vulnerable to be misdiagnosed as SIRS. Besides, noninfectious SIRS patients misdiagnosed as sepsis might get inappropriately treated with antibiotics, contributing to emerging antibiotic resistance. Hence, early diagnosis of these noninfectious SIRS patient is vital in the reduction of inappropriate antibiotic usage, consequently decreasing antibiotic resistance.
Since sepsis and SIRS are inflammatory conditions, several inflammatory cytokines have been studied for their potential diagnostic and prognostic role in sepsis. Among these, procalcitonin (PCT) has demonstrated superior sensitivity and specificity and is often used in intensive care settings. Recently, a study demonstrated that PCT can be used as a differential diagnostic characteristic between SIRS and culture negative sepsis patients.[9] However, PCT estimation remains expensive and is unavailable in many resource deficient settings. Hence, other biomarkers which are inexpensive, easily available, and enable clinicians to differentiate sepsis from SIRS would be helpful, particularly in intensive care settings toward decreasing mortality and appropriate antibiotic use.
During acute infection, nonspecific granulocytopenia has been demonstrated after intramuscular activation or injection of chemotactic factors.[10] Zappert was the first to report eosinopenia in response to acute infection.[11] Eosinophils constitute 0–6% of the peripheral white blood cells with an upper limit of 350 cells/μl and its production is regulated by granulocyte macrophage colony stimulating factor, interleukins (ILs)-3 and IL-5.[12,13,14] Absolute eosinophil count (AEC) is an economical and easily available investigation in rural and urban hospitals. A few scientists have evaluated its usefulness in differentiating between sepsis and SIRS.[15,16,17] However, their findings are not conclusive with regard to its specificity and sensitivity in the intensive care setting.
Hence, we attempted to evaluate the diagnostic accuracy of eosinopenia to differentiate sepsis from noninfectious SIRS (recent onset pancreatitis and trauma). It was compared with total leukocyte count (TLC) and PCT.
Materials and Methods
Clinical setting and study design
This was a prospective, observational, single-center study, conducted in patients with sepsis and noninfectious SIRS in a tertiary care Intensive Care Unit (ICU) setting. Patient screening, enrollment and PCT estimations were performed prospectively. TLC and AEC measurements were done as a part of the routine clinical investigation. The study was approved by the institutional ethics committee. Informed consent was obtained from legal representatives of the patients.
Patient population
All adult patients (>18 years); n = 170 admitted from the community to the ICU were screened and patients diagnosed with noninfectious SIRS, sepsis, severe sepsis or septic shock (according to the established consensus sepsis definition) were enrolled in this study.[4]
Patients transferred from other ICU's, postoperative, immunocompromised, pregnant and with malignancy or those who died or were discharged in the next 24 h were excluded from the study. Patients with bilateral pneumonia (suspected viral infection) and diagnosed tropical diseases such as malaria, dengue, leptospira, and rickettesiae were also excluded.
Patient's demographics, principal diagnosis, clinical history, and baseline characteristics were collected at the time of admission. Acute Physiology and Chronic Health Evaluation Score (APACHE II) were calculated from the worst value of the parameters within first 24 h to evaluate severity of illness in enrolled patients. Following ACCP/SCCM guidelines, two sets of blood cultures, urine culture, sputum culture (in nonintubated patients), endotracheal culture (in intubated patients), and high vaginal swab culture (where puerperal sepsis was suspected) were sent.
Blood sampling and laboratory measurement
On the day of admission, blood samples were collected for AEC, TLC and PCT estimation. To determine AEC and TLC, samples were collected in ethylenediaminetetraacetic acid containing tubes. The counts were performed by automated analyzer (Beckman Coulter, Fullerton, CA, USA). Blood samples for PCT estimation were obtained in serum evacuated separator tubes. Samples were centrifuged for the separation of serum at 3000 rpm for 10 min. PCT estimation was done with time-resolved amplified cryptate emission technology by measuring the signal that is emitted from an immunocomplex with time delay (Kryptor PCT; BRAHMS, Henningsdorf, Germany).
Patient categories
Sepsis groups
Initially, all patients with a source of clinically suspected infection, fulfilling inclusion criteria were enrolled in the sepsis group. The diagnosis of bacterial infection in these patients was done on the basis of findings of a clinical focus of infection. Intra-abdominal infection was diagnosed in case of the exudative ascitic tap with increased polymorphonuclear cell count. Bacterial pneumonia was confirmed by X-ray showing lobar infiltrate. Urosepsis was suspected with signs of urinary tract infection and with a raised leukocyte count in the urine (>10 pus cells/high-power field [hpf]), and signs of pyelonephritis by ultrasonography. Cellulitis was diagnosed by the skin signs, i.e., lesions. Puerperal sepsis was suspected in postpartum patients with signs of pelvic pain, abnormal, or foul-smelling vaginal discharge (presence of pus).[9]
After obtaining culture reports, sepsis group was further divided into culture negative and culture positive groups. A blood culture was considered positive if any significant pathogenic bacterial organism was grown from twin cultures taken from different sites. Respiratory secretions were considered positive for infection if many polymorpohonuclear cells were present along with colony count >105. Urine culture was considered positive if there were >10 pus cells/hpf, along with single organism cultured with >105 colony forming units/ml.
Noninfectious systemic inflammatory response syndrome group
Patients with two or more signs of SIRS with recent onset pancreatitis and trauma (within 24 h) without any evidence of infection were enrolled in this group.
Statistical analysis
The results are presented as mean (range) or median (25th–75th percentiles [interquartile range]). Mann–Whitney U-test was applied to compare nonparametrically distributed variables. To compare three independent variables, one-way analysis of variance (parametric distribution) or Kruskal–Wallis (nonparametric distribution) test was applied. Chi-square was used to compare categorical variables. Receiver operating characteristic curve (ROC) was plotted to calculate the area under the curve (AUC) and obtaining cutoff values. For eosinophil count, <50 cells/mm3 was taken as cutoff point. For TLC and PCT, best cutoff values were calculated. At these cutoff values sensitivities, specificities predictive values and likelihood ratios were calculated. The value of P < 0.05 was considered significant and <0.005 highly significant. All statistical analyses were performed using Statistical Package for Social Survey version 17 (SPSS Inc., Chicago, IL, USA).
Results
Demographic, clinical, and baseline characteristics of study population
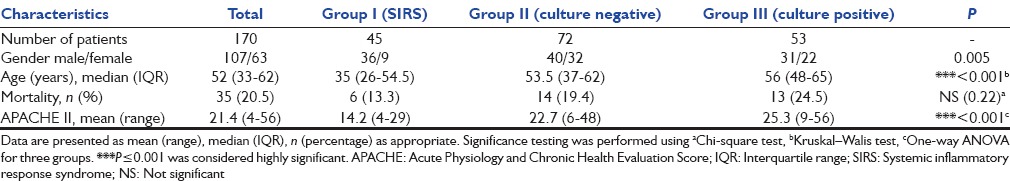
Of 170 patients enrolled, 125 were severe sepsis and septic shock patients, rest 45 were noninfectious SIRS patients. Among 45 SIRS patients enrolled, 25 were diagnosed with recent onset pancreatitis and rest 20 met with trauma in the last 24 h. Suspected sepsis patients were categorized into two groups later, depending on the culture report: As culture negative (n = 72) and culture positive (n = 53). The demographic profile and outcome of all study groups are depicted in Table 1. Number of males and females were significantly different in SIRS group as compared to both sepsis groups (P < 0.005). Median age and APACHE II scores were significantly lower in SIRS group as compared to sepsis group (P < 0.001). Overall mortality observed was 20.5%. Although mortality was different between SIRS and sepsis group, it did not achieve statistical significance [Table 1].
Table 1.
Characteristics of the study population
The initial source of suspected infection in sepsis patients was respiratory (41%) followed by abdominal (25%), urinary tract (18%).
Diagnostic performances of study parameters
Levels of study parameters
Levels were measured on the 1st day of the ICU admission and were compared between classified study groups. There was no difference observed in AEC of SIRS group when compared with all sepsis and sepsis groups (P = not significant). The median PCT and TLC levels were significantly higher in overall sepsis patients, culture negative and culture positive groups as compared to SIRS.
On the day of admission, mean AEC in all patients (sepsis and SIRS combined) was 84 cells/mm3 (median, 0 cells/mm3). Although in SIRS group, mean eosinophil count was 85 cells/mm3 (median, 0 cells/mm3) and in sepsis group, it was 77 cells/mm (median, 0 cells/mm3), but this difference was not statistically significant.
Mean TLC levels of all the patients was 16,600 cells/mm3. In SIRS group, it was 15,100 cells/mm3, whereas in the sepsis group, it was 20,600 cells/mm3. This difference was found to be statistically significant (P < 0.001). Median PCT levels were 0.98 ng/ml and 18.6 ng/ml in SIRS group and sepsis group, respectively (P < 0.001). Median levels with interquartile range in all study groups are illustrated in Figure 1. Both TLC and PCT demonstrated significant difference between SIRS and sepsis as well as both groups of culture negative and culture positive sepsis.
Figure 1.
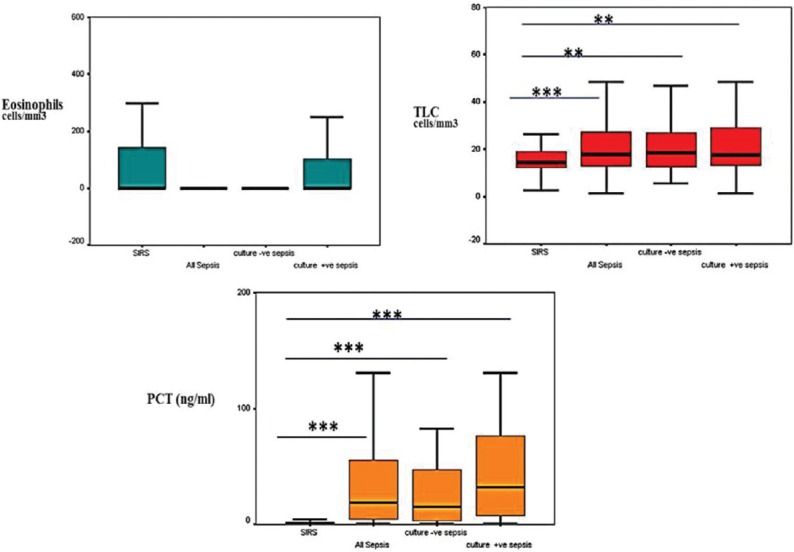
Box plot presentation of absolute eosinophil count, total leucocyte count and procalcitonin levels in different study groups, central line represent median, boxes represent 25th–75th percentiles; whiskers, 95% confidence interval. Noninfectious systemic inflammatory response syndrome group was compared with all sepsis, culture negative and culture positive sepsis groups, *P < 0.05, **P < 0.01, ***P < 0.001
Receiver operating characteristic curve analysis
To assess the differentiating ability of study parameters ROC curve analysis was performed between all sepsis groups and SIRS group.
All sepsis versus systemic inflammatory response syndrome
ROC analysis demonstrated average AUC of 0.455 for eosinopenia in differentiating sepsis from SIRS, indicating a poor discriminating power. TLC and PCT showed significant AUC of 0.640 and PCT 0.908 (average), respectively [Figure 2].
Figure 2.
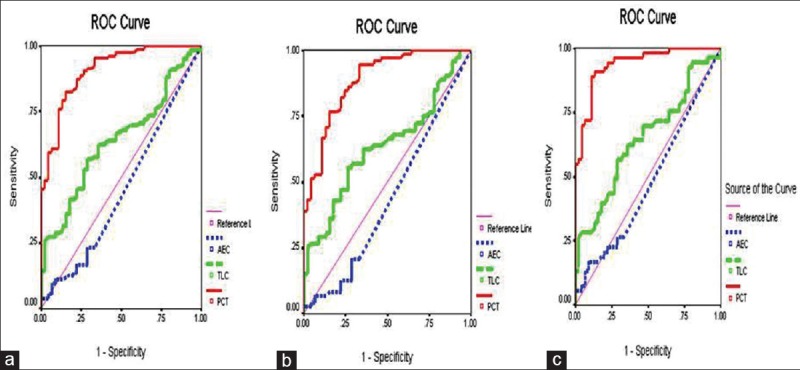
Receiver operating characteristic curves for absolute eosinophil count, total leucocyte count and procalcitonin in distinguishing systemic inflammatory response syndrome patients from all sepsis (a), culture negative sepsis (b), and culture positive sepsis (c) groups
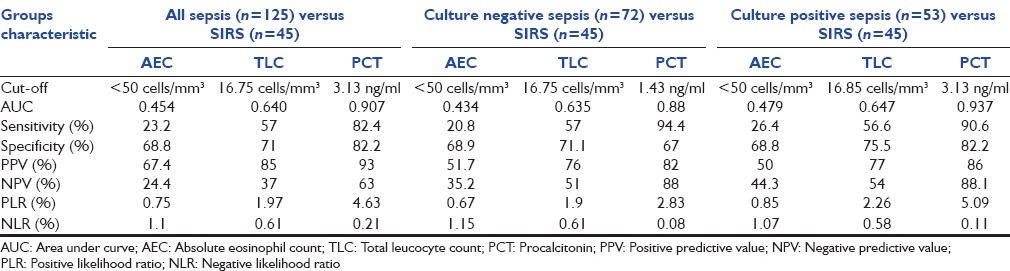
At a cutoff value of < 50 cells/mm3, AEC yielded a sensitivity of 23%, 21%, 26% (all sepsis, culture negative, culture positive sepsis groups) and specificity of 69% (all sepsis groups). TLC demonstrated best cutoff value of 16,000 cells/mm3 with a sensitivity of 57% (all sepsis groups) and specificity of 71%, 75% (all sepsis, culture positive sepsis). Best cutoff of PCT for all sepsis was 3.13 ng/ml (sepsis and culture positive) and 1.43 ng/ml with maximum sensitivity of 94% (culture negative sepsis) and specificity of 82% (all sepsis and culture positive sepsis). Results of detailed ROC analysis are tabulated in Table 2.
Table 2.
Area under curve, sensitivity, specificity, predictive values and likelihood ratios of all variables to differentiate patients with sepsis/culture negative/culture positive sepsis from systemic inflammatory response syndrome
Discussion
Early proper diagnosis and differentiation of sepsis patients from noninfectious SIRS patients in ICU setting is imperative for appropriate treatment selection and favorable outcomes. Biomarkers aid clinical judgment in this objective for intensivists. Existing biomarkers such as PCT, IL-6, and others though helpful, are however expensive and not easily available in resource-deficient settings. Exploration of additional biomarkers can aid diagnosis and differentiation as standalone parameters. This study aimed at investigating the diagnostic accuracy of eosinopenia as compared to PCT and TLC in sepsis and noninfectious causes of inflammation.
Literature shows conflicting results when studying eosinopenia as a biomarker for diagnosing infection. In this study, AEC demonstrated low sensitivity, moderate specificity and poor predictive value as compared to TLC and PCT in differentiating all sepsis, culture negative, culture positive sepsis from SIRS in comparison with PCT. Similar to our results, Smithson et al. showed no correlation between eosinopenia and infections.[18] Setterberg et al. also indicated that eosinopenia is not a valuable marker for infection.[19] On the contrary, two recent studies by Abidi et al. and Shaaban et al. showed a strong relationship between bacterial infection and eosinopenia suggesting that eosinopenia could differentiate between sepsis and noninfectious inflammation response, difficult to differentiate clinically.[15,16] This might be due to the inclusion of different patient groups in the noninfectious category as compared to our study. Patients enrolled in noninfectious groups of the above-mentioned studies (Abidi and Shaaban et al.) had a definite clinical diagnosis such as cardiac conditions, pulmonary diseases, hemorrhage, and therefore posed no difficulty in differentiation. On the other hand, our noninfectious patient group includes those patients’ group with significant SIRS, whose clinical picture posed a dilemma (recent onset pancreatitis and trauma; within 24 h).
To explain the absence of significance of eosinopenia in differentiating between sepsis and SIRS in our study, we postulate that in SIRS and sepsis, eosinopenia is secondary to the inflammatory response which is a common feature to both the groups. Initially, this eosinopenic response is a result of rapid peripheral sequestration of circulating eosinophils, probably in response to chemotactic factors, for example, C5a and fibrin factors which are released at the inflammatory site. Thus, the circulating eosinophils are chemotactically drawn to the inflammatory site, and therefore are not in circulation. Later, an additional mechanism comes into play in the form of suppression of migration of mature eosinophils from the bone marrow probably due to the release of adrenal glucocorticoids and epinephrine released by the stress of inflammation.[20,21,22]
The strength of this study is targeting the most appropriate population in the noninfectious group which is really difficult to differentiate clinically from sepsis. The prime purpose of our study was to investigate eosinopenia in sepsis diagnosis due to low cost as compared to other at present available markers. Unfortunately, we failed to find any significance of eosinopenia in differentiating sepsis from SIRS. We did not find any significant difference in eosinophil count of two groups because noninfectious causes whether it is pancreatitis or trauma; are also due to inflammation; a primary factor for causing eosinopenia.
Conclusion
PCT still remains the most promising biomarker till date. However considering the high cost of the test and its nonavailability in peripheral hospitals worldwide renders PCT a nonideal biomarker. Therefore, the urge of this study was to find a cheaper biomarker of sepsis. Due to lack of significance observed in this study, eosinophil count cannot be recommended as a differentiating marker of systemic infection vis-a-vis other noninfectious causes of inflammation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: A challenge for patients and hospitals. NCHS Data Brief. 2011;62:1–8. [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 3.van der Poll T, Opal SM. Host-pathogen interactions in sepsis. Lancet Infect Dis. 2008;8:32–43. doi: 10.1016/S1473-3099(07)70265-7. [DOI] [PubMed] [Google Scholar]
- 4.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 5.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 6.Peres Bota D, Lopes Ferreira F, Mélot C, Vincent JL. Body temperature alterations in the critically ill. Intensive Care Med. 2004;30:811–6. doi: 10.1007/s00134-004-2166-z. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 9.Anand D, Das S, Bhargava S, Srivastava LM, Garg A, Tyagi N, et al. Procalcitonin as a rapid diagnostic biomarker to differentiate between culture-negative bacterial sepsis and systemic inflammatory response syndrome: A prospective, observational, cohort study. J Crit Care. 2015;30:218.e7–12. doi: 10.1016/j.jcrc.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Bass DA, Gonwa TA, Szejda P, Cousart MS, DeChatelet LR, McCall CE. Eosinopenia of acute infection: Production of eosinopenia by chemotactic factors of acute inflammation. J Clin Invest. 1980;65:1265–71. doi: 10.1172/JCI109789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zappert J. About the occurring of eosinophilic cells in human bleeding. Z Klin Med. 1893;23:227–308. [Google Scholar]
- 12.Metcalf D, Burgess AW, Johnson GR, Nicola NA, Nice EC, DeLamarter J, et al. In vitro actions on hemopoietic cells of recombinant murine GM-CSF purified after production in Escherichia coli: Comparison with purified native GM-CSF. J Cell Physiol. 1986;128:421–31. doi: 10.1002/jcp.1041280311. [DOI] [PubMed] [Google Scholar]
- 13.Metcalf D, Begley CG, Nicola NA, Johnson GR. Quantitative responsiveness of murine hemopoietic populations in vitro and in vivo to recombinant multi-CSF (IL-3) Exp Hematol. 1987;15:288–95. [PubMed] [Google Scholar]
- 14.Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, et al. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abidi K, Khoudri I, Belayachi J, Madani N, Zekraoui A, Zeggwagh AA, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care. 2008;12:R59. doi: 10.1186/cc6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaaban H, Daniel S, Sison R, Slim J, Perez G. Eosinopenia: Is it a good marker of sepsis in comparison to procalcitonin and C-reactive protein levels for patients admitted to a critical care unit in an urban hospital? J Crit Care. 2010;25:570–5. doi: 10.1016/j.jcrc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Garnacho-Montero J, Huici-Moreno MJ, Gutiérrez-Pizarraya A, López I, Márquez-Vácaro JA, Macher H, et al. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin, and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Crit Care. 2014;18:R116. doi: 10.1186/cc13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smithson A, Perelló R, Nicolas JM. Is eosinopenia a reliable marker of sepsis? Crit Care. 2009;13:409. doi: 10.1186/cc7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Setterberg MJ, Newman W, Potti A, Smego RA., Jr Utility of eosinophil count as predictor of bacteremia. Clin Infect Dis. 2004;38:460–1. doi: 10.1086/380846. [DOI] [PubMed] [Google Scholar]
- 20.Bass DA. Behavior of eosinophil leukocytes in acute inflammation. I. Lack of dependence on adrenal function. J Clin Invest. 1975;55:1229–36. doi: 10.1172/JCI108041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bass DA. Behavior of eosinophil leukocytes in acute inflammation. II. Eosinophil dynamics during acute inflammation. J Clin Invest. 1975;56:870–9. doi: 10.1172/JCI108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiffmann E, Corcoran BA, Wahl SM. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975;72:1059–62. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]


