Abstract
A small series of 5-nitro-2-aminothiazole-based amides containing arylpiperazine-, biphenyl- or aryloxyphenyl groups in their core were synthesized and evaluated as antitrypanosomatid agents. All tested compounds were active or moderately active against Trypanosoma cruzi amastigotes in infected L6 cells and Trypanosoma brucei brucei, four of eleven compounds were moderately active against Leishmania donovani axenic parasites while none were deemed active against T. brucei rhodesiense. For the most active/moderately active compounds a moderate selectivity against each parasite was observed. There was good correlation between lipophilicity (clogP value) and antileishmanial activity or toxicity against L6 cells. Similarly, good correlation existed between clogP values and IC50 values against T. cruzi in structurally related subgroups of compounds. Three compounds were more potent as antichagasic agents than benznidazole but were not activated by the type I nitrorectusase (NTR).
Keywords: 5-Nitro-2-aminothiazoles, Type I nitroreductase, Antitrypanosomal agents, Chagas disease, Leishmania
InChIKey: MZSRZQZBRGCXDO-UHFFFAOYSA-N
Abbreviations: NTD, Neglected tropical diseases; T. brucei, Trypanosoma brucei; HAT, human African trypanosomiasis; T. cruzi, Trypanosoma cruzi; Bnz, benznidazole (N-benzyl-2-(2-nitro-1H-imidazol-1-yl)acetamide); Nfx, nifurtimox (4-(5-nitrofurfurylindenamino)-3-methylthio-morpholine-1,1-dioxide); NTR, type I nitroreductase; TbNTR, T. brucei NTR; CYP51, sterol 14α-demethylase enzyme; TcCYP51, T. cruzi CYP51; IC50, concentration for 50% growth inhibition; SI, selectivity index; SAR, structure-activity relationships
Graphical abstract
Highlights
-
•
Novel 5-nitro-2-aminothiazole-based amides were synthesized and evaluated for antiparasitic activity.
-
•
Most derivatives were active or moderately active against Trypanosoma cruzi and Trypanosoma brucei.
-
•
Some analogs demonstrated moderate antileishmanial activity in vitro.
-
•
Lipophilicity positively affects antichagasic and antileishmanial activity.
-
•
These compounds are not activated by Type I nitroreductases.
1. Introduction
American trypanosomiasis (Chagas disease), human African trypanosomiasis (HAT or sleeping sickness) and leishmaniasis are considered neglected tropical diseases (NTD) and represent a severe global health problem [1], [2]. It is estimated that together these three diseases, caused by protozoan parasitic infections, affect approximately 20 million people and are responsible for more than 110,000 deaths annually [2]. African trypanosomiasis is endemic in many sub-Saharan African countries and is caused by Trypanosoma brucei rhodesiense and T. brucei gambiense. Chagas disease, caused by Trypanosoma cruzi, is endemic in South and Central America but is now spreading worldwide, mainly due to human and vector migration [3], [4]. Leishmaniasis, caused by more than 20 Leishmania species, occurs throughout tropical and sub-tropical regions and is now spreading worldwide as an HIV co-infection [5].
Treatment of these NTD is currently based on a series of problematic drugs. Thus, nifurtimox (Nfx) and benznidazole (Bnz), the two currently used medications for Chagas disease are associated with limited efficacy, severe toxicity and long treatment requirements [6], [7]. Similarly, drugs used to treat HAT and leishmaniasis are highly toxic (e.g. melarsoprol, antimonials), may require i.v. administration (e.g. melarsoprol, suramin, DFMO, antimonials), can cause severe side effects, or are of high cost (e.g. DFMO, liposomal amphotericin B, miltefosine and paromomycin) [8], [9], [10]. Therefore, there is an urgent need for new effective, safe and affordable alternatives.
Although inhibitors of the fungal sterol 14α-demethylase enzyme (CYP51) and the orthologous enzyme T. cruzi CYP51 (TcCYP51) demonstrated promising efficacy against Chagas disease in preclinical studies [11], [12], [13], data from clinical trials using posaconazole or ravuconazole were disappointing [14], [15]. Moreover, recent evidence indicates that nitroheterocyclics might be more efficacious trypanocidal agents than CYP51 inhibitors [16], and combinations of the two may offer even a better solution [17].
We have shown that several chemical classes of 3-nitro-1H-1,2,4-triazole-based compounds exhibit excellent antichagasic activity both in vitro and in vivo [18], [19], [20], [21], [22], [23], [24], [25]. Furthermore, appreciable anti-HAT activity was also observed in vitro with several such analogs [18], [19], [20], [21], [22], [23], [24], [25] whereas in vitro antileishmanial activity was demonstrated with a sub-class of 3-nitrotriazole- and 2-nitroimidazole-based aryloxyphenylamides [25]. Nitro-activation by an oxygen-insensitive type I nitroreductase (NTR), an enzyme located in the mitochondrion of trypanosomatids and absent from most other eukaryotes, is partially responsible for the trypanocidal activity of these and other nitroheterocyclic compounds [18], [19], [21], [22], [23], [24], [25], [26], [27], [28], [29]. More recently, we have synthesized 3-nitrotriazole-based rigid amides and carbinols which act as bifunctional agents; they exert their antitrypanosomal activity upon activation by type I NTRs and by inhibiting the parasite's CYP51 enzyme [23], [25]. Interestingly, 3-nitrotriazole-based compounds are significantly more potent and less toxic than their 2-nitroimidazole-based counterparts [18], [19], [20], [21], [22], [23], [24], [25], [30].
Here we have expanded our research by investigating the role that another nitroheterocyclic ring, 5-nitro-2-aminothiazole, plays in antitrypanosomatid activity. Nitrothiazole- and nitrobenzothiazole-containing compounds exhibit antiparasitic, antibacterial, antifungal and antitubercular activities [31], [32], [33], [34]. Therefore, we have synthesized and evaluated in vitro a small series of 5-nitro-2-aminothiazole-based compounds bearing moieties that were previously proven effective in the trypanocidal activity of 3-nitrotriazole-based agents.
2. Results and discussion
2.1. Chemistry
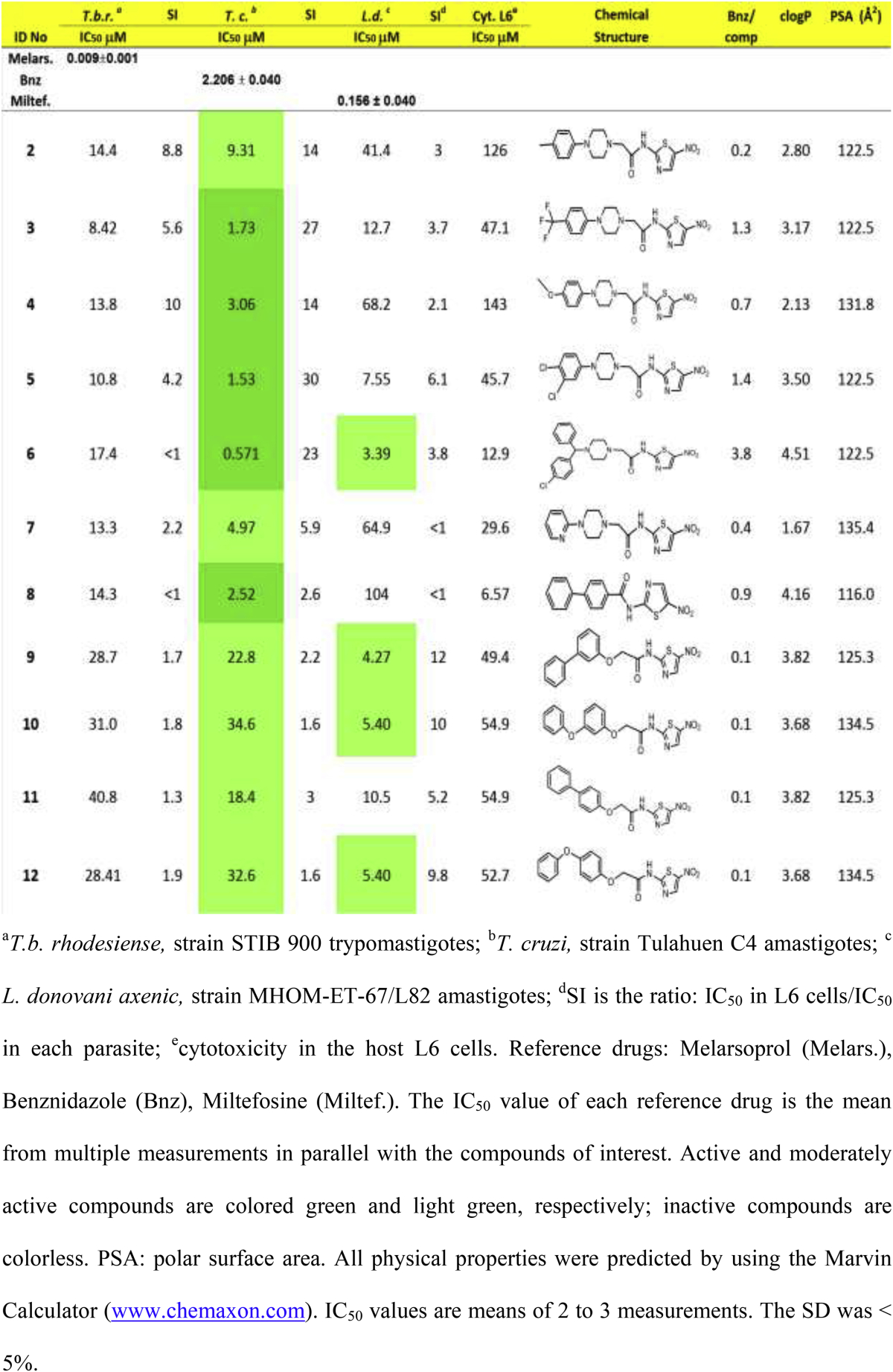
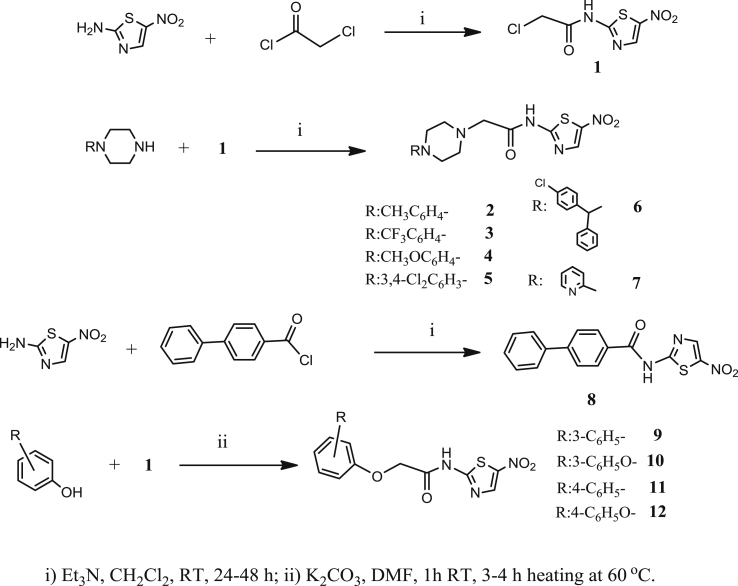
The synthesis of 5-nitro-2-aminothiazole-based compounds (Table 1) is straightforward and based on well-established chemistry, outlined in Scheme 1.
Table 1.
In vitro antiparasitic activity, host toxicity and physical properties of 5-nitro-2-amino-thiazole-based amides.
Scheme 1.
Synthesis of compounds on Table 1.
The precursor alkylchloride 1 as well as compound 8 were formed by nucleophilic substitution of 2-chloroacetyl chloride and [1,1′-biphenyl]-4-carbonyl chloride, respectively, by 5-nitro-2-aminothiazole, in the presence of triethylamine. Amides 2–7 were obtained by nucleophilic substitution of alkylchloride 1 by an appropriate piperazine at room temperature and in the presence of triethylamine. Finally, amides 9–12 were prepared by nucleophilic substitution of alkylchloride 1 by the potassium salt of an appropriate phenol in DMF, by heating for 3–4 h at 60 °C. Efforts were made to improve the yield of amides 9–12 by changing the solvent to anhydrous DMSO or CH3CN without any positive results. All final compounds and intermediates were characterized by 1H NMR (500 or 400 MHz) and HRMS.
2.2. Biological evaluation
2.2.1. Antiparasitic activity and cytotoxicity
Compounds in Table 1 were screened for antiparasitic activity against three trypanosomatids: T. cruzi, T. b. rhodesiense and Leishmania donovani. The concentration of compound that inhibits parasite growth by 50% (IC50) was calculated from dose response curves for each parasite (Table 1). In addition, compounds were tested for toxicity in L6 rat skeletal myoblasts, used as host cells for T. cruzi amastigotes, in order to calculate a selectivity index for each parasite (SI = IC50L6/IC50parasite) (Table 1). Antiparasitic activity was evaluated according to the following criteria: an IC50 of <4.0 μM, between 4.0 and 60 μM or >60 μM, designates ‘active’, ‘moderately active’ or ‘inactive’ compounds, respectively, against T. cruzi amastigotes; for blood stream form (BSF) T. b. rhodesiense, IC50 values of <0.5 μM, between 0.5 and 6.0 μM or >6.0 μM identify ‘active’, ‘moderately active’ or ‘inactive’ compounds, respectively; finally, for L. donovani amastigotes, IC50 of <1 μM, between 1.0 and 6.0 μM or >6.0 μM, provides ‘active’, ‘moderately active’ or ‘inactive’ compounds, respectively [35].
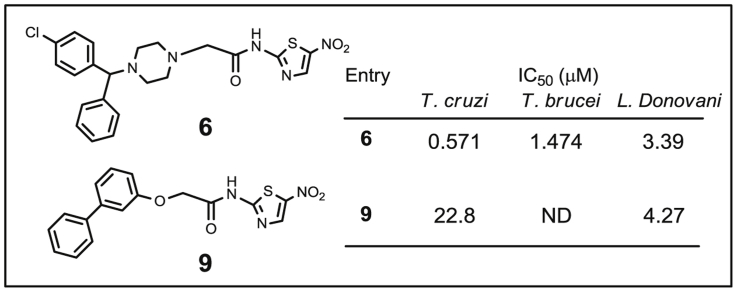
According to the criteria set above, all tested compounds in Table 1 were active or moderately active antichagasic agents (green or light green, respectively). Four compounds (6, 9, 10 and 12) were moderately active (light green) against L. donovani parasites whereas no compound demonstrated antiparasitic activity against T. b. rhodesiense. Moreover, all compounds showed PSA values > 100 Å2, which makes them highly unlikely to be capable of penetrating the blood–brain barrier and demonstrate anti-HAT activity in vivo.
Several analogs (3, 5–9) demonstrated IC50 values < 50 μM against L6 host cells, presumably due to their high lipophilicity (Table 1), resulting in low selectivity indices. However, even compounds with IC50 values > 50 μM against L6 cells demonstrated a less than ideal SI, which is desired to be ≥ 50 for T. cruzi and ≥20 for L. donovani [35].
2.2.2. SAR analysis of antichagasic activity
The compounds in Table 1 were synthesized having in mind 3-nitro-1,2,4-triazole-based analogs with known substantial trypanocidal properties, described previously by this group [20], [23], [24], [25]. Taking a closer look at the piperazine derivatives 2–7, we observe that these yielded IC50 values against T. cruzi parasites ranging from 0.571 to 9.31 μM; thus they are 1.1- to 9-fold less potent than the corresponding 3-nitrotriazole-based analogs (IC50 values 0.169–4.64 μM) [24]. Similarly, the aryl/aryloxy-derivatives 8–12 were only moderately active antichagasic agents, compared to 3-nitrotriazole-based aryloxyphenylamides which demonstrate T. cruzi IC50 values at low nM concentrations [23], [25]. Therefore, clearly 5-nitro-2-aminothiazole-based amides are less potent antichagasic agents than their 3-nitrotriazole-based analogs.
Another general observation is that the 5-nitro-2-aminothiazole-based amides are significantly more lipophilic (Table 1) than their 3-nitrotriazole-based counterparts with the latter having clogP values between −0.198 and 3.1. In addition, 5-nitro-2-aminothiazole-based amides demonstrate higher PSA values than their 3-nitrotriazole-based analogs (the latter having PSA values less than 116 [24]), which may negatively affect cell permeation [36]. These features may contribute to the higher toxicity of the nitroaminothiazoles in L6 cells and their reduced potency against the parasites (Table 1).
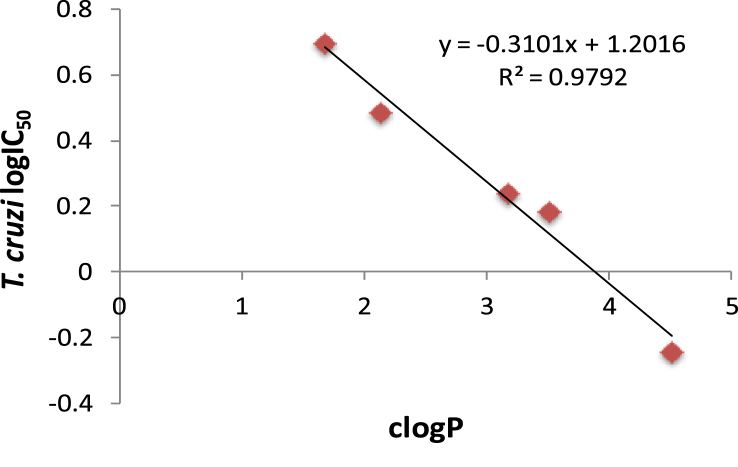
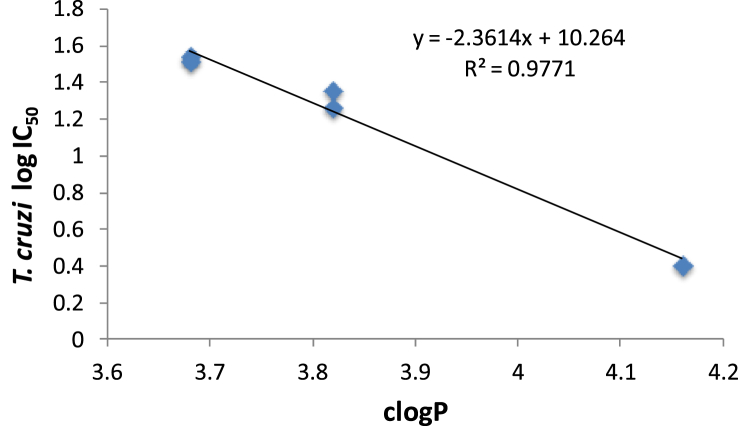
There was an excellent correlation between antichagasic activity and lipophilicity (R2 = 0.979) in the piperazine-amide subgroup of compounds 3–7 (which were active against T. cruzi) as shown in Fig. 1. Thus, the piperazine amide 6 with the highest clogP value (4.51) was the most active compound against T. cruzi, demonstrating an IC50 of 571 nM, 3.86-fold more active than Bnz (Table 1). Compound 6, however, was about 8-fold less active than its 3-nitrotriazole-based analog, in which the piperazinic ring is directly connected with the carbonyl (piperazide) and the nitrotriazole ring is connected with the carbonyl through a methylene group [24]. SAR follows the same rules observed in the 3-nitrotriazole-based piperazines and piperazides [20], [24]. Therefore, dichlorophenylpiperazine 5 was a slightly better antichagasic agent than trifluoromethylphenylpiperazine 3, the latter demonstrating better antichagasic activity than the methoxyphenylpiperazine 4 or the heteroarylpiperazine 7. It is not clear if the electronic effect of substitution plays a role in activity other than influencing the clogP value.
Fig. 1.
Correlation between antichagasic activity (log IC50 values against T. cruzi) and lipophilicity (clogP values) in 3–7, compounds that are active against T. cruzi and bear a piperazine moiety.
With regard to in the structurally related compounds 8–12, there was also excellent correlation between antichagasic activity and lipophilicity (R2 = 0.977), with the most lipophilic biphenylamide 8 having the lowest IC50 value against T. cruzi (Fig. 2). Interestingly, and despite their relatively high lipophilicity, the (phenoxy/phenyl)phenoxy-derivatives 9–12 were only moderately active antichagasic agents, in stark contrast to 3-nitrotriazole-based aryloxyphenylamides, which were exceptionally active (at low nM concentrations) against T. cruzi [25].
Fig. 2.
Correlation between antichagasic activity (log IC50 values against T. cruzi) and lipophilicity (clogP values) in the subgroup of structurally similar compounds 8–12.
2.2.3. Analysis of anti-Leishmania activity
There was no correlation between antichagasic and antileishmanial activity for compounds 2–12. Thus, compounds 9, 10 and 12 that displayed a moderate antichagasic activity (IC50 values of 23–35 μM) demonstrated high potency towards L. donovani, yielding IC50s of 4.27–5.40 μM.
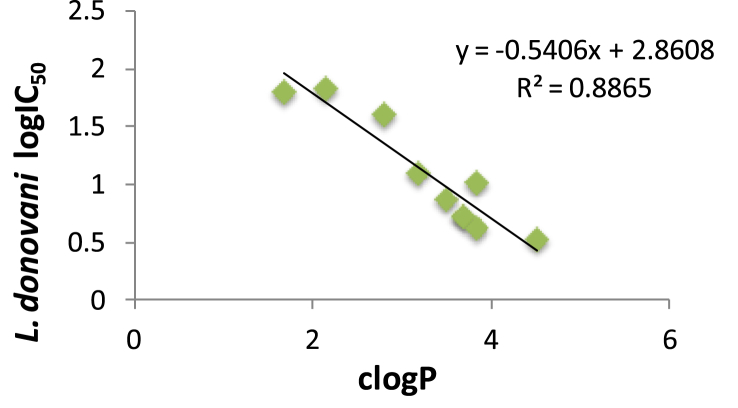
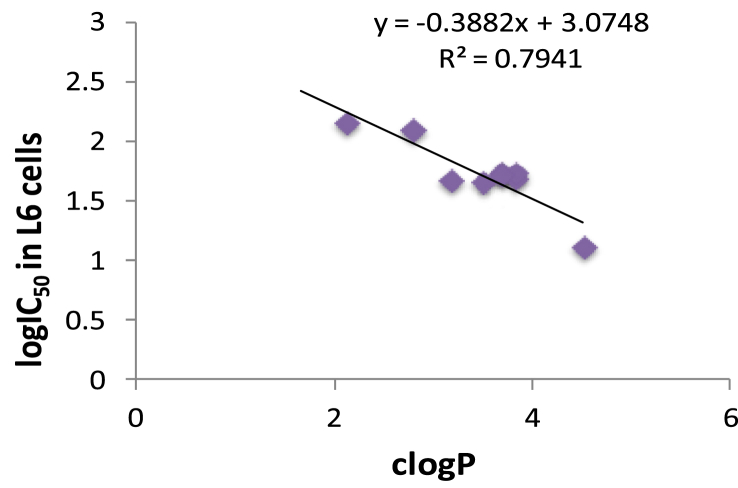
In contrast to antichagasic activity and, with the exception of the most lipophilic compound 6, the 3-nitro-2-aminothiazole-based piperazine amides 2–7 were generally less active antileishmanial agents than the (phenoxy/phenyl)phenoxy derivatives 9–12. With regard to the role of substitution in the antileishmanial activity, the same rules existed, which were mentioned above for antichagasic activity. There was good correlation between clogP and logIC50 values against L. donovani parasites for all compounds (Table 1), regardless of activity (Fig. 3). Therefore, once again, lipophilicity was very important determinant for antileishmanial activity. However, lipophilicity also resulted in relatively high toxicity with good correlation between clogP values and logIC50 values in L6 cells (Fig. 4).
Fig. 3.
Correlation between log IC50 values against L. donovani and clogP values for compounds in Table 1.
Fig. 4.
Correlation between log IC50 values in L6 cells and clogP values for compounds in Table 1.
2.2.4. The role of type I nitroreductases
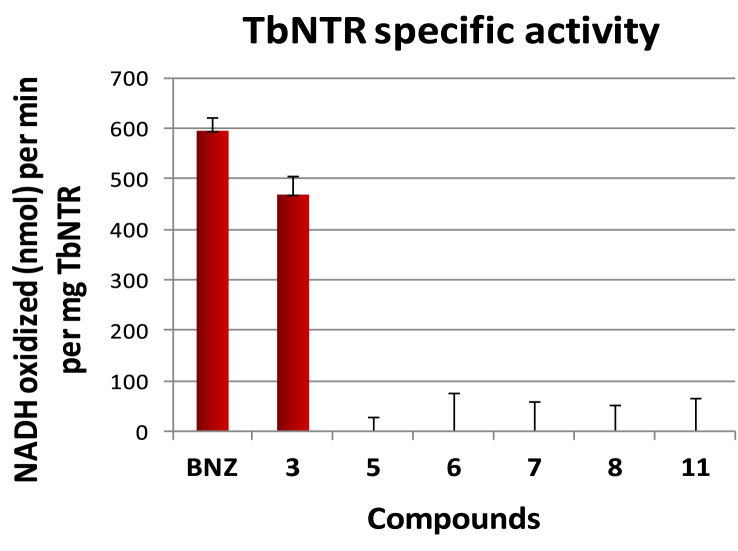
To elucidate the mechanism of action of the novel compounds in Table 1, representative derivatives (3, 5, 6, 7, 8 and 11) were evaluated as substrates of purified, recombinant TbNTR and their enzyme specific activity, measured as nmol NADH oxidized min−1 mg−1 protein, compared with that of benznidazole (Fig. 5). With the exception of compound 3, all other tested analogs were deemed to be poor TbNTR substrates, consistent with results obtained for previously studied N-substituted 5-nitro-2-aminothiazoles where the substituent had electron withdrawing groups [37]. Compound 3, which was not the most potent analog against T. cruzi amastigotes, provided a similar TbNTR specific activity to BNZ (Fig. 5).
Fig. 5.
Specific activity values are measured in nmol NADH oxidized min−1 mg−1 TbNTR. The values correspond to averages from assays performed in triplicate ± standard deviation.
To further determine whether NTR plays a role in metabolizing the substrates within the parasite, the above subset of compounds were phenotypically screened against BSF T. b. brucei expressing wild type or elevated levels of TbNTR. Compound 7, with an IC50 > 10 μM in wild type parasites was not screened against the recombinant line. For most of the remaining compounds cells overexpressing TbNTR were only 2- to 3-fold more sensitivity than controls to the agent, a relatively low shift when compared to that observed with nifurtimox. The biochemical and phenotypic screening data suggest that NTR plays little or no role in the metabolism of these compounds within the parasite itself. As these ‘non-TbNTR’ activated compounds display moderate growth inhibitory properties towards wild type T. b. brucei (Table 2) the mode of action of these compounds remains unknown although this antitrypanosomal activity does appear to be sub-species specific: The selected 5-nitrothiazole-based compounds tested are up to 12-fold more potent towards T. b. brucei than against T. b. rhodesiense.
Table 2.
Activity of selected nitrothiazoles against wild type and TbNTR overexpressing T. b. brucei parasites in the presence of tetracycline (+tet).
| Compound | IC50 value (μM) T.b. brucei |
Ratio |
||
|---|---|---|---|---|
| Wild type | TbNTR (−tet) | TbNTR (+tet) | –tet/+tet | |
| nfx | 3.980 ± 0.150 | 6.359 ± 0.119 | 0.869 ± 0.046 | 7.3 |
| 3 | 1.019 ± 0.045 | 1.460 ± 0.113 | 0.526 ± 0.024 | 2.8 |
| 5 | 1.365 ± 0.091 | 1.957 ± 0.101 | 0.747 ± 0.075 | 2.6 |
| 6 | 1.474 ± 0.104 | 1.638 ± 0.069 | 0.636 ± 0.022 | 2.6 |
| 7 | 21.575 ± 3.630 | – | – | – |
| 8 | 1.612 ± 0.396 | 4.587 ± 0.149 | 2.176 ± 0.085 | 2.1 |
| 11 | 3.753 ± 0.383 | 3.249 ± 0.142 | 2.200 ± 0.100 | 1.5 |
In conclusion, novel N-substituted 5-nitro-2-aminothiazoles with an arylpiperazine-, biphenyl- or aryloxyphenyl group in the core were active or moderately active antichagasic agents and moderately active against T. b. brucei parasites. Only one derivative, compound 6, demonstrated activity against T. cruzi amastigotes at nM concentrations and was about 4-fold more potent than BNZ. In addition, some of these compounds demonstrated a moderate antileishmanial activity against L. donovani axenic amastigotes. These particular compounds were not good substrates for type I NTR. However, more simple N-substituted 5-nitro-2-aminothiazoles were shown to be excellent substrates of type I NTR and their antiparasitic activity was increased about 10-fold in NTR overexpressing T. b. brucei [37]. Interestingly, these more simple N-acyl substituted 5-nitro-2-aminothiazoles demonstrated significantly lower clogP values than the compounds described in here [37]. Therefore, this class of compounds deserves further investigation and structural optimization may provide leads for further development.
3. Experimental
3.1. Chemistry
3.1.1. General
All starting materials and solvents were purchased from Sigma–Aldrich (Milwaukee, WI), were of research-grade quality and used without further purification. Solvents used were anhydrous and the reactions were carried out under a nitrogen atmosphere and exclusion of moisture. Melting points were determined by using a Mel-Temp II Laboratory Devices apparatus (Holliston, MA) and are uncorrected. Proton NMR spectra were obtained on a Varian Inova-500 or an Agilent Hg-400 spectrometer at 500 or 400 MHz, respectively, and are referenced to Me4Si or to the corresponding solvent, if the solvent was not CDCl3. High-resolution electrospray ionization (HRESIMS) mass spectra were obtained on a Agilent 6210 LC-TOF mass spectrometer at 11,000 resolution. Thin-layer chromatography was carried out on aluminum oxide N/UV254 or polygram silica gel G/UV254 coated plates (0.2 mm, Analtech, Newark, DE). Chromatography was carried out on preparative TLC alumina GF (1000 microns) or silica gel GF (1500 microns) plates (Analtech). All final compounds were purified by preparative TLC chromatography on silica gel or alumina plates and also checked by HPLC (≥95% purity).
3.1.2. Synthesis of 2-chloro-N-(5-nitrothiazol-2-yl)acetamide (1) [33]
A suspension of 5-nitrothiazol-2-amine (1 eq) and triethylamine (1.1 eq) in 10 mL dichloromethane was added drop wise to a dichloromethane solution (5–7 mL) of 2-chloroacetyl chloride (1.1 eq) and the reaction was left overnight at room temperature. Alternatively, the solution of 2-chloroacetyl chloride was added at once to the suspension of 5-nitrothiazol-2-amine and triethylamine. The TLC on silica gel with ethyl acetate: petroleum ether (75:25) indicated completion of the reaction. The desired product was isolated as light yellow crystals through column chromatography as above: 481 mg (81% yield).
3.1.3. General synthesis of N-(5-nitrothiazol-2-yl)acetamides 2–7
A dichloromethane solution (6 mL) of an appropriate piperazine (1 eq) and triethylamine (3 eq) was added drop wise to a suspension of 2-chloro-N-(5-nitrothiazol-2-yl)acetamide (1) in 5 mL CH2Cl2 and the reaction was kept at room temperature under a nitrogen atmosphere and stirring for 48 h. The reaction solvent was evaporated and the residue was redissolved in ethyl acetate. The inorganic salts were filtered away and the residue was chromatographed on preparative TLC silica gel plates with ethyl acetate/petroleum ether as eluent to obtain the desired pure product as a powder or crystals. Purity was also checked by HPLC and it was ≥95%.
3.1.3.1. N-(5-nitrothiazol-2-yl)-2-(4-(p-tolyl)piperazin-1-yl)acetamide (2)
Orange microcrystallic powder (62%): mp 160–161 °C (dec); 1H NMR (400 MHz, (CDCl3) δ: 8.33 (s, 1H), 7.10 (d, J = 7.6 Hz, 2H), 6.85 (d, J = 8.8 Hz, 2H), 3.38 (s, 2H), 3.23 (t, J = 4.8 Hz, 4H), 2.81 (t, J = 5.2 Hz, 4H), 2.29 (s, 3H). HRESIMS calcd for C16H20N5O3S m/z [M+H]+ 362.1281 found 362.1285.
3.1.3.2. N-(5-nitrothiazol-2-yl)-2-(4-(4-(trifluoromethyl)phenyl)piperazin-1-yl)acetamide (3)
Orange powder (55%): mp 173–175 °C; 1H NMR (400 MHz, (CDCl3) δ: 8.33 (s, 1H), 7.51 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 8.4 Hz, 2H), 3.40 (s, 2H), 3.38 (t, J = 5.2 Hz, 4H), 2.82 (t, J = 5.2 Hz, 4H). HRESIMS calcd for C16H16N8O4S m/z [M+H]+ 416.1010, found 416.1005.
3.1.3.3. 2-(4-(4-methoxyphenyl)piperazin-1-yl)-N-(5-nitrothiazol-2-yl)acetamide (4)
Bright orange powder (57%): mp 154–156 °C (dec); 1H NMR (400 MHz, (CDCl3) δ: 8.33 (s, 1H), 6.91 (d, J = 8.8 Hz, 2H), 6.86 (d, J = 9.2 Hz, 2H), 3.78 (s, 3H), 3.39 (s, 2H), 3.17 (t, J = 4.8 Hz, 4H), 2.81 (t, J = 4.8 Hz, 4H). HRESIMS calcd for C16H20N5O4S m/z [M+H]+ 378.1231 found 378.1233.
3.1.3.4. 2-(4-(3,4-dichlorophenyl)piperazin-1-yl)-N-(5-nitrothiazol-2-yl)acetamide (5)
Bright yellow microcrystals (65%): mp 169–171 °C; 1H NMR (400 MHz, (CD3COCD3) δ: 8.44 (s, 1H), 7.36 (d, J = 9.2 Hz, 1H), 7.12 (d, J = 2.8 Hz, 1H), 6.97 (dd, J = 9.2, 2.8 Hz, 1H), 3.53 (s, 2H), 3.37 (t, J = 5.2 Hz, 4H), 2.84 (t, J = 5.2 Hz, 4H). HRESIMS calcd for C15H16Cl2N5O3S m/z [M+H]+ 416.0345, 418.0317, found 416.0346, 418.0317.
3.1.3.5. 2-(4-((4-chlorophenyl)(phenyl)methyl)piperazin-1-yl)-N-(5-nitrothiazol-2-yl)acetamide (6)
Orange microcrystallic powder (66%): mp 92–95 °C; 1H NMR (400 MHz, (CDCl3) δ: 8.31 (s, 1H), 7.36–7.21 (m, 9H), 4.27 (s, 1H), 3.31 (s, 2H), 2.66 (t, J = 4.8 Hz, 4H), 2.49 (br s, 4H). HRESIMS calcd for C22H23ClN5O3S m/z [M+H]+ 472.1205, found 472.1209.
3.1.3.6. N-(5-nitrothiazol-2-yl)-2-(4-(pyridin-2-yl)piperazin-1-yl)acetamide (7)
Orange microcrystallic powder (56%): mp 175–180 °C (dec); 1H NMR (400 MHz, (CDCl3) δ: 8.33 (s, 1H), 8.21 (dd, J = 4.4, 1.2 Hz, 1H), 7.52 (dt, J = 8.4, 2.0 Hz, 1H), 6.68 (m, 2H), 3.65 (t, J = 4.8 Hz, 4H) 3.38 (s, 2H), 2.76 (t, J = 4.8, Hz, 4H). HRESIMS calcd for C14H17N6O3S m/z [M+H]+ 349.1077 found 349.1083.
3.1.4. N-(5-nitrothiazol-2-yl)-[1,1′-biphenyl]-4-carboxamide (8)
[1,1′-Biphenyl]-4-carbonyl chloride was added in portions to a suspension of 5-nitrothiazol-2-amine (1 eq) and triethylamine (2.5 eq) in 10–12 mL dichloromethane. The reaction mixture was kept at room temperature overnight under stirring and a nitrogen atmosphere. The desired product was obtained after preparative TLC on silica gel plates using ethyl acetate: petroleum ether (50:50) as eluent. Beige microcrystals (55%): mp > 230 °C; 1H NMR (400 MHz, (CD3COCD3) δ: 8.50 (s, 1H), 8.31 (d, J = 8.8 Hz, 2H), 7.92 (d, J = 8.8 Hz, 2H), 7.78 (d, J = 7.6 Hz, 2H), 7.53 (t, J = 7.6 Hz, 2H), 7.45 (t, J = 7.6 Hz, 1H). HRESIMS calcd for C16H10N3O3S m/z [M−H]− 324.0448 found 324.0464.
3.1.5. General synthesis of N-(5-nitrothiazol-2-yl)acetamides 9–12
An appropriate phenol (1.05 eq) and K2CO3 (2.1 eq) were mixed together in dry DMF (4 mL) and stirred for an hour. Then a DMF solution (4 mL) of 2-chloro-N-(5-nitrothiazol-2-yl)acetamide (1 eq) was added through a funnel and the reaction mixture was heated at 60 °C for 3–4 h. The solvent was evaporated and the residue was chromatographed on silica gel preparative TLC plates using ethyl acetate: petroleum ether mixture as an eluent. The desired product is formed in relatively small yield (≤35%) and appears immediately after the unreacted phenol on TLC. Changing the solvent to CH3CN or DMSO did not improve the yield.
3.1.5.1. 2-([1,1′-biphenyl]-3-yloxy)-N-(5-nitrothiazol-2-yl)acetamide (9)
White microcrystals (25%): mp 148–150 °C; 1H NMR (400 MHz, (CClD3) δ: 9.94 (br s, 1H), 8.36 (s, 1H), 7.60–7.33 (m, 7H), 7.21 (dd, J = 2.4, 1.6 Hz, 1H), 6.96 (dd, J = 7.2, 2.4, Hz, 1H), 4.85 (s, 2H). HRESIMS calcd for C17H14N3O4S m/z [M+H]+ 356.0700, found 356.0696.
3.1.5.2. N-(5-nitrothiazol-2-yl)-2-(3-phenoxyphenoxy)acetamide (10)
White microcrystals (34%, based on recovered phenol): mp 151–153 °C; 1H NMR (400 MHz, (CDCl3) δ: 9.84 (br s, 1H), 8.35 (s, 1H), 7.38 (t, J = 7.6 Hz, 2H), 7.30 (t, J = 8.0 Hz, 1H), 7.17 (t, J = 8.0 Hz, 1H), 7.05 (d, J = 7.6 Hz, 2H), 6.73 (dd, J = 8.0, 2.0 Hz, 1H), 6.68 (dd, J = 8.0, 2.4 Hz, 1H), 6.62 (t, J = 2.4 Hz, 1H), 4.74 (s, 2H). HRESIMS calcd for C17H14N3O5S m/z [M+H]+ 372.0648, found 372.0649.
3.1.5.3. 2-([1,1′-biphenyl]-4-yloxy)-N-(5-nitrothiazol-2-yl)acetamide (11)
Light yellow microcrystals (35%, based on recovered phenol): mp 196–198 °C; 1H NMR (400 MHz, CDCl3) δ: 8.36 (s, 1H), 7.60 (d, J = 8.8 Hz, 2H), 7.55 (dd, J = 8.0, 0.8 Hz, 2H), 7.44 (t, J = 7.2 Hz, 2H), 7.35 (t, J = 7.6 Hz, 1H), 7.06 (d, J = 8.8 Hz, 2H), 4.83 (s, 2H). HRESIMS calcd for C17H14N3O4S m/z [M+H]+ 356.070, found 356.0694.
3.1.5.4. N-(5-nitrothiazol-2-yl)-2-(4-phenoxyphenoxy)acetamide (12)
Off white microcrystals (30%): mp 185–187 °C; 1H NMR (400 MHz, (CDCl3) δ: 9.91 (br s, 1H), 8.36 (s, 1H), 7.33 (t, J = 8.0 Hz, 2H), 7.10 (t, J = 7.6 Hz, 1H), 7.05–6.95 (m, 6H), 4.77 (s, 2H). HRESIMS calcd for C17H14N3O5S m/z [M+H]+ 372.0649, found 372.0655.
3.2. Biological evaluation
3.2.1. In vitro screening
In vitro activity against T. cruzi, T. b. rhodesiense, L. donovani and cytotoxicity assessment using L6 cells (rat skeletal myoblasts) was determined using a 96-well plate format as previously described [38]. Data were analyzed with the graphic program Softmax Pro (Molecular Devices, Sunnyvale, CA, USA), which calculated IC50 values by linear regression from the sigmoidal dose inhibition curves.
3.2.2. In vitro T. brucei brucei antiproliferating assays and susceptibility studies
T. brucei brucei bloodstream form parasites were seeded at 1 × 103 mL−1 in 200 μL of growth medium containing different concentrations of a nitrothiazole or nifurtimox. Where appropriate, induction of the TbNTR was carried out by adding tetracycline (1 μg/mL). After incubation for 3 days at 37 °C, resazurin (2.5 μg per well) was added to each well and the plates incubated for a further 8 h. The cell density of each culture was determined as described before [26] and the IC50 established.
3.2.3. Enzymatic activity studies with type I NTRs
Recombinant TbNTR was prepared and assayed as previously described [39], [40]. The activity of purified his-tagged TbNTR was assessed spectrophotometrically at 340 nm using various nitrothiazole substrates (100 μM) and NADH (100 μM) with the enzyme specific activity expressed as nmol NADH oxidized min−1 mg−1 of enzyme. Benznidazole was used as control substrate.
Acknowledgments
The authors thank M. Cal, M. Jud and S. Keller (Swiss TPH) for parasite assay results and Dr. Ana Rodriguez (New York University School of Medicine) for obtaining the in vivo data. This work was supported in part by internal funds of the Radiation Medicine Department at NorthShore University HealthSystem. In addition, the Drugs for Neglected Diseases initiative (DNDi) received financial support from the Bill and Melinda Gates Foundation to perform the in vitro screenings against parasites. The donors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version, at http://dx.doi.org/10.1016/j.ejmech.2016.04.010. These data include MOL files and InChiKeys of the most important compounds described in this article.
Appendix A. Supplementary data
Mol Files
The following ZIP file contains the MOL files of the most important compounds referred to in this article.
MOL file. ZIP file containing the MOL files of the most important compounds in this article.
References
- 1.Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. 2012. p. 975. WHO Technical Report Series. [PubMed] [Google Scholar]
- 2.Stuart K., Brun R., Croft S., Fairlamb A., Gürtler R.E., McKerrow J., Reed S., Tarleton R. Kinetoplastids: related protozoan pathogens, different diseases. J. Clin. Invest. 2008;118:1301–1310. doi: 10.1172/JCI33945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez P., Dumonteil E., Betancourt Cravioto M., Bottazzi M., Tapia-Conyer R. An unfolding tragedy of Chagas disease in North America. PLoS Negl. Trop. Dis. 2013;7:e2300. doi: 10.1371/journal.pntd.0002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leslie M. Infectious diseases. A tropical disease hits the road. Science. 2011;333:934. doi: 10.1126/science.333.6045.934. [DOI] [PubMed] [Google Scholar]
- 5.van Griensven J., Carrillo E., López-Vélez R., Lynen L., Moreno J. Leishmaniasis in immunosuppressed individuals. Clin. Microbiol. Infect. 2014;20:286–299. doi: 10.1111/1469-0691.12556. [DOI] [PubMed] [Google Scholar]
- 6.DNDi-website available: http://www.dndi.org/diseases-projects/diseases/chagas/current-treatment.html.
- 7.Castro J.A., Montalto de Mecca M., Bartel L.C. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis) Hum. Exp. Toxicol. 2006;25:471–479. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- 8.Sundar S., Singh A., Rai M., Prajapati V.K., Singh A.K., Ostyn B., Boelaert M., Dujardin J.-C., Chakravarty J. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin. Infect. Dis. 2012;55(4):543–550. doi: 10.1093/cid/cis474. [DOI] [PubMed] [Google Scholar]
- 9.Romero G.A.S., Boelaert M. Control of visceral leishmaniasis in Latin America — a systematic review. PLoS Negl. Trop. Dis. 2010;4(1):e584. doi: 10.1371/journal.pntd.0000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Griensven J., Balasegaram M., Meheus F., Alvar J., Lynen L., Boelaert M. Combination therapy for visceral leishmaniasis. Lancet. Infect. Dis. 2010;10:184–194. doi: 10.1016/S1473-3099(10)70011-6. [DOI] [PubMed] [Google Scholar]
- 11.Villalta F., Dobish M.C., Nde P.N., Kleshchenko Y.Y., Hargrove T.Y., Johnson C.A., Waterman M.R., Johnston J.N., Lepesheva G.I. VNI cures acute and chronic experimental Chagas disease. J. Infect. Dis. 2013;208:504–511. doi: 10.1093/infdis/jit042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andriani G., Amata E., Beatty J., Clements Z., Coffey B.J., Courtemanche G., Devine W., Erath J., Juda C.E., Wawrzak Z., Wood J.T., Lepesheva G.I., Rodriguez A., Pollastri M.P. Antitrypanosomal lead discovery: identification of a ligand-efficient inhibitor of Trypanosoma cruzi CYP51 and parasite growth. J. Med. Chem. 2013;56:2556–2567. doi: 10.1021/jm400012e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lepesheva G.I., Hargrove T.Y., Rachakonda G., Wawrzak Z., Pomel S., Cojean S., Nde P.N., Nes W.D., Locuson C.L., Calcutt M.W., Waterman M.R., Daniels J.S., Loiseau P.M., Villalta F. VFV as a new effective CYP51 structure-derived drug candidate for Chagas disease and visceral leishmaniasis. J. Infect. Dis. 2015;212(9):1439–1448. doi: 10.1093/infdis/jiv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina I., Gόmez i Prat J., Salvador F., Treviño B., Sulleiro E., Serre N., Pou D., Roure S., Cabezos J., Valerio L., Blanco-Grau A., Sánchez-Montalvá A., Vidal X., Pahissa A. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. Engl. J. Med. 2014;370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 15.Chatelain E. Chagas disease drug discovery: toward a new era. J. Biomol. Screen. 2014 doi: 10.1177/1087057114550585. [DOI] [PubMed] [Google Scholar]
- 16.Moraes C.B., Giardini M.A., Kim H., Franco C.H., Araujo-Junior A.M., Schenkman S., Chatelain E., Freitas-Junior L.H. Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: implications for Chagas disease drug discovery and development. Sci. Rep. 2014;4:4703–4714. doi: 10.1038/srep04703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diniz L.d.F., Urbina J.A., de Andrade I.M., Mazzeti A.L., Martins T.A.F., Caldas I.S., Talvani A., Ribeiro I., Bahia M.T. Benznidazole and posaconazole in experimental Chagas disease: positive interaction in concomitant and sequential treatments. PLoS Negl. Trop. Dis. 2013;7:e2367. doi: 10.1371/journal.pntd.0002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papadopoulou M.V., Bourdin Trunz B., Bloomer W.D., McKenzie C., Wilkinson S.R., Prasittichai C., Brun R., Kaiser M., Torreele E. Novel 3-nitro-1H-1,2,4-triazole-based aliphatic and aromatic amines as anti-Chagasic agents. J. Med. Chem. 2011;54:8214–8223. doi: 10.1021/jm201215n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadopoulou M.V., Bloomer W.D., Rosenzweig H.S., Chatelain E., Kaiser M., Wilkinson S.R., McKenzie C., Ioset J.-R. Novel 3-nitro-1H-1,2,4-triazole-based amides and sulfonamides as potential anti-trypanosomal agents. J. Med. Chem. 2012;55:5554–5565. doi: 10.1021/jm300508n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulou M.V., Bloomer W.D., Rosenzweig H.S., Kaiser M., Chatelain E., Ioset J.-R. Novel 3-nitro-1H-1,2,4-triazole-bearing piperazines and 2-amino-benzothiazoles as anti-Chagasic agents. Bioorg. Med. Chem. 2013;21:6600–6607. doi: 10.1016/j.bmc.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadopoulou M.V., Bloomer W.D., Rosenzweig H.S., Ashworth R., Wilkinson S.R., Kaiser M., Andriani G., Rodriguez A. Novel 3-nitro-1H-1,2,4-triazole-based compounds as potential anti-Chagasic drugs: In vivo studies. Future Med. Chem. 2013;5:1763–1776. doi: 10.4155/fmc.13.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadopoulou M.V., Bloomer W.D., Rosenzweig H.S., Wilkinson S.R., Kaiser M. Novel nitro(triazole/imidazole)-based heteroarylamides/sulfonamides as potential antitrypanosomal agents. Eur. J. Med. Chem. 2014;87:79–88. doi: 10.1016/j.ejmech.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulou M.V., Bloomer W.D., Lepesheva G.I., Rosenzweig H.S., Kaiser M., Aguilera-Venegas B., Wilkinson S.R., Chatelain E., Ioset J.-R. Novel 3-nitrotriazole-based amides and carbinols as bifunctional anti-Chagasic agents. J. Med. Chem. 2015;58:1307–1319. doi: 10.1021/jm5015742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papadopoulou M.V., Bloomer W.D., Rosenzweig H.S., O'Shea I.P., Wilkinson S.R., Kaiser M. 3-Nitrotriazole-based piperazides as potent antitrypanosomal agents: In vitro and in vivo evaluation. Eur. J. Med. Chem. 2015;103:325–334. doi: 10.1016/j.ejmech.2015.08.042. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulou M.V., Bloomer W.D., Rosenzweig H.S., Wilkinson S.R., Kaiser M., Chatelain E., Ioset J.-R. Discovery of potent nitrotriazole-based antitrypanosomal agents: In vitro and in vivo evaluation. Bioorg. Med. Chem. 2015;23:6467–6476. doi: 10.1016/j.bmc.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson S.R., Taylor M.C., Horn D., Kelly J.M., Cheeseman I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. PNAS. 2008;105:5022–5027. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alsford S., Eckert S., Baker N., Glover L., Sanchez-Flores A., Leung K.F., Turner D.J., Field M.C., Berriman M., Horn D. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature. 2010;482:232–236. doi: 10.1038/nature10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker N., Alsford S., Horn D. Genome-wide RNAi screens in African trypanosomes identify the nifurtimox activator NTR and the eflornithine transporter AAT6. Mol. Biochem. Parasitol. 2011;176:55–57. doi: 10.1016/j.molbiopara.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson S.R., Bot C., Kelly J.M., Hall B.S. Trypanocidal activity of nitroaromatic prodrugs: current treatments and future perspectives. Curr. Top. Med. Chem. 2011;11:2072–2084. doi: 10.2174/156802611796575894. [DOI] [PubMed] [Google Scholar]
- 30.Buchanan-Kilbey G., Djumpah J., Papadopoulou M.V., Bloomer W.B., Hu L., Wilkinson S.R., Ashworth R. Evaluating the developmental toxicity of trypanocidal nitroaromatic compounds on zebrafish. Acta Trop. 2013;128:701–705. doi: 10.1016/j.actatropica.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Samadhiya P., Sharma R., Srivastava S.K. Synthesis of azetidinone derivatives of 2-amino-5-nitrothiazole and their medicinal importance. Eur. Chem. Bull. 2013;2(9):611–617. [Google Scholar]
- 32.Ballard T.E., Wang X., Olekhnovich I., Koerner T., Seymour C., Hoffman P.S., MacDonald T.L. Biological activity of modified and exchanged 2-amino-5-nitrothiazole amide analogues of nitazoxanide. Bioor. Med. Chem. Lett. 2010;20:3537–3539. doi: 10.1016/j.bmcl.2010.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saleh Y.R.H., Saadeh H.A., Kaur H., Goyal K., Sehgal R., Mubarak M.S. The synthesis of novel hybrid compounds containing 5-nitrothiazole moiety as potential antiparasitic agents. Monatsh. fuer Chem. 2015;146(12):2087–2095. [Google Scholar]
- 34.Navarrete-Vazquez G., Chavez-Silva F., Colin-Lozano B., Estrada-Soto S., Hidalgo-Figueroa S., Guerrero-Alvarez J., Mednez S.T., Reyes-Vivas H., Oria-Hernández J., Canul-Canché J., Ortiz-Andrade R., Moo-Puc R. Synthesis of nitro(benzo)thiazole acetamides and in vitro antiprotozoal effect against amitochondriate parasites Giardia intestinalis and Trichomonas vaginalis. Bioorg. Med. Chem. 2015;23:2204–2210. doi: 10.1016/j.bmc.2015.02.059. [DOI] [PubMed] [Google Scholar]
- 35.Nwaka S., Ramirez B., Brun R., Maes L., Douglas F., Ridley R. Advancing drug innovation for neglected diseases—criteria for lead progression. PLoS Negl. Trop. Dis. 2009;3:e440. doi: 10.1371/journal.pntd.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark D.E. What polar surface area ever done for drug discovery. Future Med. Chem. 2011;3(4):469–484. doi: 10.4155/fmc.11.1. [DOI] [PubMed] [Google Scholar]
- 37.O'Shea I.P., Shahed M., Aguilera-Venegas B., Wilkinson S.R. Evaluating 5-nitrothiazoles as trypanocidal agents. Antimicrob. Agents Chemother. 2015 doi: 10.1128/AAC.02006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orhan I., Sener B., Kaiser M., Brun R., Tasdemir D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar. Drugs. 2010;8:47–58. doi: 10.3390/md8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall B.S., Wu X., Hu L., Wilkinson S.R. Exploiting the drug-activating properties of a novel trypanosomal nitroreductase. Antimicrob. Agents Chemother. 2010;54:1193–1199. doi: 10.1128/AAC.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall B.S., Meredith E.L., Wilkinson S.R. Targeting the substrate preference of a type I nitroreductase to develop anti-trypanosomal quinone-based prodrugs. Antimicrob. Agents Chemother. 2012;56:5821–5830. doi: 10.1128/AAC.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MOL file. ZIP file containing the MOL files of the most important compounds in this article.