The escalating prevalence of the metabolic syndrome has coincided with the emergence of a lifestyle that replaces physical activity with overindulgence. In this setting, excess nutrients continuously bombard the major metabolic organs, leaving resident cells to cope with a steady influx of superfluous carbon fuel. The molecular consequences of energy surplus are far-reaching. Among these, growing evidence suggests carbon overload promotes lysine acetylation, a protein modification prominent in mitochondria and linked to detrimental effects on energy metabolism. Because this phenomenon of carbon stress is increasingly recognized as a key feature of aging and metabolic disease, scientists are now keenly interested in understanding the functional impacts of protein acetylation and the mechanisms that defend against them.
The best-characterized countermeasures against protein hyperacetylation are mediated by a family of NAD+-dependent deacylases known as the sirtuins, which may have evolved as a stress response mechanism to offset spurious, nonenzymatic acetylation events that occur upon increasing carbon pressure (1). This family of proteins has a wide range of biological effects on disease processes associated with aging, including cancer, neurodegeneration, and metabolic syndrome. Sirtuins remove a variety of posttranslational acyl-modifications from proteins, including acetyl-lysine modifications (2), which accumulate during prolonged high-fat feeding (3,4). Interestingly, within 1 week of feeding mice a fat-rich diet, expression of the mitochondrial sirtuin, SIRT3, increases in the liver (3), possibly to protect against aberrant protein acetylation. Consistent with this prediction, hepatic protein acetylation remains low at this time point. However, after chronic high-fat feeding (13 weeks), SIRT3 expression diminishes and hepatic hyperacetylation of proteins manifests (3). Accordingly, SIRT3 knockout (SIRT3KO) mice are more susceptible to diet-induced acetylation of mitochondrial proteins, which is accompanied by more severe obesity and glucose intolerance relative to their wild-type counterparts.
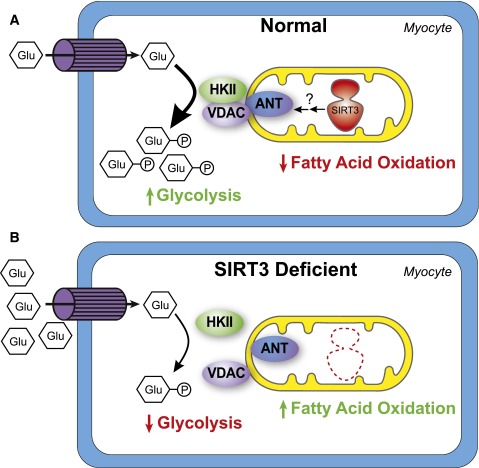
The mechanisms by which SIRT3 deficiency disrupts whole-body glucose homeostasis remain uncertain. In the current issue of Diabetes, Lantier et al. (5) begin to fill this gap by evaluating conscious SIRT3KO mice using the hyperinsulinemic-euglycemic clamp technique—the gold-standard method for measuring insulin sensitivity and glucose flux in vivo. They found that the major contributor to glucose intolerance in high-fat–fed SIRT3KO mice was impaired glucose uptake in skeletal muscle, which appeared to develop without an overt reduction in insulin signaling. The article by Lantier et al. extends previous findings by pinpointing the skeletal muscle as a principal site at which SIRT3 exerts control of glucose metabolism. Furthermore, the study attributes the poor glucose uptake in muscles of SIRT3KO mice to perturbations in the location and activity of hexokinase II (HKII), the irreversible enzyme that phosphorylates and thereby traps glucose within the myocyte. Previous work by this group and others has shown that HKII translocation to the mitochondria and its binding to the voltage-dependent anion channel (VDAC) promote enzyme activity as well as glucose uptake and oxidation (6,7,8). In the muscles of SIRT3KO mice fed a high-fat diet, the amount of HKII bound to VDAC was reduced in association with lower enzyme activity and reduced glucose uptake as compared with wild-type control mice. This impingement on intramuscular glucose phosphorylation was accompanied by a shift in mitochondrial fuel selection, such that glucose oxidation diminished while reliance on fatty acids increased (Fig. 1), which together led to a decay in glucose tolerance.
Figure 1.
SIRT3 promotes glucose uptake in skeletal muscle. A: SIRT3 promotes HKII-VDAC-ANT complex formation in myocytes, resulting in increased HKII activity and increased glucose uptake. This results in more glycolysis and less reliance on fatty acids as a fuel source. B: In the absence of SIRT3, HKII-VDAC-ANT complex formation is disrupted and HKII activity is decreased. Glucose uptake is impaired and myocytes rely more heavily on fatty acids for fuel. Glu, glucose; P, phosphorylation.
The findings by Lantier et al. (5) add to the growing complexity of how SIRT3 regulates metabolism. Previously, Hirschey et al. (9) showed that in the livers of SIRT3KO mice, hyperacetylation of long-chain acyl-CoA dehydrogenase in the fatty acid oxidation pathway led to reduced long-chain acyl-CoA dehydrogenase activity and decreased hepatic fatty acid oxidation. This resulted in hepatic steatosis, which contributed to the metabolic syndrome–like condition seen in high-fat–fed SIRT3KO mice. In addition, SIRT3KO mice had impaired glucose tolerance and a reduction in whole-body insulin sensitivity compared with controls. However, insulin signaling was not measured directly, so the exact tissues that contributed to whole-body insulin resistance were not identified.
In contrast to decreased hepatic fatty acid oxidation reported by Hirschey et al. (9), Jing et al. (10) showed that SIRT3KO mice have increased fatty acid oxidation in skeletal muscle as a result of impaired pyruvate dehydrogenase activity and reduced ability to use carbohydrates as a metabolic fuel source. This likely contributes to the glucose intolerance seen in SIRT3KO mice. Thus, the results found by Lantier et al. (5) are largely supportive of the previous data by Jing et al. (10), showing a similar switch to fatty acid oxidation in the skeletal muscle of high-fat–fed SIRT3KO mice.
Interestingly, the results by Lantier et al. (5) conflict with another study by Jing et al. (11) reporting that total body loss of SIRT3 impairs insulin signaling in muscle. While the reason for this discrepancy is unclear, the new report builds evidence that glucose intolerance can still take hold in the absence of defective insulin signaling. These observations fit with the model of distributed flux control, originally proposed by Wasserman (12), wherein the major barrier to muscle glucose uptake shifts from transport to phosphorylation once GLUT4 translocation occurs upon insulin stimulation. Moreover, because HKII consumes ATP to generate glucose-6-phosphate (G6P) and ADP, the mitochondrial-localized enzyme is thought to modulate respiratory kinetics by altering local ATP turnover rates. The report by Lantier et al. proposes that SIRT3 promotes the formation of a HKII-VDAC-adenine nucleotide translocator (ANT) complex on mitochondria, which, in turn, augments HKII activity, glucose uptake, and glucose oxidation. In the absence of SIRT3, this complex was disrupted. However, the mechanisms by which SIRT3 influences the formation of this complex are still unknown. The acetylation status of HKII, VDAC, and ANT in muscle from SIRT3KO and wild-type mice was not evaluated and will be an important future direction. Regardless, these observations together suggest that a primary defect in mitochondria is sufficient to affect glucose uptake in skeletal muscle, independent of changes in insulin signaling.
The tissue-specific roles of SIRT3 have yet to be established. SIRT3 appears to affect liver and muscle tissue in different ways and was recently reported to impart distinct actions on metabolism in fuel-producing versus fuel-consuming tissues (13). Surprisingly, Fernandez-Marcos et al. (14) found that liver- and skeletal muscle–specific SIRT3KO mice present an unremarkable metabolic phenotype when fed either a standard chow or short-term high-fat diet. Future studies performing hyperinsulinemic-euglycemic clamps in these mice are needed to determine whether glucose flux in muscle and/or liver is disrupted or whether loss of SIRT3 in multiple tissues is required for the development of insulin resistance. From a therapeutic perspective, as high-fat–fed mice have decreased SIRT3, future studies should test whether overexpressing SIRT3 in a tissue-specific manner is sufficient to prevent diet-induced insulin resistance. Also noteworthy is that humans harboring a SIRT3 single nucleotide polymorphism that results in decreased SIRT3 activity have an increased likelihood of developing metabolic syndrome (3,15). Together with the study by Lantier et al. (5), these findings suggest that strategies to increase SIRT3 activity, specifically in the skeletal muscle of obese patients with diabetes, may offer promising therapeutic potential.
Article Information
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 3081.
References
- 1.Wagner GR, Hirschey MD. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol Cell 2014;54:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KA, Green MF, Huynh FK, Wagner GR, Hirschey MD. SnapShot: mammalian sirtuins. Cell 2014;159:956–956.e1 [DOI] [PMC free article] [PubMed]
- 3.Hirschey MD, Shimazu T, Jing E, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell 2011;44:177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendrick AA, Choudhury M, Rahman SM, et al. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J 2011;433:505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lantier L, Williams AS, Williams IM, et al. SIRT3 is crucial for maintaining skeletal muscle insulin action and protects against severe insulin resistance in high-fat–fed mice. Diabetes 2015;64:3081–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasserman DH, Ayala JE. Interaction of physiological mechanisms in control of muscle glucose uptake. Clin Exp Pharmacol Physiol 2005;32:319–323 [DOI] [PubMed] [Google Scholar]
- 7.Southworth R. Hexokinase-mitochondrial interaction in cardiac tissue: implications for cardiac glucose uptake, the 18FDG lumped constant and cardiac protection. J Bioenerg Biomembr 2009;41:187–193 [DOI] [PubMed] [Google Scholar]
- 8.Golshani-Hebroni SG, Bessman SP. Hexokinase binding to mitochondria: a basis for proliferative energy metabolism. J Bioenerg Biomembr 1997;29:331–338 [DOI] [PubMed] [Google Scholar]
- 9.Hirschey MD, Shimazu T, Goetzman E, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010;464:121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jing E, O’Neill BT, Rardin MJ, et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes 2013;62:3404–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jing E, Emanuelli B, Hirschey MD, et al. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A 2011;108:14608–14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab 2009;296:E11–E21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittenhafer-Reed KE, Richards AL, Fan J, et al. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab 2015;21:637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Marcos PJ, Jeninga EH, Canto C, et al. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci Rep 2012;2:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulin R, Dromparis P, Sutendra G, et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab 2014;20:827–839 [DOI] [PubMed] [Google Scholar]