Progression of diabetic nephropathy (DN) is commonly defined by an increase in albuminuria from normoalbuminuria to microalbuminuria and from microalbuminuria to macroalbuminuria. Although early detection of DN can prevent or slow its progression, a major difficulty in inducing remission in patients with early DN is the identification of biomarkers that could help identify patients more likely to progress to end-stage renal disease (ESRD). Traditional risk factors such as albuminuria do not effectively predict DN progression, and other predictors of DN have yet to be characterized and validated. The need for discovering sensitive and easily detectable biomarkers to monitor the decline in renal function and to separate progressors from nonprogressors of DN is therefore of paramount importance.
Recently, microRNAs (miRNAs) have emerged as one such potential class of biomarkers. Mature miRNAs are a class of evolutionarily conserved, short (20–22 nucleotides long), noncoding RNA that are potent regulators of gene expression. After several synthesis and processing steps, mature miRNAs are loaded into the RNA-induced silencing complex, which directs the miRNAs to its target messenger RNAs (mRNAs). Once bound to its target mRNA, the RNA-induced silencing complex can facilitate several forms of transcriptional repression depending on the strength of the miRNA-mRNA interaction and seed-sequence/target site complementarity, ultimately resulting in the loss of protein expression (1). Thus, the recent discovery of miRNAs has clearly introduced an additional layer of intricacy to our understanding of gene regulation.
Within the past decade, a vast number of studies exploring the significant contribution of miRNAs to human health and disease have underscored the critical relevance of miRNAs to basic and translational biology. Importantly, several promising miRNA therapeutics (either replacement or inhibition therapies) have begun early-stage phase 1/2 clinical trials based on promising preclinical findings (2–4). Additionally, a rapidly growing number of studies have highlighted tissue-specific and urinary miRNAs as potential biomarkers in a number of pathological conditions (5–8).
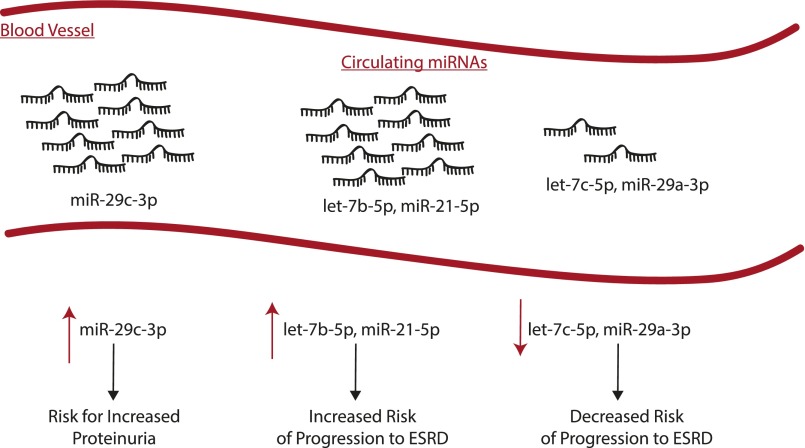
In this issue of Diabetes, Pezzolesi et al. (9) demonstrate for the first time the potential role of experimentally validated, cytokine-regulated plasma-circulating miRNAs as biomarkers for monitoring the progression of DN in subjects with type 1 diabetes (T1D). The authors integrated the existing knowledge of biologically relevant miRNAs to DN pathogenesis and progression as a screening tool to identify miRNAs that can be potentially used as clinical biomarkers. The central premise of the work was to screen levels of miRNAs regulated by transforming growth factor (TGF)-β1, a well-documented pathogenic cytokine in DN, in a set of rapidly progressing proteinuric T1D patients. The authors compared miRNA expression levels from the plasma of three groups of T1D patients: 1) rapid progressors, patients with proteinuria and diabetes but with normal renal function at the time of enrollment who progressed rapidly with a fast rate of estimated glomerular filtration rate decline (mean ± SD duration of 5.1 ± 2.8 years); 2) nonprogressors, patients with proteinuria and diabetes with normal renal function who maintained normal and stable renal function over the course of follow-up (mean ± SD duration of 11.1 ± 5.0 years); and 3) control subjects, patients with diabetes and normoalbuminuria who maintained normal and stable renal function during follow-up (mean ± SD duration of 7.4 ± 1.6 years). Using the baseline levels of known TGF-β1–regulated miRNAs, the investigators narrowed their study to five highly detectable TGF-β1–regulated miRNAs (i.e., let-7b-5p, let-7c-5p, miR-21-5p, miR-29a-3p, and miR-29c-3p). Of these five miRNAs, only circulating levels of let-7b-5p (P = 0.01) and miR-21-5p (P = 0.006) were associated with a significant increase in the risk of ESRD progression. miR-29a-3p (P = 0.0007) and let-7c-5p (P = 0.0002) were significantly associated with a greater than 50% reduction in the risk of rapid progression to ESRD. Interestingly, while levels of miR-29c-3p did not show any association with risk of rapid progression to ESRD (P = 0.68), levels of circulating miR-29c-3p were significantly increased in patients with proteinuria and diabetes from both rapid progressors (P = 0.0009) and nonprogressors (P = 0.0003) compared with normoalbuminuric control subjects (Fig. 1).
Figure 1.
Schematic depicting TGF-β1–regulated circulating miRNAs as predictors of progression of DN.
The work by Pezzolesi et al. (9) parallels previous studies done to investigate circulating miRNA levels as biomarkers for disease progression in other kidney diseases (10,11). A major strength of the study is that the investigators took advantage of patient samples from the extensively characterized Joslin cohort (12). Furthermore, the findings of the study validated conclusions from previously published preclinical studies related to the candidate miRNAs investigated (13). Additionally, while the authors acknowledge that there are indeed other potential miRNAs that may predict progression of DN, either regulated by TGF-β1 or other factors, the identification of a select group of well-characterized miRNAs should allow for future studies to pinpoint the potential role of miRNAs in the progression of DN in a much more focused fashion.
One caveat that should be taken into consideration is that there is a general tendency to extrapolate information regarding the levels of circulating miRNAs to the levels of miRNAs within the tissues, which presumably contribute to specific phenotypes. Indeed, although the five miRNAs selected for analysis by Pezzolesi et al. (9) are regulated by TGF-β1, how the kidney levels of these five miRNAs contribute to the risk of rapid progression of DN remains relatively unexplored. Another conundrum regarding the interpretation of miRNAs as biomarkers for assessing the risk of progression to ESRD in patients with DN is the classic chicken-and-egg causality dilemma as it remains unclear whether perturbation of miRNA levels in the kidney contribute to DN progression or the progression of DN alters levels of circulating miRNAs. Finally, while the current study investigated circulating plasma levels of miRNAs, a growing number of studies demonstrate the ease and robust detection of urinary miRNAs in patients with DN and other kidney pathologies (14–16). Future research would ultimately determine the most reliable method for detecting miRNA levels related to DN progression, taking ease and quantity of sample acquisition into account.
In summary, the findings presented by Pezzolesi et al. (9) merit further investigation in order to test the applicability and reproducibility in additional cohorts with T1D and type 2 diabetes. Ultimately, a prospective randomized trial is needed to establish the role of miRNAs as predictors of progression in patients with DN.
Article Information
Funding. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants RO1DK091310 and RO1DK078900.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 3285.
References
- 1.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 2009;10:126–139 [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Kelnar K, Liu B, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 2011;17:211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov 2010;9:775–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandiera S, Pfeffer S, Baumert TF, Zeisel MB. miR-122—a key factor and therapeutic target in liver disease. J Hepatol 2015;62:448–457 [DOI] [PubMed] [Google Scholar]
- 5.Kato M, Castro NE, Natarajan R. MicroRNAs: potential mediators and biomarkers of diabetic complications. Free Radic Biol Med 2013;64:85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng G. Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev 2015;81:75–93 [DOI] [PubMed] [Google Scholar]
- 7.van Empel VP, De Windt LJ, da Costa Martins PA. Circulating miRNAs: reflecting or affecting cardiovascular disease? Curr Hypertens Rep 2012;14:498–509 [DOI] [PubMed] [Google Scholar]
- 8.Jung HJ, Suh Y. Circulating miRNAs in ageing and ageing-related diseases. J Genet Genomics 2014;41:465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pezzolesi MG, Satake E, McDonnell KP, Major M, Smiles AM, Krolewski AS. Circulating TGF-β1–regulated miRNAs and the risk of rapid progression to ESRD in type 1 diabetes. Diabetes 2015;64:3285–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Zhang C, Chen H, et al. Evaluation of microRNAs miR-196a, miR-30a-5P, and miR-490 as biomarkers of disease activity among patients with FSGS. Clin J Am Soc Nephrol 2014;9:1545–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szeto CC, Li PK. MicroRNAs in IgA nephropathy. Nat Rev Nephrol 2014;10:249–256 [DOI] [PubMed] [Google Scholar]
- 12.Skupien J, Warram JH, Smiles AM, et al. The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int 2012;82:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trionfini P, Benigni A, Remuzzi G. MicroRNAs in kidney physiology and disease. Nat Rev Nephrol 2015;11:23–33 [DOI] [PubMed] [Google Scholar]
- 14.Haase M, Mertens PR. Urinary biomarkers—silver bullets to faster drug development and nephron protection. Nephrol Dial Transplant 2010;25:3167–3169 [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Kwan BC, Lai FM, Chow KM, Li PK, Szeto CC. Urinary miR-21, miR-29, and miR-93: novel biomarkers of fibrosis. Am J Nephrol 2012;36:412–418 [DOI] [PubMed] [Google Scholar]
- 16.Channavajjhala SK, Rossato M, Morandini F, et al. Optimizing the purification and analysis of miRNAs from urinary exosomes. Clin Chem Lab Med 2014;52:345–354 [DOI] [PubMed] [Google Scholar]