Abstract
For the past decade, docetaxel has remained the global standard of care for frontline treatment of metastatic castration-resistant prostate cancer (mCRPC). Until recently, there were limited options for patients with mCRPC following docetaxel failure or resistance, but now the approved treatment choices for these patients have expanded to include abiraterone acetate, cabazitaxel and enzalutamide. Additionally, the radioactive therapeutic agent radium-223 dichloride has been recently approved in patients with CRPC with bone metastases. Although each of these agents has been shown to convey significant survival benefit as a monotherapy, preclinical findings suggest that combining such innovative strategies with traditional treatments may achieve additive or synergistic effects, further augmenting patient benefit. This review will discuss the transformation of the post-docetaxel space in mCRPC, highlighting the spectrum of newly approved agents in this setting in the USA and the European Union, as well as summarizing treatments with non-chemotherapeutic mechanisms of action that have demonstrated promising results in recent phase 3 trials. Lastly, this review will address the potential of combinatorial regimens in mCRPC, including the pairing of novel immunotherapeutic approaches with chemotherapy, radiotherapy or androgen ablation.
Keywords: androgen ablation, chemotherapy, immunotherapy, post-docetaxel, prostate cancer, radiotherapy
Introduction
Prostate cancer is the most common non-skin cancer afflicting men in the Western world and represents the sixth leading cause of male cancer-related deaths worldwide.1 As such, an imperative goal of therapy for prostate cancer is prolonging overall survival (OS).
Until 2010, docetaxel chemotherapy in combination with prednisone remained the established standard of care for patients with metastatic disease,2,3 with limited options for patients following docetaxel failure. However, studies are revealing new ways to treat metastatic castration-resistant prostate cancer (mCRPC), including disease that has progressed on docetaxel therapy. These alternatives not only include novel approaches with traditional modalities such as chemotherapy and radiotherapy (RT) but also encompass further suppression of androgen signaling through alternative pathways and leveraging the immune system to promote an antitumor immune response.
As a result, the treatment paradigm for mCRPC is rapidly evolving in the USA and the European Union, and the nature of this evolution is transforming the timing of docetaxel therapy, the population that receives docetaxel, and the treatment choices for patients who progress after docetaxel therapy. This review will discuss the transformation of the treatment space for mCRPC from both US and EU perspectives, highlighting the spectrum of newly approved and investigational agents in both regions. It will also address the potential of combinatorial regimens in mCRPC, including pairings of novel immunotherapeutic strategies with traditional approaches such as chemotherapy, RT or androgen ablation.
Background: disease state and treatment paradigm
RT and prostatectomy are potentially curative for early-stage prostate cancer.4 However, for patients with later-stage disease and those who progress following definitive therapy, androgen-deprivation therapy (ADT) is used for disease control.2,5 The majority of patients are initially sensitive to ADT, but most tumors eventually become resistant to primary hormone therapy within a median time of 14–30 months.6 The efficacy of ADT is affected by several disease characteristics, including lower biopsy Gleason score, the absence of metastases and lower serum prostate-specific antigen (PSA) at initiation of ADT.7
Patients with prostate cancer that has progressed despite castrate levels of testosterone (<50 ng/ml), that is, CRPC,8 may expect a survival timeframe of 12–22 months from the time of progression.9,10 Initially, many patients with mCRPC may be asymptomatic or have mild symptoms. Commonly, prostate cancer metastasizes to bone, which is associated with symptoms of pain and skeletal-related events, including fracture or spinal compression.5 Palliation of bone metastases, along with prolongation of survival, remain key goals in the treatment of mCRPC.5
Historically, mCRPC was thought to be ‘hormone-insensitive,’ and chemotherapy with docetaxel and prednisone was the gold standard for more than a decade.2,3 When compared with mitoxantrone-based regimens, docetaxel-based regimens increased median OS to ∼16.5–19 months in patients with mCRPC.11–13 Although ∼50% of all men with mCRPC respond to docetaxel with reductions in PSA levels, the majority of responders ultimately develop resistance to this treatment.14 In fact, it has been reported that ∼70% of patients have progressive disease, as measured by rising PSA levels or by Response Evaluation Criteria in Solid Tumors (RECIST), either during or within 3 months of completing docetaxel treatment.15 The clinical challenge is when to start chemotherapy. For example, a patient with asymptomatic or mild symptomatic disease who would rather delay treatment until significant symptoms arise may risk less efficacious treatment outcomes due to more advanced disease. Docetaxel is associated with significant toxicity, which limits its use in mCRPC to instances in which the clinical benefit outweighs the adverse event (AE) profile (for example, rapidly progressing or symptomatic disease).11,12,14 Furthermore, in some patients, treatment is contraindicated due to the presence of comorbidities. In practice, <50% of those with progressive mCRPC receive docetaxel; therefore, there is a need for improved first-line therapies and more options after docetaxel failure.
Recently, randomized phase 3 trials have reported statistically significant improvements in OS in mCRPC (Table 1).11,13,15–19 These results have led to regulatory approvals of agents including sipuleucel-T (Provenge, Dendreon, Seattle, WA, USA),20 abiraterone acetate (Zytiga, Jannsen Biotech, Horsham, PA, USA),21,22 cabazitaxel (Jevtana, Sanofi-Aventis, Bridgewater, NJ, USA, and Sanofi-Aventis Groupe, Paris, France),23,24 enzalutamide (Xtandi, Astellas Pharma US, Inc., Northbrook, IL, USA),25 and radium-223 dichloride (Xofigo; Bayer Healthcare Pharmaceuticals; Wayne, NJ, USA)26 in the USA and/or the European Union.
Table 1. Completed phase 3 trials in CRPC post-docetaxel with a survival advantage.
| Investigational vs control arm | Study | Patient population | Overall survival (mo) | Mean change (mo) | Tolerability | Reference |
|---|---|---|---|---|---|---|
| Docetaxel/prednisone vs mitoxantrone/prednisonea | TAX327 | Advanced CRPC (N = 1006) | 19.2 vs 16.3 (HR = 0.79; P = 0.004) | 2.9 | AEs were more common in the docetaxel arm | Berthold;13 Tannock11 |
| Cabazitaxel/prednisone vs mitoxantrone/prednisone | TROPIC | Docetaxel-pretreated mCRPC (N = 755) | 15.1 vs 12.7 (HR = 0.70; P < 0.0001) | 2.4 | Grade ≥3 neutropenia (82 vs 58%) and diarrhea (6 vs 1%) were more frequent in the cabazitaxel vs control arm | de Bono15 |
| Abiraterone acetate/prednisone vs placebo/prednisone | COU-AA-301 | Docetaxel-pretreated mCRPC (N = 1195) | 15.8 vs 11.2 (HR = 0.74; P < 0.0001) | 4.6 | Most common grade 3–4 AEs amongst pts in the abiraterone acetate and placebo groups, respectively, were fatigue (9 vs 10%), anemia (8 vs 8%), back pain (7 vs 10%) and bone pain (6 vs 8%) | Fizazi;16 de Bono17 |
| Enzalutamide vs placebo | AFFIRM | Docetaxel-pretreated mCRPC (N = 1199) | 18.4 vs 13.6 (HR = 0.63; P < 0.001) | 4.8 | AEs more frequently reported in the enzalutamide group included fatigue, diarrhea and hot flashes; seizures were reported in 5 pts (0.6%) receiving enzalutamide | Scher18 |
| Radium-223 dichloride + BSC vs placebo/BSC | ALSYMPCA | Symptomatic CRPC with bone metastases, of whom 50% were chemotherapy-naive and 50% were post-docetaxel (N = 921) | 14.9 vs 11.3 (HR = 0.70; P = 0.00007) | 3.6 | Grade 3–4 neutropenia in 2.2 vs 0.7%, and grade 3–4 thrombocytopenia in 6.3 vs 2% in radium-223 dichloride and placebo groups, respectively | Parker19 |
Abbreviations: AE, adverse event; BSC, best supportive care; CRPC, castration-resistant prostate cancer; HR, hazard ratio; mCRPC, metastatic CRPC; mo, month; pt, patient.
Phase 3 TAX327 trial that led to docetaxel approval in advanced CRPC shown in table for comparison.
Harnessing the immune system to fight cancer
The immune system has an important role in the development of tumors, as evidenced, in part, by preclinical research showing that tumors are more aggressive when immune function is impaired. Tumors grow more rapidly in immunodeficient mice relative to controls, especially in those with deficiencies in the development or function of CD8+ cytotoxic T cells, CD4+ helper T cells or natural killer (NK) cells.27 Furthermore, tumor infiltration by T cells and NK cells has been shown to improve the prognosis of patients across tumor types.28
Through a process known as ‘immunosurveillance,’ the immune system can recognize and eradicate precursors of cancer before they become clinically apparent.29 The tumor can be completely eradicated or a state of equilibrium may be achieved between tumor immunity and tumor growth, whereby tumor growth is controlled. Unfortunately, for some, the presence of tumor cells that can avoid, resist or suppress the natural immune response may shift the equilibrium toward tumor growth.30 Tumors escape the immune system by a number of mechanisms, including the secretion of immunosuppressive factors such as transforming growth factor β (TGF-β), which inhibits an antitumor immune response, or the recruitment of regulatory T cells (Tregs) or myeloid-derived suppressor cells to the tumor microenvironment, either of which can suppress the activity of cytotoxic T cells.27
Treatments that target the immune system to enhance antitumor immunity may provide clinical benefit in cancer patients and allow the long-term suppression of tumor growth. Key approaches include vaccine therapy using peptide-based or dendritic cell-based strategies, or adoptive cell transfer therapy. Additionally, an understanding of the pathways that regulate antitumor immune responses has led to the development of immune checkpoint inhibitors as an alternative approach to treatment.30 The approval of sipuleucel-T for mCRPC provided proof of concept that immunotherapeutic approaches are a viable treatment option in this setting, with encouraging results from clinical trials of Prostvac (Bavarian Nordic, Kvistgård, Denmark), a cancer vaccine, and ipilimumab (Yervoy, Bristol-Myers Squibb, Princeton, NJ, USA, and Bristol-Myers Squibb S.r.l., Anagni, Italy),31,32 an immune checkpoint inhibitor, providing further supporting evidence.
Newly Approved Agents for mCRPC
First-line treatment
Approvals of sipuleucel-T, an autologous cellular immunotherapy product, and abiraterone acetate, an oral prodrug that blocks CYP17, provide oncologists in the USA with alternative options to first-line docetaxel.20,21 More recently, abiraterone acetate was also approved by the European Medicines Agency (EMA) in the chemotherapy-naive population.22
Sipuleucel-T is generated from a patient's own peripheral blood mononuclear cells, which are then activated ex vivo with a recombinant fusion protein comprising granulocyte/macrophage colony-stimulating factor and prostatic acid phosphatase.10 This active cellular immunotherapy was approved by the US Food and Drug Administration (FDA) in 2010 as a first-line treatment option in men with asymptomatic or minimally symptomatic mCRPC,2,20 making it the first immunotherapy to receive regulatory approval for this tumor type. The approval was based on results of the phase 3 IMPACT trial, which randomized patients to either sipuleucel-T (n = 341) or placebo (n = 171) administered intravenously every 2 weeks, for a total of three infusions.10 A significant survival benefit was demonstrated in the patients treated with sipuleucel-T relative to those in the placebo group (25.8 months vs 21.7 months, respectively; hazard ratio (HR) = 0.78, 95% confidence interval (CI) 0.61–0.98; P = 0.03); furthermore, a greater improvement in the 36-month survival rate (31 vs 23%, respectively) was evident in the sipuleucel-T arm compared with the placebo group.10 AEs occurring more frequently with sipuleucel-T than placebo included chills (54.1 vs 12.5%, respectively), pyrexia (29.3 vs 13.7%, respectively) and headache (16 vs 4.8%, respectively).10
The IMPACT trial reported an OS benefit that was observed despite no significant differences in measurements of disease progression.10 The authors surmised that this was attributable to delayed tumor responses, as observed with this and other immunotherapies in various tumor types, possibly due to the time required to activate the immune system against the tumor.10,30 Recent immunological data showed a correlation between peripheral blood immune responses to the fusion protein and OS that is consistent with the proposed immunological mechanism of action.33
Abiraterone acetate blocks CYP17, a critical enzyme in testosterone synthesis.17 Although initially approved in the post-chemotherapy setting, approval of abiraterone acetate in chemotherapy-naive disease came following positive results from the phase 3 COU-AA-302 trial, in which men with mCRPC who had not received docetaxel (n = 1088) and were treated with abiraterone acetate/prednisone trended toward improved OS relative to those who received placebo/prednisone (median not reached vs 27.2 months, respectively; HR = 0.75, 95% CI = 0.61–0.93; P = 0.01); however, the difference in OS was not statistically significant because, at the time of analysis, the median OS end point had not yet been reached.34 Furthermore, abiraterone acetate improved radiographic progression-free survival (rPFS) relative to placebo (16.5 months vs 8.3 months, respectively; HR = 0.53, 95% CI = 0.45–0.62; P < 0.001).34 Fatigue, arthralgia and peripheral edema were more commonly reported in the abiraterone acetate treatment group than in the control group; grade 3–4 mineralocorticoid-related AEs, including fluid retention (28 vs 24%), hypokalemia (17 vs 13%) and hypertension (22 vs 13%), were more frequent in patients treated with abiraterone acetate/prednisone.34 Of note, this study differed from previous chemotherapy studies in that progressive disease was predominantly defined as radiological progression rather than PSA progression.11,12,34 A post hoc follow-up analysis suggested that continued treatment (≥24 months) with abiraterone acetate/prednisone may further delay disease progression and prolong survival, with acceptable safety and tolerability.35
Post-chemotherapy treatment
In addition to the approval of sipuleucel-T and abiraterone acetate in the first-line setting, abiraterone acetate and cabazitaxel have been approved as post-chemotherapy treatment options by both the FDA and EMA,21–24 and enzalutamide was approved in the USA.25 Each of these agents addresses the unmet need of mCRPC following docetaxel failure, which further expands available treatment options for this malignancy.
Approval of abiraterone acetate in the post-chemotherapy setting in mCRPC came following results from the phase 3 COU-AA-301 trial17 that showed abiraterone acetate/prednisone demonstrated a statistically significant improvement in OS (15.8 months vs 11.2 months; HR = 0.74, 95% CI = 0.64–0.86; P < 0.0001) relative to placebo/prednisone in docetaxel-pretreated patients with mCRPC (n = 1195; randomized 2:1, experimental to control).16 Median time to PSA progression (8.5 months vs 6.6 months, respectively; P < 0.0001), median rPFS (5.6 months vs 3.6 months, respectively; P < 0.0001) and the proportion of patients who had a PSA response (29.5 vs 5.5%; P = 0.0001) were all superior in the abiraterone group relative to the placebo group.16 As observed in COU-AA-302, mineralocorticoid-related AEs were more frequent in the abiraterone acetate group than in the placebo group in this study.17
Cabazitaxel, a chemotherapeutic agent belonging to the taxane class of microtubule inhibitors, was approved by US and EU regulatory agencies23,24 in the post-chemotherapy setting following the phase 3 TROPIC study in which 755 men were randomized to receive mitoxantrone/prednisone or cabazitaxel/prednisone.15 Cabazitaxel treatment led to a statistically significantly longer OS in patients (15.1 months vs 12.7 months, respectively; HR = 0.70, 95% CI = 0.59–0.83; P < 0.0001) compared with those in the mitoxantrone group.15 Additionally, median progression-free survival (PFS) was greater in the cabazitaxel arm relative to the mitoxantrone arm (2.8 months vs 1.4 months, respectively; HR = 0.74, 95% CI = 0.64–0.86; P < 0.0001).15 The clinically significant grade ≥3 AEs that were more common in the cabazitaxel than mitoxantrone arm were neutropenia (82 vs 58%, respectively) and diarrhea (6 vs <1%, respectively).15
Enzalutamide (formerly known as MDV3100), an oral androgen receptor signaling inhibitor, was approved by the FDA for use in docetaxel-pretreated mCRPC in August 2012,25 following positive results from the phase 3 AFFIRM trial.18 Use of enzalutamide resulted in a 4.8-month improvement in OS relative to treatment with placebo (18.4 months vs 13.6 months, respectively; HR = 0.63, 95% CI = 0.53–0.75; P < 0.001) in men with mCRPC previously treated with docetaxel (n = 1199, randomized 2:1, respectively).18 Enzalutamide also demonstrated superiority for key secondary end points relative to placebo, including the proportion of patients with reduced PSA levels by ≥50% (54 vs 2%, respectively; P < 0.001), soft-tissue response rate (29 vs 4%, respectively; P < 0.001), quality-of-life response rate (43 vs 18%, respectively; P < 0.001), time to PSA progression (8.3 months vs 3.0 months, respectively; HR = 0.25; P < 0.001), rPFS (8.3 months vs 2.9 months, respectively; HR = 0.40; P < 0.001) and time to first skeletal-related event (16.7 months vs 13.3 months, respectively; HR = 0.69; P < 0.001).18 AEs that were reported more frequently in the enzalutamide group included fatigue, diarrhea and hot flashes. Additionally, seizures were reported in five patients (0.6%) receiving enzalutamide.18 As steroids have been shown to activate androgen receptor signaling in preclinical models, a post hoc analysis of the AFFIRM study investigated the effects of concomitant corticosteroid use on the efficacy of enzalutamide. Interestingly, their use resulted in reduced OS and higher rates of grade 3–4 AEs in patients treated with either enzalutamide or placebo.36
Bone-targeting RT
As discussed previously, bone is a common metastatic site in mCRPC, and bone metastases are major contributors to morbidity and disease progression. Following the FDA's approval of denosumab (Xgeva, Amgen, Inc., Thousand Oaks, CA, USA), a human monoclonal antibody against receptor activator of nuclear factor-kappa B ligand, for the prevention of skeletal-related events in patients with bone metastases from solid tumors, including mCRPC,37 additional therapeutic agents have been evaluated for their potential to positively impact both OS and the effects of bone metastases. The alpha particle-emitting radioactive therapeutic agent radium-223 dichloride has recently been approved by the FDA in patients with CRPC without visceral metastatic disease and with bone metastases.26
Radium-223 dichloride was evaluated in the phase 3 ALSYMPCA trial in 921 patients with bone metastases; half of them were chemotherapy-naive, whereas the other half had been pretreated with chemotherapy.19,38 Patients were randomized 2:1 to radium-223 or placebo and stratified by pretreatment status. Treatment with radium-223 dichloride demonstrated statistically significant improvement in OS (14.9 months vs 11.3 months, respectively; HR = 0.695, 95% CI = 0.581–0.832; P = 0.00007) compared with placebo in patients.19 Safety with radium-223 dichloride was reported as favorable, regardless of whether patients had previous docetaxel treatment, with a low incidence of myelosuppression (grade 3–4 neutropenia in 2.2 and 0.7% and grade 3–4 thrombocytopenia in 6.3 and 2% of the radium-223 dichloride and placebo groups, respectively).19,38 Notably, men treated with radium-223 dichloride experienced significantly longer time to first skeletal-related event (15.6 months vs 9.8 months, respectively; HR = 0.658, 95% CI = 0.522–0.830; P = 0.00037) relative to placebo-treated patients.19 In addition, this trial reported that radium-233 dichloride reduced pain (50 vs 62% reported pain as an AE; radium-223 dichloride vs placebo, respectively) and opioid use (36 vs 50%; radium-223 dichloride vs placebo, respectively) in patients with CRPC with bone metastases.39
The introduction of each of these new agents is likely to affect the context in which docetaxel is used. Increased use of newer therapies in the first-line setting (to the extent that they are available in a given region) is widening the interval between progression to mCRPC and initiation of chemotherapy or, in some cases, precludes docetaxel therapy entirely.40
Phase 3 Studies in The Post-Docetaxel Setting: Ipilimumab
Ipilimumab is being evaluated in two phase 3 studies in CRPC,41,42 one of which is in patients with docetaxel-pretreated mCRPC (discussed in the next section).41 The evolving treatment landscape for patients in this setting has implications for clinical trial design in the near term, as well as for patient selection in the long run. If granted marketing authorization following phase 3 evaluation, ipilimumab is expected to further diversify the armamentarium of agents used in docetaxel-pretreated mCRPC beyond abiraterone acetate, cabazitaxel and enzalutamide.
Targeting immune checkpoints, such as with ipilimumab, is an immunotherapy approach that differs from vaccines like sipuleucel-T. Rather than targeting a tumor-specific antigen to modulate the immune system against the tumor, ipilimumab, a fully human IgG1 monoclonal antibody, binds cytotoxic T-lymphocyte antigen-4 (CTLA-4) to augment antitumor immune responses, thus targeting the immune system itself. Ipilimumab was approved in 2011 at a dose of 3 mg/kg for the treatment of unresectable or metastatic melanoma by regulatory agencies in >40 countries.31,32 Notably, ipilimumab has demonstrated OS benefit in two phase 3 trials for advanced melanoma, with 19–36% of patients experiencing long-term (4-year) survival.43–45 In advanced melanoma, ipilimumab has side effects that are reflective of its immune mechanism of action, which are manageable using product-specific treatment guidelines.46,47 Furthermore, ipilimumab has shown clinical activity in a number of early clinical trials in prostate cancer.48–50 As discussed in a later section, ipilimumab is currently being investigated in a phase 3 study (CA184-043) in patients with mCRPC post-docetaxel (NCT00861614) (Table 2).41,51–63 In this study, patients receive a single dose of bone-directed RT (8 Gy), followed by either 10 mg/kg ipilimumab or placebo every 3 weeks for up to four doses, and either ipilimumab or placebo every 12 weeks as maintenance therapy. The primary end point of this trial is OS, while secondary end points include PFS, pain response and safety in both arms. Accrual was completed in 2012; this study is currently continuing to follow patients for survival.41,63 Ipilimumab is also being evaluated in patients who have not yet received docetaxel (NCT01057810).42
Table 2. Recruiting/ongoing phase 2 and 3 trials in prostate cancer with immunotherapy-combination regimens.
| Clinicaltrials.gov identifier | Phase | Treatment arm(s) | Patient population | Primary outcome measure | Status | Sponsor | |
|---|---|---|---|---|---|---|---|
| Immunotherapy combined with chemotherapy | NCT0114550851 | 2 | Vaccinia-PSA (L155)-TRICOM vaccine + docetaxel/prednisone vs Docetaxel/ | Metastatic hormone-resistant PC | OS | Ongoing | National Cancer Institute (NCI) |
| NCT0142096552 | 2 | Sipuleucel-T vs Sipuleucel-T+CT-011 vs Sipuleucel-T + CT-011 + cyclophosphamide | Advanced CRPC | Determine feasibility and immune efficacy of combinations | Recruiting | Georgia Regents University | |
| Immunotherapy combined with RT | NCT0086161441 | 3 | Ipilimumab vs placebo, each following single dose of RT | CRPC | OS | Ongoing | Bristol-Myers Squibb |
| Immunotherapy combined with androgen ablation | NCT0045046353 | 2 | Prostvac/TRICOM + flutamide vs Flutamide alone | Androgen insensitive, non-metastatic PC | Time to treatment failure | Recruiting | National Cancer Institute (NCI) |
| NCT0058375254 | 2 | 3 rounds of adenovirus/PSA vaccine vs ADT 14 days prior to beginning adenovirus/PSA vaccinations | Recurrent PC after local therapy | PSA doubling-time response | Recruiting | University of Iowa | |
| NCT0119427155 | 2 | Leuprolide acetate + ipilimumab + radical prostatectomy | PC | Longitudinal peripheral blood values | Recruiting | M.D. Anderson Cancer Center | |
| NCT0137738956 | 2 | Ipilimumab + ADT | Castrate-sensitive PC | Proportion of pts achieving a PSA ≤0.2 ng/ml at month 7 | Recruiting | M.D. Anderson Cancer Center | |
| NCT0143139157 | 2 | ADT initiated 2 wks after the last sipuleucel-T infusion vs ADT initiated 12 wks prior to the first sipuleucel-T infusion | Non-metastatic PC and a rising serum PSA after primary therapy | Change in immune response to PA2024; to determine whether ADT started before or after sipuleucel-T leads to superior augmentation of immune response to sipuleucel-T | Ongoing | Dendreon | |
| NCT0148786358 | 2 | Concurrent arm: Sipuleucel-T concurrent with abiraterone acetate/prednisone for 26 wks vs Sequential arm: Sipuleucel-T therapy followed by abiraterone acetate/prednisone. Abiraterone acetate/prednisone will start 6 wks after the last infusion of sipuleucel-T and continue for | Metastatic CRPC | Evaluate cumulative sipuleucel-T CD54 upregulation | Ongoing | Dendreon | |
| NCT0149897859 | 2 | Ipilimumab + AST | Metastatic hormone-resistant PC | Fraction of pts who achieve an undetectable PSA (≤0.2 ng/ml) | Recruiting | OHSU Knight Cancer Institute | |
| NCT0168849260 | 1/2 | Ipilimumab + abiraterone acetate + prednisone | Chemotherapy and immunotherapy-naive metastatic CRPC | Safety; PFS | Recruiting | Memorial Sloan-Kettering Cancer Center | |
| Immunotherapy–based triplet regimens | NCT0143696861 | 3 | ProstAtak vaccine + valacyclovir + RT +/-ADT vs Placebo + valacyclovir + RT +/- ADT | Localized PC | DFS | Recruiting | Advantagene, Inc. |
| NCT0169687762 | 1/2 | Degarelix acetate vs Cyclophosphamide + GVAX, followed by degarelix acetate | High-risk localized PC undergoing radical prostatectomy | Intraprostatic CD8+ T-cell infiltration; Number of pts with AEs | Recruiting | Sidney Kimmel Comprehensive Cancer Center |
Abbreviations: ADT, androgen-deprivation therapy; AE, adverse event; AST, androgen suppression therapy; CRPC, castration-resistant prostate cancer; DFS, disease-free survival; OS, overall survival; PC, prostate cancer; PFS, progression-free survival; PSA, prostate-specific antigen; pt, patient; RT, radiotherapy; wk, week.
Combinatorial Approaches to Treating Prostate Cancer
An improved understanding of the prostate cancer microenvironment is leading to more efficacious therapies, both through the improved use of existing treatments and the development of new agents. It is postulated that the most effective approaches against mCRPC may be the combination of innovative strategies with traditional treatments, or with each other, to achieve additive or synergistic effects.64 Such combinatorial strategies have been the subject of clinical investigation in mCRPC both before and after the administration of docetaxel/prednisone. Preclinical evidence has provided insight into the various mechanisms that lead to the development of prostate cancer; these pathways are not confined to the cancer epithelial cell but also involve the tumor microenvironment.65 Multiple signaling pathways provide crosstalk between epithelial cells and stromal cells to promote tumor growth and metastases, including androgen receptor signaling and immune surveillance.66
Evaluation of the molecular mechanisms of action for taxane chemotherapies and androgen ablation therapy has revealed the potential for interplay that could lead to clinical synergy between these two classes of agents. Docetaxel, for example, down-regulates androgen receptor expression in a dose-dependent manner, suggesting that the combination of docetaxel and androgen ablation could be synergistic.67 However, androgen ablation therapy has also been shown to augment class III β-tubulin expression, which is thought to impair taxane therapy.68 Maturation of data from studies evaluating the combination of taxanes and androgen ablation will clarify the situation further.
Likewise, mechanistic evaluation suggests that it may be possible to increase the effects of immunotherapies by using them concurrently with other anticancer agents. Data suggest that combining agents that modulate immunosuppression with approaches that favor antigen-specific immune responses may be beneficial in patients with cancer. For instance, of 144 patients with metastatic melanoma who received ipilimumab, those with antibody responses to the cancer antigen NY-ESO-1 at baseline were more likely to experience clinical benefit than those without an antibody response.69
As shown in Figure 1, anticancer treatments can inhibit suppressive mechanisms of tumor-induced immune tolerance, boost T- and/or B-cell responses, or stress tumor cells to increase their immunogenicity or sensitivity to lysis.28 As such, there is great interest in exploring potentially additive or synergistic regimens such as immunotherapy plus chemotherapy, RT or androgen ablation, in the clinic.
Figure 1.
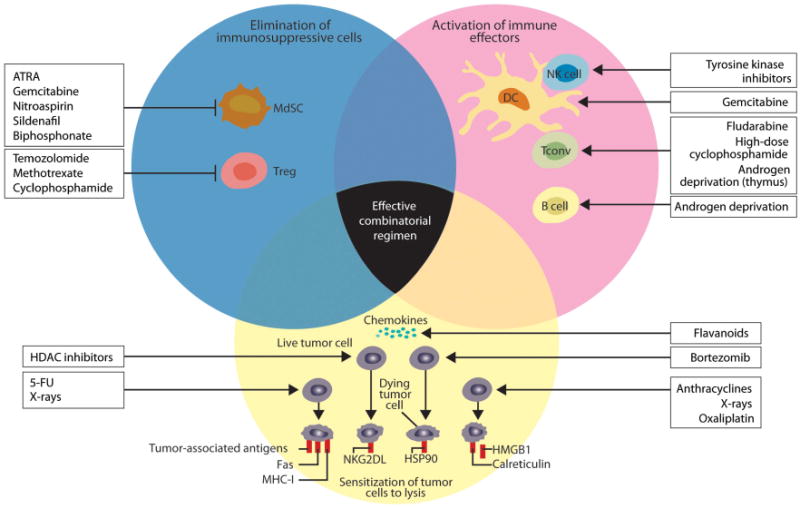
Immune potentiating mechanisms of action of chemotherapies, RT and androgen deprivation. This figure shows how anticancer drugs can activate the immune system. Agents can inhibit immunosuppressive mechanisms of tumor-induced immune tolerance, boost T- and/or B-cell responses or stress tumor cells so they become immunogenic and sensitive to lysis. It is important to note that the anticancer therapies shown apply generally to the field, and some of the agents shown are not indicated for the treatment of mCRPC. Reproduced with permission from the Journal of Clinical Investigation.28 5-FU, 5-fluorouracil; ATRA, all-trans-retinoic acid; DC, dendritic cell; HDAC, histone deacetylases; HSP90, heat shock protein 90; mCRPC, metastatic castrate-resistant prostate cancer; MdSC, myeloid-derived suppressor cell; MHC-I, major histocompatibility complex class I; HMGB1, high mobility group box 1 protein; NK, natural killer cell; NKG2DL, NK cell group 2D ligands; Tconv, conventional effectors; Treg, regulatory T cell.
Immunotherapy in combination with chemotherapy
The overexpression of numerous distinctive antigens on prostate cancer cells and cell lines makes prostate cancer well suited for active immunotherapy.70 Additionally, although chemotherapy is viewed as immunosuppressive because of its ablation of leukocytes, recent work indicates it may also have immunomodulatory effects.28,71 Prostate tumors may promote immune tolerance early in the disease course; as such, chemotherapy may help overcome this hurdle of tumor-induced immune tolerance by reducing the amount of suppressive cytokines, such as TGF-β and interleukin 6, secreted by tumor cells.28,70 Chemotherapy may also deplete Tregs and stimulate tumor-specific effector T-cell proliferation.70,72 Furthermore, following chemotherapy-induced depletion, functional cancer-specific lymphocytes may repopulate the tumor microenvironment via homeostatic proliferation. In addition, preclinical data published by Brown et al.73 suggest that a key mechanism by which homeostatic proliferation supports tumor rejection is by maintaining and/or re-establishing T-cell responsiveness.
Preclinical studies have reported that some chemotherapeutic agents, such as docetaxel and paclitaxel, promote specific immune cell types. For instance, docetaxel administration in 4T1-Neu mammary tumor-bearing mice selectively decreased myeloid-derived suppressor cell, while increasing cytotoxic T-lymphocyte (CTL) responses.74 Similar preclinical studies reported that paclitaxel enhanced the antitumor effects of a toll-like receptor 9 agonist, while also diminishing Tregs.75 Further research with murine tumor models suggests that sequencing of chemotherapy and immunotherapy may be critical. A study by Garnett et al.76 found that administration of a standard dose of docetaxel following poxvirus immunizations improved vaccine-specific immune responses in tumor-bearing mice; however, administration of docetaxel prior to immunizations did not result in benefit.
Preclinical data suggest that CTLA-4 blockade may have synergistic antitumor activity with chemotherapy,77–79 possibly because the cytotoxic effects of chemotherapeutic agents may provide a source of tumor antigens that are then presented to T cells by antigen-presenting cells. CTLA-4 blockade then promotes expansion and infiltration of tumor-primed CTLs. Additionally, chemotherapy may induce changes in tumor architecture to facilitate penetration of immunotherapeutic agents.28,80 Interestingly, Jure-Kunkel et al.78,79 reported that following treatment with CTLA-4 blockade and chemotherapy, some animals rejected a subsequent tumor rechallenge, suggesting the development of a protective immune response in certain tumor models.
Preclinical findings with immunotherapy paired with chemotherapy have translated to early clinical trials in prostate cancer (Table 3).48,81–86 Ongoing phase 2 clinical trials in prostate cancer in which immunotherapies are being combined with chemotherapy are shown in Table 2.
Table 3. Completed clinical trials in prostate cancer with immunotherapy-combination regimens.
| Phase | Patient population (N) | Treatment arm(s) | Primary end point | Results | Reference | |
|---|---|---|---|---|---|---|
| Immunotherapy combined with chemotherapy | 2 | Metastatic, androgen-independent PC (28) | Poxvirus-PSA vaccine + docetaxel/dexamethasone vs Poxvirus-PSA vaccine | Determine whether docetaxel/dexamethasone has an effect on generating an immune response to the vaccine | 3.2 mo vs 1.8 mo median time to progression in the combination arm vs vaccine alone arm, respectively | Arlen81 |
| 2 | Hormone-resistant PC, docetaxel eligible (43) | Ipilimumab + docetaxel vs Ipilimumab | Safety and activity between arms | Two pts who received ipilimumab monotherapy vs 1pt who received ipilimumab + docetaxel had confirmed PSA responses; 18 pts experienced 52 serious AEs, 10 of which were attributed to ipilimumab | Small82 | |
| Immunotherapy combined with RT | 2 | CRPC (30) | Combination immunotherapy + RT vs RT | Determine whether a PSA-specific T-cell response to the vaccine regimen could be mounted in the presence of RT | 13/17 pts who received combination immunotherapy + RT had >3-fold increase in the number of PSA-specific T cells relative to pts in the RT-alone arm, who failed to show a detectable increase in the number of PSA-specific T cells | Gulley83 |
| 1/2 | Metastatic CRPC, pre- and post-docetaxel (71) | Ipilimumab + RT vs Ipilimumab | Safety | No DLTs; treatment-related AEs and immune-related AEs were common in pts treated with or without RT | Slovin48 | |
| Immunotherapy combined with androgen ablation | 1 | Pts with post-prostatectomy recurrence of prostate cancer (6) | Vaccinia-PSA (Prostvac), after radical prostatectomy | Toxicity; serum PSA rise related to serum testosterone restoration, and immunologic effects | No DLTs, and toxicity was minimal; noteworthy variability in time required for testosterone restoration, 1 pt showed continued undetectable serum PSA (<0.2 ng/ml) for >8 mo after testosterone restoration | Sanda84 |
| 2 | Non-metastatic CRPC (42) | Prostvac-VF vs Nilutamide, with potential to crossover after PSA rise to receive combined therapies | Time to progression | Median survival exhibited a trend toward improvement for pts initially randomized to the vaccine arm (median 5.1 vs 3.4 y; P = 0.13); subset of 12 pts who initially received vaccine and then later received nilutamide suggested improved survival compared with the 8 pts who began with nilutamide and then were treated with vaccine (median 6.2 y vs 3.7 y, P = 0.045) | Madan85 | |
| 2 | Advanced chemotherapy-naive CRPC (108) | Ipilimumab + androgen ablation vs Androgen ablation | Percentage without progression at 18 mo | 55 vs 38% undetectable PSA by 3 mo in the combination arm vs androgen ablation alone arm, respectively | Tollefson86 |
Abbreviations: AE, adverse event; CRPC, castrate-resistant prostate cancer; DLT, dose-limiting toxicity; mo, month; PC, prostate cancer; PSA, prostate-specific antigen; pt, patient; RT, radiotherapy; y, year.
Immunotherapy in combination with RT
Combining immunotherapy with RT treatment regimens for prostate cancer may also result in synergy, and preliminary observations have suggested that irradiation of cancer cells can prime an antitumor immune response.87,88 This response may occur through the uptake of the damaged cancerous cells by antigen-presenting cells89 and subsequently the presentation of tumor antigens to immune cells, or possibly through the induction of a more proinflammatory microenvironment.87,90
Preclinical data also suggest that there may be synergistic antitumor activity between RT and vaccine therapy91 or CTLA-4 blockade.92–94 For example, murine experiments by Chakraborty et al.91 demonstrated synergy between local radiation of tumor and active vaccine therapy. The studies used mice transgenic for carcinoembryonic antigen (CEA) and a murine carcinoma cell line transfected with CEA. The vaccine regimen included a prime and boost strategy using CEA-TRICOM. A single dose of 8-Gy radiation to tumors induced upregulation of the death receptor Fas in situ for a number of days. Neither radiation at this dose nor vaccine therapy alone was capable of inhibiting growth of 8-day established tumors, but combining the two strategies led to a significant cure rate, and tumors exhibited a massive infiltration of T cells. Mice cured of tumors demonstrated CD4+ and CD8+ T-cell responses specific for CEA and also revealed the induction of high levels of T-cell responses to two other antigens (gp70 and p53) overexpressed in tumors, suggesting the presence of a consequential antigen cascade.91
Demaria et al.94 tested the hypothesis that the combination of RT to the primary tumor and CTLA-4 blockade can elicit antitumor immunity and thereby inhibit metastases. Using the poorly immunogenic metastatic mouse mammary carcinoma 4T1 as a model, they reported that blockade of CTLA-4 alone or use of RT alone resulted in similar survival rates to those of control mice. However, the combination of RT and CTLA-4 blockade in malignant mice led to synergistic effects, resulting in a statistically significant survival advantage.94
In an important mechanistic study, Pilones et al.93 found that invariant natural killer T (iNKT) cells, a subset with unique regulatory functions, have a critical role in regulating the response to treatment with local RT and CTLA-4 blockade. The growth of poorly immunogenic and vastly metastatic 4T1 mammary carcinoma primary tumors and lung metastases was compared in wild-type and iNKT cell-deficient (iNKT –/–) mice after they received RT and/or a monoclonal antibody against CTLA-4. The response to RT in combination with CTLA-4 blockade was substantially increased in the absence of iNKT cells, with 50% of iNKT –/– mice (vs none of wild-type animals) exhibiting complete tumor regression, long-term survival or resistance to a challenge with 4T1 cells. Additionally, tumor-infiltrating iNKT cells were greatly reduced in wild-type mice that were administered RT plus CTLA-4 blockade.93
Most recently, Dewan et al.92 reported that fractionated RT induced an abscopal effect, defined as tumor regression seen outside the field of RT, when used in combination with anti-CTLA-4 antibody in two independent carcinoma mouse models. Importantly, another study in an autochronous model of prostate cancer suggested that the timing of immunotherapy following RT may be quite restricted, which could have implications for future clinical development of combinatorial strategies.95
The concept of immunotherapy paired with RT has also been evaluated in clinical studies (Table 3) and is the subject of ongoing research (Table 2). Based on the preclinical evidence in mCRPC noted previously and antitumor responses in patients with advanced melanoma,46,47 a phase 1/2, dose-escalation trial was conducted to evaluate the use of ipilimumab alone or in combination with RT in patients with mCRPC, with or without prior chemotherapy (Table 3).48 Of 50 PSA-evaluable patients in the 10 mg/kg ± RT cohort of this trial, eight had PSA response lasting between 3 and > 13 months; furthermore, of the 28 tumor-evaluable patients receiving 10 mg/kg ± RT, 1 displayed complete response whereas 6 had stable disease.48 Encouraging results from this phase 1/2 study of ipilimumab ± RT with or without prior docetaxel treatment, which included no dose-limiting toxicities within the dose-limiting toxicities period, has led to phase 3 evaluation of this agent. As mentioned previously, in the CA184-043 trial, ipilimumab is given following a single dose of RT in an effort to prime for an initial antitumor immune response (Table 2).41,63 The results of this trial were recentely reported (ECCO 2014); the study did not meet its primary endpoint of an increase in overall survival, but did meet a secondary endpoint of improved progression free survival (PFS).
As shown in Table 2, a phase 3 study is investigating the use of ProstAtak (AdV-tk + valacyclovir), an investigational agent that kills tumor cells and elicits an antitumor vaccine effect, in combination with standard external beam RT with or without ADT for intermediate–high-risk localized prostate cancer (NCT01436968).61 This study has an estimated enrollment of 700 patients and has a primary end point of disease-free survival.61
Immunotherapy in combination with androgen ablation
Evidence suggests that in aged mice, androgen ablation can result in regeneration of the thymus and the appearance of naive T cells in the peripheral blood.96,97 In humans, androgen ablation correlates with increased infiltration of CD4+ T cells into the prostate gland.96,98
Early clinical trials in prostate cancer have evaluated the use of androgen ablation with immunotherapy agents such as Prostvac and ipilimumab (Table 3), while a number of current phase 2 trials are examining the clinical benefit of pairing androgen ablation with agents such as ipilimumab and sipuleucel-T, amongst others (Table 2). Of note, intriguing preliminary data from an ongoing phase 2 trial investigating the regimen of ipilimumab with the hormonal agent leuprolide acetate in pre-surgery prostate cancer patients found that this combination affects T-cell responses, including a measurable increase in the frequency of T cells expressing inducible costimulator molecules (NCT01194271).55,99 Recent results reporting early data regarding an ongoing phase 2 trial suggest that the combination of ipilimumab and ADT in castration-sensitive prostate cancer patients significantly increases the frequency of T cells expressing inducible costimulator (ICOS), as well as the levels of memory T-cell markers (NCT01377389).56,100 Additionally, preliminary results from a phase 2 sequencing trial with sipuleucel-T and ADT in men with biochemically recurrent prostate cancer suggest that tumor-specific T-cell responses and immune responses are augmented when sipuleucel-T is given after rather than prior to ADT (NCT01431391).57,101 Follow-up analyses will determine whether improved immune responses correlate with clinical parameters.101
A phase 2 study investigating the use of sipuleucel-T with concurrent or sequential abiraterone acetate plus prednisone (NCT01487863)58 recently reported interim results, which suggest that sipuleucel-T can be successfully manufactured during concurrent abiraterone acetate/prednisone administration.102 The potency and prime boost effect were similar to sipuleucel-T monotherapy. However, pending the release of mature data, it is not yet known how concurrent or sequential abiraterone acetate/prednisone administration impacts the efficacy of sipuleucel-T.102
Combining Agents with Novel Mechanisms of Action
Preclinical and early clinical studies of immunotherapy in combination with therapeutic modalities such as chemotherapy, RT or androgen ablation have revealed enhancement of endogenous or induced antitumor immune responses, providing support for future clinical evaluation of such pairings.64 There is much interest in determining whether other modalities such as cryoablation would result in additive or synergistic effects when paired with immunotherapy, as cryotherapy has shown promising systemic antitumor effects when combined with other anticancer agents such as chemotherapy.103 Of note, with the emergence of checkpoint blockade strategies, the ability to combine multiple immunotherapy agents with divergent mechanisms of action has become an intriguing area of scientific research. The aim is to optimize immunotherapy by targeting different aspects of the immune response.104 Given the recent phase 1 safety and activity findings regarding use of an antibody to block programmed death-1, an inhibitory receptor expressed by T cells, in various tumor types,105 there is interest in combining cancer vaccines with such immune checkpoint blockade antibodies to induce synergistic antitumor responses.104 Another approach involves the use of histone deacetylase inhibitors that can prime tumor cells for eradication by the immune system. This is achieved through multiple mechanisms, including the upregulation of surface molecules such as major histocompatibility complex class I/II as well as costimulatory (CD80, CD86) and adhesion molecules.106 This upregulation may reduce the ability of tumor cells to evade immune detection. Furthermore, histone deacetylase inhibitors have been shown to downregulate Treg cells and augment immunotherapy in animal models of renal and prostate cancers.107
Challenges in The mcrpc Treatment Landscape
Although advances in the biology of prostate cancer have vastly enhanced knowledge and expanded the pool of agents with demonstrated efficacy, medical oncologists face key challenges in defining the optimal treatment paradigm for this malignancy. First-line administration of non-chemotherapy agents such as sipuleucel-T and abiraterone acetate, rather than docetaxel, has dramatically altered the idea of the ‘typical’ patient with mCRPC. With the variety of newly approved agents in mCRPC, administration of docetaxel is expected to be delayed or even omitted, when possible, in favor of agents with a more desirable risk:benefit profile.40 It is possible that in the coming years the use of docetaxel as a therapeutic ‘landmark’ may erode away, with treatment decisions relying even more heavily on an individual patient's prior responses to hormonal treatment and immunotherapy.
Additionally, there is a lack of head-to-head trials comparing existing standards of care and new or emerging treatments. Direct comparison of treatments will offer greater insight into the clinical activity of each agent. Furthermore, with the approval of abiraterone acetate, cabazitaxel and enzalutamide in the post-chemotherapy setting, there are at least three effective agents in this arena; therefore, choosing a comparator for future trials remains a huge hurdle.
Given the diverse mechanisms of action for each of the agents approved for mCRPC since 2010, determining optimal sequence and combinations of agents will be essential to the management of prostate cancer. For any two therapeutic approaches with non-overlapping mechanisms of action, there exists the potential for cultivating synergistic improvements in outcome, but there is also the potential for compounded toxicity profiles when the agents are used in combination. In some cases, there is a specific preclinically or clinically supported rationale for evaluating two agents or classes concurrently or sequentially with one another. For example, as enzalutamide does not need to be given with corticosteroids,25 it may be a more suitable partner for immunotherapy than docetaxel. Likewise, the low rate of myelosuppression observed with radium-223 dichloride in the ALSYMPCA study,19 as previously discussed, suggests that it may be a better pairing with immunotherapy than standard RT. Additionally, in the European Union, abiraterone acetate is commonly administered with the mineralocorticoid receptor antagonist eprenolone rather than with prednisone, which may be important in terms of optimal combinations with immunotherapy, as practitioners seek to avoid the immunosuppressive effects of steroids so that patients can achieve maximal clinical benefit.
Of note, it is not clear whether sequencing of agents will result in superior survival of patients with mCRPC. Recent data in docetaxel-pretreated patients with mCRPC suggest that therapy with either enzalutamide or abiraterone acetate post-cabazitaxel results in prolonged OS compared with those who received enzalutamide or abiraterone acetate pre-cabazitaxel treatment (65 months vs 39 months (from first dose of docetaxel), respectively); prospective trials are required to confirm these results.108 In certain cases, administration of agents simultaneously rather than sequentially may be more efficacious. Given that preliminary results from a trial in which enzalutamide was given after abiraterone acetate and docetaxel suggested possible reduced efficacy in mCRPC patients,109 it is hypothesized that simultaneous administration of these agents may lead to improved benefit, a combination that is currently being evaluated clinically in mCRPC (NCT01650194).110
To this end, a long-term goal in the field is to distinguish clinical biomarkers that will aid in the selection of patients who will benefit from specific therapies, which is critical in tailoring individualized treatment regimens. It was previously shown that circulating tumor cells are prognostic biomarkers, both before and after treatment, in patients treated with taxanes,111 and evaluation of circulating tumor cells as predictive biomarkers is now underway for newly approved agents. As immunotherapy continues to emerge as a treatment modality in mCRPC, it is likely that immunoprofiling will have a greater role in diagnosis, prognostic assessment and therapeutic strategy.112 Although immunotherapy in mCRPC is effective in patients whose disease is chemotherapy-naive and in those pretreated with chemotherapy, in the sipuleucel-T and ipilimumab trials, only a subset of patients in each of these settings experienced therapeutic benefit.10,48 We do not yet have a biomarker to determine which of these patients will exhibit the greatest benefit. Earlier use of secondary androgen ablation in the pre-chemotherapy space begs the question of whether patients who have received abiraterone acetate prior to chemotherapy would benefit from re-treatment with abiraterone acetate following failure of docetaxel. Again, data are sparse and biomarkers for androgen sensitivity are lacking, although it is possible that testosterone levels may indicate receptiveness to androgen ablation following docetaxel.
Conclusions
Worldwide, one of the most significant challenges posed in prostate cancer management has been establishing treatment for a patient who no longer responds to primary androgen ablation therapy and has further progressed on docetaxel-based chemotherapy. Our improved understanding of the complex biology that underscores the malignancy of prostate cancer has led to clinical investigation of innovative approaches such as hormonal therapy, radiopharmaceuticals, immunotherapy and newer chemotherapy, which offer clinicians more options to manage metastatic disease. Ipilimumab is currently being evaluated for efficacy in the post-docetaxel setting in a phase 3 trial. If approved, ipilimumab is expected to further transform the treatment landscape for mCRPC.
The expanding arsenal of treatments for mCRPC has shifted the paradigm for late-line prostate cancer therapy, which has substantial implications for the trial design of future therapies seeking approval in this setting. There has also been a shift in the profile of the typical mCRPC patient, as a result of increased choices for first-line therapy. The potential of combinatorial regimens in mCRPC, including the pairing of novel immunotherapeutic approaches with chemotherapy, RT or androgen ablation, will continue this cycle of evolution, which will help to alleviate the current clinical challenge of navigating the most appropriate use of the newer available agents.
Acknowledgments
Professional medical writing and editorial assistance was provided by Ami P. Modi, PhD, at StemScientific, and funded by Bristol-Myers Squibb (BMS).
Footnotes
Note Added in Proof: Overall survival data on this trial were recently reported at ECCO; the trial did not meet its primary endpoint of an increase in overall survival.
Conflict of Interest: CG Drake has served as a consultant for Amplimmune, BMS, CoStim, Dendreon and Pfizer, and has patents/potential royalties licensed by Amplimmune. P Sharma has served as a paid consultant for BMS, Dendreon, Helsinn, Jounce and MedImmune, serves on the board of directors for Society for Immunotherapy of Cancer and has stock in Jounce. W Gerritsen serves on advisory boards for Aglaia Biomedical Ventures, Amgen, Bayer, BMS, Ipsen, Janssen, Merck and Sanofi, and has received speaker's honoraria from Astellas, BMS and Janssen.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.NCCN. [Accessed March 25, 2013];NCCN Clinical Practice Guidelines in Oncology–Prostate Cancer. 2012 1 Available at http://www.nccn.org. [Google Scholar]
- 3.Horwich A, Parker C, Bangma C, Kataja V and on behalf of the ESMO Guidelines Working Group. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v129–v133. doi: 10.1093/annonc/mdq174. [DOI] [PubMed] [Google Scholar]
- 4.Abdollah F, Schmitges J, Sun M, Jeldres C, Tian Z, Briganti A, et al. Comparison of mortality outcomes after radical prostatectomy versus radiotherapy in patients with localized prostate cancer: a population-based analysis. Int J Urol. 2012;19:836–844. doi: 10.1111/j.1442-2042.2012.03052.x. [DOI] [PubMed] [Google Scholar]
- 5.Maluf FC, Smaletz O, Herchenhorn D. Castration-resistant prostate cancer: systemic therapy in 2012. Clinics. 2012;67:389–394. doi: 10.6061/clinics/2012(04)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Risk M, Corman JM. The role of immunotherapy in prostate cancer: an overview of current approaches in development. Rev Urol. 2009;11:16–27. [PMC free article] [PubMed] [Google Scholar]
- 7.Ross RW, Xie W, Regan MM, Pomerantz M, Nakabayashi M, Daskivich TJ, et al. Efficacy of androgen deprivation therapy in patients with advanced prostate cancer: association between Gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer. 2008;112:1247–1253. doi: 10.1002/cncr.23304. [DOI] [PubMed] [Google Scholar]
- 8.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Chau CH, Figg WD. Challenges to improved therapeutics for metastatic castrate resistant prostate cancer: from recent successes and failures. J Hematol Oncol. 2012;5:35. doi: 10.1186/1756-8722-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. for the IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 11.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. for the TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;35:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 12.Petrylak DP, Tangen CM, Hussain MH, Lara Jr PN, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 13.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 14.Marín-Aguilera M, Codony-Servat J, Kalko SG, Fernández PL, Bermudo R, Buxo E, et al. Identification of docetaxel resistance genes in castration-resistant prostate cancer. Mol Cancer Ther. 2012;11:329–339. doi: 10.1158/1535-7163.MCT-11-0289. [DOI] [PubMed] [Google Scholar]
- 15.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. for the TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration- resistant prostate cancer progressing after docetaxel treatment: a randomized open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 16.Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. for the COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 17.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. for the COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. for the AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 19.Parker C, Nilsson S, Heinrich D, O'Sullivan JM, Fossa SD, Chodacki A, et al. Updated analysis of the phase III, double-blind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA); Presented at the 2012 American Society of Clinical Oncology Annual Meeting; Chicago, IL, USA. 1–5 June 2012; abstract LBA4512. [Google Scholar]
- 20.Provenge (sipuleucel-T) (package insert) Dendreon Corporation; Seattle, WA: 2011. [Google Scholar]
- 21.Zytiga (abiraterone acetate) (package insert) Centocor Ortho Biotech, Inc; Horsham, PA, USA: 2012. [Google Scholar]
- 22.Zytiga (abiraterone acetate) (summary of product characteristics) Janssen-Cilag International N.V; Beerse, Belgium: 2012. [Google Scholar]
- 23.Jevtana (cabazitaxel) (package insert) Sanofi-Aventis; Bridgewater, NJ, USA: 2013. [Google Scholar]
- 24.Jevtana (cabazitaxel) (summary of product characteristics) Sanofi-Aventis Groupe; Paris, France: 2013. [Google Scholar]
- 25.Xtandi (enzalutamide) (package insert) Astellas Pharma US, Inc; Northbrook, IL, USA: 2012. [Google Scholar]
- 26.Xofigo (radium Ra 223 dichloride) (package insert) Bayer HealthCare Pharmaceuticals Inc; Wayne, NJ, USA: 2013. [Google Scholar]
- 27.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 30.Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–546. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 31.US Yervoy (ipilimumab) (package insert) Bristol-Myers Squibb Company; Princeton, NJ: 2013. [Google Scholar]
- 32.EU Yervoy (ipilimumab) (package insert) Bristol-Myers Squibb S.r.l; Anagni, Italy: 2012. [Google Scholar]
- 33.Sheikh NA, Petrylak D, Kantoff P, Dela Rosa C, Stewart FP, Kuan LY, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62:137–147. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan CJ, Smith MR, deBono JS, Molina A, Logothetis CJ, de Souza P, et al. for the COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2012;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathkopf DE, Smith MR, De Bono JS, Logothetis C, Shore N, De Souza PL, et al. Long-term safety and efficacy analysis of abiraterone acetate (AA) plus prednisone (P) in metastatic castration-resistant prostate cancer (mCRPC) without prior chemotherapy (COU-AA-302) J Clin Oncol. 2013;31:310s. abstract 5009. [Google Scholar]
- 36.Scher HI, Fizazi K, Saad F, Chi KN, Taplin M, Sternberg CN, et al. Impact of on-study corticosteroid use on efficacy and safety in the phase III AFFIRM study of enzalutamide, an androgen receptor inhibitor. J Clin Oncol. 2013;31 abstract 6. [Google Scholar]
- 37.Xgeva (denosumab) (package insert) Amgen Inc; Thousand Oaks, CA, USA: 2013. [Google Scholar]
- 38.Vogelzang NJ, Helle SI, Johannessen DC, O'Sullivan JM, Garcia-Vargas JE, Gillies C, et al. Efficacy and safety of radium-223 dichloride (Ra-223) in castration-resistant prostate cancer (CRPC) patients with bone metastases who did or did not receive prior docetaxel (D) in the phase III ALSYMPCA trial. J Clin Oncol. 2013;31:324s. abstract 5068. [Google Scholar]
- 39.Nilsson S, Sartor AO, Bruland OS, Fang F, Aksnes AK, Parker C. Pain analyses from the phase III randomized ALSYMPCA study with radium-223 dichloride (Ra-223) in castration-resistant prostate cancer (CRPC) patients with bone metastases. J Clin Oncol. 2013;31:317s. abstract 5038. [Google Scholar]
- 40.Higano D, Saad F, Somer B, Curti B, Petrylak D, Drake CG, et al. A phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration- resistant prostate cancer (CRPC); Presented at the 2009 Genitourinary Cancers Symposium; Orlando, FL, USA. 26–28 February 2009; abstract LBA150. [Google Scholar]
- 41.Clinical Trials.gov NCT00861614. [Accessed 4 June 2013];Study of immunotherapy to treat advanced prostate cancer. http://clinicaltrials.gov/ct2/show/NCT00861614?term=00861614&rank=1.
- 42.Clinical Trials.gov NCT01057810. [Accessed 4 June 2013];Phase 3 study of immunotherapy to treat advanced prostate cancer. http://clinicaltrials.gov/ct2/show/NCT01057810?term=NCT01057810&rank=1.
- 43.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maio M, Bondarenko I, Robert C, Thomas L, Garbe C, Testib A, et al. Four-year survival update for metastatic melanoma (MM) patients (pts) treated with ipilimumab (IPI) + dacarbazine (DTIC) on phase 3 study CA184-024. Ann Oncol. 2012;23(Suppl 9):ix367. abstract 1127P. [Google Scholar]
- 45.Lebbe C, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, et al. Five-year survival rates for patients (pts) with metastatic melanoma (MM) treated with ipilimumab (IPI) in phase II trials. Ann Oncol. 2012;23(Suppl 9):ix363. doi: 10.1093/annonc/mdt161. abstract 1116PD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 48.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 51.Clinical Trials.gov NCT01145508. [Accessed June 4, 2013];Docetaxel and prednisone with or without vaccine therapy in treating patients with metastatic hormone-resistant prostate cancer. http://clinicaltrials.gov/ct2/show/NCT01145508?term=NCT01145508&rank=1.
- 52.Clinical Trials.gov NCT01420965. [Accessed 4 June 2013];Sipuleucel-T, CT-011, and cyclophosphamide for advanced prostate cancer. http://clinicaltrials.gov/ct2/show/NCT01420965?term=NCT01420965&rank=1.
- 53.Clinical Trials.gov NCT00450463. [Accessed June 4, 2013];Vaccine therapy with PROSTVAC/TRICOM and flutamide versus flutamide alone to treat prostate cancer. http://clinicaltrials.gov/ct2/show/NCT00450463?term=NCT00450463&rank=1.
- 54.Clinical Trials.gov NCT00583752. [Accessed June 4, 2013];Phase II study of adenovirus/PSA vaccine in men with recurrent prostate cancer after local therapy APP21. http://clinicaltrials.gov/ct2/show/NCT00583752?term=00583752&rank=1.
- 55.Clinical Trials.gov NCT01194271. [Accessed June 4, 2013];Neoadjuvant ipilimumab in prostate cancer. http://clinicaltrials.gov/ct2/show/NCT01194271?term=NCT01194271&rank=1.
- 56.Clinical Trials.gov NCT01377389. [Accessed 4 June 2013];Ipilimumab + androgen deprivation therapy in prostate cancer. http://clinicaltrials.gov/ct2/show/NCT01377389?term=NCT01377389&rank=1.
- 57.Clinical Trials.gov NCT01431391. [Accessed 4 June 2013];Sequencing of sipuleucel-T and ADT in men with non-metastatic prostate cancer. http://clinicaltrials.gov/ct2/show/NCT01431391?term=NCT01431391&rank=1.
- 58.Clinical Trials.gov NCT01487863. [Accessed 4 June 2013];Concurrent versus sequential treatment with sipuleucel-t and abiraterone in men with metastatic castrate resistant prostate cancer (mCRPC) http://clinicaltrials.gov/ct2/show/NCT01487863?term=NCT01487863&rank=1.
- 59.Clinical Trials.gov NCT01498978. [Accessed 4 June 2013];Ipilimumab in combination with androgen suppression therapy in treating patients with metastatic hormone-resistant prostate cancer. http://clinicaltrials.gov/ct2/show/NCT01498978?term=NCT01498978&rank=1.
- 60.Clinical Trials.gov identifier NCT01688492. [Accessed 4 June 2013];Combining ipilimumab with abirater-one acetate plus prednisone in chemotherapy and immunotherapy-naïve patients with progressive metastatic castration-resistant prostate cancer. http://clinicaltrials.gov/ct2/show/NCT01688492?term=NCT01688492&rank=1.
- 61.Clinical Trials.gov NCT01436968. [Accessed June 4, 2013];Phase 3 study of ProstAtak with standard radiation therapy for localized prostate cancer (PrTK03) http://clinicaltrials.gov/ct2/show/NCT01436968?term=NCT01436968&rank=1.
- 62.Clinical Trials.gov NCT01696877. [Accessed June 4, 2013];A neoadjuvant study of androgen ablation combined with cyclophosphamide and GVAX vaccine for localized prostate cancer. http://clinicaltrials.gov/ct2/show/NCT01696877?term=NCT01696877&rank=1.
- 63.Drake CG, Scher HI, Bossi A, van den Eertwegh AJM, McHenry B, Fitzmaurice TF, et al. CA184-043: A randomized, double-blind, phase III trial comparing ipilimumab versus placebo following a single dose of radiotherapy in patients with castration-resistant prostate cancer who have received prior treatment with docetaxel. J Clin Oncol. 2012;30(suppl) abstract TPS4689. [Google Scholar]
- 64.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corn PG. The tumor microenvironment in prostate cancer: elucidating molecular pathways for therapy development. Cancer Manag Res. 2012;4:183–193. doi: 10.2147/CMAR.S32839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dayyani F, Gallick GE, Logothetis CJ, Corn PG. Novel therapies for metastatic castrate-resistant prostate cancer. J Natl Cancer Inst. 2011;103:1665–1675. doi: 10.1093/jnci/djr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuroda K, Liu H, Kim S, Guo M, Navarro V, Bander NH. Docetaxel down-regulates the expression of androgen receptor and prostate-specific antigen but not prostate-specific membrane antigen in prostate cancer cell lines: Implications for PSA surrogacy. Prostate. 2009;69:1579–1585. doi: 10.1002/pros.21004. [DOI] [PubMed] [Google Scholar]
- 68.Terry S, Ploussard G, Allory Y, Nicolaiew N, Boissière-Michot F, Maillé P, et al. Increased expression of class III β-tubulin in castration-resistant human prostate cancer. Br J Cancer. 2009;101:951–956. doi: 10.1038/sj.bjc.6605245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA. 2011;108:16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Slovin SR. Toward maximizing immunotherapy in metastatic castration-resistant prostate cancer—rationale for combinatorial approaches using chemotherapy. Front Oncol. 2012;2:43. doi: 10.3389/fonc.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma Y, Conforti R, Aymeric L, Locher C, Kepp O, Kroemer G, et al. How to improve the immunogenicity of chemotherapy and radiotherapy. Cancer Metastasis Rev. 2011;30:71–82. doi: 10.1007/s10555-011-9283-2. [DOI] [PubMed] [Google Scholar]
- 72.McDonnell AM, Nowak AK, Lake RA. Contribution of the immune system to the chemotherapeutic response. Semin Immunopathol. 2011;33:353–367. doi: 10.1007/s00281-011-0246-z. [DOI] [PubMed] [Google Scholar]
- 73.Brown IE, Blank C, Kline J, Kacha AK, Gajewski TF. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J Immunol. 2006;177:4521–4529. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- 74.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vicari AP, Luu R, Zhang N, Patel S, Makinen SR, Hanson DC, et al. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother. 2009;58:615–628. doi: 10.1007/s00262-008-0586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mokyr MB, Kalinichenko T, Gorelik L, Bluestone JA. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 1998;58:5301–5304. [PubMed] [Google Scholar]
- 78.Jure-Kunkel MN, Masters G, Girit E, Dito G, Lee FY. Antitumor activity of anti-CTLA-4 monoclonal antibody (mAb) in combination with ixabepilone in preclinical tumor models. J Clin Oncol. 2008;26(15 suppl):144s. abstract 3048. [Google Scholar]
- 79.Lee FL, Jure-Kunkel MN, Salvati ME. Synergistic activity of ixabepilone plus other anticancer agents: preclinical and clinical evidence. Ther Adv Med Oncol. 2011;3:11–25. doi: 10.1177/1758834010386402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nowak A, Robinson B, Lake R. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–4496. [PubMed] [Google Scholar]
- 81.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Small E, Higano C, Tchekmedyian N, Sartor O, Stein B, Young R, et al. Randomized phase II study comparing 4 monthly doses of ipilimumab (MDX-010) as a single agent or in combination with a single dose of docetaxel in patients with hormone-refractory prostate cancer. J Clin Oncol. 2006;24(suppl 18):4609. [Google Scholar]
- 83.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 84.Sanda MG, Smith DC, Charles LG, Hwang C, Pienta KJ, Schlom J, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 85.Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tollefson MK, Karnes RJ, Thompson RH, Granberg CF, Hillman DW, Breau RH, et al. A randomized phase II study of ipilimumab with androgen ablation compared with androgen ablation alone in patients with advanced prostate cancer; Presented at the American Society of Clinical Oncology 2010 Genitourinary Cancers Symposium; San Francisco, CA, USA. 5–7 March 2010; abstract 168. [Google Scholar]
- 87.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 89.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 90.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 91.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 92.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pilones KA, Kawashima N, Yang AM, Babb JS, Formenti SC, Demaria S. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res. 2009;15:597–606. doi: 10.1158/1078-0432.CCR-08-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 95.Harris TJ, Hipkiss EL, Borzillary S, Wada S, Grosso JF, Yen HR, et al. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate. 2008;68:1319–1329. doi: 10.1002/pros.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Antonarakis ES, Drake CG. Combining immunological and androgen-directed approaches: an emerging concept in prostate cancer immunotherapy. Curr Opin Oncol. 2012;24:258–265. doi: 10.1097/CCO.0b013e32835205a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 98.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao J, Sun J, Ward JF, Rao P, Troncoso P, Araujo JC, et al. Combination therapy with anti-CTLA-4 plus leuprolide acetate in the pre-surgical setting of patients with regional, high-risk prostate cancer. Cancer Res. 2012;72(Suppl 8) abstract no. 4388. [Google Scholar]
- 100.Aparicio A, Sun J, Tang DN, Arap W, Araujo J, Corn P, et al. A phase II study of ipilimumab plus androgen deprivation therapy in castration-sensitive prostate carcinoma. Cancer Res. 2012;72(Suppl 8) abstract no. 5368. [Google Scholar]
- 101.Antonarakis ES, Kibel AS, Adams G, Karsh LI, Elfiky A, Shore ND, et al. A randomized phase II study evaluating the optimal sequencing of sipuleucel-T and androgen deprivation therapy (ADT) in biochemically recurrent prostate cancer (BRPC): immune results. J Clin Oncol. 2013;31:312s. abstract 5016. [Google Scholar]
- 102.Small EJ, Lance RS, Redfern CH, Millard FE, Gardner TA, Karsh LI, et al. A randomized phase II trial of sipuleucel-T with concurrent or sequential abiraterone acetate (AA) plus prednisone (P) in metastatic castrate-resistant prostate cancer (mCRPC) J Clin Oncol. 2013;31:319s. doi: 10.1158/1078-0432.CCR-15-0079. abstract 5047. [DOI] [PubMed] [Google Scholar]
- 103.Levy MY, Sidana A, Chowdhury WH, Solomon SB, Drake CG, Rodriguez R, et al. Cyclophosphamide unmasks an antimetastatic effect of local tumor cryoablation. J Pharmacol Exp Ther. 2009;330:596–601. doi: 10.1124/jpet.109.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dickinson M, Johnstone RW, Prince HM. Histone deacetylase inhibitors: potential targets responsible for their anti-cancer effect Invest New Drugs. 2010;28(Suppl 1):S3–S20. doi: 10.1007/s10637-010-9596-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen L, Ciesielski M, Ramakrishnan S, Miles KM, Ellis L, Sotomayor P, et al. Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS One. 2012;7:e30815. doi: 10.1371/journal.pone.0030815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Angelergues A, Maillet D, Flechon A, Ozguroglu M, Mercier F, Guillot A, et al. Prognostic factors of survival in patients with metastatic castration resistant prostate cancer (mCRPC) treated with cabazitaxel: sequencing might matter. J Clin Oncol. 2013;31:323s. abstract 5063. [Google Scholar]
- 109.Stevenson R, Ford D, Zarkar AM, Glaholm J, Porfiri E, Tew A, et al. The sequential use of abiraterone and enzalutamide (MDV3100) in castrate resistant prostate cancer patients: experience from Birmingham, United Kingdom. J Clin Oncol. 2013;31 abstract e16048. [Google Scholar]
- 110.Clinical Trials.gov identifier NCT01650194. [Accessed 4 June 2013];A study to determine safety and tolerability of enzalutamide (MDV3100) in combination with abiraterone acetate in bone metastatic castration-resistant prostate cancer patients. http://clinicaltrials.gov/ct2/show/NCT01650194?term=NCT01650194&rank=1.
- 111.Danila DC, Pantel K, Fleisher M, Scher HI. Circulating tumors cells as biomarkers: progress toward biomarker qualification. Cancer J. 2011;17:438–450. doi: 10.1097/PPO.0b013e31823e69ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McCourt CM, Boyle D, James J, Salto-Tellez M. Immunohistochemistry in the era of personalised medicine. J Clin Pathol. 2013;66:58–61. doi: 10.1136/jclinpath-2012-201140. [DOI] [PubMed] [Google Scholar]


