Abstract
Fast skeletal muscle Troponin T (TNNT3) is an important component of the skeletal muscle contractile machinery. The pre-mRNA encoding TNNT3 is alternatively spliced and changes in the pattern of TNNT3 splice form expression are associated with alterations in thin filament calcium sensitivity and force production during muscle contraction, thereby regulating muscle function. Interestingly, during aging, muscle force/cross sectional area is reduced, suggesting that loss of mass does not completely account for the impaired muscle function that develops during the aging process. Therefore, in the present study, we tested the hypothesis that age- and changes in muscle loading are associated with alterations in TNNT3 alternative splicing in the rat gastrocnemius muscle. We found that the relative abundance of several TNNT3 splice forms varied significantly with age among 2, 9, and 18-month old rats, and the pattern correlated with changes in body weight rather than muscle mass. Hindlimb immobilization for 7 days resulted in dramatic alterations in splice form relative abundance such that the pattern was similar to that observed in lighter animals. Remobilization for 7 days restored the splicing pattern toward that observed in the non-immobilized limb, even though muscle mass had not yet begun to recover. In conclusion, the results suggest that TNNT3 pre-mRNA alternative splicing is rapidly (i.e. within days) modulated in response to changes in the load placed on the muscle. Moreover, the results show that restoration of TNNT3 alternative splicing to control patterns is initiated prior to an increase in muscle mass.
Keywords: aging, disuse atrophy, TNNT3, alternative splicing, immobilization, remobilization
Introduction
Troponin T is a component of the troponin complex that controls actin-myosin cross-bridge formation and force production during muscle contraction (Farah et al. 1995; Reinach et al. 1997; Perry 1998; Gordon et al. 2000). In skeletal muscle of insects (Marden et al. 1999; Marden et al. 2001; Nongthomba et al. 2007; Schilder et al. 2007; Marden et al. 2008; Singh et al. 2014) and mammals (Medford et al. 1984; Pan et al. 1992; Briggs et al. 1996; Ogut et al. 1999; Stefancsik et al. 2003; Gallon et al. 2006; Schilder et al. 2011; Schilder et al. 2012), Troponin T (TNT) and fast Troponin T (TNNT3), respectively, are expressed as multiple isoforms, and the pattern of isoform expression is largely determined by alternative splicing of the Troponin T precursor mRNA (pre-mRNA) encoding the protein. Interestingly, in both insects (Marden et al. 2008) and rodents (Schilder et al. 2011), alternative splicing of the Troponin T pre-mRNA varies in a quantitatively precise fashion with variation in (or changes to) body weight. Thus, as body weight increases, there is a rapid (within 5 d) and highly predictable quantitative shift in the relative abundance of specific Troponin T splice forms (Schilder et al. 2011). There are at least 12 TNNT3 splice forms that can be divided into two categories based on whether they include mutually exclusive exon 16 (referred to here as TNNT3α splice forms) or 17 (referred to here as TNNT3β splice forms). In rats, three TNNT3α splice forms are present in the gastrocnemius muscle, and the relative abundance of all three is consistently increased in heavier compared to lighter animals. In contrast, of the nine TNNT3β splice forms identified in the gastrocnemius, the relative abundance of seven is consistently reduced as body weight increases.
Changes in the pattern of TNNT3 alternative splicing that manifest with increasing body weight are thought to be an important component of the increase in muscle force and power output needed to maintain mobility in heavier compared to lighter individuals (Schachat et al. 1987; Greaser et al. 1988; Pan et al. 1992; Reiser et al. 1992; Marden et al. 1999; Ogut et al. 1999; Marden et al. 2001; MacFarland et al. 2002; Chaudhuri et al. 2005; Gallon et al. 2006). Muscle performance is regulated by TNNT3 in part through the differential effects of various splice forms on calcium sensitivity (Chaudhuri et al. 2005; Gallon et al. 2006). At the molecular level, the C-terminal region of TNNT3 interacts with sarcomere components such as tropomyosin, and troponin C that play important roles in Ca2+ sensitivity. Because the C-terminus of Tnnt3 differs between the α and β splice forms, they have distinctive effects on Ca2+ sensitivity during muscle contraction (Chaudhuri et al. 2005; Gallon et al. 2006). For example, TNNT3α has higher affinity for tropomyosin and interacts more strongly with troponin C, when compared to TNNT3β (Pan et al. 1992; Wu et al. 1995; Chaudhuri et al. 2005). Consequently, in the presence of TNNT3α, the affinity of troponin C for Ca2+ is three-fold higher than in the presence of TNNT3β. Similarly, Gallon et al. showed that reconstitution of rat psoas muscle fibers with TNNT3α resulted in increased Ca2+ sensitivity, whereas reconstitution with TNNT3β led to a decrease in Ca2+ sensitivity (Gallon et al. 2006). Therefore, relevant to the present study, increased abundance of TNNT3α splice forms is associated with improved calcium sensitivity and force production during muscle contraction (Chaudhuri et al. 2005; Gallon et al. 2006).
Although body weight is an important determinant of TNNT3 alternative splicing, recent studies suggest that other mechanisms also exist to modulate its splicing pattern. For example, in insect flight muscle, body weight-induced TNT alternative splicing can be attenuated by dietary manipulation (Marden et al. 2008). Similarly, in a genetic model of obesity, body weight-inappropriate TNNT3 pre-mRNA alternative splicing was observed in rats, and impaired alternative splicing may thus contribute to the deficit in body weight specific muscle performance often associated with obesity (Schilder et al. 2011). A decline in muscle performance is also observed in the elderly (Allman et al. 2004; Horner et al. 2011; Goldspink 2012; Moore et al. 2014) or after periods of inactivity (Callahan et al. 2014; Miller et al. 2014), e.g. during limb immobilization (Greenhaff 2006; de Boer et al. 2007). In part, reduced force production under these conditions is a result of loss of muscle mass. However, in some cases, the decrease in force production is greater in magnitude than the loss of muscle mass, and relative force production (force/muscle mass) is disproportionally reduced (Mitchell et al. 2012; Miller et al. 2013). Whether or not changes in the relative abundance of TNNT3 splice forms play a role in such alterations is unknown. Therefore, in the present study we tested the hypothesis that age- and muscle unloading are associated with alterations in TNNT3 pre-mRNA alternative splicing in rat gastrocnemius muscle. In these studies, we assessed the relative abundance of TNNT3 splice forms in gastrocnemius muscle of 2-, 9-, and 18-month old rats. We also examined the effect of unilateral hindlimb immobilization for 7 d, and remobilization for a subsequent 7 d, on gastrocnemius muscle TNNT3 splice form abundance. Overall, the results demonstrate that increased load is associated with increased relative abundance of TNNT3α splice forms that have previously been shown to be associated with greater calcium sensitivity and force production (Chaudhuri et al. 2005; Gallon et al. 2006), and that this relationship is disrupted when the load on the muscle is reduced. Moreover, we find that 7 d of remobilization (i.e. reloading) restores the pattern of TNNT3 alternative splicing toward that observed in the control hindlimb independent of changes in muscle mass.
Materials and Methods
Animal Studies
The morphological data and muscle samples were obtained from a previous study (Kelleher et al. 2014) that is briefly described here. Male Sprague Dawley rats at 2-, 9-, and 18-months of age were obtained from Charles River laboratories and housed under conditions of 12 hr light/dark cycle with ad libitum access to food and water. Unilateral hindlimb immobilization was carried out by application of a fiberglass cast to one hindlimb as previously described. All immobilized rats were casted for 7 days. One group of 9-month old rats was subjected to a 7-day immobilization period followed by a 7-day remobilization period after cast removal. Younger (i.e. 2-month old) rats were not used in remobilization studies because their body weight would increase significantly during the 2-week experimental period, making interpretation of any changes in TNNT3 alternative splicing complicated. The gastrocnemius muscle was harvested from anesthetized rats, weighed, frozen between aluminum blocks pre-cooled in liquid nitrogen, and stored at −80°C prior to analysis. In all experiments, the muscle from the contralateral non-immobilized hindlimb was analyzed as the control. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Penn State College of Medicine.
Quantification of TNNT3 splice forms
The methods used for RNA isolation, reverse transcription, and PCR analysis as well as the sequence of the primers used to quantify TNNT3 splice forms has been previously published (Schilder et al. 2011). Briefly, the frozen gastrocnemius muscle was pulverized under liquid nitrogen and total RNA was isolated using Trizol reagent as per the manufacturer’s protocol (Invitrogen). cDNA was prepared using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems), and PCR was carried out using GoTaq DNA polymerase (Promega) with a fluorescein-labeled forward primer and two reverse primers as previously described (Schilder et al. 2011). The fluorescein-labeled PCR amplicons were analyzed by capillary electrophoresis (3730XL DNA Analyzer, Applied Biosystems) and the fragment size of the PCR amplicons was determined using the 1200 LIZ internal size standard (Applied Biosystems). The peak height value for each PCR amplicon was obtained by analysis using Peak Scanner software (Applied Biosystems). This method (Schilder et al. 2011) was previously used to identify PCR amplicons corresponding to all 12 TNNT3 splice forms in rat gastrocnemius muscle based on fragment size (Supplementary Figure S1). The relative abundance of each TNNT3 splice form was calculated as the ratio of its detected peak height to the total of all peak heights.
Statistical Analysis
Statistical analyses were performed using Prism 6 (GraphPad Software, Inc.) and JMP Pro 10 (SAS Institute Inc.). The effects of age on TNNT3 splice form relative abundance were evaluated using one-way ANOVA over the three age groups. Multiple comparison error rates were controlled with Tukey’s post-hoc test. The effect of immobilization and interaction with age were determined using a two-way nested random effects model (two-way nested ANOVA) to analyze TNNT3 splice form relative abundance in the immobilized limb relative to the contralateral non-immobilized limb. The nested random effects model analyzes the relative abundance of the TNNT3 splice forms by nesting the limb (immobilized or non-immobilized) within the animal to control for between animal heterogeneity by considering each animal as an experimental unit. Principal component analysis was applied to reduce the 12-dimensional data sets and the first two principal components were included in the analyses. Comparisons between immobilized and non-immobilized muscles or remobilized and non-immobilized muscles from the same animal were performed using paired Student’s t-test. Statistical significance was set at p<0.05. Data are represented as mean ± SEM.
Results
Effect of age
The age of the animals used in this study was chosen based on results of a previous one from our laboratory (Kimball et al. 2004). Thus, 2-month old rats were selected as representative of young growing animals, 9-month old rats as representative of mature adults, and 18-month old rats were selected because age-related loss of muscle mass begins to manifest at approximately this age. In agreement with the earlier study, the relative mass (i.e. muscle mass/body mass ratio) of the gastrocnemius was not different in 2- and 9-month old rats, but was 14% (p<0.05) lower in 18-month compared to 9-month old rats (Table 1).
Table 1.
Body and gastrocnemius mass.
| Rat age | Body mass (g) | Muscle mass (g) | Muscle mass / Body mass (× 10,000) | ||
|---|---|---|---|---|---|
| Immob or recov limb | Control limb | Immob or recov limb | Control limb | ||
| 2 m | 320.5 ± 5.3 | 1.196 ± 0.044*#+ | 1.809 ± 0.027#+ | 37.3 ± 1.32*# | 56.5 ± 5.07+ |
| 9 m | 456.6 ± 4.4 | 2.213 ± 0.069* | 2.603 ± 0.097 | 49.4 ± 1.42*+ | 55.5 ± 2.03+ |
| 9 m (recov) | 414.0 ± 16.6 | 1.466 ± 0.073*# | 2.287 ± 0.075 | 35.4 ± 1.13*# | 55.5 ± 1.93 |
| 18 m | 537.5 ± 21.4 | 2.106 ± 0.091* | 2.543 ± 0.093 | 39.33 ± 1.33*# | 47.5± 1.46# |
Rats, 2-, 9-, and 18-months of age, had one hindlimb immobilized for 7 days, and an additional group of 9-month old animals had their casts removed after 7 days and were allowed to remobilize for an additional 7 days (recov).
p < 0.05 compared to control limb;
p < 0.05 compared to equivalent limb in 9 month old rats;
p < 0.05 compared to equivalent limb in 18 month old rats. The data in this table has been previously published (Kelleher et al. 2015), and permission to reproduce it here was granted by the publisher.
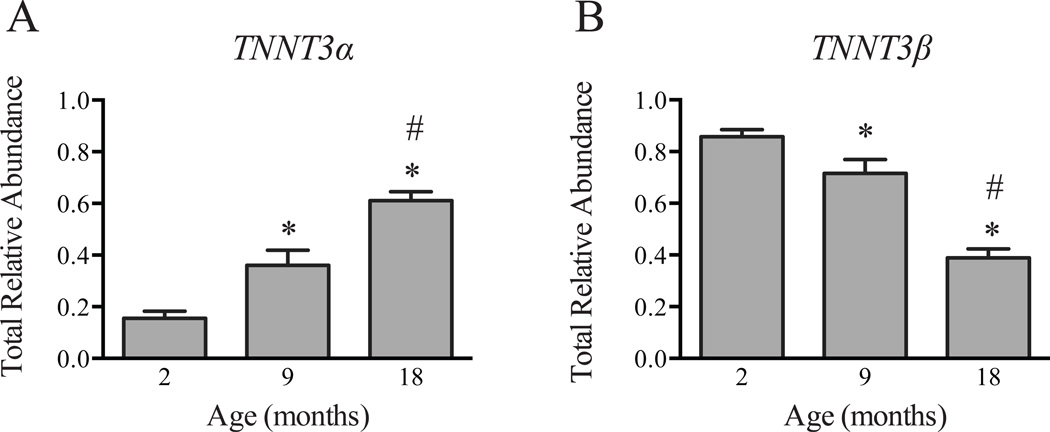
Significant differences were observed in the relative abundance of 10 out of 12 TNNT3 splice forms among the three age groups (Table 2). As illustrated in Fig. 1A, the total relative abundance of TNNT3α splice forms in the gastrocnemius muscle exhibited a continuous increase in expression from 2- to 18-months of age whereas the total relative abundance of TNNT3β splice forms exhibited the opposite pattern of change, i.e. there was a decline in total relative abundance with increasing age (Fig. 1B). Principal component analysis revealed that the first two principal components (PC1 and PC2) accounted for 97% (PC1 and PC2 captured 74.5% and 22.5%, respectively) of the variation in the dataset and PC1 was significantly influenced by age (Table 2).
Table 2.
Effect of age on TNNT3 splice forms.
| Tnnt3 splice forms | Relative abundance | Effect of Age | |||
|---|---|---|---|---|---|
| One-way ANOVA | |||||
| 2-month | 9-month | 18-month | F statistic | p value | |
| α1 | 0.120 ± 0.024 | 0.305 ± 0.052* | 0.509 ± 0.033*# | 26.01 | < 0.0001 |
| α2 | 0.020 ± 0.003 | 0.037 ± 0.006 | 0.072 ± 0.003*# | 35.52 | < 0.0001 |
| α3 | 0.016 ± 0.001 | 0.019 ± 0.001 | 0.029 ± 0.002*# | 24.04 | < 0.0001 |
| β1 | 0.020 ± 0.002 | 0.008 ± 0.001* | 0.005 ± 0.001* | 23.21 | < 0.0001 |
| β2 | 0.009 ± 0.001 | 0.008 ± 0.001 | 0.014 ± 0.002# | 4.985 | 0.0198 |
| β3 | 0.360 ± 0.023 | 0.371 ± 0.048 | 0.264 ± 0.022 | 2.731 | 0.0936 |
| β4 | 0.023 ± 0.005 | 0.034 ± 0.007 | 0.017 ± 0.003 | 2.326 | 0.1279 |
| β5 | 0.084 ± 0.013 | 0.015 ± 0.006* | 0.003 ± 0.001* | 25.99 | < 0.0001 |
| β6 | 0.084 ± 0.004 | 0.055 ± 0.010* | 0.045 ± 0.005* | 8.911 | 0.0023 |
| β7 | 0.120 ± 0.011 | 0.047 ± 0.011* | 0.025 ± 0.003* | 27.84 | < 0.0001 |
| β8 | 0.044 ± 0.007 | 0.023 ± 0.005* | 0.004 ± 0.001* | 15.43 | 0.0003 |
| β9 | 0.119 ± 0.015 | 0.034 ± 0.010* | 0.013 ± 0.002* | 25.59 | < 0.0001 |
| PC1 | * | *# | 24.95 | < 0.0001 | |
| PC2 | 0.3716 | 0.6958 | |||
The mean ± S.E.M relative abundance of the TNNT3 splice forms in gastrocnemius muscle of 2-, 9-, and 18-month old rats is presented. The first two principal components and the results from one-way ANOVA for age effects are shown. Statistically significant differences (p<0.05) determined by Tukey’s post-hoc testing are indicated. n=6–7 per group.
compared to 2-month old rats;
compared to 9-month old rats.
Figure 1. Effect of age on TNNT3 alternative splicing patterns.
The relative abundance of the 12 TNNT3 splice forms in gastrocnemius muscle of 2-, 9-, and 18-month old rats was assessed by a combination of PCR analysis and capillary electrophoresis as described under “Methods”. The total relative abundance of (A) TNNT3α and (B) TNNT3β splice forms is shown. Statistical significance (p<0.05) was determined by one-way ANOVA and Tukey’s post-hoc test. n=6–7 per group. * compared to 2-month old rats; # compared to 9-month old rats.
Effect of immobilization
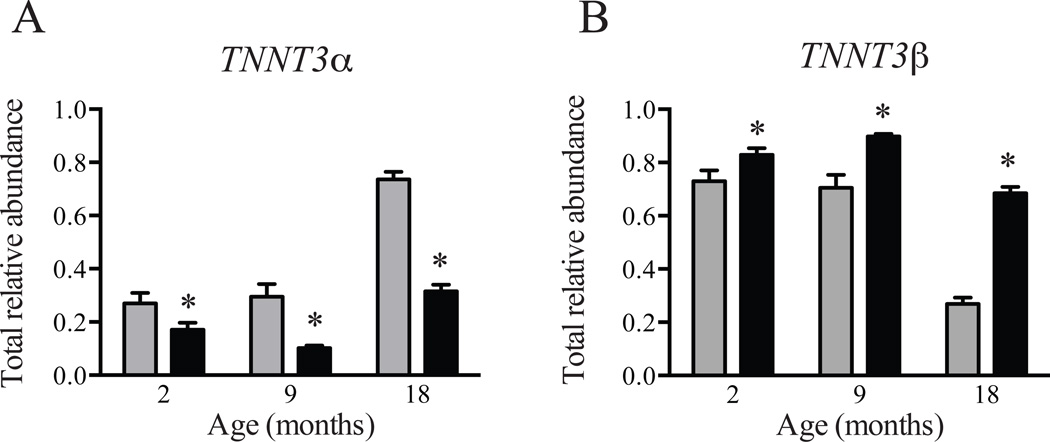
A subset of 2, 9, and 18-month old rats was subjected to 7 days of unilateral hindlimb immobilization (Kelleher et al. 2014). As shown in Table 3, TNNT3 splice form relative abundance in the gastrocnemius muscle was altered in response to both age and immobilization. The total relative abundance of TNNT3α splice forms (Fig. 2A) was significantly decreased and the total relative abundance of TNNT3β splice forms (Fig. 2B) was significantly increased in the immobilized limb of 2-, 9- and 18-month old rats when compared to the control non-immobilized limb of the same animal. Similar to results observed in control rats that had not been immobilized (Table 2), PC1 and PC2 accounted for 97% (88.6% and 8.4%, respectively) of the variation in the dataset. PC1 captured most of the variation in the entire dataset and was significantly affected by both age and immobilization (Table 3). Two-way nested ANOVA indicated a significant interaction effect for age and immobilization (Table 3). The decrease in total relative abundance of TNNT3α splice forms in response to immobilization in 9- and 18-month old rats (64% and 57% decrease respectively) was greater than in 2-month old rats (35% decrease, p<0.05). Similarly, the immobilization-induced change in total relative abundance of TNNT3β splice forms was greater in 18-month old rats (163% increase) than in 2- or 9-month old rats (14% or 29% increase respectively, p<0.05).
Table 3.
Effect of age and immobilization on TNNT3 splice forms.
|
TNNT3 splice forms |
Relative Abundance | |||||
| 2-month | 9-month | 18-month | ||||
| Control limb | Immobilized limb |
Control limb | Immobilized limb |
Control limb | Immobilized limb |
|
| α1 | 0.197 ± 0.033 | 0.114 ± 0.018* | 0.240 ± 0.040 | 0.080 ± 0.008* | 0.586 ± 0.026 | 0.274 ± 0.034* |
| α2 | 0.038 ± 0.005 | 0.031 ± 0.004 | 0.030 ± 0.007 | 0.012 ± 0.001 | 0.093 ± 0.006 | 0.054 ± 0.007* |
| α3 | 0.028 ± 0.004 | 0.025 ± 0.004 | 0.024 ± 0.003 | 0.009 ± 0.001* | 0.048 ± 0.006 | 0.027 ± 0.004* |
| β1 | 0.015 ± 0.001 | 0.019 ± 0.001* | 0.010 ± 0.001 | 0.013 ± 0.001 | 0.004 ± 0.001 | 0.006 ± 0.001* |
| β2 | 0.018 ± 0.002 | 0.017 ± 0.002 | 0.013 ± 0.003 | 0.006 ± 0.001 | 0.022 ± 0.004 | 0.015 ± 0.002 |
| β3 | 0.322 ± 0.017 | 0.315 ± 0.013 | 0.340 ± 0.026 | 0.467 ± 0.019 | 0.162 ± 0.010 | 0.378 ± 0.028* |
| β4 | 0.029 ± 0.003 | 0.081 ± 0.009* | 0.023 ± 0.006 | 0.074 ± 0.017* | 0.010 ± 0.002 | 0.051 ± 0.007* |
| β5 | 0.054 ± 0.008 | 0.025 ± 0.002* | 0.038 ± 0.005 | 0.017 ± 0.001* | 0.005 ± 0.001 | 0.004 ± 0.001 |
| β6 | 0.088 ± 0.007 | 0.108 ± 0.005* | 0.062 ± 0.006 | 0.105 ± 0.004* | 0.032 ± 0.004 | 0.092 ± 0.008* |
| β7 | 0.096 ± 0.006 | 0.099 ± 0.006 | 0.081 ± 0.015 | 0.097 ± 0.010 | 0.019 ± 0.002 | 0.052 ± 0.004* |
| β8 | 0.034 ± 0.006 | 0.054 ± 0.006* | 0.023 ± 0.002 | 0.041 ± 0.006* | 0.004 ± 0.001 | 0.013 ± 0.002* |
| β9 | 0.098 ± 0.011 | 0.110 ± 0.006 | 0.056 ± 0.008 | 0.077 ± 0.005* | 0.015 ± 0.002 | 0.034 ± 0.003* |
| TNNT3 splice form | Two-way ANOVA (nested) | |||||
| Effect of Age | Effect of Immobilization | Age * Immobilization | ||||
| F statistic | p value | F statistic | p value | F statistic | p value | |
| α1 | 65.1542 | <.0001 | 52.5714 | <.0001 | 10.7655 | 0.0003 |
| α2 | 44.7273 | <.0001 | 19.4619 | 0.0001 | 4.8226 | 0.0153 |
| α3 | 9.9045 | 0.0005 | 13.0311 | 0.0011 | 2.4487 | 0.1035 |
| β1 | 76.5313 | <.0001 | 11.5078 | 0.002 | 0.1905 | 0.8275 |
| β2 | 5.2331 | 0.0112 | 4.237 | 0.0483 | 0.7098 | 0.4998 |
| β3 | 30.0513 | <.0001 | 31.4339 | <.0001 | 19.5423 | <.0001 |
| β4 | 6.8984 | 0.0034 | 61.1599 | <.0001 | 0.3459 | 0.7104 |
| β5 | 38.6427 | <.0001 | 21.6376 | <.0001 | 6.1588 | 0.0057 |
| β6 | 19.8269 | <.0001 | 60.6869 | <.0001 | 5.9859 | 0.0065 |
| β7 | 54.4016 | <.0001 | 9.0167 | 0.0054 | 2.8995 | 0.0706 |
| β8 | 38.1622 | <.0001 | 17.4841 | 0.0002 | 0.9474 | 0.3991 |
| β9 | 79.9899 | <.0001 | 9.757 | 0.0039 | 0.2294 | 0.7964 |
| Total TNNT3α | 62.8523 | <.0001 | 49.4036 | <.0001 | 10.2252 | 0.0004 |
| Total TNNT3β | 65.0894 | <.0001 | 51.161 | <.0001 | 10.5915 | 0.0003 |
| PC1 | 60.6813 | <.0001 | 54.0919 | <.0001 | 13.224 | <.0001 |
| PC2 | 33.7262 | <.0001 | 0.9318 | 0.3421 | 8.3441 | 0.0013 |
The mean ± S.E.M relative abundance of the TNNT3 splice forms in gastrocnemius muscle from immobilized and contralateral non-immobilized (control) limbs of 2-, 9-, and 18-month old rats is presented. The first two principal components and the results from two-way nested ANOVA for age, immobilization and interaction (Age * Immobilization) effects are shown. Statistically significant changes (p<0.05) determined by paired Student’s t-test are indicated. n=6–7 per group.
compared to the control limb for that particular age group.
Figure 2. Effect of unilateral hindlimb immobilization on TNNT3 alternative splicing patterns.
The total relative abundance of (A) TNNT3α and (B) TNNT3β splice forms in the gastrocnemius muscle in the immobilized (black bars) and contralateral non-immobilized (grey bars) hindlimbs of 2-, 9-, and 18-month old rats is shown. Statistical significance (p<0.05) was determined by paired Student’s t-test. n=6–7 per group. * compared to the contralateral non-immobilized limb for that particular age group.
Effects of immobilization followed by remobilization
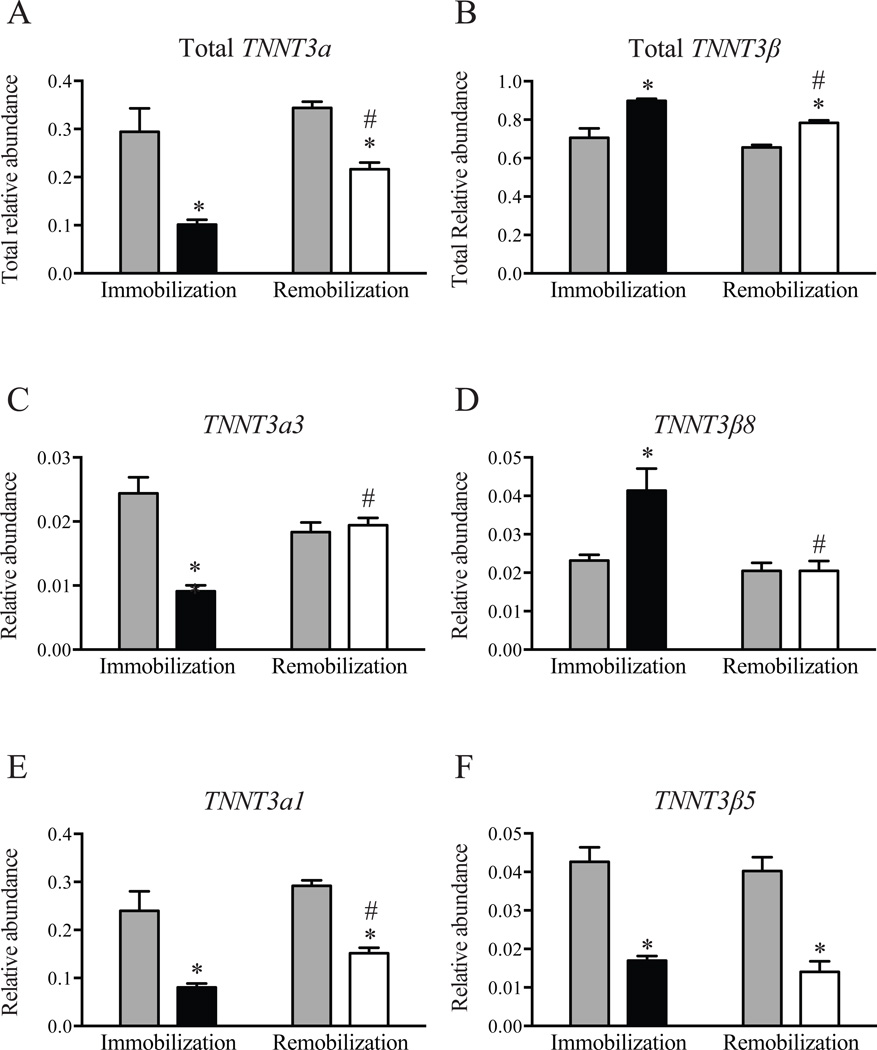
To assess the effect of remobilization on TNNT3 pre-mRNA alternative splicing, a separate group of 9-month old rats was subjected to unilateral hindlimb immobilization for 7 d after which the cast was removed and the animals were allowed to recover for 7 d (Kelleher et al. 2014). This ‘Remobilization’ group was compared to the ‘Immobilization’ group that included 9-month old rats casted for 7d. Similar to results presented in Fig. 2, immobilization resulted in decreased total relative abundance of TNNT3α splice forms (Fig. 3A) and increased total relative abundance of TNNT3β splice forms (Fig. 3B). Remobilization was associated with an increase in TNNT3α (Fig. 3A) and a decrease in TNNT3β (Fig. 3B) splice form total relative abundance, when compared to the immobilized hindlimb. However, the response to remobilization varied among specific splice forms, with some exhibiting complete restoration to the relative abundance observed in the control non-immobilized hindlimb (e.g. TNNT3α3 (Fig. 3C) and TNNT3β8 (Fig. 3D)), some exhibiting partial restoration (e.g. TNNT3α1 (Fig. 3E)), and others exhibiting no change when compared to the immobilized hindlimb (e.g. TNNT3β5 (Fig. 3F)).
Figure 3. Effect of unilateral hindlimb immobilization and remobilization on TNNT3 alternative splicing patterns.
The effect of 7d of remobilization following 7 d of immobilization on the total relative abundance of (A) TNNT3α and (B) TNNT3β splice form abundance was assessed as described under “Methods”. The relative abundance of (C) TNNT3α3, (D) TNT3β8, (E) TNNT3α1, and (F) TNT3β5 are also shown. Grey bars, non-immobilized hindlimb; black bars, immobilized hindlimb; open bars, remobilized hindlimb. Statistical significance (p<0.05) was determined by Student’s t-test. n=5–6 per group. * compared to the contralateral non-immobilized limb, paired test; # compared to the immobilized limb of the ‘Immobilization’ group, unpaired test.
Discussion
We recently demonstrated that alternative splicing of the TNNT3 pre-mRNA in rat gastrocnemius muscle varies with changes in body weight associated with normal growth in a precise and quantitative fashion (Schilder et al. 2011). However, the heaviest rats examined in the previous study weighed less than 350 g, and thus the body weight of the youngest rats used in the present study was similar to that of the oldest rats used in the previous study. The present study extends the earlier one and shows that total relative abundance of TNNT3α splice forms significantly increases and total relative abundance of TNNT3β splice forms decreases in older, heavier rats. Indeed, in younger, lighter rats, TNNT3β splice forms accounted for >80% of TNNT3, whereas in older, heavier rats, TNNT3β splice form relative abundance was reduced to <40% of TNNT3. Moreover, the adjustments to gastrocnemius muscle TNNT3 pre-mRNA alternative splicing correlated with muscle loading despite a significant decrease in relative gastrocnemius mass with age (i.e. at 18-months of age) observed in these animals (Kelleher et al. 2014). Thus, the mechanism that controls modulation of TNNT3 pre-mRNA alternative splicing that occurs in response to variation in muscle loading appears to operate across a much wider range of body weights than reported previously (Schilder et al. 2011), that is, from rats weighing less than 100 g to more than 600 g. We propose that these adjustments to TNNT3 pre-mRNA alternative splicing represent the output of a compensatory, load-sensitive mechanism that is activated in the muscle in an attempt to (at least partially) maintain function in the face of reduced muscle mass.
There is some precedent for such a mechanism. For example, the multifunctional protein titin (TTN) in cardiac muscle is thought to act as a sarcomere-based stretch sensor (Granzier et al. 2007) and it is also alternatively spliced in response to mechanical stress (e.g. due to cardiac disease, Miller et al. 2004; Granzier et al. 2007), affecting cardiac muscle stiffness. Moreover, Akt/mTOR activity is thought to regulate titin alternative splicing in response to hormonal (e.g. insulin) signaling in cardiac muscle (Linke et al. 2010). Interestingly, Akt activity is also affected by mechanical stimulation of skeletal muscle cells (Hornberger et al. 2005; Atherton et al. 2009) and our group recently demonstrated a role for Akt signaling in stretch-induced changes in troponin T alternative splicing in C2C12 culture (Schilder et al. 2012). Consistent with a possible role for Akt in regulation of TNNT3 alternative splicing are the findings in our previous study {Kelleher, 2015 #5186} that the relative phosphorylation of Akt on Ser473 is higher in 2-month old than in 9 or 18-month old rats, and that phosphorylation of the kinase is lower in the immobilized compared to the contralateral non-immobilized hindlimb of 2-month old rats. In that study we also found that Akt phosphorylation was elevated in the immobilized leg 7 d after removal of the cast compared to the contralateral control limb. However, it is unlikely that Akt is the sole mediator of altered TNNT3 alternative splicing because no difference in phosphorylation of the kinase was observed in response to immobilization in 9 or 18-month old rats, although in the present study TNNT3 alternative splicing was altered independent of age.
While a role for Akt remains highly speculative and does not exclude involvement of systemically released factors (e.g. cytokines, hormones), current information is consistent with a muscle (cell) based sensor-effector system that is sensitive to quantitative variation in load and can modulate alternative splicing of troponin T and likely other sarcomere proteins. Consistent with this idea is the finding in our previous study (Schilder et al. 2011) that artificial increases in body weight by means of externally attached loads have the same effect on TNNT3 alternative splicing as an equal change in actual body weight, and the observation that TNNT3 protein isoform expression is altered in the soleus muscle in response to unloading caused by hindlimb suspension (Stevens et al. 2002; Yu et al. 2007). Indeed, in the present study, the TNNT3 pre-mRNA alternative splicing pattern in the immobilized hindlimb muscle was similar to that observed in much younger, lighter rats, with an overall decrease in total relative abundance of TNNT3α splice forms and increased total relative abundance of TNNT3β splice forms compared to non-immobilized hindlimbs. For example, the relative abundance of the TNNT3α3 splice form in the gastrocnemius from the immobilized hindlimb of 18-month old rats was the same as that in the non-immobilized hindlimb of 2-month old rats. While we did not test this specifically, the difference in TNNT3 alternative splicing between the immobilized and non-immobilized hindlimbs likely contributes to a decrease in contractility and force production in the atrophied muscles, given the known effects of TNNT3α and β splice forms on muscle calcium sensitivity (Gallon et al. 2006). In contrast, after a 7 day recovery period, the pattern of TNNT3 pre-mRNA alternative splicing was partially restored to that observed in the non-immobilized hindlimb. Interestingly, although the recovery of TNNT3 pre-mRNA alternative splicing patterns started within 7 days of remobilization, gastrocnemius muscle mass had not yet begun to recover (Kelleher et al. 2014). Indeed, muscle mass continued to decline during the recovery period in these animals. Therefore, the remobilization-induced changes in TNNT3 pre-mRNA alternative splicing may be an important component in restoration of muscle contractility and performance prior to restoration of mass, and controlled by intracellular pathways independent of those that control muscle mass.
In the present study, TNNT3α abundance increased, whereas TNNT3β abundance decreased in older compared to younger rats. In contrast, in a recent study (Coble et al. 2015), we reported that the relative abundance of TNNT3α decreased in older individuals whereas TNNT3β abundance was increased. We believe that the most likely explanation for the difference is that in the previous study there was no difference in body weight in the older compared to the younger subjects whereas in the present study the body weight of the older rats was significantly greater compared to the younger ones. Because TNNT3 alternative splicing is directly modulated in response to changes in body weight-induced changes in muscle loading, potential age-related changes may have been masked in the present study by the higher body weight of the older compared to the younger animals. It is also possible that the changes in relative abundance of the different splice forms observed in the previous study might manifest in rats older than the ones used in the present study. This possibility could be explored in future studies.
Age and inactivity-related changes in pre-mRNA alternative splicing have been reported for other genes. For example, aging impairs mechanical strain-induced alternative splicing of the insulin-like growth factor I pre-mRNA (Goldspink 2006; Goldspink 2012). In muscles of older compared to younger animals, the expression of an insulin-like growth factor I splice variant referred to as mechano growth factor (MGF) is diminished. Moreover, muscle atrophy due to inactivity in animals subjected to hindlimb suspension is associated with changes in alternative splicing of the peroxisome proliferator-activated receptor Gamma Coactivator 1-α (PGC-1α) pre-mRNA, an important regulator of expression of genes involved in energy production in skeletal muscle (Stevenson et al. 2003). Whether or not such alterations occur through mechanisms similar to or distinct from the ones involved in controlling TNNT3 pre-mRNA alternative splicing is currently unknown. Delineation of the mechanism(s) involved in TNNT3 alternative splicing, and the signaling pathways that regulate the process, may provide insight into the control of alternative splicing of pre-mRNAs encoding other proteins. However, delineation of such mechanisms involved are likely to be difficult due to the large number of proteins that mediate and control the splicing process {Hoskins, 2012 #5187} coupled with a current lack of knowledge concerning the signaling pathways that regulate TNNT3 alternative splicing.
In summary, the present study provides further evidence for the existence of a mechanism(s) that converts quantitative information regarding muscle loading into appropriate adjustments to muscle sarcomere gene regulation and also suggests that mechanisms controlling sarcomere composition may respond more quickly than those controlling overall muscle mass adjustments to re-loading after a period of disuse.
Supplementary Material
Acknowledgments
The authors thank Sharon Rannels, Holly Lacko, Lydia Kutzler, Dr. Andrew Kelleher, and Dr. Bradley Gordon for their help in performing the animal studies presented here. We also acknowledge the Penn State Genomics Core Facility at University Park, PA for services provided in quantification of TNNT3 splice forms. The work was supported in part by Abbott Nutrition and a grant from NIH (DK-094141).
Abbreviations
- IGF-1
insulin-like growth factor 1
- mTORC1
mechanistic target of rapamycin complex 1
- MGF
mechano growth factor
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1- α
- Pre-mRNA
precursor mRNA
- SR proteins
serine/arginine rich proteins
- Tnnt3
fast skeletal muscle Troponin T
Footnotes
Author disclosures
The authors declare that there are no conflicts of interest.
References
- Allman BL, Rice CL. An age-related shift in the force-frequency relationship affects quadriceps fatigability in old adults. J Appl Physiol (1985) 2004;96:1026–1032. doi: 10.1152/japplphysiol.00991.2003. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Szewczyk NJ, Selby A, Rankin D, Hillier K, Smith K, Rennie MJ, Loughna PT. Cyclic stretch reduces myofibrillar protein synthesis despite increases in FAK and anabolic signalling in L6 cells. J Physiol. 2009;587:3719–3727. doi: 10.1113/jphysiol.2009.169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MM, Schachat F. Physiologically regulated alternative splicing patterns of fast troponin T RNA are conserved in mammals. Am J Physiol. 1996;270:C298–C305. doi: 10.1152/ajpcell.1996.270.1.C298. [DOI] [PubMed] [Google Scholar]
- Callahan DM, Miller MS, Sweeny AP, Tourville TW, Slauterbeck JR, Savage PD, Maugan DW, Ades PA, Beynnon BD, Toth MJ. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J Physiol. 2014;592:4555–4573. doi: 10.1113/jphysiol.2014.279034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri T, Mukherjea M, Sachdev S, Randall JD, Sarkar S. Role of the fetal and alpha/beta exons in the function of fast skeletal troponin T isoforms: correlation with altered Ca2+ regulation associated with development. J Mol Biol. 2005;352:58–71. doi: 10.1016/j.jmb.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Coble J, Schilder RJ, Berg A, Drummond MJ, Rasmussen BB, Kimball SR. Influence of ageing and essential amino acids on quantitative patterns of troponin T alternative splicing in human skeletal muscle. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2015;40:788–796. doi: 10.1139/apnm-2014-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J Physiol. 2007;583:1079–1091. doi: 10.1113/jphysiol.2007.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah CS, Reinach FC. The troponin complex and regulation of muscle contraction. FASEB J. 1995;9:755–767. doi: 10.1096/fasebj.9.9.7601340. [DOI] [PubMed] [Google Scholar]
- Gallon CE, Tschirgi ML, Chandra M. Differences in myofilament calcium sensitivity in rat psoas fibers reconstituted with troponin T isoforms containing the alpha- and beta-exons. Arch Biochem Biophys. 2006;456:127–134. doi: 10.1016/j.abb.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Impairment of IGF-I gene splicing and MGF expression associated with muscle wasting. Int J Biochem Cell Biol. 2006;38:481–489. doi: 10.1016/j.biocel.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Age-related loss of muscle mass and strength. J Aging Res. 2012;2012:158279. doi: 10.1155/2012/158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Granzier H, Radke M, Royal J, Wu Y, Irving TC, Gotthardt M, Labeit S. Functional genomics of chicken, mouse, and human titin supports splice diversity as an important mechanism for regulating biomechanics of striated muscle. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;293:R557–R567. doi: 10.1152/ajpregu.00001.2007. [DOI] [PubMed] [Google Scholar]
- Greaser ML, Moss RL, Reiser PJ. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chains. J Physiol. 1988;406:85–98. doi: 10.1113/jphysiol.1988.sp017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhaff PL. The molecular physiology of human limb immobilization and rehabilitation. Exerc Sport Sci Rev. 2006;34:159–163. doi: 10.1249/01.jes.0000240017.99877.8a. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Armstrong DD, Koh TJ, Burkholder TJ, Esser KA. Intracellular signaling specificity in response to uniaxial vs. multiaxial stretch: implications for mechanotransduction. American Journal of Physiology - Cell Physiology. 2005;288:C185–C194. doi: 10.1152/ajpcell.00207.2004. [DOI] [PubMed] [Google Scholar]
- Horner AM, Russ DW, Biknevicius AR. Effects of early-stage aging on locomotor dynamics and hindlimb muscle force production in the rat. J Exp Biol. 2011;214:3588–3595. doi: 10.1242/jeb.055087. [DOI] [PubMed] [Google Scholar]
- Kelleher AR, Pereira SL, Jefferson LS, Kimball SR. REDD2 Expression in Rat Skeletal Muscle Correlates with Nutrient-Induced Activation of mTORC1: Responses to Aging, Immobilization, and Remobilization. Am J Physiol Endocrinol Metab. 2014 doi: 10.1152/ajpendo.00341.2014. ajpendo.00341.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher AR, Pereira SL, Jefferson LS, Kimball SR. REDD2 expression in rat skeletal muscle correlates with nutrient-induced activation of mTORC1: responses to aging, immobilization, and remobilization. Am J Physiol Endocrinol Metab. 2015;308:E122–E129. doi: 10.1152/ajpendo.00341.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, O'Malley JP, Anthony JC, Crozier SJ, Jefferson LS. Assessment of biomarkers of protein anabolism in skeletal muscle during the life span of the rat: sarcopenia despite elevated protein synthesis. Am J Physiol Endocrinol Metab. 2004;287:E772–E780. doi: 10.1152/ajpendo.00535.2003. [DOI] [PubMed] [Google Scholar]
- Linke WA, Kruger M. The giant protein titin as an integrator of myocyte signaling pathways. Physiology (Bethesda, Md.) 2010;25:186–198. doi: 10.1152/physiol.00005.2010. [DOI] [PubMed] [Google Scholar]
- MacFarland SM, Jin JP, Brozovich FV. Troponin T isoforms modulate calcium dependence of the kinetics of the cross-bridge cycle: studies using a transgenic mouse line. Arch Biochem Biophys. 2002;405:241–246. doi: 10.1016/s0003-9861(02)00370-3. [DOI] [PubMed] [Google Scholar]
- Marden JH, Fescemyer HW, Saastamoinen M, MacFarland SP, Vera JC, Frilander MJ, Hanski I. Weight and nutrition affect pre-mRNA splicing of a muscle gene associated with performance, energetics and life history. J Exp Biol. 2008;211:3653–3660. doi: 10.1242/jeb.023903. [DOI] [PubMed] [Google Scholar]
- Marden JH, Fitzhugh GH, Girgenrath M, Wolf MR, Girgenrath S. Alternative splicing, muscle contraction and intraspecific variation: associations between troponin T transcripts, Ca(2+) sensitivity and the force and power output of dragonfly flight muscles during oscillatory contraction. J Exp Biol. 2001;204:3457–3470. doi: 10.1242/jeb.204.20.3457. [DOI] [PubMed] [Google Scholar]
- Marden JH, Fitzhugh GH, Wolf MR, Arnold KD, Rowan B. Alternative splicing, muscle calcium sensitivity, and the modulation of dragonfly flight performance. Proc Natl Acad Sci U S A. 1999;96:15304–15309. doi: 10.1073/pnas.96.26.15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford RM, Nguyen HT, Destree AT, Summers E, Nadal-Ginard B. A novel mechanism of alternative RNA splicing for the developmentally regulated generation of troponin T isoforms from a single gene. Cell. 1984;38:409–421. doi: 10.1016/0092-8674(84)90496-3. [DOI] [PubMed] [Google Scholar]
- Miller MK, Granzier H, Ehler E, Gregorio CC. The sensitive giant: the role of titin-based stretch sensing complexes in the heart. Trends in cell biology. 2004;14:119–126. doi: 10.1016/j.tcb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME, Ades PA, Maughan DW, Palmer BM, Toth MJ. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol (1985) 2013;115:1004–1014. doi: 10.1152/japplphysiol.00563.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi: 10.3389/fphys.2014.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AZ, Caturegli G, Metter EJ, Makrogiannis S, Resnick SM, Harris TB, Ferrucci L. Difference in muscle quality over the adult life span and biological correlates in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62:230–236. doi: 10.1111/jgs.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nongthomba U, Ansari M, Thimmaiya D, Stark M, Sparrow J. Aberrant splicing of an alternative exon in the Drosophila troponin-T gene affects flight muscle development. Genetics. 2007;177:295–306. doi: 10.1534/genetics.106.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogut O, Granzier H, Jin JP. Acidic and basic troponin T isoforms in mature fast-twitch skeletal muscle and effect on contractility. Am J Physiol. 1999;276:C1162–C1170. doi: 10.1152/ajpcell.1999.276.5.C1162. [DOI] [PubMed] [Google Scholar]
- Pan BS, Potter JD. Two genetically expressed troponin T fragments representing alpha and beta isoforms exhibit functional differences. J Biol Chem. 1992;267:23052–23056. [PubMed] [Google Scholar]
- Perry SV. Troponin T: genetics, properties and function. J Muscle Res Cell Motil. 1998;19:575–602. doi: 10.1023/a:1005397501968. [DOI] [PubMed] [Google Scholar]
- Reinach FC, Farah CS, Monteiro PB, Malnic B. Structural interactions responsible for the assembly of the troponin complex on the muscle thin filament. Cell Struct Funct. 1997;22:219–223. doi: 10.1247/csf.22.219. [DOI] [PubMed] [Google Scholar]
- Reiser PJ, Greaser ML, Moss RL. Developmental changes in troponin T isoform expression and tension production in chicken single skeletal muscle fibres. J Physiol. 1992;449:573–588. doi: 10.1113/jphysiol.1992.sp019102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachat FH, Diamond MS, Brandt PW. Effect of different troponin T-tropomyosin combinations on thin filament activation. J Mol Biol. 1987;198:551–554. doi: 10.1016/0022-2836(87)90300-7. [DOI] [PubMed] [Google Scholar]
- Schilder RJ, Kimball SR, Jefferson LS. Cell-autonomous regulation of fast troponin T pre-mRNA alternative splicing in response to mechanical stretch. Am J Physiol Cell Physiol. 2012;303:C298–C307. doi: 10.1152/ajpcell.00400.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder RJ, Kimball SR, Marden JH, Jefferson LS. Body weight-dependent troponin T alternative splicing is evolutionarily conserved from insects to mammals and is partially impaired in skeletal muscle of obese rats. J Exp Biol. 2011;214:1523–1532. doi: 10.1242/jeb.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder RJ, Marden JH. Parasites, proteomics and performance: effects of gregarine gut parasites on dragonfly flight muscle composition and function. J Exp Biol. 2007;210:4298–4306. doi: 10.1242/jeb.011114. [DOI] [PubMed] [Google Scholar]
- Singh SH, Kumar P, Ramachandra NB, Nongthomba U. Roles of the troponin isoforms during indirect flight muscle development in Drosophila. J Genet. 2014;93:379–388. doi: 10.1007/s12041-014-0386-8. [DOI] [PubMed] [Google Scholar]
- Stefancsik R, Randall JD, Mao C, Sarkar S. Structure and sequence of the human fast skeletal troponin T (TNNT3) gene: insight into the evolution of the gene and the origin of the developmentally regulated isoforms. Comp Funct Genomics. 2003;4:609–625. doi: 10.1002/cfg.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L, Bastide B, Kischel P, Pette D, Mounier Y. Time-dependent changes in expression of troponin subunit isoforms in unloaded rat soleus muscle. Am J Physiol Cell Physiol. 2002;282:C1025–C1030. doi: 10.1152/ajpcell.00252.2001. [DOI] [PubMed] [Google Scholar]
- Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol. 2003;551:33–48. doi: 10.1113/jphysiol.2003.044701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu QL, Jha PK, Du Y, Leavis PC, Sarkar S. Overproduction and rapid purification of human fast skeletal beta troponin T using Escherichia coli expression vectors: functional differences between the alpha and beta isoforms. Gene. 1995;155:225–230. doi: 10.1016/0378-1119(94)00846-k. [DOI] [PubMed] [Google Scholar]
- Yu ZB, Gao F, Feng HZ, Jin JP. Differential regulation of myofilament protein isoforms underlying the contractility changes in skeletal muscle unloading. Am J Physiol Cell Physiol. 2007;292:C1192–C1203. doi: 10.1152/ajpcell.00462.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.