Abstract
OBJECTIVE
A common variant rs236918 in the PCSK7 gene has the strongest association with iron homeostasis and is related to insulin resistance. Dietary carbohydrate (CHO) modulates the genetic effect on insulin resistance. We examined whether 2-year weight-loss diets modify the effect of PCSK7 genetic variants on changes in fasting insulin levels and insulin resistance in a randomized, controlled trial.
RESEARCH DESIGN AND METHODS
Data were analyzed in the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial, which is a randomized, controlled 2-year weight-loss trial using diets that differed in macronutrient proportions. PCSK7 rs236918 was genotyped in 730 overweight or obese adults (80% whites) in this trial. We assessed the progression in fasting insulin and glucose levels, and insulin resistance by genotypes.
RESULTS
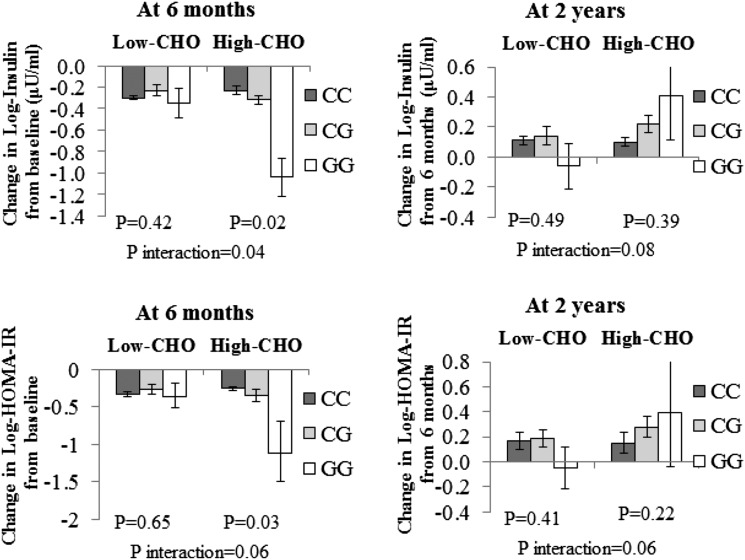
During the 6-month weight-loss phase, the PCSK7 rs236918 G allele was significantly associated with greater decreases in fasting insulin levels in the high–dietary CHO group (P for interaction = 0.04), while the interaction for changes in HOMA-insulin resistance (HOMA-IR) (P for interaction = 0.06) did not reach significant levels in white subjects. The G allele was significantly associated with a greater decrease in fasting insulin levels and HOMA-IR in response to high dietary CHO levels (P = 0.02 and P = 0.03, respectively). From 6 months to 2 years (weight-regain phase), the interactions became attenuated due to the regaining of weight (P for interactions = 0.08 and 0.06, respectively). In addition, we observed similar and even stronger results in the whole-study samples from the trial.
CONCLUSIONS
Our data suggest that PCSK7 genotypes may interact with dietary CHO intake on changes in insulin sensitivity in the white Americans.
Introduction
Compelling evidence has shown that elevated body iron stores might be associated with insulin resistance (1) and type 2 diabetes risk (2). Elevated iron stores may interfere with hepatic insulin extraction, leading to peripheral hyperinsulinemia (3). Several epidemiological studies (4,5) have revealed that genetic variants in iron store–related pathways were directly associated with or interacted with diets in relation to type 2 diabetes. A recent genome-wide association study (GWAS) (6) identified a locus near the proprotein convertase subtilisin/kexin type 7 gene (PCSK7) (rs236918), which shows the strongest relation with markers of body iron stores. The function of PCSK7 in iron homeostasis was demonstrated in studies by Guillemot et al. (7) and Schwienbacher et al. (8), in which it was found that PCSK7 modulated hepcidin expression by directly influencing soluble hemojuvelin levels. In addition, PCSK7 may act as an important mediator of adipocyte differentiation (9), and potentially affect obesity and related metabolic disorders such as insulin resistance. However, whether PCSK7 genotype is related to insulin resistance or type 2 diabetes risk remains unclear.
Previous gene-diet interaction analysis has shown that dietary carbohydrate (CHO) modulates the effect of genetic variants on insulin resistance (10). In addition, high-CHO feeding restored PCSK subtype mRNA expression after 24 h of fasting in mice (11). Therefore, in the current study, we aimed to examine whether the PCSK7 genotype affects long-term changes in fasting insulin and insulin resistance, and to test whether a 2-year weight-loss diet might modify the effects of PCSK7 genotype on the long-term changes in insulin resistance over the course of the intervention. The identification of gene-diet interactions may help to elucidate the mechanisms of the development of insulin resistance.
Research Design and Methods
Study Participants
The Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial (clinical trial reg. no. NCT00072995, clinicaltrials.gov) is a 2-year randomized clinical trial comparing the effects of energy-reduced diets with different compositions of fat, protein, and CHO on weight change, which was conducted at Boston, MA, and Baton Rouge, LA, in 2004–2007. The study was approved by the human subjects committee at the Harvard School of Public Health and Brigham and Women’s Hospital, Boston, MA; the Pennington Biomedical Research Center of the Louisiana State University, Baton Rouge, LA; and a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. All participants gave written informed consent. Detailed information on the study design and methods has been previously described (12). Major criteria for study exclusion were the presence of diabetes or unstable cardiovascular disease, the use of medications that affect body weight, and insufficient motivation. A total of 811 overweight or obese subjects (BMI ≥25 and ≤40 kg/m2) who were 30–70 years of age were randomly assigned to one of the following four diets; the target percentages of energy derived from fat, protein, and CHO in the four diets were, respectively, 20%, 15%, and 65%; 20%, 25%, and 55%; 40%, 15%, and 45%; and 40%, 25%, and 35%. In the current study, participants from the diet groups were combined for the comparison of low-CHO diets (35% and 45%) and high-CHO diets (55% and 65%), and for the comparison of the lowest-CHO diets (35%) and the highest-CHO diets (65%). In the POUNDS LOST trial, the high-CHO/low-CHO diets are indeed the same as the low-fat/high-fat diets. After 2 years, 80% of the participants (n = 645) had completed the trial. Among those participants who had genotyping data (n = 730), 594 participants actually completed the trial. To assess the dietary adherence across the intervention, dietary intake was assessed in a random sample of 50% of the participants, by a review of the 5-day diet record at baseline and by 24-h recall during a telephone interview on 3 nonconsecutive days at 6 months and at 2 years.
Measurements
Body weight and waist circumference were measured in the morning before breakfast at baseline, 6 months, and 2 years. Height was measured at baseline. BMI was calculated as weight (in kilograms)/height2 (in square meters). Fasting blood samples were collected at baseline, 6 months, and 2 years; and serum glucose and insulin levels were measured at the clinical laboratory at the Pennington Biomedical Research Center. Insulin resistance was estimated by HOMA-insulin resistance (HOMA-IR) calculated by the following equation: (fasting insulin [in microunits per milliliter] × [fasting glucose [in milligrams per deciliter]/18.01])/22.5 (13).
Genotyping
DNA was extracted from the buffy coat fraction of centrifuged blood using the QIAamp Blood Kit (Qiagen, Chatsworth, CA). Single nucleotide polymorphism (SNP) rs236918 in the PCSK7 gene was selected because the PCSK7 association with soluble transferrin receptor generation and/or iron homeostasis was the most significant (rs236918) in a meta-analysis of five GWASs (6). The SNP was genotyped successfully in 730 of 811 total participants using the OpenArray SNP Genotyping System (BioTrove, Woburn, MA). The genotype success rate was 99% in available DNA samples. Replicated quality control samples (10%) were included in every genotyping plate with >99% concordance (14).
Statistical Analysis
In the final analysis, we included only those participants who had genotype data (n = 730). The primary end points for this study were changes in fasting insulin levels and insulinresistance over the course of the intervention. General linear models (PROC GLM) for continuous variables and the χ2 test (PROC FREQ) for categorical variables were applied for the comparison according to genotype groups at baseline. We compared the changes in the primary end points, biomarkers of adherence, and nutrient intakes across genotype groups at 6 months and 2 years using generalized linear models. To test for interactions, we examined genotype and genotype-diet interactions as independent predictors of changes in the weight-loss phase (from baseline to 6 months) and weight-regain phase (patients generally regain lost weight from 6 months to 2 years) (12), adjusted for age, sex, ethnicity, baseline BMI, weight change, and the baseline value for the respective outcome trait in the generalized linear models. All reported P values were nominal. A two-sided test and a P value of 0.05 were considered statistically significant. We used Quanto version 1.2.4 (University of Southern California, Los Angeles, CA; http://hydra.usc.edu.gxe) to estimate the detectable effect sizes of genotype-diet interactions. The study had 80% power to detect the gene-diet interaction by accounting for a 16.7% change in fasting insulin levels, and an 18.4% change in HOMA-IR at a significance level of 0.05. Statistical analyses were performed with SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Characteristics of Study Population
Baseline characteristics of participants according to the PCSK7 rs236918 genotype are presented in Table 1. The minor allele frequency (G allele) was 0.12 in the total population. The genotype frequencies were significantly different by ethnicity but not sex. Body weight, BMI, waist circumference, fasting glucose and insulin levels, and HOMA-IR were not related to PCSK7 genotype at baseline. The association of PCSK7 genotype with fasting glucose and insulin levels and HOMA-IR was not significant after further adjustment of baseline BMI. No associations of genotype with weight loss and waist loss at 6 months and 2 years were observed.
Table 1.
Characteristics by PCSK7 gene variant (rs236918) at baseline and changes at 6 months and 2 years in participants in the POUNDS LOST study
| Characteristics | CC (n = 566) | CG (n = 153) | GG (n = 11) | P value* |
|---|---|---|---|---|
| Age, years | 50.6 ± 9.4 | 51.8 ± 9.0 | 50.6 ± 9.0 | 0.46 |
| Sex | 0.05 | |||
| Female | 344 (77.3) | 98 (22.0) | 3 (0.7) | |
| Male | 222 (77.9) | 55 (19.3) | 8 (2.8) | |
| Race or ethnicity | <0.0001 | |||
| White | 473 (81.27) | 102 (17.5) | 7 (1.2) | |
| Black | 76 (67.9) | 35 (31.3) | 1 (0.9) | |
| Hispanic | 15 (60.0) | 9 (36.0) | 1 (4.0) | |
| Asian or other | 2 (18.2) | 7 (63.6) | 2 (18.2) | |
| Dietary intake per day | ||||
| Energy, kcal | 1,958 ± 529 | 2,009 ± 658 | 2,192 ± 610 | 0.24 |
| CHO, % | 45 ± 8 | 44 ± 7 | 44 ± 8 | 0.06 |
| Fat, % | 37 ± 6 | 37 ± 6 | 37 ± 7 | 0.41 |
| Protein, % | 18 ± 3 | 18 ± 4 | 18 ± 5 | 0.45 |
| Weight, kg | 92.9 ± 15.6 | 94.5 ± 15.5 | 91.4 ± 11.5 | 0.45 |
| BMI, kg/m2 | 32.5 ± 3.9 | 33.1 ± 3.7 | 31.9 ± 3.2 | 0.32 |
| Insulin (lU/mL)† | 12.1 ± 7.8 | 12.8 ± 7.6 | 12.6 ± 7.0 | 0.41 |
| HOMA-IR† | 2.8 ± 2.0 | 3.0 ± 1.9 | 2.9 ± 1.7 | 0.38 |
| Insulin change† | ||||
| At 6 months | −0.26 ± 0.45 | −0.26 ± 0.45 | −0.25 ± 0.52 | 0.88 |
| 2 years | −0.15 ± 0.46 | −0.12 ± 0.43 | −0.10 ± 0.68 | 0.72 |
| HOMA-IR change† | ||||
| 6 months | −0.28 ± 0.50 | −0.29 ± 0.49 | −0.25 ± 0.58 | 0.54 |
| 2 years | −0.12 ± 0.50 | −0.10 ± 0.46 | −0.06 ± 0.79 | 0.63 |
| Weight loss at 6 months, kg | −6.8 ± 5.6 | −6.2 ± 5.4 | −3.2 ± 5.6 | 0.14 |
| Waist loss at 6 months, cm | −6.9 ± 6.2 | −6.8 ± 5.8 | −3.1 ± 5.2 | 0.47 |
| Weight loss at 2 years, kg | −4.0 ± 7.4 | −4.1 ± 7.1 | 0.2 ± 6.3 | 0.58 |
| Waist loss at 2 years, cm | −5.7 ± 7.6 | −5.6 ± 7.6 | −2.2 ± 7.2 | 0.56 |
Data are expressed as the mean ± SD or n (%) .
P values were calculated by χ2 test for categorical variables, and by F tests after adjustment for age, sex, and ethnicity for continuous variables.
These variables were log transformed before analysis. The model was adjusted for baseline BMI.
Table 2 shows dietary intake and adherence markers of the participants in white subjects. We assessed the biomarkers to confirm the dietary adherence. There were no significant differences in mean values of nutrient intakes and biomarkers of adherence at 6 months and 2 years across the PCSK7 rs236918 genotype (P > 0.05). Total energy, fat, protein, and CHO levels, and changes in urinary nitrogen levels and respiratory quotient (biomarkers of adherence) confirmed that differences in macronutrient intake among the groups were consistent with those recoded in the dietary reports, and that participants modified their intakes of macronutrients in the direction of the goals, although the targets were not fully achieved (12). Dietary intake and adherence markers of the participants in the whole population are shown in Supplementary Table 1.
Table 2.
Nutrient intake and biomarkers of adherence according to PCSK7 rs236918 at 6 months and 2 years in white subjects
| At 6 months |
At 2 years |
|||||
|---|---|---|---|---|---|---|
| CC | CG | GG | CC | CG | GG | |
| Dietary intake per day* | ||||||
| Energy, kcal | 1,631 ± 480 | 1,674 ± 524 | 1,713 ± 261 | 1,565 ± 500 | 1,507 ± 494 | 1,700 ± 634 |
| CHO, % | 50 ± 10 | 48 ± 11 | 41 ± 4 | 49 ± 10 | 47 ± 11 | 43 ± 8 |
| Fat, % | 30 ± 8 | 31 ± 8 | 35 ± 4 | 31 ± 8 | 31 ± 9 | 38 ± 6 |
| Protein, % | 20 ± 4 | 22 ± 6 | 21 ± 6 | 20 ± 4 | 20 ± 6 | 21 ± 4 |
| Biomarkers of adherence | ||||||
| Urinary nitrogen, g† | 11.6 ± 4.7 | 12.1 ± 3.9 | 12.0 ± 6.3 | 11.9 ± 4.6 | 12.5 ± 4.2 | 11.9 ± 4.4 |
| Respiratory quotient‡ | 0.84 ± 0.04 | 0.84 ± 0.05 | 0.84 ± 0.03 | 0.83 ± 0.04 | 0.83 ± 0.05 | 0.84 ± 0.04 |
Data are expressed as the mean ± SD.
Data were included for 5–268 participants per diet group at 6 months, and 4–140 participants at 2 years.
Data were included for 8–420 participants per diet group at 6 months, and 9–362 participants at 2 years.
Data were included for 8–420 participants per diet group at 6 months, and 5–292 participants at 2 years.
Genotype Effects on Change in Insulin Resistance During Weight-Loss Phase (0–6 Months)
We primarily analyzed the data in the white subgroup (80% of the study samples). During the 6-month intervention, we found a significant interaction between PCSK7 rs236918 genotype and dietary CHO levels on changes in fasting insulin levels after adjustment for age, sex, ethnicity, weight change, and baseline values for respective phenotypes (P for interaction = 0.04), while the interaction for changes in HOMA-IR (P for interaction = 0.06) did not reach a significant level (Fig. 1). In the adjusted model, the G allele was significantly associated with a greater decrease in fasting insulin levels and HOMA-IR in response to high dietary CHO levels (P = 0.02 and P = 0.03, respectively). Further adjustment for baseline BMI and dietary protein LEVELS in the model yielded similar results. We further analyzed the effects of the interaction of the PCSK7 rs236918 genotype and the two most extreme CHO diets (35% vs. 65%) on fasting insulin levels and insulin resistance. We did not observe significant results after adjustment for age, sex, ethnicity, baseline BMI, weight change, and baseline values for respective phenotypes.
Figure 1.
Effects of PCSK7 rs236918 genotype and CHO diets on changes and reversion in fasting insulin levels and HOMA-IR during the 2-year intervention in white Americans. Fasting insulin levels and HOMA-IR were log transformed before analysis. P values are adjusted for age, sex, ethnicity, weight change, and baseline values for respective phenotypes.
In the whole-study samples, we found similar and even stronger interactions between PCSK7 rs236918 genotype and dietary CHO on changes in fasting insulin levels and HOMA-IR (both P for interactions = 0.001). In the adjusted model, the G allele was significantly associated with a greater decrease in fasting insulin levels and HOMA-IR in response to high dietary CHO levels (P = 0.004 and P = 0.012, respectively), whereas opposite genotype effects on changes in insulin levels and insulin resistance were observed in the low–dietary CHO group during the 6-month intervention (P = 0.002 and P = 0.008, respectively) (Supplementary Fig. 1).
Genotype Effects on Change in Insulin Resistance During Weight-Regain Phase (7–24 Months)
In the white subgroup, between 7 and 24 months, the interactions between PCSK7 rs236918 genotype and dietary CHO on reversion in fasting insulin level (P for interaction = 0.08) and HOMA-IR (P for interaction = 0.06) became attenuated after adjustment for age, sex, ethnicity, baseline BMI, weight change, and baseline values for respective phenotypes (Fig. 1). No interactions were observed between the PCSK7 genotype and other macronutrients.
In the whole-study samples, we found significant interactions between PCSK7 rs236918 and dietary CHO on a return to or toward baseline levels of fasting insulin and HOMA-IR after adjustment for age, sex, ethnicity, weight change, and baseline values for respective phenotypes (P for interactions = 0.054 and 0.034, respectively). In the adjusted model, the G allele was associated with greater reversion of fasting insulin levels and HOMA-IR in response to high dietary CHO levels (P = 0.028 and P = 0.027, respectively) (Supplementary Fig. 1). However, we did not observe significant differences in reversion of fasting insulin levels and HOMA-IR between genotypes in response to low dietary CHO levels from 6 months to 2 years (P = 0.459 and P = 0.375, respectively). Further analysis of the interaction between PCSK7 rs236918 genotype and the two most extreme CHO diets on insulin level and insulin resistance yielded significant results (P for interaction = 0.01 and 0.006, respectively) after adjustment for age, sex, ethnicity, baseline BMI, weight change, and baseline values for respective phenotypes.
Trajectory of Changes in Fasting Insulin and HOMA-IR
We then used linear mixed models to assess the genotype by time effect over the 2-year intervention. We found that study participants with the G allele had a greater improvement of fasting insulin levels and HOMA-IR than those without the G allele across the 2-year intervention in the high–dietary CHO group in the white and whole populations. Interestingly, participants with the G allele had greater increases of fasting insulin levels and HOMA-IR than those without the G allele from 6 months to 2 years during the intervention in the high–dietary CHO group (Supplementary Fig. 2).
Conclusions
In the 2-year randomized weight-loss intervention trial, we observed that dietary CHO modified the effect of PCSK7 rs236918 on changes in fasting insulin levels in the white subgroup and in the whole-study samples. Individuals with the PCSK7 rs236918 G allele might benefit more from reductions in fasting insulin levels and insulin resistance than those without this allele by choosing a high dietary CHO level during the weight-loss phase.
PCSK7 rs236918 was recently identified to have the strongest association with markers of body iron store (6), which has been associated with insulin resistance and the risk of T2D (1,2,15). PCSK7 might affect the iron stores (16) and subsequently interfere with hepatic insulin extraction, leading to hyperinsulinemia (3,15).
During the weight-loss phase, we observed that the effect of PCSK7 rs236918 on changes in fasting insulin levels was significantly modified by dietary CHO. The participants with PCSK7 rs236918 G allele had a greater decrease in fasting insulin when consuming a high-CHO diet. The mechanism is not fully understood. However, CHO-rich foods with low glycemic index were used in the present intervention. A previous study (17) has shown that consumption of low–glycemic index foods reduces the risk of type 2 diabetes. A rodent study (11) found that high–dietary CHO refeeding restored PCSK subtype mRNA expression after 24 h of fasting. Therefore, we hypothesize that the CHO diet with low glycemic index might differentially regulate the gene expression in the PCSK7-involved pathway or in insulin-related metabolic pathways according to the genotypes. To date, there are no functional data supporting a causal variant in the PCSK7 gene. Our further assessment showed that the PCSK7 variant rs236918 was not in linkage disequilibrium with GWAS-identified loci in 11q that might influence insulin-related traits. Therefore, further studies are required to detect the causal variant in the future.
During weight-regain phase, the rs236918 G allele was marginally associated with greater reversion of insulin levels and HOMA-IR toward baseline. As we observed previously, the genotype effects on insulin resistance (14), blood pressure (18), and body weight (19) showed “reversion” patterns during weight regain in the POUNDS LOST trial. This reversion may be partly due to diminished adherence to dietary intervention, similar to other weight-loss trials (20–22). Our data suggest that individuals with the G allele might be more sensitive to the alterations in dietary intake regarding changes in insulin sensitivity.
To the best of our knowledge, this is the first study to investigate interactions between a PCSK7 genetic variant and dietary CHO on insulin resistance in a large and long-term randomized weight-loss trial. There is no evidence that PCSK7 genetic variant rs236918 tags in other ethnicities; therefore, we analyzed the white Americans separately, and subsequently combined all ethnicities as well. Our findings suggest a new insight into the use of PCSK7 genetic variant in improving personalized dietary interventions for individuals with diabetes. Importantly, this large sample trial had a high rate of retention and the sensitivity to detect small changes in body weight. Furthermore, the population was diverse with respect to age, income, and geography. Thus, the findings should be directly applicable to clinical recommendations and the development of population-wide recommendations by public health officials (12). However, several limitations need to be considered when interpreting our findings. First, it is difficult to distinguish which macronutrient plays the key role behind the observed interactions because increased CHO intake reflects decreased fat intake. Thus, either CHO or dietary fat may modulate the effect of the PCSK7 rs236918. Second, the power to detect the effect of genotype on insulin resistance in response to the real difference in macronutrients intake was reduced because of the decline of adherence to various diets after 6 months, which is similar to most of the long-term diet intervention trials reported (14,23). Finally, 80% of the participants in our study are white, and further studies are required to determine whether our findings are generalizable to other ethnic groups. Furthermore, the minor allele frequency of rs236918 varies across ethnicities; we acknowledge that further PCSK7 genotype–directed clinical trials are required to replicate our findings in diverse populations, although the randomized clinical trial is thought to be the ideal model to test gene-environment interactions.
In conclusion, we found that individuals with the PCSK7 rs236918 G allele might obtain more benefit from the improvement of insulin resistance during the 6-month intervention. Future intervention studies on the basis of specific genotypes (e.g., PCSK7 rs236918) are needed to evaluate the significance of using genetic information in guiding dietary interventions. Our findings lend support to the need for personalized nutrition advice in the future.
Article Information
Acknowledgments. The authors thank all participants in the trial for their dedication and contribution to the research.
Funding. This study was supported by grants from the National Heart, Lung, and Blood Institute (HL-071981), the Boston Obesity Nutrition Research Center (DK-46200), the National Institute of Diabetes and Digestive and Kidney Diseases (DK-091718), the National Natural Science Foundation of China (NNSFC30972453), and the Program for New Century Excellent Talents in University (NCET-10–0420). L.Q. was the recipient of an American Heart Association Scientist Development Award (0730094N).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.H. and Q.Q. contributed to the study concept and design; the acquisition, analysis, and interpretation of the data; the statistical analysis; and the drafting and critical revision of the manuscript. J.H. contributed to the study concept and design; the acquisition, analysis, and interpretation of the data; and the drafting and critical revision of the manuscript. Y.L. contributed to the study concept and design and the critical revision of the manuscript. G.A.B. and J.R. contributed to the study concept and design and the critical revision of the manuscript and were involved in the collection and analysis of the data and the funding of the initial project. F.M.S. contributed to the study concept and design, the critical revision of the manuscript, and administration, material support, and study supervision and was involved in the collection and analysis of the data and the funding of the initial project. L.Q. contributed to the study concept and design; the acquisition, analysis, and interpretation of the data; the drafting and critical revision of the manuscript; and administration, material support, and study supervision. L.Q. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00072995, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-0473/-/DC1.
References
- 1.Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care 2004;27:2422–2428 [DOI] [PubMed] [Google Scholar]
- 2.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 2004;291:711–717 [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini E. Insulin resistance, iron, and the liver. Lancet 2000;355:2181–2182 [DOI] [PubMed] [Google Scholar]
- 4.Qi L, Meigs J, Manson JE, et al. HFE genetic variability, body iron stores, and the risk of type 2 diabetes in U.S. women. Diabetes 2005;54:3567–3572 [DOI] [PubMed] [Google Scholar]
- 5.Gan W, Guan Y, Wu Q, et al. Association of TMPRSS6 polymorphisms with ferritin, hemoglobin, and type 2 diabetes risk in a Chinese Han population. Am J Clin Nutr 2012;95:626–632 [DOI] [PubMed] [Google Scholar]
- 6.Oexle K, Ried JS, Hicks AA, et al. Novel association to the proprotein convertase PCSK7 gene locus revealed by analysing soluble transferrin receptor (sTfR) levels. Hum Mol Genet 2011;20:1042–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillemot J, Canuel M, Essalmani R, Prat A, Seidah NG. Implication of the proprotein convertases in iron homeostasis: proprotein convertase 7 sheds human transferrin receptor 1 and furin activates hepcidin. Hepatology 2013;57:2514–2524 [DOI] [PubMed] [Google Scholar]
- 8.Schwienbacher C, Serafin A, Zanon A, Pramstaller PP, Pichler I, Hicks AA. Involvement of proprotein convertase PCSK7 in the regulation of systemic iron homeostasis. Hepatology 2013;58:1860–1861 [DOI] [PubMed] [Google Scholar]
- 9.Croissandeau G, Basak A, Seidah NG, Chrétien M, Mbikay M. Proprotein convertases are important mediators of the adipocyte differentiation of mouse 3T3-L1 cells. J Cell Sci 2002;115:1203–1211 [DOI] [PubMed] [Google Scholar]
- 10.Smith CE, Arnett DK, Corella D, et al. Perilipin polymorphism interacts with saturated fat and carbohydrates to modulate insulin resistance. Nutr Metab Cardiovasc Dis 2012;22:449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costet P, Cariou B, Lambert G, et al. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J Biol Chem 2006;281:6211–6218 [DOI] [PubMed] [Google Scholar]
- 12.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 14.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2011;124:563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrielsen JS, Gao Y, Simcox JA, et al. Adipocyte iron regulates adiponectin and insulin sensitivity. J Clin Invest 2012;122:3529–3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelsen SM, Christian JL. Site-specific cleavage of BMP4 by furin, PC6, and PC7. J Biol Chem 2009;284:27157–27166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–2267 [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Qi Q, Liang J, Hu FB, Sacks FM, Qi L. Neuropeptide Y promoter polymorphism modifies effects of a weight-loss diet on 2-year changes of blood pressure: the preventing overweight using novel dietary strategies trial. Hypertension 2012;60:1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu M, Qi Q, Liang J, et al. Genetic determinant for amino acid metabolites and changes in body weight and insulin resistance in response to weight-loss diets: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2013;127:1283–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003;348:2082–2090 [DOI] [PubMed] [Google Scholar]
- 21.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005;293:43–53 [DOI] [PubMed] [Google Scholar]
- 22.Shai I, Schwarzfuchs D, Henkin Y, et al.; Dietary Intervention Randomized Controlled Trial (DIRECT) Group Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229–241 [DOI] [PubMed] [Google Scholar]
- 23.Qi Q, Xu M, Wu H, et al. IRS1 genotype modulates metabolic syndrome reversion in response to 2-year weight-loss diet intervention: the POUNDS LOST trial. Diabetes Care 2013;36:3442–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]