Abstract
We describe the first case, to our knowledge, of disseminated Mycobacterium bovis Bacillus Calmette-Guérin infection in a child with Bare Lymphocyte Syndrome type II after undergoing hematopoietic stem cell transplantation (HSCT). The patient presented 30 days post HSCT with fever and lymphadenitis. Lymph node, blood, and gastric aspirates were positive for M. bovis. The patient received a prolonged treatment course with a combination of isoniazid, levofloxacin, and ethambutol. Her course was further complicated by granulomatous lymphadenitis and otitis media associated with M. bovis that developed during immune suppression taper and immune reconstitution. Ultimately, the patient recovered fully, in association with restoration of immune function, and has completed 12 months of therapy.
Keywords: BCG infection, Bare Lymphocyte Syndrome II, HSCT, pediatric transplant, Mycobacterium bovis
The Bacillus Calmette-Guérin (BCG) vaccine is created from a live, attenuated strain of Mycobacterium bovis. It is usually given at birth to infants in areas where tuberculosis is endemic, to prevent disseminated and central nervous system tuberculosis. Although rare, disseminated infection following BCG vaccine (also known as BCG-osis) is a serious and potentially life-threatening complication that occurs predominantly in children with compromised immunity (1–5). Furthermore, when patients with primary immune deficiency disorders inadvertently receive the BCG vaccine, subclinical infections may occur that may then be “unmasked” when recovery of immune responses ensues. This phenomenon of opportunistic infection-associated immune reconstitution inflammatory syndrome (IRIS) has been well described in children with human immunodeficiency virus (HIV) after response to antiretroviral therapy (2, 6). A different type of IRIS, known as paradoxical IRIS, has also been described where the recovery of pathogen-specific immune responses during antiretroviral therapy leads to a characteristic syndrome of recurrent inflammation (fever, elevated C-reactive protein), and enlargement of preexisting lesions and/or development of new lesions (lymph nodes, pleuritis) that are culture-negative for microorganisms (7–9). These subtypes of IRIS are being increasingly recognized after solid organ transplantation and hematopoietic stem cell transplantation (HSCT; 8, 10).
Bare Lymphocyte Syndrome type II (BLS-II), a member of the heterogeneous severe combined immunodeficiency (SCID) group, is caused by a genetic deficiency in major histocompatibility complex (MHC) class II expression on antigen-presenting cells. This condition leads to a combined immunodeficiency with defective CD4 T-cell development and a lack of T-helper cell-dependent antibody production by B cells. Although numbers of circulating B lymphocytes are normal, humoral immunity is severely impaired as well.
More than 150 patients with BLS-II have been reported worldwide (11). The disease is passed on with an autosomal recessive inheritance pattern resulting from defects in several distinct transacting regulatory factors that are required for the expression of MHC class II genes. The deficiency can be subclassified into 4 complementation groups: Mutations in CITA (complementation group A), RFXANK (group B), RFX5 (group C), and RFXAP (group D) genes have been identified. Children affected by this condition are predisposed to recurrent bacterial, viral, fungal, and protozoan infections starting within the first year of life. Although some children reach puberty, and a few survive into adulthood, the majority die before the age of 10 years (12, 13). As with other combined immunodeficiency disorders, HSCT is currently the only available curative treatment, and is best completed before development of complications that result in severe end-organ damage (11, 14–16). Successful HSCT outcomes are limited by a high incidence of primary graft failure along with severe graft-versus-host disease (GVHD) and regimen-related toxicities (14, 16–18).
Unlike children with other primary immune deficiency disorders, children with BLS-II do not seem to develop severe infections after vaccination with BCG (19), and disseminated BCG after HSCT has not been reported (20). To our knowledge, this is the first case of disseminated BCG infection reported in a patient with BLS-II.
Case presentation
A 23-month-old girl with BLS-II was transferred from the Kingdom of Saudi Arabia to Rainbow Babies and Children’s Hospital for an allogeneic HSCT. She was born full term and received her immunizations according to schedule (including BCG at birth) and was well until 6 months of age, when she presented with severe pneumonia complicated by respiratory failure. There-after, she had multiple recurrent infections including impetigo, oral thrush, esophageal candidiasis, sinusitis, pneumonia, and otitis media, with chronic diarrhea and failure to thrive.
Upon transfer to our center, genetic evaluation demonstrated a homozygous mutation in exon 6 (362A>T;Asp121Val) RFXANK gene, confirming the diagnosis of BLS-II. Analysis of MHC class I and MHC class II expression on cells using flow cytometric analysis revealed an absence of MHC class II on CD19+ cells, which was consistent with the diagnosis of BLS-II. The expression of MHC class I was normal. Her laboratory data showed a normal white cell count of 9.1 × 109/L with absolute lymphocyte count of 3.47 × 109/L, CD4: 0.729 × 109/L, CD8: 0.8333 × 109/L, and immunoglobulins IgG 428 mg/dL, IgA <6 mg/dL, and IgM <5 mg/dL.
Given her primary diagnosis and its associated infectious sequelae, along with the availability of a 7/8 human leukocyte antigen-matched related donor (her biological father), the patient underwent a pre-HSCT evaluation including computed tomography (CT) imaging. Chest CT revealed subsegmental areas of opacity in the right upper lobe, left upper lobe, and right mid-lung field, as well as left axillary lymphadenopathy. To further evaluate these findings, bronchoscopy was performed and cultures obtained, which were positive for Moraxella catarrhalis but negative for acid-fast bacilli (AFB) by stain and culture. CT of the sinuses revealed opacification of maxillary sinuses, bilateral mastoid air cells, and middle ear cavities. Given these findings, nasal endoscopic irrigation of maxillary sinuses was performed and bilateral pressure equalization (PE) tubes were placed. Inner ear cultures grew β-lactam-negative Haemophilus species, while sinus cultures grew Corynebacterium species, Stenotrophomonas maltophilia, and Haemophilus species. Based on the lung, sinus, and ear culture results, the patient completed a 14-day course of oral ciprofloxacin.
In addition to hepatomegaly visualized by CT of the abdomen and pelvis, the patient had persistent transaminitis. Therefore, a liver biopsy was performed, and histology showed chronic portal hepatitis with scattered portal and periportal granulomas that stained negative for AFB.
In preparation for the allogeneic HSCT, the patient received reduced-intensity conditioning with targeted-dose intravenous busulfan (0.8 mg/kg every 6 h × 8 doses, goal area under the curve of 1000 µMol × min) fludarabine (30 mg/m2 × 5 doses), and rabbit anti-thymocyte globulin (2.5 mg/kg × 3 doses). Unmanipulated bone marrow from her 7/8 human leukocyte antigen-matched (mismatched at the DRB1 loci) father was used as the stem cell graft. GVHD prophylaxis included tacrolimus (goal level 8–12 ng/mL) and mycophenolate mofetil (15 mg/kg every 8 h), which were started 3 days before HSCT graft infusion (day [D] 0). The initial transplant course was complicated by moderate-severe sinusoidal obstruction syndrome of the liver and subsequent primary graft failure on D42.
The patient subsequently underwent a second HSCT using alemtuzumab (10 mg × 4 doses), fludarabine (30 mg/m2 × 4 doses), and a single fraction total body irradiation (200 cGy) on D–1 (21) and granulocyte colony-stimulating factor mobilized peripheral blood stem cells from the original related donor. Single-agent tacrolimus was used as GVHD prophylaxis. The patient had an uneventful immediate post-transplant course and achieved neutrophil engraftment on D9.
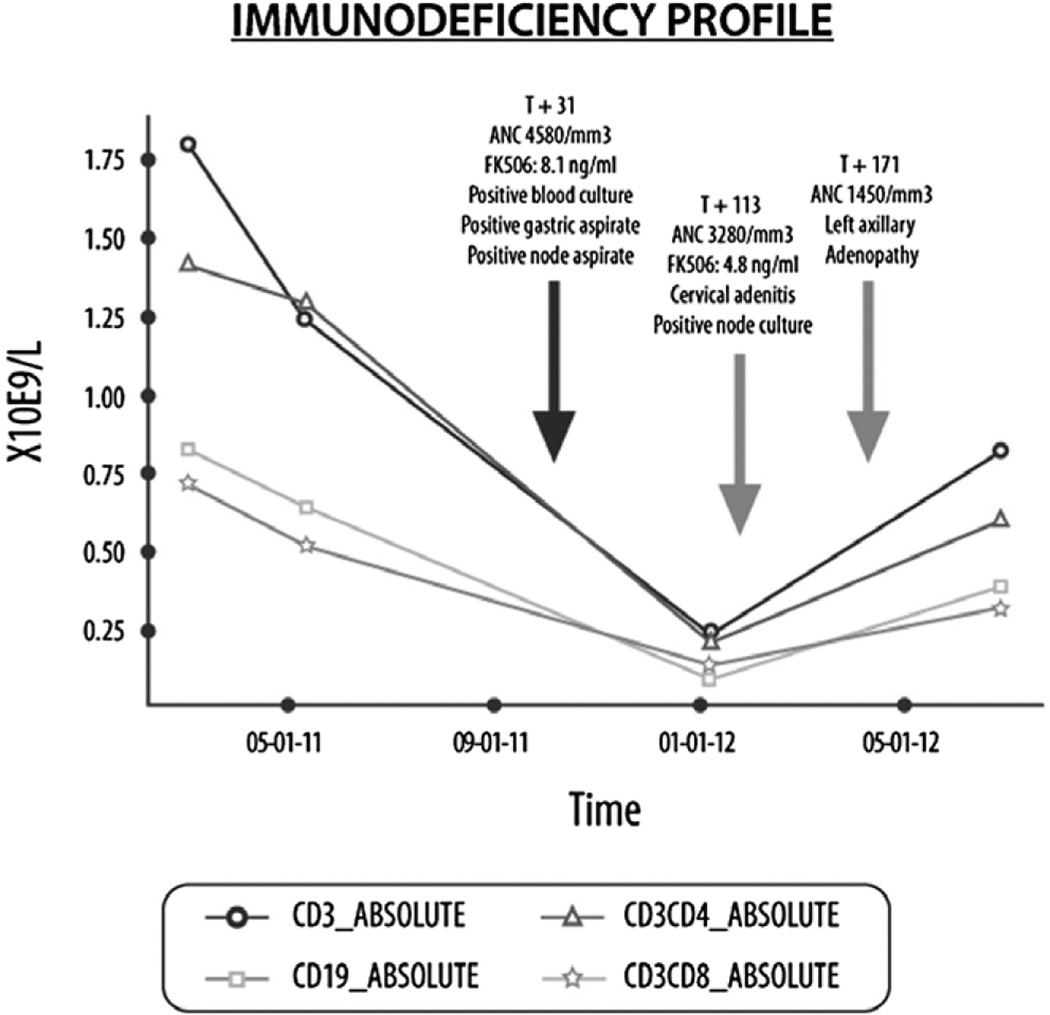
On D30 of the second HSCT procedure, the patient developed fevers and a large fluctuant left cervical lymph node. At this time, the absolute neutrophil count was 4.58 × 109/L and absolute lymphocyte count was 0.260 × 109/L. Incision and drainage of the lymph node was performed. Stains of the muco-purulent fluid were positive for AFB, and subsequent culture and polymerase chain reaction results identified M. bovis/BCG that was susceptible to isoniazid (INH), rifampin, and ethambutol, and resistant to pyrazinamide. Cultures of blood and gastric aspirates also yielded M. bovis/BCG. The patient was started initially on INH, ethambutol, and levofloxacin, a rifampin-sparing regimen, to avoid induction of tacrolimus metabolism (22). Five weeks after starting therapy, elevations of alanine aminotransferase and aspartate aminotransferase were noted and reached levels of 5 times the upper limit of normal. At that time, the INH and other potential liver toxic medications were stopped, with subsequent improvement of the liver enzymes. INH was later successfully reintroduced with no subsequent hepatitis.
On D113, the left cervical fluctuant lymph node had recurred and a left middle-ear mass attached to the PE tubes was seen. The mass was excised and the lymph node aspirated in the operating room; however, the PE tubes were retained. The mass showed multiple granulomas with AFB present (Fig. 1) and subsequently M. bovis/BCG was isolated and found to have a susceptibility profile similar to the initial isolate. Lymph node fluid culture was negative. Rifampin was added to the regimen and a tacrolimus taper was begun.
Fig. 1.
Lymphocyte subsets and clinical symptoms after hematopoietic stem cell transplantation. ANC, absolute neutrophil count; FK506, tacrolimus.
Approximately 1 month later (D152), the patient developed a sinus tract at the site of original biopsy with persistent drainage requiring curettage. At this time the PE tubes were removed, and intraoperative stains and cultures from the sinus tract were negative.
Three weeks later (D171), the patient again presented with an enlarged and purple-colored left axillary node. A positron-emission tomography scan showed increased fludeoxyglucose activity in the area of the enlarged lymph nodes, with no additional areas of abnormal uptake. A left axillary lymphadenectomy was performed on D207. The mass appeared to be scrofulous on gross examination. Microscopically, granulomas were present throughout the tissue, but stains and cultures were negative for AFB and other organisms.
The patient continued to do well postoperatively. After a 4-month taper, immune suppression was discontinued, and the patient was medically cleared to return to the Kingdom of Saudi Arabia to complete 12 months of anti-M. bovis/BCG therapy.
Discussion
BCG is recommended by the World Health Organization for infants in developing countries to prevent severe manifestations of tuberculosis. The vaccine is generally safe in patients with normal immune systems and complications are rare, with an estimated incidence of disseminated BCG disease of 0.1–4.3 cases per 1 million vaccinated children (3, 23). However, cellular immune deficiency has been identified as a major risk factor for disseminated disease development, which is often fatal in vulnerable children despite treatment with appropriately directed therapy (3, 4, 23, 24). While BCG vaccination is contraindicated in immune compromised children, the majority of patients are immunized at birth, before the diagnosis of immune deficiency is made.
Other than in HIV-infected patients, severe localized and disseminated BCG disease has been reported in patients with SCID variants and can be the first indication of a compromised immune system (5). Despite BLS-II being a combined cellular and humoral immune deficiency disorder, children with BLS-II rarely develop adverse reactions to BCG vaccination (5, 11, 19, 20). In one study looking at 35 patients with BLS-II, 7 patients were immunized with the BCG vaccine but did not develop disseminated disease. The absence of BCG infection was thought to be attributed to the presence of residual immunity conveyed by CD8+ T cells and natural killer cells in this patient population (11). Our patient carried the diagnosis of BLS-II and had a history of several opportunistic infections, but no evidence of a clinical BCG infection was found during the extensive pre-transplant evaluation. Patients with BLS-II have normal MHC class I molecules and normal or low CD8+ cells. The role of CD8+ in early control of mycobacterial infections is not completely clear, but these cells may be stimulated early in mycobacterial infections leading to the production of interferon gamma, which is pivotal in the control of these infections (25).
Allogeneic HSCT is the only curative treatment for BLS-II. The use of chemotherapy in the conditioning regimen and immune suppression for GVHD prophylaxis further compromises host immunity during the peri-transplant period, thereby increasing the risk for disseminated BCG infection in previously immunized patients. On the other hand, children with congenital immune deficiencies who develop disseminated BCG infection are at high risk of death, and HSCT may be a life-saving procedure as restoration of the immune system, in addition to anti-mycobacterial agents, may ultimately lead to control of the infection (26–29).
It is unclear how long BCG can persist at vaccination sites or elsewhere. A recent study showed that 4 weeks after vaccination with BCG, microorganisms persisted in the vaccination site in about half of healthy volunteers but were cleared in most by 3 months (30). Others have reported disseminated BCG infection in patients with HIV 30 years post vaccination indicating that viable organisms may persist for long periods of time in the immunocompromised host (3, 8). Our patient was almost 2 years of age at the time of HSCT and had never had evidence of a BCG-related complication. It is likely that she developed disseminated BCG infection 30 days following her second HSCT procedure, around the time of extreme immune suppression that resulted from GVHD prophylaxis in combination with severe, prolonged neutropenia and lymphopenia from the conditioning regimen. Given the medical history, we assumed that the infection was caused by the BCG inoculum. The patient’s isolate was sent to the CDC, who identified the strain as BCG/M. bovis, which had the pncA His57Asp mutation that confers resistance to pyrazinamide, typical of BCG/M. bovis strains. However, absolute confirmation of our assumption would require access to the vaccine strain used in Saudi Arabia.
We searched medical literature written in the English language for reports on patients who developed BCG infections after HSCT and found 10 publications (20, 26–28, 31–36; Table 1). The cases described were 19 patients with primary immune deficiency disorders (16 children with SCID/Omenn’s and 3 with other disorders). All patients had received the BCG vaccine as part of their routine immunizations in infancy. Eleven children had evidence of BCG infection prior to HSCT that ranged from local inflammation at the vaccination site to disseminated disease. In 8 children, disseminated disease developed after HSCT, 3 of which had no evidence of BCG infection before HSCT. The timing between HSCT and the development of the BCG infection ranged from 1 to 18 months, but most patients had reactivation of the disease within 4 months post HSCT.
Table 1.
Bacillus Calmette-Guérin (BCG) infections after hematopoietic stem cell transplantation in children with primary immune deficiency
| Author (Reference) | Immune deficiency |
Gender/age at transplant (months) |
BCG infection prior to transplant |
Treatment/ prophylaxis before transplant |
Type of transplant |
BCG infection after transplant |
Timing between transplant and BCG infections |
Duration of treatment |
|---|---|---|---|---|---|---|---|---|
| Aytekin et al. (26) | PNP | M/23 | No | INH + ciprofloxacin prophylaxis |
MRD BMT, no conditioning |
Disseminated BCG: Pre-auricular, left axillary lymphadenitis; crural, intramuscular, and retroperitoneal abscesses |
Early post engraftment, days 45 and 210 post transplant |
30 months |
| Amayiri et al. (20) | 6 patients with SCID |
3 M/3 F 5–33 |
In 4 patients | Unknown | PBSCT MRD | Skin cellulitis and lymphadenitis in all 6 and disseminated in 1 patient |
NA | 1–20 months |
| Amayiri et al. (20) | 3 Non-SCID (Griscelli syndrome, Omenn’s syndrome, Chediak– Higashi) |
2 M, 1 F/4–16 | In 1 patient | Unknown | 2 BMT MRD 1 UCB |
3 developed disease, not specified |
NA | NA |
| Bacalhau et al. (31) | SCID | M/NA | Disseminated BCG/splenic nodules |
INH + RIF + ETM continued through transplant + splenectomy |
T-cell depleted MUD BMT |
Disseminated BCG: Nodules at the BCG inoculation site, enlarged thoracic and abdominal lymph nodes, fever, leukocytosis |
3 months | NA |
| Bernatowska et al. (32) | SCID | NA | Inflammation at BCG |
RIF | BMT | Disseminated BCG: Tuberculomas osteomyelitis, multiple liver lesions |
2 months | 12 months |
| Bernatowska et al. (32) | SCID | NA | Inflammation at BCG |
INH until 3 months post transplant |
BMT | Inflammation at BCG site and lymphadenitis |
4 months | 12 months |
| Heyderman et al. (33) | Omenn | F/4 | No | None | BMT | Suppurative lymphadenopathy right groin |
90 days | 9 months |
| Ikincioğullari et al. (27) | SCID | F/5 | No | INH prophylaxis | MRD BMT | Disseminated BCG: Dactylitis, soft tissue hand abcess; hepatosplenomegaly; perivertebral abscess |
1 month, 6 months, and 18 months post transplant |
36 months |
| Jaing et al. (34) | SCID | M/5 | Disseminated BCG/skin nodules and pneumonia |
INH + RIF + ETM + ciprofloxacin |
UCB 2 loci mismatched |
Ulceration of BCG scar | 1 month | 1 year |
| Minegishi et al. (35) | SCID | M/7 | No | None | SBA | Disseminated BCG: Ulcer at BCG site, neck, abdomen, legs, hematuria |
15 weeks | NA |
| McKenzie and Roux (28) | SCID | M/8 | Swelling at BCG site |
INH + RIF + PZA | MRD BMT | Disseminated BCG: Fever, subcutaneous nodules at BCG site and on the thighs, and a patchy erythematous rash on trunk and limbs |
6 days, and lesions continued to appear intermittently until 7 months after transplant |
At least 7 months |
| Skinner et al. (36) | SCID | F/7 | Disseminated BCG hepatitis and severe anemia |
INH + rifampicin | BMT mismatched T-cell depleted |
Disseminated: Hepatitis |
1 month with massive splenic granulomas, and hypersplenism developing at 6 months |
Prolonged therapy (through 2nd transplant) |
PNP, purine nucleoside phosphorylase deficiency; M, male; INH, isoniazid; MRD, matched related donor; BMT, bone marrow transplant; SCID, severe combined immunodeficiency; F, female; PBSCT, peripheral blood stem cell transplant; NA, not available; UCB, umbilical cord transplant; RIF, rifampin; ETM, ethambutol; MUD, matched unrelated donor; SBA, soy bean agglutinin-fractionated histoincompatible, maternal marrow; PZA, pyrazinamide.
Taper of immune suppression and the recovery of absolute lymphocyte counts (Fig. 1) in our patient also coincided with the development of inflammatory BCG adenitis that was related to IRIS; a left cervical followed by axillary lymphadenitis developed >90 days after the initiation of anti-BCG treatment. Biopsy failed to grow mycobacteria in culture, and histopathologic examination showed inflammatory granulomas.
This syndrome of inflammatory BCG adenitis associated with immune reconstitution following allogeneic HSCT was first described by Searle et al. (8) in 2010. They reported 4 patients treated with HSCT in infancy for malignant conditions who had been vaccinated with BCG at birth. All 4 children developed painful ipsilateral lymphadenopathy post transplantation, occurring at the time of increasing T-cell numbers.
At this time, it is unclear how to best manage children with IRIS caused by BCG. In patients with HIV, from whom most of the experience emanates, needle aspiration, surgical debridement, and systemic corticosteroids in addition to anti-mycobacterial treatment have been used, but some cases have resolved spontaneously without intervention or antibiotics (2, 8, 37). Three of the 4 patients reported by Searle et al. (8) were treated with steroids (2 additionally were treated with INH and rifampin while on steroids) and 1 received no intervention. Because our patient had disseminated disease at onset, we treated her aggressively with antimicrobial therapy, surgical debridement, and discontinuation of immune suppression, which resulted in a full recovery.
To date, no set guidelines exist for the use of anti-mycobacterial prophylaxis in patients with a history of BCG vaccination at birth, who ultimately require allogeneic HSCT for immune deficiency. Two children without evidence of BCG infection before HSCT received INH or INH/ciprofloxacin and, despite this prophylaxis, developed disseminated disease post transplant (26, 27).
In conclusion, patients with primary immune deficiency disorders including BLS-II, who have a history of BCG vaccination at birth, should be considered at high risk for disseminated BCG infection following HSCT, even in the absence of clinical or subclinical disease pre-transplant. No clear data are available to recommend the use of peri-transplant prophylaxis, but this should be considered in high-risk patients on a case-by-case basis. A history of BCG disease pre-transplant, including disseminated BCG, should not be a contraindication for HSCT, as successful hematopoietic and immune reconstitution with functional donor cells is likely critical to successful treatment. Finally, inflammatory BCG adenitis associated with IRIS can be observed in patients after HSCT, and mimics that reported in HIV patients receiving antiretroviral therapy. The clinician should be alerted to these manifestations as the patient’s immune system reconstitutes.
Abbreviations
- AFB
acid-fast bacilli
- BCG
Bacillus Calmette-Guérin
- BLS-II
Bare Lymphocyte Syndrome type II
- CT
computed tomography
- D
day
- GVHD
graft-versus-host disease
- HIV
human immunodeficiency virus
- HSCT
hematopoietic stem cell transplantation
- INH
isoniazid
- IRIS
immune reconstitution inflammatory syndrome
- MHC
major histocompatibility complex
- PE
pressure equalization
- SCID
severe combined immunodeficiency
Footnotes
Author contributions: R.F.A. and B.E.G. cared for the patient, reviewed the literature, and were primarily responsible for writing the manuscript. M.R.J., L.C., R.E., J. Auletta, and J. Arnold all contributed to the care of the patient and reviewed the manuscript. K.R.C. contributed to and supervised the care of the patient and contributed to and oversaw the completed manuscript.
References
- 1.Grange JM. Complications of bacille Calmette-Guerin (BCG) vaccination and immunotherapy and their management. Commun Dis Public Health. 1998;1(2):84–88. [PubMed] [Google Scholar]
- 2.Hesseling AC, Rabie H, Marais BJ, et al. Bacille Calmette-Guerin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis. 2006;42(4):548–558. doi: 10.1086/499953. [DOI] [PubMed] [Google Scholar]
- 3.Talbot EA, Perkins MD, Silva SF, Frothingham R. Disseminated bacille Calmette-Guerin disease after vaccination: case report and review. Clin Infect Dis. 1997;24(6):1139–1146. doi: 10.1086/513642. [DOI] [PubMed] [Google Scholar]
- 4.Afshar Paiman S, Siadati A, Mamishi S, Tabatabaie P, Khotaee G. Disseminated Mycobacterium bovis infection after BCG vaccination. Iran J Allergy Asthma Immunol. 2006;5(3):133–137. [PubMed] [Google Scholar]
- 5.Norouzi S, Aghamohammadi A, Mamishi S, Rosenzweig SD, Rezaei N. Bacillus Calmette-Guerin (BCG) complications associated with primary immunodeficiency diseases. J Infect. 2012;64(6):543–554. doi: 10.1016/j.jinf.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulware DR, Callens S, Pahwa S. Pediatric HIV immune reconstitution inflammatory syndrome. Curr Opin HIV AIDS. 2008;3(4):461–467. doi: 10.1097/COH.0b013e3282fe9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddow LJ, Easterbrook PJ, Mosam A, et al. Defining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis. 2009;49(9):1424–1432. doi: 10.1086/630208. [DOI] [PubMed] [Google Scholar]
- 8.Searle E, Patel H, Vilar FJ, et al. Inflammatory BCG adenitis associated with immune reconstitution following allogeneic haematopoietic stem cell transplant in infancy. Pediatr Blood Cancer. 2010;54(1):166–169. doi: 10.1002/pbc.22143. [DOI] [PubMed] [Google Scholar]
- 9.Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M IeDEA Southern and Central Africa. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(4):251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun HY, Singh N. Opportunistic infection-associated immune reconstitution syndrome in transplant recipients. Clin Infect Dis. 2011;53(2):168–176. doi: 10.1093/cid/cir276. [DOI] [PubMed] [Google Scholar]
- 11.Ouederni M, Vincent QB, Frange P, et al. Major histocompatibility complex class II expression deficiency caused by a RFXANK founder mutation: a survey of 35 patients. Blood. 2011;118(19):5108–5118. doi: 10.1182/blood-2011-05-352716. [DOI] [PubMed] [Google Scholar]
- 12.Reith W, Steimle V, Mach B. Molecular defects in the bare lymphocyte syndrome and regulation of MHC class II genes. Immunol Today. 1995;16(11):539–546. doi: 10.1016/0167-5699(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 13.Klein C, Lisowska-Grospierre B, LeDeist F, Fischer A, Griscelli C. Major histocompatibility complex class II deficiency: clinical manifestations, immunologic features, and outcome. J Pediatr. 1993;123(6):921–928. doi: 10.1016/s0022-3476(05)80388-9. [DOI] [PubMed] [Google Scholar]
- 14.Picard C, Fischer A. Hematopoietic stem cell transplantation and other management strategies for MHC class II deficiency. Immunol Allergy Clin North Am. 2010;30(2):173–178. doi: 10.1016/j.iac.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Small TN, Qasim W, Friedrich W, et al. Alternative donor SCT for the treatment of MHC class II deficiency. Bone Marrow Transplant. 2013;48(2):226–232. doi: 10.1038/bmt.2012.140. [DOI] [PubMed] [Google Scholar]
- 16.Al-Mousa H, Al-Shammari Z, Al-Ghonaium A, et al. Allogeneic stem cell transplantation using myeloablative and reduced-intensity conditioning in patients with major histocompatibility complex class II deficiency. Biol Blood Marrow Transplant. 2010;16(6):818–823. doi: 10.1016/j.bbmt.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Renella R, Picard C, Neven B, et al. Human leucocyte antigen-identical haematopoietic stem cell transplantation in major histocompatability complex class II immunodeficiency: reduced survival correlates with an increased incidence of acute graft-versus-host disease and pre-existing viral infections. Br J Haematol. 2006;134(5):510–516. doi: 10.1111/j.1365-2141.2006.06213.x. [DOI] [PubMed] [Google Scholar]
- 18.Siepermann M, Gudowius S, Beltz K, et al. MHC class II deficiency cured by unrelated mismatched umbilical cord blood transplantation: case report and review of 68 cases in the literature. Pediatr Transplant. 2011;15(4):E80–E86. doi: 10.1111/j.1399-3046.2010.01292.x. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Mustapha I, Ben-Farhat K, Guirat-Dhouib N, et al. Clinical, immunological and genetic findings of a large Tunisian series of major histocompatibility complex class II deficiency patients. J Clin Immunol. 2013;33(4):865–870. doi: 10.1007/s10875-013-9863-8. [DOI] [PubMed] [Google Scholar]
- 20.Amayiri N, Al-Zaben A, Ghatasheh L, Frangoul H, Hussein AA. Hematopoietic stem cell transplantation for children with primary immunodeficiency diseases: single center experience in Jordan. Pediatr Transplant. 2013;17(4):394–402. doi: 10.1111/petr.12081. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed N, Leung KS, Rosenblatt H, et al. Successful treatment of stem cell graft failure in pediatric patients using a submyeloablative regimen of campath-1H and fludarabine. Biol Blood Marrow Transplant. 2008;14(11):1298–1304. doi: 10.1016/j.bbmt.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Glotzbecker B, Duncan C, Alyea E, 3rd, Campbell B, Soiffer R. Important drug interactions in hematopoietic stem cell transplantation: what every physician should know. Biol Blood Marrow Transplant. 2012;18(7):989–1006. doi: 10.1016/j.bbmt.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Sadeghi-Shanbestari M, Ansarin K, Maljaei SH, et al. Immunologic aspects of patients with disseminated bacille Calmette-Guerin disease in north-west of Iran. Ital J Pediatr. 2009;35:42. doi: 10.1186/1824-7288-35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang LH, Shyur SD, Weng JD, Shin-Chi, Tzen CY, Huang FY. Disseminated Bacille Calmette-Guerin disease as the initial presentation of X-linked severe combined immunodeficiency–a case report. Asian Pac J Allergy Immunol. 2005;23(4):221–226. [PubMed] [Google Scholar]
- 25.Camuset G, Lefebvre N, Christmann D, et al. Disseminated bacille Calmette-Guerin infection in two patients with CD8+ T-cell lymphopenia. Eur Respir J. 2009;34(5):1199–1201. doi: 10.1183/09031936.00066609. [DOI] [PubMed] [Google Scholar]
- 26.Aytekin C, Yuksek M, Dogu F, et al. An unconditioned bone marrow transplantation in a child with purine nucleoside phosphorylase deficiency and its unique complication. Pediatr Transplant. 2008;12(4):479–482. doi: 10.1111/j.1399-3046.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 27.Ikincioğullari A, Doğu F, Ciftci E, et al. An intensive approach to the treatment of disseminated BCG infection in a SCID patient. Bone Marrow Transplant. 2002;30(1):45–47. doi: 10.1038/sj.bmt.1703578. [DOI] [PubMed] [Google Scholar]
- 28.McKenzie RH, Roux P. Disseminated BCG infection following bone marrow transplantation for X-linked severe combined immunodeficiency. Pediatr Dermatol. 2000;17(3):208–212. doi: 10.1046/j.1525-1470.2000.01754.x. [DOI] [PubMed] [Google Scholar]
- 29.Lin CJ, Wang SC, Ku CL, Kao JK, Chen M, Liu CS. Successful unrelated cord blood stem cell transplantation in an X-linked chronic granulomatous disease patient with disseminated BCG-induced infection. Pediatr Neonatol. 2013 May 13; doi: 10.1016/j.pedneo.2013.04.001. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Minassian AM, Satti I, Poulton ID, Meyer J, Hill AV, McShane H. A human challenge model for Mycobacterium tuberculosis using Mycobacterium bovis bacille Calmette-Guerin. J Infect Dis. 2012;205(7):1035–1042. doi: 10.1093/infdis/jis012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bacalhau S, Freitas C, Valente R, et al. Successful handling of disseminated BCG disease in a child with severe combined immunodeficiency. Case Rep Med. 2011;2011:527569. doi: 10.1155/2011/527569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernatowska EA, Wolska-Kusnierz B, Pac M, et al. Disseminated bacillus Calmette-Guerin infection and immunodeficiency. Emerg Infect Dis. 2007;13(5):799–801. doi: 10.3201/eid1305.060865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heyderman RS, Morgan G, Levinsky RJ, Strobel S. Successful bone marrow transplantation and treatment of BCG infection in two patients with severe combined immunodeficiency. Eur J Pediatr. 1991;150(7):477–480. doi: 10.1007/BF01958426. [DOI] [PubMed] [Google Scholar]
- 34.Jaing TH, Lee WI, Lin TY, Huang JL, Chen SH, Chow R. Successful unrelated mismatched cord blood transplantation in an infant with severe combined immunodeficiency and Mycobacterium bovis bacillus Calmette-Guerin disease. Pediatr Transplant. 2006;10(4):501–504. doi: 10.1111/j.1399-3046.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- 35.Minegishi M, Tsuchiya S, Imaizumi M, et al. Successful transplantation of soy bean agglutinin-fractionated, histoincompatible, maternal marrow in a patient with severe combined immunodeficiency and BCG infection. Eur J Pediatr. 1985;143(4):291–294. doi: 10.1007/BF00442303. [DOI] [PubMed] [Google Scholar]
- 36.Skinner R, Appleton AL, Sprott MS, et al. Disseminated BCG infection in severe combined immunodeficiency presenting with severe anaemia and associated with gross hypersplenism after bone marrow transplantation. Bone Marrow Transplant. 1996;17(5):877–880. [PubMed] [Google Scholar]
- 37.Puthanakit T, Oberdorfer P, Punjaisee S, Wannarit P, Sirisanthana T, Sirisanthana V. Immune reconstitution syndrome due to bacillus Calmette-Guerin after initiation of antiretroviral therapy in children with HIV infection. Clin Infect Dis. 2005;41(7):1049–1052. doi: 10.1086/433177. [DOI] [PMC free article] [PubMed] [Google Scholar]