Abstract
Macrophages are present in nearly all tissues and are critical for development, homeostasis, and regeneration. Resident tissue macrophages of bone, termed osteal macrophages, are recently classified myeloid cells that are distinct from osteoclasts. Osteal macrophages are located immediately adjacent to osteoblasts, regulate bone formation, and play diverse roles in skeletal homeostasis. Genetic or pharmacological modulation of macrophages in vivo results in significant bone phenotypes, and these phenotypes depend on which macrophage subsets are altered. Macrophages are also key mediators of osseous wound healing and fracture repair, with distinct roles at various stages of the repair process. A central function of macrophages is their phagocytic ability. Each day, billions of cells die in the body and efferocytosis (phagocytosis of apoptotic cells) is a critical process in both clearing dead cells and recruitment of replacement progenitor cells to maintain homeostasis. Recent data suggest a role for efferocytosis in bone biology and these new mechanisms are outlined. Finally, although macrophages have an established role in primary tumors, emerging evidence suggests that macrophages in bone support cancers which preferentially metastasize to the skeleton. Collectively, this developing area of osteoimmunology raises new questions and promises to provide novel insights into pathophysiologic conditions as well as therapeutic and regenerative approaches vital for skeletal health.
Keywords: MACROPHAGE, EFFEROCYTOSIS, BONE FORMATION, FRACTURE REPAIR, SKELETAL METASTASIS
Macrophages
Macrophages (Greek for “big eaters”) are mononuclear myeloid lineage cells originally known for their protective role eliminating undesired pathogens, and their recruitment from peripheral blood to quickly mediate inflammation and infection. Their origin and attributed functions have been evolving to highlight their unique and vital role in the metabolism of nearly all tissues. Macrophages play diverse roles in many physiological processes including glucose, lipid, amino acid, and iron metabolism and alteration of macrophage function can result in disease.(1) Most organs and tissues contain a population of resident macrophages that are adapted to their local environment and perform critical tissue-specific functions supporting homeostasis.(2) For example, microglia, tissue macrophages of the brain, participate in immune surveillance and scavenge for damaged neuronal processes and debris. Likewise, tissue macrophages in the liver, Kupffer cells, participate in debris removal and regulate iron homeostasis. An important and distinguishing quality of macrophages is their highly plastic nature and ability to rapidly adapt to local environmental cues. As such, the intrinsic variation in the local environment throughout a tissue also dictates that there are multiple subsets of resident tissue macrophages within a particular organ. The spleen highlights this diversity, and features both red-pulp and white-pulp macrophages located in distinct regions of the same organ. Of interest for this review, the skeleton also features its own resident tissue macrophage population, distinct from osteoclasts, that plays a similarly important, yet unique role in bone homeostasis.
Osteal Macrophages Are Located Adjacent to Osteoblasts and Support Bone Formation
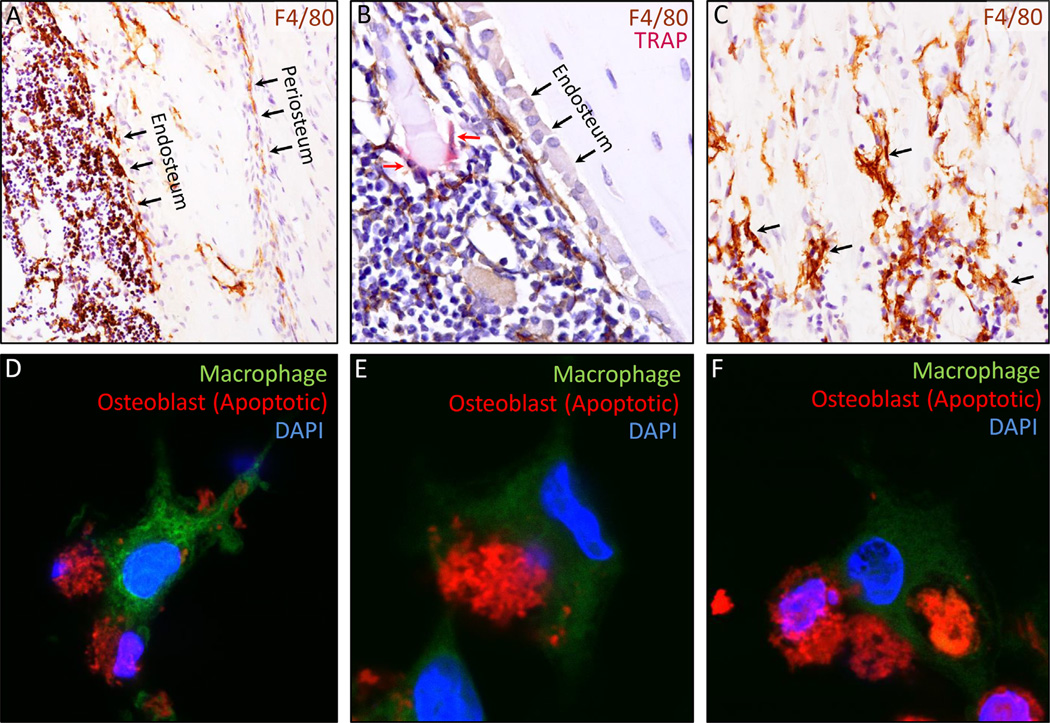
Although osteoclasts are classically viewed as the “resident macrophages” of bone, a recently characterized resident population of non-osteoclast macrophages in the skeleton has been shown to play diverse roles in bone biology(3) (Fig. 1A–C). The specific location of these osteal macrophages (aka “osteomacs”) in bone strongly suggests their potential to regulate bone formation and homeostasis. Frequent distribution of macrophages (F4/80+) near the bone surface were initially observed in the 1980s.(4) This work was extended by Chang and colleagues,(3) who demonstrated that osteal macrophages are not only found on bone surfaces intercalated within resting osteal tissue, but are notably located immediately adjacent to mature osteoblasts at sites of active bone modeling. Impressively, over 75% of osteoblasts on the endosteal surface of cortical bone were covered by a canopy like structure of F4/80+, CD68+, Mac-3+, but TRAP− osteal macrophages.(5) They also described a similar population of macrophages in adult human bone. The location of macrophages next to osteoblasts in vivo suggested they may play a role in support of bone formation and bone biology.
Fig. 1.
(A–C) Immunohistochemical staining (brown) of macrophages (F4/80+) in 4-week-old C57/B6 mice highlight their location in cortical and trabecular bone, and location immediately next to osteoblasts. (A) Macrophages (F4/80+) are located on the periosteal and endosteal surfaces of cortical bone, as well as in the marrow space. (B) Macrophages are located immediately adjacent to osteoblasts and line the bone formation surface, as shown here on the endosteal surface. This image was co-stained with TRAP (red stain) to mark osteoclasts and shows that despite sharing same lineage, TRAP+ cells (red arrows) are not F4/80+, and vice versa. (C) Similar to cortical bone, macrophages are also present throughout the trabecular secondary spongiosa (black arrows highlight examples of several positive cells). The distal growth plate is located immediately above the pictured field of view. (D–F) The goal of these images are to highlight the efferocytosis process of an apoptotic osteoblastic cell from initial apoptotic cell recognition by the macrophage to total cell engulfment. Primary murine macrophages were cultured for 7 days with M-CSF and stained green with CFSE. Osteoblastic (MC4) cells were stained deep red, and subsequently induced to undergo apoptosis with UV light (30 min). Deep red–stained apoptotic osteoblast-like cells were then cocultured with green macrophages, which underwent efferocytosis of the apoptotic osteoblastic cells over 5 to 10 hours. The efferocytosis process is highlighted, showing initial macrophage recognition of an apoptotic osteoblastic cell (D), engulfment (E), and finally an apoptotic osteoblastic cell totally engulfed by a macrophage (F). CFSE = carboxyfluorescein succinimidyl ester.
Although the location of macrophages in vivo suggested a potential role of macrophage support of bone formation, coculture of macrophages and osteoblasts established a functional link in vitro.(3) An underappreciated aspect of primary cell culture experiments is the heterogeneous nature of the cell population, and the role of macrophages in primary cultures of osteoblasts is a case in point. Macrophages (F4/80+) typically comprise 15% to 20% of the cells when murine bone marrow is harvested. Similar levels (11% to 17%) of F4/80+ macrophages have been found in primary calvarial osteoblastic cell preparations.(3) To assess the functional role of macrophages on osteoblasts, Chang and colleagues(3) took advantage of the heterogeneous nature of primary osteoblast cultures. Calvarial osteoblast culture was carried out per status quo, and also performed when macrophage cells (F4/80+) were removed from the primary calvarial osteoblast culture via a magnetic sorting technique. Removal of macrophages from primary osteoblast cultures significantly reduced osteoblast mineralization and gene expression (eg, osteocalcin). Similarly, addition of either isolated bone macrophages or in vitro–generated bone marrow macrophages to purified (macrophage-depleted) calvarial osteoblast cultures was required for mineralization in response to a physiologic Ca2+ anabolic stimulus.(3) Purified (macrophage-depleted) osteoblasts alone did not increase mineralization when calcium was added. The ability of monocytes and macrophages to facilitate mineralization has been extended to human cells where monocytes were shown to induce osteoblastogenesis in human mesenchymal stem cells (MSCs) by activation of STAT3.(6) In addition, oncostatin M produced by macrophages was identified as a specific macrophage factor that supports the osteoblastic potential of human MSCs.(7) Interestingly, further evidence of the ability of macrophages to support mineralization has surfaced in other fields. When vascular smooth muscle cells or calcifying vascular cells are cocultured with macrophages, increased mineralization is observed.(8,9) These data highlight that macrophages are not only located in close proximity to osteoblasts in vivo, but also have the capacity to support their function.
Parallel in vivo experiments examined the effect of macrophage removal in animal models and further established a functional role of macrophages in support of bone formation. To examine the role of macrophages in vivo, the Mafia (macrophage Fas-induced apoptosis) mouse model was used, which inducibly ablates macrophages and monocyte lineage cells upon injection of the AP20187 dimerizing compound.(10,11) When macrophages were ablated in Mafia mice, osteoblast surfaces and bone formation were greatly reduced, bolstering the suggestion that macrophages support bone formation with in vivo evidence.(3,12) This finding has been extended to other macrophage depletion models such as treatment with clodronate liposomes.(12) Although aforementioned models are confounded by systemic macrophage effects, exogenous G-CSF, acting via G-CSF receptor expressed on macrophages,(13) more specifically depletes macrophages in bone and bone marrow and similarly suppresses osteoblasts.(12,14) The number of macrophages in the spleen did not decrease in response to the same G-CSF treatment.(15)
Related studies examining the bone phenotype after 6 weeks of partial macrophage depletion in skeletally mature Mafia mice revealed reduced bone mass relative to controls.(16) Interestingly, no changes in osteoclast surface were observed in these same mice, indicating that despite sharing a similar myeloid lineage, the regulation of these differentiated cells can be uncoupled. As a cautionary note, care must be taken when using the Mafia mouse model to emphasize the role of macrophages on bone while minimizing significant osteoclast effects. Detailed dosing regimens have been developed to achieve that goal.(16) Interestingly, because macrophages were purposely reduced but not completely ablated in the Mafia mouse during this long duration, and the osteoclast surface was unchanged, it may suggest that osteoclastogenesis is preferentially preserved when there are limited myeloid lineage cells. Macrophage deletion with a lysozyme M (Lys-M) driven cre recombinase transgenic model also leads to reduced bone mass and additionally reinforces a macrophage role in the regulation of bone homeostasis.(17) This work further showed the role of macrophage depletion on bone during development, because the reduced trabecular bone mass observed at 3 months of age was not present at birth.(17) Collectively, these data show that reducing macrophages yields significant low bone mass phenotypes.
Although the Mafia model showed that reduction of the macrophage population decreases bone formation and bone mass, converse experiments which amplify the macrophage population also imply a macrophage role in bone biology. Specifically, systemic treatment of mice with a broad spectrum pro-myeloid factor, macrophage colony-stimulating factor (CSF)-1, increases bone mass and bone formation.(18–20) Although the increase in bone formation with systemic CSF-1 has been attributed to the increase in bone remodeling, the direct role of osteal macrophages in support of bone formation suggests that other factors could mediate this finding.
Further defining the role of macrophages on bone anabolism is the skeletal response to anabolic intermittent parathyroid hormone (iPTH) therapy. The increase in bone formation with iPTH also coincides with expansion of osteal macrophages on the periosteal and endosteal surface of cortical bone in mice.(16) When macrophage-depleted Mafia mice were treated with iPTH, the expected anabolic response was not observed, suggesting that osteal macrophages are a necessary cell type to support PTH anabolism. Thus, macrophages are not only necessary to support bone formation in homeostatic conditions, but also support the bone formation effect of a U.S. Food and Drug Administration (FDA)-approved osteoporosis therapy.
Thus far, discussion of “osteal macrophages” has largely focused on macrophages that line the bone surface. However, other macrophages are present in bone. For example, a large population of macrophages exist in the bone marrow, and macrophages (F4/80+) typically represent 15% to 20% of these cells. Other actions attributed to these myeloid cells include HSC niche and hematopoietic cell maintenance.(12,15) Although these macrophages within the bone marrow are not located directly adjacent to osteoblasts, they may also regulate bone mass by secretion of trophic factors that create a bone marrow microenvironment supporting bone anabolism or catabolism. Identification of a unique marker for osteal macrophages that line the bone surface has remained elusive, making it difficult for studies to decouple the role of ostealmacrophages from macrophages within the marrow and throughout the body, as well as from myeloid cells that become osteoclasts.
Macrophages are a highly plastic cell type and consideration of their various subtypes within the bone microenvironment is useful for determining their specific role in bone homeostasis. These differences are likely even more prominent during the complex and highly dynamic nature of osseous wound healing outlined in the next section. In fracture repair, all three types (inflammatory macrophages, macrophages in bone marrow, and osteal macrophages on the bone surface) play featured roles at various time points during bone healing. The timing and relative contribution of the varied macrophage types that participate in osseous wound healing are important to delineate.
Osteal Macrophages Are Key Regulators of Osseous Wound Healing
Bone repair requires both removal of damaged tissues and a prolonged regenerative response that achieves anatomical and functional restoration of bone. A bone injury is rapidly detected by innate immune cells, particularly macrophages. These macrophages initiate a cascade of events that culminate in replacement of the injury-induced hematoma with a vascularized fibrous connective tissue known as granulation tissue.(21) Anabolic mechanisms are initiated within this granulation tissue and progress to achieve formation of a structurally viable periosteal callus or intraosseous bone bridge, the mechanism being dictated by fracture biomechanics. This temporary bone structure is then slowly remodeled to return normal bone architecture and strength properties.(22) Although macrophage contribution to this paradigm during the initial inflammatory phase has been broadly accepted and indirectly supported,(23) definitive evidence has been lacking. The Mafia mouse model provided a tool to test this paradigm in bone injury models that heal via intramembranous ossification(24) or endochondral ossification–mediated periosteal callus formation.(25) When macrophage depletion was initiated at the time of bone injury, it resulted in catastrophic failure of bone repair in both models.(24,25) This outcome was validated using alternative macrophage targeting approaches(17,24) and was not recapitulated when osteoclasts were specifically targeted.(24,26) More recent work in zebrafish has shown that macrophage depletion compromises tail fin regeneration and bony ray patterning, confirming a role of macrophages in osseous wound healing in a distinct animal model.(27) Taken together, these data suggest macrophages are essential for the initiation of bone repair, independent of the type of bone formation involved.
Accumulating evidence supported that macrophage contributions to bone repair were unlikely to be restricted to the initial inflammatory phase of healing. This evidence included: (1) characterization of osteal macrophages (2) evidence supporting macrophage contribution to collagen deposition and mineralization in nonosseous tissues(28); (3) demonstration of macrophage presence within repair-associated tissues during the early anabolic phase in both humans(29) and animal models(24,25,30); and (4) the roles of macrophages in soft-tissue injury spanning from the initial response to complete tissue regeneration.(31) To test this, macrophage depletion in the Mafia mouse model was timed so that macrophages were unaffected during the initial inflammatory events, but depleted at the transition to the anabolic repair phase. This approach indicated that macrophages support bone formation during intramembranous(24) and endochondral osseous healing.(25) Using the tibial injury model Guihard and colleagues(32) showed that macrophage production of the anabolic molecule oncostatin M and subsequent STAT3 activation contributed significantly to robust bone healing. This provided the first evidence unraveling macrophage proanabolic molecular mechanisms during osseous healing and further validated direct macrophage contributions to bone repair. Macrophage promotion of bone formation is unlikely to be restricted to a single molecule with in vivo evidence confirming macrophage potential to express bone morphogenetic protein (BMP)-2,(33) BMP-4,(34) and Wnt family members.(16) Dissection of both the direct and indirect actions of fracture-associated macrophages is needed.
Both resident osteal macrophages(3,12,16,17,24,25,32,35) and inflammatory macrophages(6,7,16,23,25,36,37) can influence osteoblast anabolic function, and both are present in fracture repair tissues.(24,25) During intramembranous ossification, a relatively simple macrophage dynamic is established with osteal macrophages predominating within the woven bone bridge, whereas inflammatory macrophages associate with damaged tissue at the injury site periphery.(24) During endochondral callus formation, inflammatory macrophages are anatomically positioned to support many key events in the inflammation and early anabolic phases of fracture repair.(25) Osteal macrophages were distributed throughout the callus near maturing bone in the late anabolic and remodeling phases.(24,25) Consequently there appears to be specific spatial and temporal distribution dynamics of macrophage subsets during facture repair. It has yet to be confirmed if these distribution dynamics underpin coordination and transition of fracture repair from inception to completion.
Deciphering the phase-specific and site-specific contributions of various macrophage subsets will be important for appropriately harnessing the potential of macrophages for therapeutic regenerative approaches. The biomechanics of fracture healing impacts the inflammatory response, and alters the number of macrophages at the fracture site, with fewer macrophages associated with intramembranous repair.(38) It is not clear whether fracture biomechanics influence only the total number, or also alters the type of macrophage subset involved. It is possible that the specific macrophage type could be a trigger in the decision of whether intramembranous or endochondral bone formation will predominate. Of interest, communication between osteocytes and macrophages has been proposed in other model systems.(39)
Fracture healing is often delayed in older patients,(40) and declines in fracture healing with age have been observed in murine models.(41) Although the reason for these differences may be in part intrinsic to changes in bone cells, mechanical environment, progenitor MSC pools, or vasculature, it may also reflect changes in the origin and nature of regulatory cells such as macrophages. In mice it has been demonstrated that rejuvenating marrow in 12-month-old mice with inflammatory cells from 4-week-old animals accelerates and improves fracture healing.(42) Although suggestive, these data do not prove that macrophages are specifically responsible for healing. Extending this concept, recent work from parabiosis experiments demonstrated that youthful circulating factors from young (4-month-old) mice significantly improve fracture healing outcomes in aged (20-month-old) mice via β-catenin signaling.(43)
Given that macrophages are dynamic and pliable, they represent an attractive therapeutic target for improving fracture repair outcomes. However, there are also challenges given the potentially fine line between productive versus destructive macrophage actions.25 Preclinical evidence supports that use of CSF-1(44) can significantly and specifically increase injury-associated osteal macrophages, but not inflammatory macrophages(24) or osteoclasts,(24,25) and enhance bone healing.(24,25,45) Moreover, an antibody against the CSF-1 receptor depletes the resident subset of monocytes as well as tissue-associated and tumor-associated macrophages, but does not inhibit inflammation.(46) The specificity of the shift in macrophages with systemic CSF-1 treatment provides more weight to speculation that the increase in bone mass is not solely due to elevated remodeling.(18–20) Consequently, cautious but optimistic progression of this therapeutic avenue is justified. Additional remaining questions include whether perturbations in macrophage function and/or subset dominance underlie impaired fracture healing or systemic bone disease, including bone fragility associated with chronic inflammation,(47–51) infection,(52,53) anti-inflammatory treatment,(54) and age.(55)
A Potential Efferocytosis Role for Osteal Macrophages in Bone Biology
The professional phagocytic “big eater” macrophage persona plays an essential role in the development and homeostasis of many tissues. In addition to phagocytosing bacteria, debris, or other danger-associated particles, macrophages also clear dead cells. Dead cells arise by way of apoptosis (programmed cell death) or necrosis (in case of acute injury). Apoptosis in the bone community is frequently considered for its potentially negative effects, such as the case with osteocytes, which leave behind vacant lacunae. However, apoptotic cell death is not only a frequent and normal physiologic feature, but its occurrence is critical for the development and maintenance of nearly all organs. Subsequent and swift removal of these apoptotic cells is essential, and failure to do so can yield a hazardous microenvironment associated with inflammation and autoimmune disease.(56–58)
Efferocytosis (reviewed in Poon and colleagues,(59) Hochreiter-Hufford and Ravichandran,(60) Ravichandran,(61) and Martin and colleagues(62) is the removal/phagocytosis of apoptotic cells and the biological importance of efferocytosis becomes immediately apparent when the amount of cell turnover in the body is considered. Every second, roughly one million cells die in the human body; and by extension, we turnover billions of cells each day. Put another way, it has been estimated that without efferocytic clearance of apoptotic cells, an 80-year-old person would have two tons of bone marrow and a 10-mile-long intestine.(63,64) Thus, clearance of apoptotic cells is important to make way for, and help recruit, new cells. Despite the incredibly large amount of cell turnover in the body, the relative paucity of dead cells in vivo suggests that efferocytosis must occur at an equally rapid pace.
It would be remiss to not make a general point regarding interpretation of apoptotic cell staining (eg, TUNEL+, AnnexinV+, etc.) as it relates to efferocytosis. The traditional interpretation of TUNEL+ staining is that it indicates the amount of apoptosis in the cell type of interest. That is to say, a researcher who suspects increased chondrocyte cell death in vivo may perform TUNEL staining, observe no difference in TUNEL+ chondrocytes, and conclude that apoptosis was unchanged. Although a seemingly rational interpretation, this can be an over simplified conclusion. The resultant data may more directly indicate the remarkable efficiency of macrophage efferocytosis rather than the degree of apoptosis.(65) Keeping this consideration in mind when interpreting in vivo apoptotic cell staining data is crucial for identifying the true mechanisms underlying a phenotype. Put simply, the incredibly efficient nature of efferocytosis can cover up real differences in apoptosis. And conversely, compromised macrophage efferocytosis can result in the presence and staining of more apoptotic cells in vivo despite no change in apoptosis.
Although discussion of macrophages in bone biology thus far has largely focused on their presence, the functional efferocytic role of macrophages in the skeleton is an area of increasing interest. Moreover, the phenotypes present in several recent studies where cell death and/or macrophage phagocytic capacity were altered support a potential role for macrophage efferocytosis in bone biology.
The contrasting bone phenotypes in models of broad macrophage ablation versus specifically targeting the phagocytic subpopulation highlight the phagocytic function of macrophages. The Mafia mouse model ablates a broad range of the macrophage lineage (as selected by the c-Fms promoter) beginning with early-stage monoblasts, reduces CD68+ phagocytic macrophages, and shows decreased bone formation with a low bone mass phenotype.(3,16) Similar observations have been made when broad macrophage depletion is achieved via LysM-cre expression relatively early in the myeloid lineage.(17) However, specifically targeting the phagocytic macrophage population using the clodronate liposome model paradoxically revealed an opposing phenotype of increased bone mass.(16) Briefly, in this model, toxic clodronate is packaged inside tiny liposomes, which are self-selectively ingested by phagocytic macrophages upon intraperitoneal injection.(66) Thus, phagocytic macrophages are specifically targeted. Although acute treatment with clodronate liposomes showed depletion of multiple macrophage subsets in bone accompanied by a rapid and short-term loss of osteoblasts, persistent (6 week) treatment resulted in increased CD68+ phagocytic macrophages.(16) A similar rebound and increase was seen in CD11b+F4/80+Ly6G− cells, which are enriched for traditional bone marrow macrophages, after acute clodronate liposome treatment.(12) The resurgence in phagocytic macrophages in the clodronate liposome model is likely afforded because earlier stage macrophages are maintained (unlike the Mafia mouse model), as well as the physiologic need to specifically replenish these phagocytic cells. Increased CD68+ macrophages with clodronate liposome treatment is suggestive of a general activation of the phagocytic macrophage system. In support of this, gene expression indicated stimulation of phagocytic, alternatively-activated “M2-like” macrophages (MER, MFG-E8, MRC1, MSR1, CD36, IL-10, and ARG1) with clodronate liposome treatment, despite no change in general inflammatory gene expression (IL-12, TNFα, IL-1β, and iNOS).(16) Importantly, the increased bone mass with clodronate liposome treatment may be explained by this shift in macrophages supporting a bone marrow microenvironment conducive to anabolism. Elevated protein levels of TGF-β, and gene expression of Wnt-10b and Wnt-3a, were observed in the marrow of mice treated with clodronate liposomes, all factors that may support the high bone mass phenotype.(16)
A contrasting response to iPTH treatment was observed with broad macrophage depletion (Mafia) versus altering the phagocytic subset (clodronate liposomes).(16) Although broad macrophage depletion diminished the anabolic response to iPTH, mice treated with clodronate liposomes paradoxically showed a greater gain in bone mass with iPTH than controls. Although the exact cellular and molecular mechanisms mediating iPTH anabolism are not completely known, these data support specific roles for macrophage subsets facilitating iPTH anabolism. PTH further accentuated the increases in gene expression of TGF-β, Wnt-3a, and Wnt-10b.(16) Given this observation, and that macrophages(67,68) and osteoclasts(69) express Wnts, studies which implicate T-cell–produced Wnt-10b as a mediator of iPTH anabolism are of particular interest.(70,71)
The effect of iPTH in models of increased cell death further identifies the act of efferocytic cell clearance in supporting a bone marrow microenvironment conducive to anabolism. That irradiation induces cell death has long been known, and inducing apoptosis is the explicit purpose of irradiation therapy.(72) However, it has been shown that treatment of non-lethally irradiated mice with iPTH resulted in greater increases in bone mass than normal (non-irradiated) mice treated with iPTH.(73) Although data from non-lethally irradiated bone confirmed a reduction in marrow cellularity, there was simultaneously an expansion in the CD68+ phagocytic macrophage population with irradiation, paralleling the clodronate liposome model. The induction of vast cell apoptosis by irradiation demands a robust phagocytic response. Moreover, similar factors that may potentiate the anabolic response of iPTH were upregulated in the marrow of irradiated bones, including TGF-β.(73) In fact, when TGF-β was inhibited, the increased impact of iPTH in irradiated mice was lost. Collectively, these data suggest that stimulating phagocytic macrophages and efferocytosis by induction of cell death results in a marrow microenvironment that supports iPTH anabolism. However, the outcome may vary depending on the level of cell death and specific stimuli, with lethal irradiation showing proinflammatory outcomes and negative impacts on bone health.(74) Interestingly, bone marrow mechanical ablation results in osteoinductive effects that are more prominent with iPTH treatment, suggesting that the induction of cell death supports a renewal of bone formation likely linked to macrophage activity.(75)
Detailed histologic study examining the fate of osteoblasts estimates approximately ~15% become osteocytes and ~30% become bone lining cells.(76) The remaining ~40% to 70% are unaccounted for and presumably these cells undergo apoptosis and are subsequently cleared.(76,77) Thus, the predominant fate of osteoblasts is apoptosis, a largely invisible process from a histological basis due to the efficiency of macrophage cell clearance. Efferocytosis of apoptotic osteoblastic cells by macrophages in vitro highlights the process from initial macrophage recognition through total osteoblastic cell engulfment (Fig. 1D–F).
Several factors strongly suggest a role for osteal macrophages efferocytosing osteoblasts: (1) it has been estimated that most osteoblasts undergo apoptosis; (2) osteal macrophages are located immediately adjacent to osteoblast bone formation surfaces; and (3) macrophages are highly phagocytic cells. Thus, macrophages are efficiently positioned to readily efferocytose osteoblasts once they display cell death surface markers. Outlined below (and in Fig. 2) is a working model of a new role for macrophage efferocytosis in bone biology.
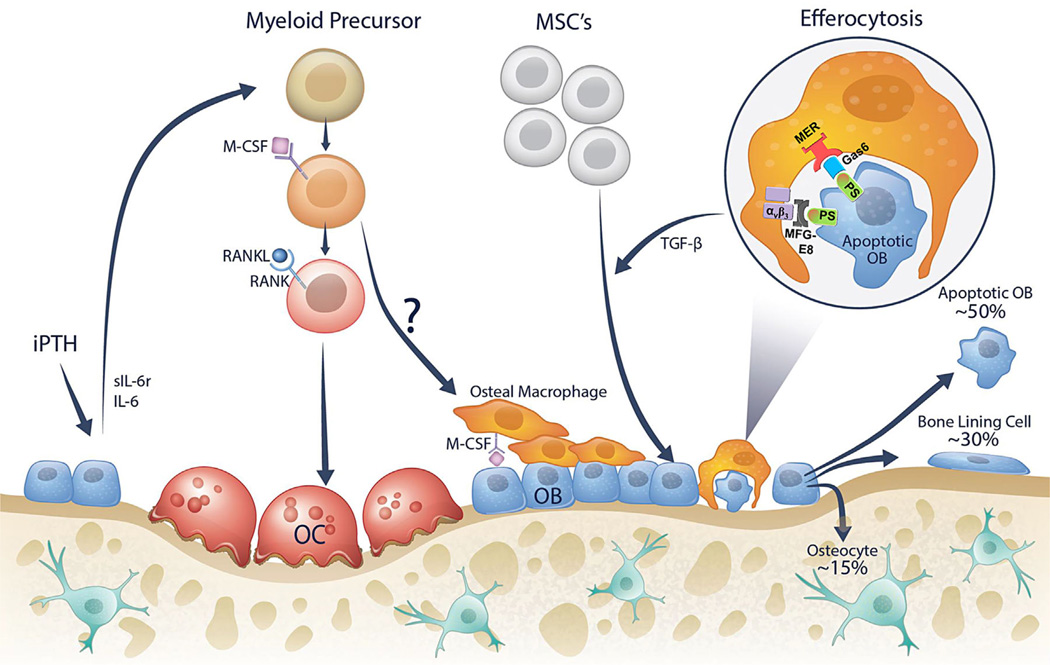
Fig. 2.
Osteal macrophages are located on the bone surface and found immediately adjacent to osteoblasts and support bone formation. Intermittent PTH (iPTH) treatment has been shown to induce osteoblastic expression of factors such as IL-6 and sIL-6r, which support expansion of the myeloid cell population (osteal macrophage and osteoclast precursors). The question mark indicates the relatively unknown source of osteal macrophages on the bone surface. At the end of an osteoblast life cycle it has three fates: (1) ~15% become embedded in the bone matrix as osteocytes; (2) ~30% become quiescent bone lining cells; and (3) the remaining ~40% to 70% likely die by apoptosis. Apoptotic osteoblasts are efficiently cleared by macrophages in a process called efferocytosis. The engulfment process of efferocytosis facilitated by expression of “eat-me” signals including PS on apoptotic cells, which are attached to macrophage proteins such as αvβ3 or Mer by linking proteins MFG-E8 or Gas6. Other efferocytosis signals have been identified but are not depicted here. Moreover, the efferocytosis process is generally associated with macrophage production of specific proteins such as TGF-β. These factors may facilitate continued bone modeling by replenishing the osteoblast population from progenitor cells. OB = osteoblast; OC = osteoclast; IL-6 = interleukin-6; sIL-6R = soluble interleukin-6 receptor; M-CSF = macrophage colony stimulating factor, RANK(L) = receptor activator of nuclear factor κB (ligand); TGF-β = transforming growth factor beta; PS = phosphatidylserine; MFG-8 = milk fat globule-EGF factor 8; αvβ3= alpha-V beta-3 integrin; Gas6 = growth arrest-specific 6; MER(tk) = receptor tyrosine kinase MerTK; MSCs = mesenchymal stromal cells.
The osteoclast-osteoblast coupling of bone remodeling and recruitment of mesenchymal progenitor cells to the bone surface and their subsequent differentiation into osteoblasts is a topic of great interest in the bone field. Recent studies have shown that factors released from the bone matrix by osteoclasts, such as TGF-β (78,79) and IGF-1,(80) are important mediators of osteoblast progenitor cell recruitment. Likewise, factors released from the osteoclast itself, such as sphingosine-1 phosphate,(69,81) CTHRC1,(82) and Wnt/BMP signaling(69) have been shown to regulate subsequent bone formation.
The proposed model (Fig. 2) suggests that efferocytosis of apoptotic osteoblasts may be another important source of factors which facilitate the subsequent influx of progenitor cells and new osteoblasts. As prototypic life cycles dictate, when a cell dies it is important that it is replaced with a new cell to maintain function. To signal phagocytic macrophages and facilitate engulfment and efferocytosis, apoptotic cells display “find-me” and “eat-me” signals.(59–62) Important “eat-me” molecules expressed on an apoptotic osteoblast include phosphatidylserine (PS), which can be linked to integrins or Mer (receptor tyrosine kinase MerTK) on a phagocytic macrophage via milk-fat globule-EGF factor 8 (MFG-E8) or Gas6, respectively. The process of efferocytosis is not only associated with removal of apoptotic cells, but also the secretion of specific factors associated with attracting progenitor cells. Notably, efferocytosis has been associated with macrophage secretion of TGF-β, an established chemoattractant of progenitor cells, in other tissues.(83) The anabolic agent PTH has been associated with changes in efferocytosis, and shown to induce expression of resolvins D1 and D2, which facilitate macrophage efferocytosis of apoptotic osteoblasts.(84) In addition, iPTH treatment leads to increased osteal macrophages, and has further been shown to expand precursor myeloid cells via IL-6 and sIL-6r.(85) Collectively, this model suggests a new process by which progenitor cells are recruited to the bone surface to maintain bone homeostasis. Importantly, this process is not necessarily confined to remodeling events as depicted (Fig. 2), and may also regulate modeling surfaces throughout the skeleton. Further studies will be critical to validate this model.
Macrophages Regulate Skeletal Metastasis and Other Pathologic Conditions
Several cancers have a predilection to metastasize to the skeleton, in particular breast and prostate cancer.(86) The prevailing paradigm is that a “vicious cycle” of skeletal metastasis is established in bone, which is dependent on bone resorption, providing factors that support tumor growth.(87) Among bone marrow-derived cells in the tumor microenvironment in bone, myeloid cells are plentiful and hence likely interface with tumor cells during their establishment and growth. Tumor-associated macrophages (TAMs) have been studied extensively in the context of a primary tumor and have been found to facilitate tumor establishment and growth.(88) Much less is known of the role of macrophages in the microenvironment of the skeletal metastatic lesion.
The presence of a tumor in the skeleton in experimental models as well as in advanced human prostate cancer is associated with increased numbers of CD206+ M2-like macrophages.(89,90) Depleting macrophages via gene targeted or pharmacologic approaches restricts tumor growth in bone, substantiating the importance of myeloid cells in the marrow and highlighting that the role of macrophages in bone may be more instrumental to tumor growth than the bone itself.(90) Because macrophages have been implicated in bone formation, as well as their osteoclast lineage partners implicated in bone resorption, it will be important to delineate the contributions of macrophage lineage cells in the bone formative, resorptive, and mixed lesions attributed to skeletal metastases.
Most of the interest in the area of macrophages and cancer has centered on myeloid cells relative to cytokines they produce and/or immune reactions they orchestrate. In bone, tumor-derived PTHrP drives myeloid cell recruitment via osteoblast produced CCL2.(91) CCL2 levels are high in the bone microenvironment and are associated with poor prognoses in primary breast tumors.(92) Metastatic breast and prostate carcinoma have increased immunostaining for macrophages versus primary cancer, and in particular, increased CD68, a phagocytic capacity marker of cells infiltrating the metastatic lesion.(93,94) It is likely that these cells support tumors via known mechanisms such as angiogenesis as well as unexplored mechanisms. Macrophages have been long implicated in their proangiogenic role in supporting tumorigenesis.(95) Still, very little has been investigated relative to a macrophage-specific role in promoting tumor establishment and growth in the bone marrow microenvironment despite the clear potential for therapeutic targeting.(96) Experimentally, modulating the bone microenvironment to increase numbers of myeloid cells and myelogenic cytokines such as CCL2 creates an environment receptive for prostate cancer skeletal metastasis.(97) CSF-1 is a potent chemokine and growth factor for regulating proliferation and differentiation of monocytes, macrophages, and osteoclasts. Tumor cells secrete CSF-1 as do osteoblasts in the microenvironment of the skeletal metastatic lesion. Emerging evidence suggests therapeutic targeting of the CSF-1 R will improve patient outcomes via reducing tumor associated macrophages in primary tumors.(98)
Interestingly, primary functions of macrophages involve phagocytosis and efferocytosis, yet these are often overlooked relative to tumorigenesis. Other than the ability of myeloid cells to differentiate to osteoclasts supporting tumor growth, little is known regarding myeloid cells, phagocytosis, efferocytosis, and skeletal metastasis. Like many other tumor strategies, efferocytosis may be a candidate approach whereby tumors may hijack a physiologic process to benefit their establishment and growth. The bone marrow provides a rich source of myeloid cells and hence tumor cells entering the marrow face a very different environment than the primary site. Rapidly growing tumors also have high rates of apoptosis to which macrophages utilizing distinct receptor signaling pathways will respond via efferocytosis. One such factor, MFG-E8 acts as a bridge protein that facilitates efferocytosis and is associated with suppression of proinflammatory responses.(99) Efferocytosis of apoptotic prostate cancer cells was associated with increased MFG-E8 and resulted in M2, protumorigenic cell polarization.
Several rare fibrohistiocytic clinical conditions suggest a potential role for macrophages in their osteal phenotypes. Langerhans cell histiocytosis (LCH), Erdheim-Chester (ECD), and Rosai-Dorfman are related fibrohistiocytic diseases that can present with both osteolytic and osteosclerotic lesions,(100–105) and notably some of the initially osteolytic lesions can spontaneously heal. CD68+ staining and macrophage presence is a common feature across these three conditions.(102,106,107) Given the pliable nature of macrophages and their location in these lesions, it is possible that macrophages regulate the osteal phenotype. Although one might postulate that “classically-activated” inflammatory macrophages favor osteolysis and “alternatively-activated” pro-healing macrophages favor osteosclerosis, it has been suggested that IL-10 produced by alternatively-activated macrophages maintains LCH cells in an immature state associated with bone lesions.(106,108)
Although it is difficult to separate the pro-osteoclastogenic aspects of myeloid cell expansion, the implications of macrophages in pathologies associated with bone are growing. Therapeutic interventions should focus on specific aspects of macrophage function that support pathology versus broader cell targeting approaches that may compromise protective and regenerative functions of macrophages.
Future and Research Directions
Present knowledge of osteal macrophages has been outlined, including their support of bone formation, fracture healing, potential role of efferocytosis in bone biology, and regulation of skeletal metastasis. The study of osteal macrophages is a relatively new area of research, representing a prime example of the importance of osteoimmunology, and many important and exciting research questions remain. Most osteal macrophage research to date has focused on the effect of the presence of macrophages, and the phenotypes that arise by their depletion or stimulation in a variety of animal models. However, identification of the specific mechanisms by which the presence of osteal macrophages support bone homeostasis and regeneration remains a question of critical importance. That is, what factors do osteal macrophages express and secrete which regulate the effect of their presence? Another important research question centers around better defining the osteal macrophage population and identification of specific markers. Presently, osteal macrophages are largely identified by their specific histologic location combined with the expression of very general macrophage markers. However, macrophages are a highly plastic cell and exist as a continuum of subtypes within many tissues, with bone marrow being an exemplary site of this phenomenon. Although this makes precise distinction and sustained targeting of osteal macrophages challenging, the identification of specific osteal macrophage markers would facilitate development of more refined animal models that will help determine their specific role. Indeed, current tools to study osteal macrophages utilize general markers that alter macrophages throughout the bone marrow and body, and the lack of specific osteal macrophage markers can limit conclusive power. Current macrophage models also have the potential to alter related cell types such as osteoclasts, or innate immune lymphoid cells, which could influence observed phenotypes. The development of more discerning animal models and identification of specific osteal macrophage markers would provide tools to answer many important questions such as the difference between osteal macrophages and other tissue-associated macrophages.
The prospect of improving patient outcomes and designing new therapies is the eventual goal of nearly all biomedical research, and future work in macrophage bone biology will play a critical role in achieving this translational goal. By better understanding the specific types of macrophages present in bone combined with the specific factors they secrete, therapies can be intelligently designed. Importantly, the diverse spectrum of macrophages and their pliable nature demands respect, because macrophages can have both regenerative and destructive roles. Specifically targeting not only the appropriate macrophage subset but in a site-specific manner will be critical to achieve optimal desired outcomes.
Acknowledgments
This work was supported in part by the National Institutes of Health Grants DK053904 and CA093900 (to LKM); Cancer Council Queensland Grant (APP1084224 to ARP); and Mater Foundation support (to ARP). We acknowledge Megan Michalski for assistance with acquiring the confocal in vitro efferocytosis images and, along with Amy Koh, Dr. Liza Raggatt and Dr. Hernan Roca for insightful discussion. We also acknowledge Dr. Kylie Alexander’s assistance in performing immunohistochemistry. We also acknowledge Stephanie O’Neil for creating the initial draft of Fig. 2.
Authors’ roles: BPS, ARP, and LKM wrote the manuscript.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15(4):432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang MK, Raggatt L-J, Alexander KA, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181(2):1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 4.Hume DA, Loutit JF, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J Cell Sci. 1984;66:189–194. doi: 10.1242/jcs.66.1.189. [DOI] [PubMed] [Google Scholar]
- 5.Wu AC, Raggatt LJ, Alexander KA, Pettit AR. Unraveling macrophage contributions to bone repair. Bonekey Rep. 2013;2:373. doi: 10.1038/bonekey.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolaidou V, Wong MM, Redpath AN, et al. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS One. 2012;7(7):e39871. doi: 10.1371/journal.pone.0039871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guihard P, Danger Y, Brounais B, et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30(4):762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- 8.Shioi A, Katagi M, Okuno Y, et al. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ Res. 2002;91(1):9–16. doi: 10.1161/01.res.0000026421.61398.f2. [DOI] [PubMed] [Google Scholar]
- 9.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105(5):650–655. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 10.Burnett SH, Kershen EJ, Zhang J, et al. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;75(4):612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 11.Burnett SH, Beus BJ, Avdiushko R, Qualls J, Kaplan AM, Cohen DA. Development of peritoneal adhesions in macrophage depleted mice. J Surg Res. 2006;131(2):296–301. doi: 10.1016/j.jss.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116(23):4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 13.Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208(2):251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkler IG, Pettit AR, Raggatt LJ, et al. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26(7):1594–1601. doi: 10.1038/leu.2012.17. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen RN, Forristal CE, Raggatt LJ, et al. Mobilization with granulocyte colony-stimulating factor blocks medullar erythropoiesis by depleting F4/80+VCAM1+CD169+ER-HR3+Ly6G+ erythroid island macrophages in the mouse. Exp Hematol. 2014;42(7):547–561. e4. doi: 10.1016/j.exphem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Cho SW, Soki FN, Koh AJ, et al. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc Natl Acad Sci USA. 2014;111(4):1545–1550. doi: 10.1073/pnas.1315153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vi L, Baht GS, Whetstone H, et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res. 2015;30(6):1090–1102. doi: 10.1002/jbmr.2422. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd SA, Yuan YY, Simske SJ, Riffle SE, Ferguson VL, Bateman TA. Administration of high-dose macrophage colony-stimulating factor increases bone turnover and trabecular volume fraction. J Bone Miner Metab. 2009;27(5):546–554. doi: 10.1007/s00774-009-0071-9. [DOI] [PubMed] [Google Scholar]
- 19.Gow DJ, Sauter KA, Pridans C, et al. Characterisation of a novel Fc conjugate of macrophage colony-stimulating factor. Mol Ther J Am Soc Gene Ther. 2014;22(9):1580–1592. doi: 10.1038/mt.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garceau V, Balic A, Garcia-Morales C, et al. The development and maintenance of the mononuclear phagocyte system of the chick is controlled by signals from the macrophage colony-stimulating factor receptor. BMC Biol. 2015;13:12. doi: 10.1186/s12915-015-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pape H-C, Marcucio R, Humphrey C, Colnot C, Knobe M, Harvey EJ. Trauma-induced inflammation and fracture healing. J Orthop Trauma. 2010;24(9):522–525. doi: 10.1097/BOT.0b013e3181ed1361. [DOI] [PubMed] [Google Scholar]
- 22.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol. 2008;19(5):459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Xing Z, Lu C, Hu D, et al. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3(7–8):451–458. doi: 10.1242/dmm.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander KA, Chang MK, Maylin ER, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011;26(7):1517–1532. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 25.Raggatt LJ, Wullschleger ME, Alexander KA, et al. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol. 2014;184(12):3192–3204. doi: 10.1016/j.ajpath.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 26.McDonald MM, Dulai S, Godfrey C, Amanat N, Sztynda T, Little DG. Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone. 2008;43(4):653–662. doi: 10.1016/j.bone.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Petrie TA, Strand NS, Tsung-Yang C, Rabinowitz JS, Moon RT. Macrophages modulate adult zebrafish tail fin regeneration. Development. 2014;141(13):2581–2591. doi: 10.1242/dev.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183(5):1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrew JG, Andrew SM, Freemont AJ, Marsh DR. Inflammatory cells in normal human fracture healing. Acta Orthop Scand. 1994;65(4):462–466. doi: 10.3109/17453679408995493. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Yu YY, Lieu S, et al. MMP9 regulates the cellular response to inflammation after skeletal injury. Bone. 2013;52(1):111–119. doi: 10.1016/j.bone.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014;20(8):857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 32.Guihard P, Boutet M-A, Brounais-Le Royer B, et al. Oncostatin M, an inflammatory cytokine produced by macrophages, supports intramembranous bone healing in a mouse model of tibia injury. Am J Pathol. 2015;185(3):765–775. doi: 10.1016/j.ajpath.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Muller PA, Koscso B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158(2):300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kan L, Liu Y, McGuire TL, et al. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009;27(1):150–156. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes TJ, Hodge JM, Singh PP, et al. Cord blood-derived macrophage-lineage cells rapidly stimulate osteoblastic maturation in mesenchymal stem cells in a glycoprotein-130 dependent manner. PLoS One. 2013;8(9):e73266. doi: 10.1371/journal.pone.0073266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyons FG, Al-Munajjed AA, Kieran SM, et al. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials. 2010;31(35):9232–9243. doi: 10.1016/j.biomaterials.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 37.Pirraco RP, Reis RL, Marques AP. Effect of monocytes/macrophages on the early osteogenic differentiation of hBMSCs. J Tissue Eng Regen Med. 2013;7(5):392–400. doi: 10.1002/term.535. [DOI] [PubMed] [Google Scholar]
- 38.Hankemeier S, Grassel S, Plenz G, Spiegel HU, Bruckner P, Probst A. Alteration of fracture stability influences chondrogenesis, osteogenesis and immigration of macrophages. J Orthop Res. 2001;19(4):531–538. doi: 10.1016/S0736-0266(00)00044-9. [DOI] [PubMed] [Google Scholar]
- 39.Asada N, Katayama Y, Sato M, et al. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell. 2013;12(6):737–747. doi: 10.1016/j.stem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Exp Gerontol. 2006;41(11):1080–1093. doi: 10.1016/j.exger.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Lopas LA, Belkin NS, Mutyaba PL, Gray CF, Hankenson KD, Ahn J. Fractures in geriatric mice show decreased callus expansion and bone volume. Clin Orthop. 2014;472(11):3523–3532. doi: 10.1007/s11999-014-3829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xing Z, Lu C, Hu D, Miclau T, Marcucio RS. Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. J Orthop Res. 2010;28(8):1000–1006. doi: 10.1002/jor.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baht GS, Silkstone D, Vi L, et al. Exposure to a youthful circulation rejuvenates bone repair through modulation of β-catenin. Nat Commun. 2015;6:7131. doi: 10.1038/ncomms8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hume DA, MacDonald KPA. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonistsof CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119(8):1810–1820. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- 45.Sarahrudi K, Mousavi M, Grossschmidt K, et al. The impact of colony-stimulating factor-1 on fracture healing: an experimental study. J Orthop Res. 2009;27(1):36–41. doi: 10.1002/jor.20680. [DOI] [PubMed] [Google Scholar]
- 46.MacDonald KPA, Palmer JS, Cronau S, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116(19):3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 47.Nikolaou VS, Efstathopoulos N, Kontakis G, Kanakaris NK, Giannoudis PV. The influence of osteoporosis in femoral fracture healing time. Injury. 2009;40(6):663–668. doi: 10.1016/j.injury.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 48.Hernandez RK, Do TP, Critchlow CW, Dent RE, Jick SS. Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database. Acta Orthop. 2012;83(6):653–660. doi: 10.3109/17453674.2012.747054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schipper LG, Fleuren HWHA, van den Bergh JPW, Meinardi JR, Veldman BAJ, Kramers C. Treatment of osteoporosis in renal insufficiency. Clin Rheumatol. 2015;34(8):1341–1345. doi: 10.1007/s10067-015-2883-4. [DOI] [PubMed] [Google Scholar]
- 50.Green E, Lubahn JD, Evans J. Risk factors, treatment, and outcomes associated with nonunion of the midshaft humerus fracture. J Surg Orthop Adv. 2005;14(2):64–72. [PubMed] [Google Scholar]
- 51.McLean RR. Proinflammatory cytokines and osteoporosis. Curr Osteoporos Rep. 2009;7(4):134–139. doi: 10.1007/s11914-009-0023-2. [DOI] [PubMed] [Google Scholar]
- 52.Reikerås O, Shegarfi H, Wang JE, Utvåg SE. Lipopolysaccharide impairs fracture healing: an experimental study in rats. Acta Orthop. 2005;76(6):749–753. doi: 10.1080/17453670510045327. [DOI] [PubMed] [Google Scholar]
- 53.Reikerås O, Wang JE, Foster SJ, Utvåg SE. Staphylococcus aureus peptidoglycan impairs fracture healing: an experimental study in rats. J Orthop Res. 2007;25(2):262–266. doi: 10.1002/jor.20274. [DOI] [PubMed] [Google Scholar]
- 54.Simon AM, O’Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am. 2007;89(3):500–511. doi: 10.2106/JBJS.F.00127. [DOI] [PubMed] [Google Scholar]
- 55.Slade Shantz JA, Yu Y-Y, Andres W, Miclau T, Marcucio R. Modulation of macrophage activity during fracture repair has differential effects in young adult and elderly mice. J Orthop Trauma. 2014;28(Suppl 1):S10–S14. doi: 10.1097/BOT.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6(5):280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 57.Baumann I, Kolowos W, Voll RE, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46(1):191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 58.Shao W-H, Cohen PL. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(1):202. doi: 10.1186/ar3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poon IKH, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14(3):166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5(1):a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207(9):1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin CJ, Peters KN, Behar SM. Macrophages clean up: efferocytosis and microbial control. Curr Opin Microbiol. 2014;17:17–23. doi: 10.1016/j.mib.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death in disease: mechanisms and emerging therapeutic concepts. N Engl J Med. 2009;361(16):1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melino G. The sirens’ song. Nature. 2001;412(6842):23. doi: 10.1038/35083653. [DOI] [PubMed] [Google Scholar]
- 65.Thorp E, Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol. 2009;86(5):1089–1095. doi: 10.1189/jlb.0209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174(1–2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 67.Lin S-L, Li B, Rao S, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107(9):4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boulter L, Govaere O, Bird TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18(4):572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008;105(52):20764–20769. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terauchi M, Li J-Y, Bedi B, et al. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009;10(3):229–240. doi: 10.1016/j.cmet.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J-Y, Walker LD, Tyagi AM, Adams J, Weitzmann MN, Pacifici R. The sclerostin-independent bone anabolic activity of intermittent PTH treatment is mediated by T-cell-produced Wnt10b. J Bone Miner Res. 2014;29(1):43–54. doi: 10.1002/jbmr.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogawa K, Yoshioka Y, Isohashi F, Seo Y, Yoshida K, Yamazaki H. Radiotherapy targeting cancer stem cells: current views and future perspectives. Anticancer Res. 2013;33(3):747–754. [PubMed] [Google Scholar]
- 73.Koh AJ, Novince CM, Li X, Wang T, Taichman RS, McCauley LK. An irradiation-altered bone marrow microenvironment impacts anabolic actions of PTH. Endocrinology. 2011;152(12):4525–4536. doi: 10.1210/en.2011-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quach JM, Askmyr M, Jovic T, et al. Myelosuppressive therapies significantly increase pro-inflammatory cytokines and directly cause bone loss. J Bone Miner Res. 2015;30(5):886–897. doi: 10.1002/jbmr.2415. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Q, Miller C, Bible J, et al. Additive effects of mechanical marrow ablation and PTH treatment on de novo bone formation in mature adult rats. Cells. 2012;1(4):1168–1181. doi: 10.3390/cells1041168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parfitt AM. Bone-forming cells in clinical conditions. In: Hall B, editor. Bone. Vol 1. Osteoblast osteocyte. Boca Raton, FL: CRC Press; 1990. pp. 351–429. [Google Scholar]
- 77.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13(5):793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 78.Tang Y, Wu X, Lei W, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15(7):757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu X, Pang L, Lei W, et al. Inhibition of Sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell. 2010;7(5):571–580. doi: 10.1016/j.stem.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xian L, Wu X, Pang L, et al. Matrix IGF-1 regulates bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18(7):1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lotinun S, Kiviranta R, Matsubara T, et al. Osteoclast-specific cathepsin K deletion stimulates S1P–dependent bone formation. J Clin Invest. 2013;123(2):666–681. doi: 10.1172/JCI64840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takeshita S, Fumoto T, Matsuoka K, et al. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest. 2013;123(9):3914–3924. doi: 10.1172/JCI69493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao YQ, Freire-de-Lima CG, Schiemann WP, Bratton DL, Vandivier RW, Henson PM. Transcriptional and translational regulation of TGF-β production in response to apoptotic cells. J Immunol. 2008;181(5):3575–3585. doi: 10.4049/jimmunol.181.5.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCauley LK, Dalli J, Koh AJ, Chiang N, Serhan CN. Cutting edge: parathyroid hormone facilitates macrophage efferocytosis in bone marrow via proresolving mediators resolvin D1 and resolvin D2. J Immunol. 2014;193(1):26–29. doi: 10.4049/jimmunol.1301945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cho SW, Pirih FQ, Koh AJ, et al. The soluble interleukin-6 receptor is a mediator of hematopoietic and skeletal actions of parathyroid hormone. J Biol Chem. 2013;288(10):6814–6825. doi: 10.1074/jbc.M112.393363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11(6):411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guise TA, Mundy GR. Cancer and bone. Endocr Rev. 1998;19(1):18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- 88.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 89.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284(49):34342–3454. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soki FN, Cho SW, Kim YW, et al. Bone marrow macrophages support prostate cancer growth in bone. Oncotarget. doi: 10.18632/oncotarget.6042. Forthcoming. Epub 2015 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X, Loberg R, Liao J, et al. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 2009;69(4):1685–1692. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steiner JL, Murphy EA. Importance of chemokine (CC-motif) ligand 2 in breast cancer. Int J Biol Markers. 2012;27(3):e179–e185. doi: 10.5301/JBM.2012.9345. [DOI] [PubMed] [Google Scholar]
- 93.Lindholm PF, Lu Y, Adley BP, et al. Role of monocyte-lineage cells in prostate cancer cell invasion and tissue factor expression. Prostate. 2010;70(15):1672–1682. doi: 10.1002/pros.21202. [DOI] [PubMed] [Google Scholar]
- 94.Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett. 2013;332(1):3–10. doi: 10.1016/j.canlet.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 95.Polverini PJ, Leibovich SJ. Induction of neovascularization in vivo and endothelial proliferation in vitro by tumor-associated macrophages. Lab Invest. 1984;51(6):635–642. [PubMed] [Google Scholar]
- 96.Laurent J, Touvrey C, Botta F, Kuonen F, Ruegg C. Emerging paradigms and questions on pro-angiogenic bone marrow-derived myelomonocytic cells. Int J Dev Biol. 2011;55(4–5):527–534. doi: 10.1387/ijdb.103228jl. [DOI] [PubMed] [Google Scholar]
- 97.Park SI, Liao J, Berry JE, et al. Cyclophosphamide creates a receptive microenvironment for prostate cancer skeletal metastasis. Cancer Res. 2012;72(10):2522–2532. doi: 10.1158/0008-5472.CAN-11-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sullivan AR, Pixley FJ. CSF-1R signaling in health and disease: a focus on the mammary gland. J Mammary Gland Biol Neoplasia. 2014;19(2):149–159. doi: 10.1007/s10911-014-9320-1. [DOI] [PubMed] [Google Scholar]
- 99.Soki FN, Koh AJ, Jones JD, et al. Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. J Biol Chem. 2014;289(35):24560–24572. doi: 10.1074/jbc.M114.571620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363–368. doi: 10.1111/j.1600-0609.2006.00648.x. [DOI] [PubMed] [Google Scholar]
- 101.Kamizono J, Okada Y, Shirahata A, Tanaka Y. Bisphosphonate induces remission of refractory osteolysis in Langerhans cell histiocytosis. J Bone Miner Res. 2002;17(11):1926–1928. doi: 10.1359/jbmr.2002.17.11.1926. [DOI] [PubMed] [Google Scholar]
- 102.Mazor RD, Manevich-Mazor M, Shoenfeld Y. Erdheim-Chester disease: a comprehensive review of the literature. Orphanet J Rare Dis. 2013;8:137. doi: 10.1186/1750-1172-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Resnick D, Greenway G, Genant H, Brower A, Haghighi P, Emmett M. Erdheim-Chester disease. Radiology. 1982;142(2):289–295. doi: 10.1148/radiology.142.2.7054816. [DOI] [PubMed] [Google Scholar]
- 104.Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19–73. [PubMed] [Google Scholar]
- 105.Demicco EG, Rosenberg AE, Björnsson J, Rybak LD, Unni KK, Nielsen GP. Primary Rosai-Dorfman disease of bone: a clinicopathologic study of 15 cases. Am J Surg Pathol. 2010;34(9):1324–1333. doi: 10.1097/PAS.0b013e3181ea50b2. [DOI] [PubMed] [Google Scholar]
- 106.Geissmann F, Lepelletier Y, Fraitag S, et al. Differentiation of Langerhans cells in Langerhans cell histiocytosis. Blood. 2001;97(5):1241–1248. doi: 10.1182/blood.v97.5.1241. [DOI] [PubMed] [Google Scholar]
- 107.Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002;69(1):67–71. doi: 10.1002/ajh.10008. [DOI] [PubMed] [Google Scholar]
- 108.Rizzo FM, Cives M, Simone V, Silvestris F. New insights into the molecular pathogenesis of Langerhans cell histiocytosis. Oncologist. 2014;19(2):151–163. doi: 10.1634/theoncologist.2013-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]