Abstract
Objective
To evaluate the effects of an egg breakfast on lunchtime energy intake in children (age 4–6 years) and adolescents (age 14–17 years).
Methods
In 2 randomized crossover trials, participants received either an egg breakfast or an isocaloric bagel breakfast. In both trials, subsequent lunchtime energy intake was the primary outcome. The trial with adolescents also measured each participant’s serum ghrelin, serum peptide YY (PYY), and self-assessment of appetite rated using a visual analog scale.
Results
Lunchtime food intakes after egg and bagel breakfasts were not significantly different for either age group. Visual analog scale ratings of hunger and satiety were also not different between the 2 treatments in adolescents. Consumption of the egg breakfast led to a significant increase in serum PYY levels (p = 0.0001) in adolescents. However, increased levels of PYY were not correlated with reduced food intake.
Conclusion
Short-term food intake in children and adolescents is not differentially altered by an egg breakfast compared to a bagel breakfast.
Keywords: eggs, obesity, appetite, PYY, ghrelin
INTRODUCTION
Childhood obesity is a growing problem in the United States and many countries around the world. Currently 17% of children and adolescents in the United States are considered obese, over 3 times more than in the 1960s [1,2]. However, treatment options are more limited for this vulnerable age group than for adults. Drug therapies and bariatric surgery are not commonly recommended, and restrictive dieting can be problematic during growth and development. One of the major avenues to combat obesity is to alter energy intake, and there are many factors that influence children’s eating behaviors. Thus, dietary interventions that modulate satiety and food consumption may offer pragmatic approaches for reducing energy intake.
Foods that increase satiety can result in decreased energy intake later in the day [3]. Eggs are a highly nutritious and commonly consumed breakfast item in the United States. They are a good source of protein, with one large egg providing approximately 6 g of protein [4]. Some studies have linked high-protein diets with enhanced weight loss [5–7], but the findings are somewhat controversial, because other studies have reported total energy intake to be more important than the macronutrient composition of the diet [8–10]. We and others have previously shown that consuming an egg breakfast increases satiety and reduces lunchtime food intake in adults [11–14]. This effect may be mediated by alterations in appetite hormones such as ghrelin and peptide YY (PYY).
Ghrelin, a hormone produced by the stomach, is believed to have appetite-stimulating effects. Ghrelin levels rise during states of energy deficiency and decrease in response to feeding. Intravenous infusion of ghrelin causes increased energy intake at subsequent meals [15,16]. PYY, a hormone produced by the ileum and colon, may have appetite-suppressing effects. In initial adult human studies, intravenous infusion of PYY decreased subsequent food intake [17,18]. Dietary manipulations that increase endogenous PYY levels such as high-protein diets may aid in increasing satiety [19]. Studies in adults have shown that both ghrelin and PYY responses vary depending on the macronutrient composition of the meal. Meals high in carbohydrates generally have the most suppressive effect on ghrelin levels immediately following meal ingestion, but meals high in protein tend to result in lower serum concentrations for longer periods of time, resulting in a lower area under the curve [20–23]. High-fat and high-protein meals have been shown to induce prolonged increases in PYY levels [24].
Thus far, few studies have examined how certain foods affect satiety and appetite hormone levels in children. Egg breakfast consumption has been shown to suppress levels of ghrelin and increase levels of PYY in adults [12,25]. Our objective was to determine whether consuming an egg breakfast would reduce subsequent food intake in children and adolescents.
MATERIALS AND METHODS
Participants and Study Design
The study was approved by the Institutional Review Board at Pennington Biomedical Research Center and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from the legal guardians of all subjects prior to study initiation. The study consisted of 2 separate randomized crossover trials. The first trial consisted of children, 4–6 years of age, recruited from a local preschool. All study procedures were performed at the preschool between February and April 2012. Eligible participants were free of chronic disease and had no known allergies or sensitivities to eggs, wheat, dairy, or soy. Children were randomly assigned to receive an egg or bagel breakfast on their first test day (Table 1). Care was taken to ensure that the children’s daily routine was kept as close to normal as possible. Study personnel verified that all participants were fasting upon arrival and no food items were available between breakfast and lunch. Breakfast was provided to children in their regular classroom as usual. Children were required to eat the entire breakfast as provided, and 3 hours later they received an ad libitum lunch. Lunch was provided in the cafeteria, which was their normal lunch area. Children were seated together at tables in their normal configuration and monitored so that they did not exchange food. Food intake was covertly determined by weighing back leftovers immediately after lunch. One week later, study procedures were repeated and children received the breakfast they did not consume on the first test day.
Table 1.
Breakfast Compositiona
| Item | Weight (g) | Energy (kcal) | Protein (g) | Fat (g) | Carbohydrates (g) |
|---|---|---|---|---|---|
| Bagel breakfast | |||||
| Bagel | 35.5 | 93 | 3.3 | 0.5 | 18.6 |
| Cream | 14.0 | 50 | 1.0 | 4.5 | 1.0 |
| Yogurt | 42.5 | 25 | 1.2 | 0.0 | 4.8 |
| Total | 92.0 | 168 | 5.5 | 5.0 | 24.3 |
| Egg breakfast | |||||
| Scrambled | 61.0 | 102 | 6.8 | 7.4 | 1.3 |
| Toast | 25.0 | 64 | 1.6 | 0.8 | 12.2 |
| Jelly | 8.5 | 5 | 0.0 | 0.0 | 2.5 |
| Total | 94.5 | 171 | 8.4 | 8.3 | 16.1 |
Portion sizes shown are for children (4–6 years old). Adolescents (14–17 years old) received twice the amount shown.
The second trial consisted of adolescents, 14–17 years of age, recruited from the greater Baton Rouge, Louisiana, area. All study procedures were performed at Pennington Biomedical Research Center between June and August 2012. Eligible participants were free of chronic disease, not actively attempting to lose weight, and had no known allergies or sensitivities to eggs, wheat, dairy, or soy. Adolescents were randomly assigned to receive an egg or bagel breakfast on their first test day. Participants were admitted to the inpatient unit of Pennington Biomedical Research Center, and an intravenous was inserted for blood collection. Participants were required to eat all food provided at breakfast. Blood was collected before breakfast and 30 and 180 minutes after breakfast completion to measure appetite hormone levels. Adolescents also rated their hunger and fullness levels before breakfast and 30, 60, 120, and 180 minutes after breakfast completion using electronic visual analog scales. Three hours after breakfast, adolescents were provided with an ad libitum lunch and instructed to eat as much or as little as they wanted and to stop when they were comfortably full. Food intake was covertly determined by weighing back leftovers immediately after lunch. Study procedures were repeated one week later with the other breakfast. Because of the setting of this trial, participants were in private rooms and ate alone but were accompanied by a parent or legal guardian.
The randomization sequence was generated by a statistician using a series of permuted blocks of 4 treatment sequences with 2 of each type (bagel, egg or egg, bagel). The study coordinator enrolled participants, and the study dietitian assigned participants to their treatment sequence using the randomization sequence.
Meal Composition
All meals were prepared and served by the Pennington Biomedical Metabolic Kitchen staff. Breakfast compositions are shown in Table 1. Breakfasts were matched for total calories and energy density. The bagel breakfast provided 13% of calories from protein, 58% from carbohydrate, and 27% from fat. The egg breakfast provided 20% of calories from protein, 37% from carbohydrate, and 43% from fat. The ad libitum lunch consisted of baked chicken, macaroni and cheese, green beans, mandarin oranges, rolls, and 1% milk (Table 2). The lunch meals were served on trays at a dining table.
Table 2.
Lunch Compositiona
| Item | Weight (g) | Energy (kcal) | Protein (g) | Fat (g) | Carbohydrates (g) |
|---|---|---|---|---|---|
| Chicken breast | 140 | 183 | 35.0 | 5.0 | 0.0 |
| Macaroni and cheese | 140 | 296 | 8.5 | 12.8 | 36.5 |
| Green beans | 68 | 19 | 1.0 | 0.1 | 4.4 |
| Mandarin oranges | 126 | 77 | 0.6 | 0.1 | 20.4 |
| Dinner roll | 48 | 150 | 4.0 | 3.0 | 28.0 |
| Milk (1%) | 244 | 102 | 8.2 | 2.4 | 12.2 |
| Total | 766 | 827 | 57.3 | 23.4 | 101.4 |
Portion sizes shown are what were offered to children (4–6 years old). Adolescents (14–17 years old) were offered 1600 kcal.
PYY and Ghrelin Concentrations
Total PYY was measured via radioimmunoassay according to the manufacturer’s instructions (Millipore, Billerica, MA). Acylated ghrelin was measured via radioimmunoassay according to the manufacturer’s instructions (Linco Research, Saint Charles, MO).
Statistical Analysis
The primary outcome of the study was ad libitum lunchtime food intake. Sample size was determined based on a previous study in adults [11]. The planned study called for enrolling 22 children and 16 adolescents with at least 80% power to detect as statistically significant a minimal average egg/bagel breakfast difference of 23.3 and 81 kcal of food intake at lunch, respectively, for children and adolescents. Post hoc calculations revealed that the final sample size with 13 children and 15 adolescents, less than originally planned, provided at least 80% power for detecting egg/breakfast difference of 31.5 and 85 kcal, respectively. Secondary outcomes included visual analog scale ratings of hunger and fullness, serum PYY levels, and serum ghrelin levels. Statistical analysis was performed using SAS 9.2 (SAS Institute Inc., Cary, NC). Data were analyzed using a mixed model with repeated measures. The fixed effects were test day effects, treatment effects, treatment sequence (order) effects, time effects, and the interaction between time and treatment, where signify interaction indicates substantial variation in treatment differences across time. The random effects were subjects within treatment sequence groups. Differences were considered statistically significant at p < 0.05. Only subjects who completed the study were included in the final analyses.
RESULTS
Subjects
Twenty-five children enrolled in the first trial and were randomly assigned to treatment sequence. Twelve participants were dropped because they did not finish breakfast as required by the study protocol. Thirteen children (6 males and 7 females; all Caucasian; age = 5 years) completed the study.
Sixteen adolescents enrolled in the second trial and were randomly assigned to treatment sequence. One subject dropped because he was unwilling to eat the foods provided. Fifteen adolescents completed the study and their characteristics are shown in Table 3.
Table 3.
Subject Characteristics of Teenagers (n = 15)
| Age (years) | 15.6 ± 1.1 |
|---|---|
| Female | 9 (60%) |
| Ethnic origin | |
| White | 11 (73%) |
| Black | 4 (27%) |
| Height (cm) | 169.7 ± 5.1 |
| Weight (kg) | 72.5 ± 22.6 |
| Overweight or obesea | 6 (40%) |
Defined as >85th body mass index–for-age percentile.
Food Intake
Food intake results are shown in Fig. 1. In total, adolescents consumed more calories than children due to the larger size of their required breakfast (170 vs 339 kcal). On average, adolescents consumed 903 ± 100 kcal and children consumed 699 ± 36 kcal. Lunchtime food intake was not significantly different between the egg and bagel breakfast conditions in either trial. Children consumed 536 ± 36 kcal and 523 ± 37 kcal at lunch after a bagel or egg breakfast, respectively. Adolescents consumed 571 ± 99 kcal and 556 ± 104 kcal at lunch after a bagel or egg breakfast, respectively.
Fig. 1.
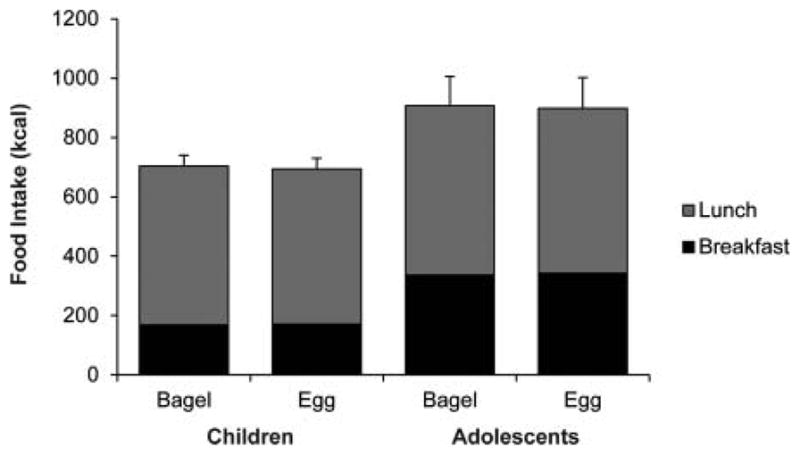
Food intake. Data for children and adolescents are summarized as means ± SE (children n = 13, adolescents n = 15).
Visual Analog Scale Ratings
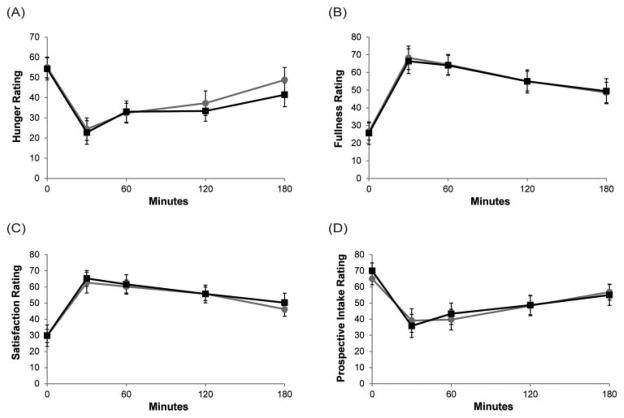
For adolescents, subjective ratings of hunger, satiety, fullness, and prospective food intake were not different between breakfasts (Fig. 2A–D).
Fig. 2.
Visual analog scale ratings of (A) hunger, (B) fullness, (C) satisfaction, and (D) prospective food intake. Black line: Egg breakfast; Grey line: Bagel breakfast. Data for adolescents are summarized as means ± SE (n = 15).
Appetite Hormones
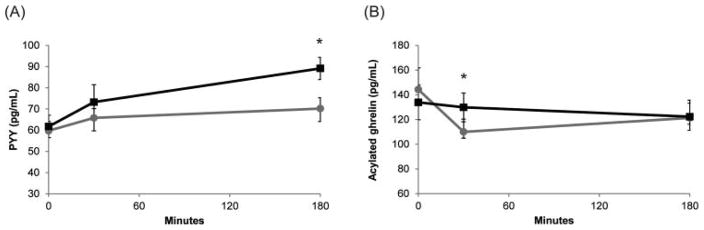
Serum PYY increased 50.7% ± 6.1% 180 min after egg breakfast consumption, whereas it only increased 19.4% after bagel breakfast consumption (p < 0.0002, Fig. 3A). Acylated ghrelin was unchanged when adolescents consumed the egg breakfast. However, consumption of the bagel breakfast led to a significant decrease in acylated ghrelin at 30 minutes post-breakfast (p < 0.002, Fig. 3B). Levels of ghrelin and PYY were not significantly correlated with visual analog scale ratings (data not shown).
Fig. 3.
Levels of appetite hormones (A) PYY and (B) acylated ghrelin. Black line: Egg breakfast, Grey line: Bagel breakfast. Data for adolescents are summarized as means ± SE (n = 15). *p < 0.001.
DISCUSSION
Our study was designed to compare the effects of 2 conventional whole food breakfasts on satiety in children and adolescents. The total weight and energy content were carefully matched, but the breakfasts differed in macronutrient content. The egg breakfast contained higher levels of both protein and fat (20% and 43% vs 13% and 27% for the bagel breakfast). Though the difference in protein content was relatively small relative to other studies [26,27], our goal was to examine the effects of whole foods on satiety rather than levels of a specific macronutrient.
This study indicates that compared to a bagel breakfast, consuming an egg breakfast did not alter satiety or subsequent food intake despite a substantial increase in serum PYY in adolescents. This seems to indicate that even though consuming eggs elicits a PYY response, it does not necessarily translate into altered satiety or food intake in adolescents. Indeed, previous studies have observed that in children PYY tends to increase after a meal, especially with meals that are high in protein or fat [28,29]. However, there was no correlation between hormone levels and appetite ratings [28,30,31].
Several other hormones are believed to be involved in the satiety response, including ghrelin. In adults, ghrelin stimulates appetite and decreases postprandially and then subsequently rises over time. We observed that the ghrelin response in adolescents varied with the macronutrient composition of the meal. Consuming a bagel breakfast elicited a response similar to that seen in adults with a postprandial decrease followed by a rise over time. However, when an egg breakfast was consumed, ghrelin did not change significantly over time. The literature on the ghrelin response to meal feeding in children is quite mixed. Some studies report that ghrelin is unresponsive to meal feeding [28,29,32,33]. Others report responses similar to those observed in adults with ghrelin transiently decreasing postprandially [30,31,34,35]. As with PYY, there was no observed correlation between ghrelin levels and appetite ratings.
Given the results of our study and previous studies observing no correlations between appetite hormone levels and subjective hunger ratings, these data would suggest that children may not be as sensitive to alterations in appetite hormone levels [28,30,31]. Though food consumption may elicit a hormonal response in children and adolescents, this does not appear to translate into altered food intake. There may be other hormones or systems that play more important roles in appetite regulation during this period of development.
Our study was limited in that we did not power the study to stratify participants by body weight category. There is evidence in both adults and children that suggests that appetite hormones are less responsive to meal feeding in obese subjects [18,32,33]. However, due to the limited number of studies and small sample sizes, it is difficult to draw any broad conclusions regarding how the appetite hormone response differs between lean and obese children. Another limitation of the study was that 12 of the children had to be dropped from the study because they did not consume the whole breakfast. We felt that this was necessary to ensure the integrity of the lunchtime meal test data. However, there may also be underlying differences between completers and noncompleters, such as food aversions or breakfast skippers vs normal breakfast eaters. Unfortunately, we did not determine their reasons for not completing breakfast because we were trying to be as unobtrusive as possible to their normal school day.
In summary, consuming an egg breakfast increased serum PYY levels in adolescents. However, this did not affect subjective hunger ratings or food intake at lunch. More research is needed to define the roles of different foods on satiety and appetite hormone levels in children during their development.
Acknowledgments
FUNDING
This study was funded by a grant from the American Egg Board/Egg Nutrition Center and in part by a Nutrition Obesity Research Center grant NIH 2P30DK072476 from NIDDK, Botanical Research Center grant P50AT002776 from NCCAM and ODS, and 1 U54 GM104940 from NIGMS, which funds the Louisiana Clinical and Translational Science Center. The sponsor did not participate in the analysis or interpretation of the data.
Footnotes
AUTHOR CONTRIBUTIONS
F.L.G. and N.V.D. designed the study. A.G.L. and R.S.P. carried out the experiments and collected the data. H.H. and W.D.J. analyzed the data. A.G.L. wrote the article. All authors reviewed and approved the final article. A.G.L., R.S.P., H.H., W.D.J., F.L.G., and N.V.D. have no additional conflicts of interest to declare.
This trial is registered at ClinicalTrials.gov NCT01530061.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among U.S. children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Troiano RP. Changes in the distribution of body mass index of adults and children in the us population. Int J Obes Relat Metab Disord. 2000;24:807–818. doi: 10.1038/sj.ijo.0801232. [DOI] [PubMed] [Google Scholar]
- 3.Holt SH, Miller JC, Petocz P, Farmakalidis E. A satiety index of common foods. Eur J Clin Nutr. 1995;49:675–690. [PubMed] [Google Scholar]
- 4.U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Beltsville, MD: U.S. Department of Agriculture; 2012. Release 25. [Google Scholar]
- 5.Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, Martinez JA, Handjieva-Darlenska T, Kunesova M, Pihlsgard M, Stender S, Holst C, Saris WH, Astrup A. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–2113. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Layman DK, Evans EM, Erickson D, Seyler J, Weber J, Bagshaw D, Griel A, Psota T, Kris-Etherton P. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J Nutr. 2009;139:514–521. doi: 10.3945/jn.108.099440. [DOI] [PubMed] [Google Scholar]
- 7.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, Purnell JQ. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41–48. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- 8.de Souza RJ, Bray GA, Carey VJ, Hall KD, LeBoff MS, Loria CM, Laranjo NM, Sacks FM, Smith SR. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the pounds lost trial. Am J Clin Nutr. 2012;95:614–625. doi: 10.3945/ajcn.111.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifton PM, Keogh JB, Noakes M. Long-term effects of a high-protein weight-loss diet. Am J Clin Nutr. 2008;87:23–29. doi: 10.1093/ajcn/87.1.23. [DOI] [PubMed] [Google Scholar]
- 10.Noakes M, Keogh JB, Foster PR, Clifton PM. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr. 2005;81:1298–1306. doi: 10.1093/ajcn/81.6.1298. [DOI] [PubMed] [Google Scholar]
- 11.Vander Wal JS, Marth JM, Khosla P, Jen KL, Dhurandhar NV. Short-term effect of eggs on satiety in overweight and obese subjects. J Am Coll Nutr. 2005;24:510–515. doi: 10.1080/07315724.2005.10719497. [DOI] [PubMed] [Google Scholar]
- 12.Ratliff J, Leite JO, de Ogburn R, Puglisi MJ, VanHeest J, Fernandez ML. Consuming eggs for breakfast influences plasma glucose and ghrelin, while reducing energy intake during the next 24 hours in adult men. Nutr Res. 2010;30:96–103. doi: 10.1016/j.nutres.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Fallaize R, Wilson L, Gray J, Morgan LM, Griffin BA. Variation in the effects of three different breakfast meals on subjective satiety and subsequent intake of energy at lunch and evening meal. Eur J Nutr. 2013;52(4):1353–9. doi: 10.1007/s00394-012-0444-z. [DOI] [PubMed] [Google Scholar]
- 14.Vander Wal JS, Gupta A, Khosla P, Dhurandhar NV. Egg breakfast enhances weight loss. Int J Obes (Lond) 2008;32:1545–1551. doi: 10.1038/ijo.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, Ghatei MA, Small C, Bloom SR. Ghrelin increases food intake in obese as well as lean subjects. Int JObes (Lond) 2005;29:1130–1136. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 16.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 17.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone pyy(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 18.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide yy3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 19.Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide yy in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Erdmann J, Lippl F, Schusdziarra V. Differential effect of protein and fat on plasma ghrelin levels in man. Regul Pept. 2003;116:101–107. doi: 10.1016/s0167-0115(03)00195-2. [DOI] [PubMed] [Google Scholar]
- 21.Erdmann J, Topsch R, Lippl F, Gussmann P, Schusdziarra V. Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J Clin Endocrinol Metab. 2004;89:3048–3054. doi: 10.1210/jc.2003-031610. [DOI] [PubMed] [Google Scholar]
- 22.Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 2008;93:1971–1979. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tannous dit El Khoury D, Obeid O, Azar ST, Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Ann Nutr Metab. 2006;50:260–269. doi: 10.1159/000091684. [DOI] [PubMed] [Google Scholar]
- 24.Helou N, Obeid O, Azar ST, Hwalla N. Variation of postprandial pyy 3-36 response following ingestion of differing macronutrient meals in obese females. Ann Nutr Metab. 2008;52:188–195. doi: 10.1159/000138122. [DOI] [PubMed] [Google Scholar]
- 25.Bayham BE, Greenway FL, Johnson WD, Dhurandhar NV. A randomized trial to manipulate the quality instead of quantity of dietary proteins to influence the markers of satiety. J Diabetes Complications. 2014;28(4):547–52. doi: 10.1016/j.jdiacomp.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Latner JD, Schwartz M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite. 1999;33:119–128. doi: 10.1006/appe.1999.0237. [DOI] [PubMed] [Google Scholar]
- 27.Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr. 2006;83:89–94. doi: 10.1093/ajcn/83.1.89. [DOI] [PubMed] [Google Scholar]
- 28.Lomenick JP, Melguizo MS, Mitchell SL, Summar ML, Anderson JW. Effects of meals high in carbohydrate, protein, and fat on ghrelin and peptide yy secretion in prepubertal children. J Clin Endocrinol Metab. 2009;94:4463–4471. doi: 10.1210/jc.2009-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misra M, Tsai PM, Mendes N, Miller KK, Klibanski A. Increased carbohydrate induced ghrelin secretion in obese vs normal-weight adolescent girls. Obesity (Silver Spring) 2009;17:1689–1695. doi: 10.1038/oby.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maffeis C, Surano MG, Cordioli S, Gasperotti S, Corradi M, Pinelli L. A high-fat vs a moderate-fat meal in obese boys: Nutrient balance, appetite, and gastrointestinal hormone changes. Obesity (Silver Spring) 2010;18:449–455. doi: 10.1038/oby.2009.271. [DOI] [PubMed] [Google Scholar]
- 31.Stock S, Leichner P, Wong AC, Ghatei MA, Kieffer TJ, Bloom SR, Chanoine JP. Ghrelin, peptide yy, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90:2161–2168. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 32.Lomenick JP, Clasey JL, Anderson JW. Meal-related changes in ghrelin, peptide yy, and appetite in normal weight and overweight children. Obesity (Silver Spring) 2008;16:547–552. doi: 10.1038/oby.2007.129. [DOI] [PubMed] [Google Scholar]
- 33.Bellone S, Castellino N, Broglio F, Rapa A, Vivenza D, Radetti G, Bellone J, Gottero C, Ghigo E, Bona G. Ghrelin secretion in childhood is refractory to the inhibitory effect of feeding. J Clin Endocrinol Metab. 2004;89:1662–1665. doi: 10.1210/jc.2003-031207. [DOI] [PubMed] [Google Scholar]
- 34.Mittelman SD, Klier K, Braun S, Azen C, Geffner ME, Buchanan TA. Obese adolescents show impaired meal responses of the appetite-regulating hormones ghrelin and pyy. Obesity (Silver Spring) 2010;18:918–925. doi: 10.1038/oby.2009.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maffeis C, Bonadonna RC, Consolaro A, Vettor R, Banzato C, Silvagni D, Bogoni G, Pellegrino M, Tato L. Ghrelin, insulin sensitivity and postprandial glucose disposal in overweight and obese children. Eur J Endocrinol. 2006;154:61–68. doi: 10.1530/eje.1.02055. [DOI] [PubMed] [Google Scholar]