Abstract
Purpose
To summarize the study design, operational strategies and procedures of the Chinese American Eye Study (CHES), a population-based assessment of the prevalence of visual impairment, ocular disease, and visual functioning in Chinese Americans.
Methods
This population-based, cross-sectional study, included 4,570 Chinese, 50 years and older, residing in the city of Monterey Park, California. Each eligible participant completed a detailed interview and eye examination. The interview included an assessment of demographic, behavioral, and ocular risk factors and health-related and vision-related quality of life. The eye examination included measurements of visual acuity, intraocular pressure, visual fields, fundus and optic disc photography, a detailed anterior and posterior segment examination, and measurements of blood pressure, glycosylated hemoglobin levels, and blood glucose levels.
Results
The objectives of the CHES are to obtain prevalence estimates of visual impairment, refractive error, diabetic retinopathy, open-angle and angle-closure glaucoma, lens opacities, and age-related macular degeneration in Chinese-Americans. In addition, outcomes include effect estimates for risk factors associated with eye diseases. Lastly, CHES will investigate the genetic determinates of myopia and glaucoma.
Conclusion
The CHES will provide information about the prevalence and risk factors of ocular diseases in one of the fastest growing minority groups in the United States.
INTRODUCTION
Chinese-Americans are one of the fastest growing populations in the United States (U.S.) (48% increase from 1990-20001). While visual impairment and other major ocular conditions are a significant public health burden in middle-aged and older Americans, Chinese-Americans exhibit a very different pattern of eye disease than other racial/ethnic groups and are largely underrepresented in studies of eye disease. East Asian and U.S. studies1, 2 indicate that persons of Chinese descent are more likely to have myopia, myopic retinopathy, angle-closure glaucoma, lens opacities, exudative age-related macular degeneration (AMD), and diabetic macular edema than African Americans, Latinos, or non-Hispanic whites. However, there are no large, population-based studies of age-related eye diseases in Chinese-Americans. As this fast-growing segment of the U.S. population ages, the burden of age-related eye disease will likely increase. Therefore, it is important to characterize the public health burden of eye diseases in this ethnic group and to understand the etiology (i.e., the risk relationships) of these diseases for preventive interventions.
Building on nearly 15 years of ocular epidemiologic research by the same research team on other NIH-funded, population-based studies, the Chinese-American Eye Study (CHES) collected data in an area with the largest concentration of Chinese-Americans in the U.S. CHES was designed to address the lack of data in Chinese-Americans aged 50 years and older. This paper summarizes the study design and procedures used in CHES.
STUDY DESIGN
This 5-year, population-based study, funded by the National Eye Institute, was designed to assess the prevalence of visual impairment, myopia, glaucoma, diabetic retinopathy (DR), age-related macular degeneration (AMD), and lens opacities in 4,570 non-institutionalized Chinese-Americans, aged 50 years and older, in the city of Monterey Park in Los Angeles County, and to determine risk indicators associated with these ocular diseases. Eligible individuals were invited to participate in the study. Institutional review board/ethics committee approval was obtained from the University of Southern California Medical Center Institutional Review Board. All study procedures adhered to recommendations of the Declaration of Helsinki.
SPECIFIC AIMS
CHES had three specific aims:
-
(1)
To determine the prevalence of visual impairment and major eye diseases in a non-institutionalized, population-based sample of Chinese-Americans, aged 50 years and older, in Los Angeles County.
Age-specific prevalence rates of the following diseases will be determined:
-
a)
visual impairment and cause-specific prevalence of visual impairment
-
b)
refractive errors, including myopia, high myopia, astigmatism, and anisometropia
-
c)
open-angle glaucoma (OAG) and angle-closure glaucoma (ACG), angle closure and narrow angles
-
d)
early and late AMD
-
e)
DR, non-diabetic retinopathy and myopic retinopathy
-
f)
lens opacities (cortical, nuclear, posterior subcapsular (PSC), and mixed);
-
a)
-
(2)To examine risk factors/indicators associated with (a) myopia, high myopia, and myopic retinopathy, (b) DR and non-diabetic retinopathy, (c) early and late AMD, (d) angle closure and narrow angles, glaucomatous visual field loss, and (e) lens opacities
-
a)Socio-demographic factors, biometric measures and nuclear lens opacification
-
b)Age, gender, waist/hip ratio, duration of diabetes, glycosylated hemoglobin (HbA1c), high blood pressure, refractive error and cataract surgery
-
c)Markers of microvascular disease (retinal arteriole, venule caliber and arteriole tortuosity and cardiovascular risk factors
-
d)Refractive and biometric measures (axial length, keratometry, noncycloplegic and cycloplegic measures of anterior chamber depth and lens thickness, and OCT based anterior chamber angle measurements) and structural ocular characteristics
-
e)Diabetes status, high blood pressure, smoking and education
-
a)
-
(3)
To examine potentially unique genetic associations of myopia and glaucoma, which have distinctive clinical profiles in this racial/ethnic group.
ORGANIZATIONAL STRUCTURE
CHES resource centers included the Study Coordinating Center, the Survey Research Center, the Data Management and Analysis Center, the Local Eye Examination Center, and the Ocular Epidemiology Reading Center. Advisory groups included: 1) the Internal Advisory Group, which met weekly to review the study progress and to address scientific and methodological issues; 2) the Community Advisory Group, which met at least twice a year to ensure community involvement and obtain advice from community leaders; and 3) the Data Monitoring and Oversight Committee, which met once a year to provide additional external oversight to the study.
STUDY AREA
The study area, within the city of Monterey Park, California, comprised fifteen census tracts. This area was chosen because (1) a high proportion of Chinese-Americans resided in the area; (2) the population of the fifteen census tracts was large enough to obtain robust prevalence estimates; (3) there was strong support and encouragement from community leaders; (4) the nearby LAC/USC Medical Center can provide necessary emergency care and (5) the demographic characteristics of the population were similar to those of Chinese populations in Los Angeles County, the State of California, and the US (Table 1). CHES collected the household enumeration data on eligible residents. The preliminary distribution can be found in Table 1.
Table 1.
Socio-demographic characteristics of the Chinese population Monterey Park, Los Angeles County, California and the U.S
| Monterey Park | LA County | California | U.S. | |
|---|---|---|---|---|
| All Ethnic Groups (n) | 60,051 | 9,519,338 | 33,871,648 | 281,421,906 |
| Chinese (%) | 41 | 3 | 3 | 1 |
| Chinese | n=24,758 | n=329,352 | n=980,642 | n=2,432,58 5 |
| (%) | (%) | (%) | (%) | |
| Age ≥50 | 33 | 26 | 27 | 24 |
| Age distribution | ||||
| 50-59 | 35 | 43 | 41 | 44 |
| 60-69 | 28 | 27 | 28 | 29 |
| 70-79 | 24 | 21 | 21 | 19 |
| 80+ | 13 | 9 | 9 | 8 |
| Gender | ||||
| Female | 52 | 52 | 52 | 52 |
| Male | 48 | 48 | 48 | 48 |
| Education status | ||||
| Less than high school | 32 | 24 | 23 | 23 |
| High school graduate | 15 | 13 | 12 | 13 |
| Some college or more | 21 | 20 | 19 | 16 |
| College graduate or more | 32 | 43 | 46 | 48 |
| Unemployed (≥ 16 years old) | 3 | 3 | 3 | 3 |
| Household Income | ||||
| $ 0 - $ 24,999 | 36 | 29 | 24 | 26 |
| $ 25,000 - $ 49,999 | 26 | 23 | 20 | 22 |
| $ 50,000 - $ 99,999 | 25 | 29 | 31 | 30 |
| $100,000 and over | 13 | 19 | 25 | 22 |
Source: Files SF2 and SF4, Census of Population and Housing
SAMPLE SIZE CONSIDERATIONS AND SAMPLING METHOD
Primary consideration for determining the study's sample size was the need to obtain robust estimates of the prevalence of the specified ocular diseases, blindness, and visual impairment. The secondary consideration was to have adequate sample size to detect significant relationships between risk factors and ocular disease. CHES estimated that a sample size of 4,570 is adequate to allows us to obtain adequate power and age-specific prevalence estimates for specific ocular conditions and to detect clinically significant odds ratios associated with various risk factors (Table 2). Table 2 provided the relative standard errors (RSE) for our study sample size. RSE is the standard error divided by the mean and expressed as a percentage. The data with the lower relative standard error has a more precise measurement since there is less variance around the mean. The sample size calculations were binomial sampling, which presumed that disease occurred independently among respondents, and no clustering was assumed. In the Baltimore Eye Survey and LALES, an adjustment for clustering did not significantly change the prevalence estimates. The sample size calculations also assumed that all participants in the same age group had the same probability of disease. Since these assumptions were never completely met, the true precision may be slightly less than shown (i.e., the relative standard errors may be slightly higher than those shown in Table 2). The sampling frame for CHES was all households within 15 census tracts of Monterey Park, California. CHES enumerated all households within this area and identified census tracts that yield eligible residents who are Chinese Americans and 50 years and older.
Table 2.
Age-specific Percent RSE† for Selected Ocular Diseases, Visual Impairment and Blindness (n=4570)
| Age Group (yr.) | Any AMD (%) | Early AMD (%) | Late AMD (%) | Any Cataract or Cataract Surgery (%) | Any Nuclear Cataract (%) | Any Cortical Cataract (%) | Any Posterior Subcapsular Cataract (%) |
|---|---|---|---|---|---|---|---|
| 50-59 | 35.3* | 35.4* | -- | 4.2 | 6.7 | 5.6 | 13.2 |
| 60-69 | 17.8 | 20.0 | 40.2* | 1.8 | 3.1 | 3.4 | 8.8 |
| 70-79 | 12.6 | 13.2 | 42.7* | 1.1 | 1.8 | 2.7 | 6.5 |
| 80+ | 8.7 | 11.0 | 16.6 | 1.4 | 2.3 | 3.4 | 8.4 |
| Age Group (yr.) | All Glaucoma (%) | POAG (%) | PACG + PAC (%) | NA (%) | Any DR (%) | Vision-threatening DR (%) |
|---|---|---|---|---|---|---|
| 50-59 | 23.8 | 35.4* | 15.1 | 8.7 | 12.7 | 24.0 |
| 60-69 | 21.1 | 28.4 | 13.8 | 8.1 | 11.9 | 27.8 |
| 70-79 | 13.3 | 18.9 | 11.6 | 7.2 | 10.6 | 15.4 |
| 80+ | 11.2 | 16.3 | 13.0 | 9.3 | 13.7 | 28.9 |
| Age Group (yr.) | Myopia (%) | High Myopia (%) | Myopic Retinopathy (%) | Visual Impairment (%) | Blindness (%) |
|---|---|---|---|---|---|
| 50-59 | 4.2 | 11.4 | 26.3 | 14.5 | 35.4* |
| 60-69 | 4.4 | 12.3 | 28.4 | 9.0 | 23.1 |
| 70-79 | 4.0 | 11.3 | 19.7 | 4.4 | 12.2 |
| 80+ | 4.9 | 14.5 | 25.4 | 4.2 | 11.2 |
Relative Standard Error (RSE) is the ratio of the standard error to the point estimate.
AMD=age-related macular degeneration; POAG=primary open angle glaucoma; PAC=primaryangle closure; PACG=primaryangle closure glaucoma; DR=diabetic retinopathy.
Data below NCHS standards, RSE > 30.
PILOT STUDY
A pilot study was conducted to determine the feasibility and potential participation rate among Chinese-Americans in areas neighboring the study area. A CHES questionnaire was developed to obtain the following information: Ocular Disease History and Eye Service Use, General Health, General Health Service Use, Insurance, Medication Usage, Cognitive Abilities Screener, Tobacco and Alcohol Intake, Demographics, Dietary Supplements Intake, History of Falls and Fractures, and Acculturation (SL-ASIA). The CHES study questionnaire was translated into Chinese by experienced bilingual translators and reviewed and back-translated by a second bilingual group with expertise in questionnaire design. For the pilot study, a sample of 50 individuals aged 50 years and older from the area surrounding Monterey Park was asked to complete the questionnaire. Three bilingual trained interviewers administered the questionnaire to each of the individuals and noted the responses on the questionnaire. The pilot data were sent to the Data Management and Analysis Center where they were reviewed for completeness, coded and entered into a database. Forty-eight of the 50 participants (96%) completed the questionnaire. Of the 50 individuals who were asked to participate, 41 (82%) self-identified themselves as being Chinese-American. No difficulties with understanding the survey items with respect to the respondents’ lives or experiences were reported or detected on explicit questioning. Of the 41 self-identified Chinese-American participants in the pilot study, 35 (85%) indicated that they would be willing to participate in the study and to undergo a complete eye examination. Thus, in the pilot study, we determined that 1) we were able to obtain data from the Chinese-American population neighboring the proposed study area; 2) we were able to analyze and draw conclusions from the data collected by this survey instrument; 3) a high proportion of Chinese reported vision/ocular problems; 4) a high proportion of individuals were willing to participate in a complete eye examination..
OPERATIONAL STRATEGIES
Ascertainment of Eligibility
Household residence was determined using the U.S. Census definition1: a resident is anyone who considers this home as his or her permanent residence and lives in the household at least 6 months/year. The eligibility criteria for CHES was (1) being a resident in one of the 15 tracts in Monterey Park, (2) being 50 years or older on the date of the household screening, and (3) self-identifying as of Chinese descent, including individuals who stated that a) parents' or grandparents’ native language was a Chinese dialect; or b) parents or grandparents had Chinese surnames.
Recruitment Strategies
The strategies CHES utilized to ensure a good response rate were a positive overall experience, refusal conversion techniques, a well-trained and -supported field staff that was familiar with the neighborhoods, incentive programs, and extensive study awareness. To spread awareness, we met with community and religious leaders (elected officials, ministers, priests, etc.), held community informational meetings, invited local media coverage, made presentations at churches, community/senior centers, parks and libraries, held booth at local festivals/health fairs, cooperated with local medical providers, published articles in local Chinese newspapers, and presented information on radio shows. To accommodate participants who worked full-time, clinics were open evenings and weekends. In addition, the Local Eye Examination Center was established in Alhambra, near the residential area of participants.
Challenges
-
1)
To accommodate language barriers and trust issues, since many participants are first-generation immigrants who do not speak English, all field and clinic staff are multilingual in different Chinese dialects.
-
2)
Because many older Chinese do not drive and have no transportation to get to the clinic for an appointment, CHES offered free transportation to and from the clinic.
-
3)
There were some locked buildings in this community that the study team couldn't get access to initially. In order to increase the community awareness, CHES reached out to the community leadership groups and organizations and assembled a Community Advisory Group, which consisted of elected officials, local media experts, non-profit organization directors, building managers and representatives from the home associations.
-
4)Another challenge CHES encountered was trust within the community. It usually required multiple visits to build rapport with potential participants. CHES administrated different approaches to overcome this challenge:
- Utilizing field interviewers of similar cultural backgrounds (i.e. language, geographical location) with targeted participants.
- conducting community awareness activities via different venues
- providing refusal aversion/conversion training to field interviewers
CHES Clinical Information System
CHES Clinical Information System integrates data from (1) the Battelle Survey Research Center home interview using the Battelle BLAISE/ACCESS database system (Battelle, St. Louis, MO), and (2) the Local Eye Examination Center clinical examination and clinical questionnaire, using the Common Application Framework Extensible (CAFÉ) database system.
Using the Battelle BLAISE/ACCESS database system, interviewers collect screener questionnaires via Computer-Assisted Personal Interviewing (CAPI). All question and answer categories appear on a computer screen, optional phrasing is automatically tailored by the computer for each interview, and skip logic and consistency checks are built directly into the system to reduce errors. All data collected on this system are uploaded and cleaned before being delivered to the CHES database.
CAFÉ was developed at USC's Norris Comprehensive Cancer Center and used in CHES for clinical data collection and management. CAFÉ includes (a) customized data entry screens, (b) common vocabulary for drop-down lists, (c) validation checks, (d) role-based security, and (e) dashboards and reminders, (f) query-based reports, and (g) data export/import capabilities for data analysis.
Field Work and In-Home Survey Procedures
The Survey Research Center screened each household to identify eligible participants. When potentially eligible subjects were encountered, the interviewers would introduce the study, address respondent concerns, obtain basic information and informed consent, conduct a computer-assisted in-home interview, and schedule study participants for a complete eye examination at the Local Eye Examination Center. An interviewer's first contact with each household and respondent was extremely important and set the stage for the rest of the study and often determined whether or not an individual agreed to participate.
Once contact was made with a person over age 18 who lived in the household, interviewers would determine subject eligibility to participate in the study and establish rapport with the family. Data collected during home interview included 1) sociodemographics (birthdate, gender, race/ethnic group, education, income level, and occupation), 2) medical and ocular history, and surgeries; family history of any systemic and ocular diseases, 3) history of smoking and alcohol consumption, 4) use of eye care services, 5) cognitive evaluation, and 6) level of acculturation (the Suinn-Lew Asian Self-Identity Acculturation Scale3, 4). Interviewers used the participants' preferred language (English, Mandarin, Cantonese, or other Chinese dialects) for interviews. All instruments were tested and validated in both Mandarin and Cantonese. The home interview usually took 30-40 minutes to complete.
In-Clinic Ocular Exam and Interview Procedures
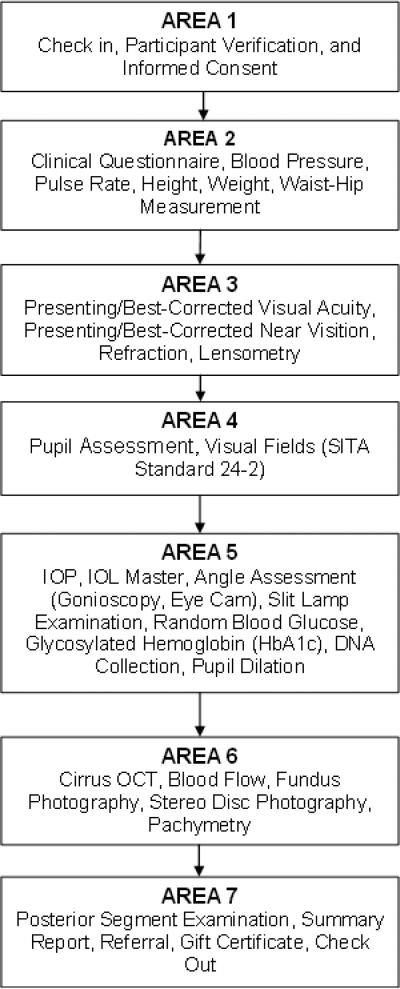
Figure 1 depicts the flow of the data collection process. The clinical examination included an in-clinic interview focused on quality-of-life questions and a clinical examination to determine the prevalence of various eye conditions and the cause of visual impairment/visual loss. During examination, data were entered directly into the CHES CAFÉ database. Standardized data collection procedures for participants included a series of measurements taken by ophthalmic technicians, an interviewer, and an ophthalmologist. The in-clinic examination consisted of the following procedures:
-
(1)
Registration and verification of name, birthdate, age, gender, residency and completion of informed consent.
-
(2)
In-clinic questionnaire: Questions on quality of life and visual functioning asked in the in-clinic interview are described in Appendix II. Quality of life was assessed using the Short Form 12-item Health Survey, a standardized questionnaire that has been validated in other populations. Self-reported visual functioning was assessed using the 25-item National Eye Institute Visual Functioning Questionnaire, which yields 12 vision-specific subscales. Both questionnaires were administered in either English or Chinese.
-
(3)
Measurements of height, weight, waist-hip ratio, pulse rate, and blood pressure (Baum Roll by Mobile Aneroid 1150NL, Copiague, NY).
-
(4)
Distance presenting and best-corrected visual acuity (VA) using the Early Treatment Diabetic Retinopathy Study (ETDRS) charts and the Lea symbol charts for illiterate participants (VA measurements were attempted at 1 meter for participants who read fewer than 20 letters at 4 meters).5
-
(5)
Near presenting and best-corrected VA using the Modified ETDRS Near Vision Acuity Chart (Precision Vision, La Salle, IL).5
-
(6)
Lensometry14 to verify the correct prescription in participants’ eyeglasses.
-
(7)
Automated refraction with a Humphrey Automatic Refractor (Carl Zeiss Meditec, Dublin, CA) if the presenting VA was not 20/20 in either eye.
-
(8)
Subjective refraction if the participant read less than 20/20 on the automated refractor.
-
(9)
The contrast sensitivity test at 1 meter using the PELLI-ROBSON contrast sensitivity charts (Haag-Streit, Essex, CM 20 2TT, UK).6 The photopic (85-120 foot candles) and the mesopic (0-3 foot candles) contrast sensitivity for both eyes were measured with each participant's habitual correction.
-
(10)
Iris color grading using the Iris Color Classification System7.
-
(11)
Pupil assessment with the Rosenbaum pupil screener (pocket version) (Wilson Ophthamic, Mustangm, OK).
-
(12)
Visual field evaluation using Swedish Interactive Threshold Algorithm (SITA) Standard C24-2 test (Carl Zeiss Humphrey Field Analyzer II 750).8
-
(13)
Ocular biometry measurement using laser interference biometry (Zeiss IOL Master, version 5.4) to obtain axial length, anterior chamber depth, and corneal curvature radii for both eyes..
-
(14)
Intraocular pressure (IOP) measurement using Goldmann applanation tonometry (Haag-Streit, Mason, OH).9
-
(15)
Slit lamp (Haag-Streit, Mason, OH) anterior ocular segment examinations.
-
(16)
Gonioscopy, using a modified Shaffer classification (according to the angle structures visible),10 which has been used in several major epidemiologic studies of angle-closure glaucoma.11-13
-
(17)
Anterior segment optical coherence tomography (AS-OCT) (CASIA SS-1000, Tomey Corporation, Magoya, Japan) to acquire a three-dimensional image of the anterior ocular segment.
-
(18)
EyeCam (Clarity Medical Systems, Pleasanton, CA) to acquire an en-face image of the anterior chamber angle in each quadrant for both eyes
-
(19)
Pupil dilation (tropicamide and phenylephrine). Participants were dilated with one drop of tropicamide (1%) and one drop of phenylephrine (2.5%) in each eye. If the participant was not sufficiently dilated 10 minutes after the first dose, the drops would be repeated. If after 10 additional minutes the participant's eyes were still not adequately dilated, a third set of drops would be instilled. If the participant was known to be allergic to tropicamide, alternative drops, such as phenylephrine would be used. If the participant was allergic to phenylephrine, then only tropicamide would be used. If the participant was allergic to both tropicamide and phenylephrine, alternative drops such as cyclopentolate would be used. If a participant states that they are allergic to eye drops, no drops are instilled.(20) Measurements of HbA1c (DCA Vantage Analyzer, Siemens Healthcare, Norwood, MA), random blood glucose, total cholesterol, high density lipoprotein, low-density lipoprotein, and triglycerides (Cholestech LDX System, Alene, Waltham, MA).
-
(21)
Collection and storage of blood and saliva (Oragene DNA, DNA Genotek Inc., Ottawa, Canada) for future DNA analysis (a separate written informed consent was obtained).
-
(22)
Heidelberg Retinal Tomography (HRT III) (Heidelberg Engineering, Carlsbad, CA) of the optic disc.
-
(23)
Cirrus OCT (Carl Zeiss Meditec, Dublin, CA) of the retinal nerve fiber layer and macular retinal thickness. 14, 15
-
(24)
Measurement of retinal blood flow by Doppler Fourier domain OCT (Doppler FD-OCT) (Optovue, Fremont, CA).
-
(25)
Stereoscopic disc photography by optic nerve camera (Nidek 3DX, Nidek Inc., Fremont, CA).
-
(26)
Fundus photography using the Topcon TRC digital 50EX retinal camera (Topcon Corp. of America, Paramus, NJ) (3 stereoscopic fields on all nondiabetic participants; 7 stereoscopic fields on all diabetic participants).16 Fundus grading for AMD, DR, and retinal vessel caliber data performed at the OERC at Madison, Wisconsin.
-
(27)
Ultrasonic A-scan/pachymeter using DGH 4000b (DGH Technology, Inc., Exton, PA) for central corneal thickness, axial length, and lens thickness.
-
(28)
LOCS II lens opacity grading, including any gradable PSC, nuclear, or cortical lens opacity. 17
-
(29)
Dilated fundus examination with direct and indirect ophthalmoscopy.
-
(30)
Post-dilation IOP measurement.
Figure 1.
Flowchart of Clinical examination/Questionnaire Procedures
After examination, the ophthalmologist discussed the results of all procedures and diagnoses with the participant and determined whether the participant needed further follow-up and care. The ophthalmologist also determined if this follow-up should be emergent, urgent, or routine, depending on the results of the clinical examination. Participants were referred to local providers if follow-up was needed. Participants with no eye care provider were given a list of all providers in the area and/or an appointment to obtain care. The participant received an easily readable report discussing the findings of the examination, including the participant's blood pressure, current visual acuity, intraocular pressure, blood glucose and HbA1c levels. The participant was also given a report that advised him/her whether further medical and eye care was needed. Attached to this report was a second detailed report, including all the results of the examination. The participant was asked to give this report to his/her eye/medical care provider so that the provider can further understand the reason for referral.
The in-clinic exam took 2.5 – 3.5 hours to complete depending on individuals. Participants received a $25 gift card to a local store for compensating their time to complete the eye examination.
Outcome Measures
CHES will provide robust estimates of the prevalence of eye disease and will evaluate the risks associated with each eye condition (noted below); CHES will also examine genetic associations with myopia, primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG).
Visual Impairment
CHES used two definitions: 1) The World Health Organization (WHO) definition of best-corrected VA (BCVA) of worse than 20/60 in the better eye and 2) U.S. definition of best corrected VA of worse than 20/40 (including 20/40) in the better eye.18 Analyses of visual impairment will utilize all four strata: 1) presenting distance vision; 2) presenting near vision; 3) best-corrected distance vision; and 4) best-corrected near vision.
Blindness was defined in two ways 1) The WHO definition: BCVA of worse than 20/400 in the better eye and 2) the U.S. definition of legal blindness: BCVA of 20/200 or worse in the better eye.18
Myopia was defined as a spherical equivalent (SE) of <−0.5D (and alternatively as <−1.0D). High myopia was defined as SE of <−5.0D. Hyperopia was defined as SE worse than >+0.5D. Anisometropia was defined as SE difference between the right and left eyes >1.0D. Astigmatism was analyzed in minus cylinders and defined as a <−0.5D of cylinder, without reference to the axis.
Myopic Retinopathy
CHES adopted the definitions from the Blue Mountains Eye Study,11 including specific signs in the presence of myopia: staphyloma, lacquer cracks, Fuchs’ spot, and myopic chorioretinal thinning or atrophy.
Occludable angles were defined as posterior, usually pigmented trabecular meshwork visible for <90° without indentation.
Primary Angle-Closure Suspect (PACS) was defined as eyes with occludable angle, but with normal IOP and no peripheral anterior synechiae (PAS).
Primary Angle-Closure (PAC) was defined as PACS eyes with IOP >= 21 mmHg or PAS >=0.5 clock hour extension with no evidence of glaucomatous optic nerve damage.
Primary Angle-Closure Glaucoma (PACG) was defined as PAC eyes with evidence of glaucomatous optic nerve damage.
Primary Open-Angle Glaucoma (POAG)
Because of the heterogeneity of definitions previously used in population-based research, CHES adopted two definitions: (1) the International Society for Geographical & Epidemiological Ophthalmology (ISGEO) “standardized” scheme,19 and (2) the expert panel approach.
For the expert panel approach, a two-step process will be used to determine the diagnosis of Olen-Angle Glaucoma (OAG). First, two glaucoma specialists will evaluate all available clinical and history data, grade both optic disc photographs and visual fields independently and determine the presence or absence of OAG using guidelines specified below. If the two glaucoma specialists agree on the diagnosis, that diagnosis will be assigned to each specific eye of each participant. If the two disagree, a third glaucoma specialist will assess the data; agreement between two of the three glaucoma specialists will be used to assign the diagnosis for each eye of each participant. Additionally, the Principal Investigator will perform a confirmatory review of all cases of OAG.
Definite OAG requires:
- The presence of an open angle, congruent, characteristic, or compatible glaucomatous visual field abnormality; and
- Evidence of characteristic or compatible glaucomatous optic disc damage in at least one eye after ophthalmologic exclusion of other possible causes.
Probable OAG requires the presence of an open angle and one of the following in at least one eye:
- A congruent, characteristic, or compatible glaucomatous visual field abnormality and the absence of optic disc data; or
- Characteristic or compatible glaucomatous optic disc damage and the absence of visual field data; or
- A combination of visual field and optic disc abnormalities characteristic of or compatible with glaucoma in the opinion of the glaucoma specialist.
Other glaucoma is defined as evidence of definite or probable glaucoma and of secondary glaucoma such as neovascular glaucoma or traumatic glaucoma.
Lens Opacities were defined using the Lens Opacities Classification System II (LOCS II).17, 20
All lens changes: include the presence, in at least 1 eye, of:
- Any gradable PSC, nuclear, or cortical lens opacity (i.e., LOCS II grade ≥ 2); or
- Lens opacities that are too advanced to grade (such as hypermature lens opacities); or
- Evidence of previous lens opacity surgery, including aphakia or presence of an intraocular lens.
Any type of lens opacity
This definition will include the presence, in at least one eye, of any gradable PSC, nuclear, or cortical lens opacity (i.e., LOCS II ≥ 2). The prevalence of any type of lens opacity will be based on participants with a gradable LOCS II finding in the relevant region. In these analyses, participants with more than one type of opacity may be included in more than one category
Single and mixed types of lens opacities
This restrictive definition includes the presence (in an individual) of single or mixed opacities. Participants will be considered to have a single type of opacity, categorized as PSC only, nuclear only, or cortical only, if that is the only type present. This definition results in four mutually exclusive categories (PSC only, nuclear only, cortical only, and mixed). The prevalence of single and mixed types of lens opacities will be based on participants with gradable LOCS II findings in all regions, (i.e., PSC, nuclear, and cortical regions.)
Diabetic Retinopathy was defined as retinopathy in persons with diabetes mellitus. Grading protocols for DR are modifications of the ETDRS adaptation of the modified Airlie House classification of DR. Eyes were graded according to the following criteria: (1) no DR (levels 10–13) or (2) any DR (levels 14–85). DR was then classified as (1) nonproliferative DR (mild [levels 14–20], moderate [levels 31–43], or severe [levels 47–53]) or (2) proliferative DR (levels 60–85).
Diabetes Mellitus
A history of diabetes and undergoing treatment with oral hypoglycemic medications, insulin, or diet alone; or HbA1c ≥ 6.5%.
Age-Related macular degeneration
The assessment of drusen characteristics and other signs of AMD was accomplished by the Wisconsin AMD Classification system.21 CHES determined the prevalence of each of the specific lesions. Prevalence of early AMD (presence of soft indistinct drusen, retinal pigment epithelial depigmentation, or increased retinal pigment) or late AMD (exudative macular degeneration or pure geographic atrophy) was determined.
Early AMD
soft drusen >125 μm, any drusen with pigmentary abnormalities, in the absence of late lesions and not associated with a non-age-related process.
Late AMD
presence of macular fluid, lipid, hemorrhage, geographic atrophy, and disciform scar not associated with other conditions, e.g., DR, venous occlusive disease, and trauma.
Polypoidal choroidal vasculopathy (PCV)
is a peculiar form of choroidal neovascularization with features that distinguish it from typical wet AMD. In all eyes with neovascular changes, CHES will go back to the participant's primary retinal specialist (if present) and confirm if PCV is present. CHES will also inquire if indocyanine green (ICG) angiography had been performed. If not, CHES will then refer the participant for an ICG angiogram, which will be sent to the OERC for diagnosis.
Quality Control Procedures
Quality control (QC) procedures were implemented throughout the study to ensure the maximum accuracy and completeness of the final data.
To ensure that each interviewer was collecting data in an accurate, professional manner, a five percent validation of each interviewer's work were completed.
Five percent of the in-clinic questionnaires were validated via follow-up telephone calls three months after the examination. Specific questions included a few key questions from the original interview that were unlikely to have different answers (for example, do you drive?) were asked again. The study also asked questions on the politeness of the project staff and on the participants’ willingness to participate in future studies.
Several variables measured in the clinic examination were re-measured, including blood pressure, presenting visual acuity, visual field, IOP, random blood glucose, HbA1c and lens grading.
- The variables of blood pressure, IOP, random blood glaucose and HbA1c were measured twice for QC purpose.
- Presenting visual acuity was QC'd throughout the study. Any participant with presenting visual acuity 20/40 (mild impairment) or worse had another visual acuity test done by a second technician, masked to the results of the first technician. If the QC result differed by 5 letters, QC was repeated and the best results were recorded.
- For a one-week period of every six month, all participants who had normal SITA Standard visual fields in both eyes underwent a QC SITA Standard in one of the two normal eyes. If the results for the QC SITA Standard visual field were normal, no further testing was done. If the results were unreliable, the participant repeated the SITA Standard. If the second SITA Standard was unreliable, no further testing was done. If the second SITA Standard was abnormal, the participant would undergo a third SITA Standard; regardless of the outcome of the third SITA Standard no more tests would be performed. If the results of the QC SITA Standard were abnormal, the participant would perform a second SITA Standard. If the results from the second SITA Standard were abnormal, no more tests would be performed. If the results of the second SITA Standard were normal or unreliable, the SITA Standard would be repeated a third time.
- The reproducibility of LOCS II grading was evaluated throughout the CHES data collection period by two ophthalmologists. The assessment consisted of independent gradings performed at the slit lamp on the same 50 eyes every 6 months. Reproducibility was measured by the proportional weighted kappa statistic and percent agreement for each opacity type (nuclear opacities, cortical opacities, and PSC opacities).
The LEEC database incorporated data checks into the program at different parts of the examination; and error-trapping procedures were built into the system to clean the data electronically. QC procedures at the OERC focused on reducing systematic and random errors in the grading procedures. At the DMAC, QC methods were implemented by error-trapping systems built into the CAFÉ databases. Once all databases from the SRC, LEEC, and OERC were merged, further QC was performed using different SAS programs, such as checking of commonly shared variables across all databases, checking dates, and sorting and checking the data for duplicate records.
Statistical Analysis
SAS (ver. 9.2, SAS Institute Inc., Cary, NC) will be used for all statistical analyses. Age-specific prevalence estimates of blindness, visual impairment, lens opacities, myopia, glaucoma, DR, and AMD will be calculated as the ratio of the number of cases to the number of participants evaluated within a given age group (50-59, 60-69, 70-79, 80+ years). Gender-specific prevalence estimates will also be calculated. The direct standardization method will be used to determine age-adjusted prevalence estimates of lens opacities, glaucoma, DR and AMD. CHES will use the age distribution of Chinese-Americans in the U.S. as the standard age distribution. For each prevalence estimate, 95% confidence intervals will be reported.
To measure the degree to which traditional and risk factors may be associated with a greater prevalence of myopia, ACG and OAG, AMD, DR and lens opacities, CHES will conduct full-cohort analyses to measure the degree to which risk factors may be associated with various outcomes. In general, the analyses will proceed from descriptive/univariate analysis to more complicated multivariate approaches. Logistic regression analyses (both univariate and stepwise selection) will be conducted to relate the likelihood of ocular disease to each variable of interest (e.g., retinal arteriole diameter, artery vein ratio anterior chamber depth) after adjusting for various confounders (e.g., age, gender, smoking status, systemic hypertension). For categorical analyses of continuous variables, cut-points will be based on the overall distribution among cases and controls. For logistic regression analyses, maximum likelihood estimates of the odds ratio and 95% confidence intervals will be calculated for each dichotomous risk indicator Xi found to be statistically significant. For continuous risk indicators, the odds ratio and 95% confidence interval will be estimated by and where Δ = the incremental difference in the risk indicator between cases and controls.
To examine potentially unique genetic associations of AMD and glaucoma, CHES will use categorical and quantitative traits (e.g. AMD scale, CCT and IOP). Clinical scores will be analyzed for each eye, stratified by worse eye or better eye after adjusting for covariates (e.g., age, gender, etc.).Single marker and haplotype analyses will be conducted for each gene, after adjusting for population stratification.
Single marker analyses
Two basic methods are used to test for a disease-causing allele: 1) determine if an allele is more frequent in cases vs. controls; 2) determine if there is departure from Hardy-Weinberg equilibrium (HWE) proportions. Using person counts for the former test is better since it does not assume HWE. Provided we can assume that DNA quality is the same in cases and controls, it is preferable to test for the difference in HWD between cases and controls. The Cochran–Armitage trend test has power for the class of models for which the HWD trend test has absolutely no power, i.e., multiplicative penetrances; however, the HWD trend test determines location more precisely in those situations where it does have power. Song and Elston proposed a weighted average of the two statistics, which tends to have good properties regardless of the unknown mode of inheritance. To calculate the Cochran-Armitage trend test statistic for haplotypes, haplotype frequencies can be estimated (separately for cases and controls) by various methods – e.g., expectation-maximization (EM) algorithm to obtain maximum likelihood estimates and their variances.
Haplotype Analyses
We will also combine the markers into haplotypes, which can be more powerful than performing single-marker tests of association for each marker in a haplotype. The EM algorithm will be used to estimate haplotype frequencies using the HAPFREQS program. In order to avoid combining multiple markers into haplotypes that may result in sparse χ2 contingency tables, we will also obtain empirical p-values using the CLUMP program, which are more reliable in this instance.
Study Strengths and Limitations
CHES is the first comprehensive, population-based study to examine the prevalence of ocular conditions in Chinese Americans. Study strengths include cultural tailoring of study methods and numerous QC, protocol-driven, and computer-based procedures to minimize error rates. Comprehensive and standardized questionnaires will facilitate assessment of risk indicators and the impact of ocular conditions on quality of life. The population-based design of CHES facilitates the generalizability of the study findings. A potential study limitation is inaccurate self-reporting of treatment history, history of ocular surgery, and risk exposures. Accuracy of self-reporting can be validated by obtaining medical records from the participants' eye care providers to confirm the nature, timing, and duration of treatment and to minimize errors in self-reporting of these treatment variables. Additionally, self-reporting is reasonably accurate when assessing alcohol use, healthcare utilization, and number of comorbidities.22-24
To our knowledge, CHES is the largest population-based study of eye health in Chinese Americans. Data from CHES will help inform vision scientists, eye care providers, community health educators, and health policy makers on the burden of eye problems in this specific population and help focus resources on the most pressing, treatable conditions in this group.
Acknowledgments
Financial Support: This work was supported by grant EY-017337 from the National Eye Institute, National Institutes of Health, Bethesda, MD and by an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY; Rohit Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar
Appendix I. The Chinese American Eye Study Group
University of Southern California
Rohit Varma, MD, MPH (Principal Investigator); Mina Torres, MS (Project Director); Chunyi Hsu, MPH (Project Manager); Stanley P. Azen, PhD (Co–Principal Investigator); Rucha Desai, MS; David Dinh, BA; Ruzhang Jiang, MD; Jie Sun, MD, PhD, MPH; Dandan Wang, MD; YuPing Wang, COT; Justine Wong, BA; Shuang Wu, MS
Battelle Survey Research Center
Lisa V. John, PhD; Michelle Cheng, MS
Ocular Epidemiology Reading Center, School of Medicine and Public Health, University of Wisconsin
Ronald Klein, MD, MPH; Lisa M. Grady, BS; Stacy M. Meuer, BS; Michael W. Neider, BA, FOPS
Appendix II.
Questionnaires Administered in the Chinese American Eye Study
| Sections | Questions |
|---|---|
| In-home | |
| A. Eye Service Use and Ocular Disease History | Glasses/contact lenses use, eye service use, history and family history of cataracts and cataract surgery, history and family history of glaucoma and glaucoma care, history and family history of macular degeneration and care, history and family history of diabetes and diabetes care |
| B. General health | History of arthritis, stroke, high blood pressure, angina, heart condition/disease, heart failure or enlarged heart, asthma, skin or other type of cancer, back problems, deafness or trouble hearing, depression, hypercholesterolemia, thyroid condition and other major health problem |
| C. Medications | History of use of birth control pills, women's health, female hormones, steroids, anti-inflammatory drugs, statins |
| D. General health service use | Health service use , barriers of health care |
| E. Insurance | Health insurance status, vision insurance status |
| F. Tobacco/alcohol | History of tobacco and alcohol usage. |
| G. Acculturation | Cultural identity |
| H. Demographics | Ethnic background, parents’ names and languages, grandparents’ ethnic background, marital status, educational level, occupation, household income |
| I. Montreal Cognitive Assessment (MOCA) | Cognitive ability |
| J. Future contact | Future contact information. |
| In-clinic | |
| A. Health-related quality of life | Short Form 12-item Health Status Questionnaire, self-rating of current health status, health-related quality of life |
| B. General Vision status | NEI-VFQ-25, self-rating of current vision-related function. |
| C. Falls and fractures | History of falls |
| D. Physical activity | Frequency of physical activity |
* NEI-VFQ = National Eye Institute Visual Functioning Questionnaire.
Appendix III.
Methods Used to Define End Points in the Chinese American Eye Study
| End Point | Method |
|---|---|
| Visual acuity | (a)Distance vision using ETDRS charts at 4 m (b)Near vision using the logarithmic Near Vision chart |
| Lens opacities | LOCS II grading at the slit lamp |
| Age-related macular degeneration | Masked grading of stereoscopic fundus photographs from 3 standard fields using a modification of the Wisconsin Age-Related macular degeneration Classification System |
| Diabetes | Determined by history of diabetes and undergoing treatment with oral hypoglycemic medications, insulin, or diet alone; or measurements of Hb A1c using the DCA 2000+ System (Bayer Corp., Tarrytown, NY) |
| Diabetic retinopathy | Masked grading of stereoscopic fundus photographs from 7 standard fields using a modified Airlie House classification scheme |
| Open-angle glaucoma | Determined by consensus between 3 glaucoma specialists from stereoscopic optic disc photographs, Humphrey automated visual fields (SITA Standard C24-2) and clinical history and examination |
| Angle-closure glaucoma | Determined by consensus between 3 glaucoma specialists from stereoscopic optic disc photographs, Humphrey automated visual fields (SITA Standard C24-2), Swept Source Anterior Segment Optical Coherence Tomograthy (AS-OCT), EyeCam, and clinical history and examination |
| Vision-related quality of life | NEI-VFQ-25 |
| Health-related quality of life | Short Form 12-item Health Survey |
ETDRS = Early Treatment Diabetic Retinopathy Study; Hb = hemoglobin; LOCS II = Lens Opacities Classification System II; NEI-VFQ-25 = 25-item National Eye Institute Visual Functioning Questionnaire; SITA = Swedish interactive thresholding algorithm.
Footnotes
Financial Disclosure: The authors have no proprietary interests or conflicts of interest related to this submission.
References
- 1.Barnes JSBC. The Asian population: 2000, Census 2000 brief. U.S. Census Bureau, U.S. Department of Commerce, Economics and Statistic Administration; Washington, D.C: 2011. p. v. [Google Scholar]
- 2.Chen THC. Asian Americans. In: Breslow L, editor. Encyclopedia of Public Health. Vol. 1. New York: 2002. [Google Scholar]
- 3.Suinn RKG, Ahuna C. The Suinn-Lew Asian Self-Identity Acculturation Scale: Cross-Cultural Information. Journal of Multicultural Counseling and Development. 1995;23(3):139–48. [Google Scholar]
- 4.Suinn RKG, Ahuna C. The Suinn-Lew Asian Self-Identity Acculturation Scale: Concurrent and Factorial Validation. Educational and Psychological Measurement. 1992;52(4):1041–6. [Google Scholar]
- 5.Ferris FL, 3rd, Bailey I. Standardizing the measurement of visual acuity for clinical research studies: Guidelines from the Eye Care Technology Forum. Ophthalmology. 1996;103(1):181–2. doi: 10.1016/s0161-6420(96)30742-2. [DOI] [PubMed] [Google Scholar]
- 6.Elliott DB, Sanderson K, Conkey A. The reliability of the Pelli-Robson contrast sensitivity chart. Ophthalmic Physiol Opt. 1990;10(1):21–4. [PubMed] [Google Scholar]
- 7.Seddon JM, Sahagian CR, Glynn RJ, et al. Evaluation of an iris color classification system. The Eye Disorders Case-Control Study Group. Invest Ophthalmol Vis Sci. 1990;31(8):1592–8. [PubMed] [Google Scholar]
- 8.Johnson CA. Standardizing the measurement of visual fields for clinical research: Guidelines from the Eye Care Technology Forum. Ophthalmology. 1996;103(1):186–9. doi: 10.1016/s0161-6420(96)30740-9. [DOI] [PubMed] [Google Scholar]
- 9.Elsheikh A, Wang D, Kotecha A, et al. Evaluation of Goldmann applanation tonometry using a nonlinear finite element ocular model. Ann Biomed Eng. 2006;34(10):1628–40. doi: 10.1007/s10439-006-9191-8. [DOI] [PubMed] [Google Scholar]
- 10.Scheie HG. Width and pigmentation of the angle of the anterior chamber; a system of grading by gonioscopy. AMA Arch Ophthalmol. 1957;58(4):510–2. doi: 10.1001/archopht.1957.00940010526005. [DOI] [PubMed] [Google Scholar]
- 11.Foster PJ, Baasanhu J, Alsbirk PH, et al. Glaucoma in Mongolia. A population-based survey in Hovsgol province, northern Mongolia. Arch Ophthalmol. 1996;114(10):1235–41. doi: 10.1001/archopht.1996.01100140435011. [DOI] [PubMed] [Google Scholar]
- 12.Arkell SM, Lightman DA, Sommer A, et al. The prevalence of glaucoma among Eskimos of northwest Alaska. Arch Ophthalmol. 1987;105(4):482–5. doi: 10.1001/archopht.1987.01060040052031. [DOI] [PubMed] [Google Scholar]
- 13.Salmon JF, Mermoud A, Ivey A, et al. The prevalence of primary angle closure glaucoma and open angle glaucoma in Mamre, western Cape, South Africa. Arch Ophthalmol. 1993;111(9):1263–9. doi: 10.1001/archopht.1993.01090090115029. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Martin E, Pinilla I, Idoipe M, et al. Intra and interoperator reproducibility of retinal nerve fibre and macular thickness measurements using Cirrus Fourier-domain OCT. Acta Ophthalmol. 89(1):e23–9. doi: 10.1111/j.1755-3768.2010.02045.x. [DOI] [PubMed] [Google Scholar]
- 15.Keane PA, Mand PS, Liakopoulos S, et al. Accuracy of retinal thickness measurements obtained with Cirrus optical coherence tomography. Br J Ophthalmol. 2009;93(11):1461–7. doi: 10.1136/bjo.2008.155846. [DOI] [PubMed] [Google Scholar]
- 16.Group DRSR Report 7: a modification of the Airlie House classification of diabetic retinopathy. Invest Ophthalmol Vis Sci. 1981;21(1 Pt 2):210–26. [PubMed] [Google Scholar]
- 17.Chylack LT, Jr., Leske MC, McCarthy D, et al. Lens opacities classification system II (LOCS II). Arch Ophthalmol. 1989;107(7):991–7. doi: 10.1001/archopht.1989.01070020053028. [DOI] [PubMed] [Google Scholar]
- 18.Organization WH. Blindness and Visual Disability. Part IV of VII. 2011:v. [Google Scholar]
- 19.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leske MC, Connell AM, Wu SY, et al. Prevalence of lens opacities in the Barbados Eye Study. Arch Ophthalmol. 1997;115(1):105–11. doi: 10.1001/archopht.1997.01100150107018. [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 22.Kehoe R, Wu SY, Leske MC, Chylack LT., Jr. Comparing self-reported and physician-reported medical history. Am J Epidemiol. 1994;139(8):813–8. doi: 10.1093/oxfordjournals.aje.a117078. [DOI] [PubMed] [Google Scholar]
- 23.Ritter PL, Stewart AL, Kaymaz H, et al. Self-reports of health care utilization compared to provider records. J Clin Epidemiol. 2001;54(2):136–41. doi: 10.1016/s0895-4356(00)00261-4. [DOI] [PubMed] [Google Scholar]
- 24.Lee DJ, Markides KS, Ray LA. Epidemiology of self-reported past heavy drinking in Hispanic adults. Ethn Health. 1997;2(1-2):77–88. doi: 10.1080/13557858.1997.9961817. [DOI] [PubMed] [Google Scholar]
- 25.Seah SK, Wong TY, Foster PJ, et al. Prevalence of lens opacity in Chinese residents of Singapore: the tanjong pagar survey. Ophthalmology. 2002;109(11):2058–64. doi: 10.1016/s0161-6420(02)01221-6. [DOI] [PubMed] [Google Scholar]
- 26.Foster PJ, Oen FT, Machin D, et al. The prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol. 2000;118(8):1105–11. doi: 10.1001/archopht.118.8.1105. [DOI] [PubMed] [Google Scholar]
- 27.Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol. 2001;85(11):1277–82. doi: 10.1136/bjo.85.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113(3):373–80. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141(3):446–55. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baasanhu J, Johnson GJ, Burendei G, Minassian DC. Prevalence and causes of blindness and visual impairment in Mongolia: a survey of populations aged 40 years and older. Bull World Health Organ. 1994;72(5):771–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Saw SM, Foster PJ, Gazzard G, Seah S. Causes of blindness, low vision, and questionnaire-assessed poor visual function in Singaporean Chinese adults: The Tanjong Pagar Survey. Ophthalmology. 2004;111(6):1161–8. doi: 10.1016/j.ophtha.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 32.Wong TY, Foster PJ, Hee J, et al. Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest Ophthalmol Vis Sci. 2000;41(9):2486–94. [PubMed] [Google Scholar]
- 33.Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2002;109(4):704–11. doi: 10.1016/s0161-6420(01)01024-7. [DOI] [PubMed] [Google Scholar]