Abstract
Emerging evidence challenges the long-held paradigm that the healthy bladder is sterile. These discoveries may provide new opportunities to address important women’s health conditions, including pre-term labor and delivery, urinary tract infections and common forms of urinary incontinence. Traditional tools for urinary bacterial assessment, including urinary dipsticks and standard urine cultures, have significant limitations that restrict the information available to clinicians. For example, the standard urine culture does not detect slow growing bacteria that die in the presence of oxygen. Two new, complementary tools, however, can detect these and other organisms, permitting a more complete characterization of bacterial communities within the female bladder. Obstetrician-gynecologists should become familiar with these new approaches (expanded quantitative urine culture and 16S rRNA gene sequencing), which can detect previously unrecognized organisms. These advances are making it possible to answer previously intractable scientific and clinical questions.
Traditional nomenclature used to describe the bacterial status in the bladder is quite dated and unsuited for the emerging information about the bacterial milieu of the female urinary tract. In the context of the sterile bladder paradigm, clinicians have learned about “uropathogens,” “asymptomatic bacteriuria,” and “urinary tract infection.” Given that the lower urinary tract is not sterile, these terms should be re-evaluated.
Clinicians can already benefit from the emerging knowledge regarding urinary organisms that have previously gone undetected or unappreciated. For example, in some subpopulations of women with urinary symptoms, existing data suggests that the urinary bacterial community may be associated with women’s health conditions of interest.
This clinical opinion highlights the inadequacies of the current tools for urinary bacterial assessment, describes the new assessment tools, explains the current interpretation of the resulting data, and proposes potential clinical uses and relevance. A new world is opening to our view, giving us the opportunity to better understand urinary bacteria and the bladder in which they live. This new knowledge has significant potential to improve patient care in obstetrics and gynecology.
Key Words/Short Phrases: 16S rRNA sequencing, asymptomatic bacteriuria, microbiome, microbiota, urgency urinary incontinence, urine culture, urinary health, urinary tract infection
Condensation
This Opinion reviews the emerging evidence that challenge clinical ideas about the bacterial environment in the female bladder may have clinical relevance.
For decades, clinicians have used a small set of tools to assess the bacterial milieu within the bladder. These tools have included office-based urinary dipsticks, formal urinalyses, and standard urine cultures in order to rule out the presence of uropathogens responsible for conditions such as urinary tract infection and the less well-understood phenomenon of asymptomatic bacteriuria. New discoveries made using novel methods have highlighted the limitations of this traditional toolkit, unveiled problems with our nomenclature, and revealed flaws in our assumptions. In this clinical opinion, we will describe the limitations of current testing and the difficulties with current nomenclature. We will present some new techniques and their associated scientific nomenclature. As these new approaches may soon enter the clinical care algorithm, we will describe how the new data are derived and displayed. Finally, we will present some of the new findings. Our goal is to equip practicing clinicians with the concepts and vocabulary needed to assess the emerging research and clinical algorithms regarding characterization of bacteria in the female urinary tract.
Clinicians have a general awareness of microbial communities (microbiota) in diverse human anatomical sites, e.g. skin, mouth, bowel, and vagina. To characterize many of these bacterial communities, the National Institutes of Health initiated the Human Microbiome Project (HMP), which has spawned overwhelming evidence that these microbiota contribute to diverse human health and disease states1–3. The terms “microbiome” and “microbiota” are often used interchangeably. In this manuscript, “microbiota” will refer to the microorganisms that exist within a niche, while “microbiome” will refer to the collection of all their genomes.
The HMP and other studies of the human microbiome typically identify bacteria and eukaryotic microbes on the basis of their DNA. Pioneered by microbial ecologists who needed a way to identify organisms that could not be cultured in the laboratory, these culture-independent, DNA-based approaches are extremely powerful precisely because they do not require isolation of the bacterium. These DNA-based approaches generally take advantage of the 16S rRNA gene, which encodes an essential component of the ribosome. Because of this essentiality, most of the 16S rRNA gene is highly conserved. Between conserved regions, however, some stretches of DNA can evolve. The sequence differences in these hypervariable regions serve as a measure of evolutionary distance and thus can be used to determine phylogenetic relatedness. All nine known hypervariable regions (V1–V9) of the 16S rRNA gene contain differences (called polymorphisms) that can be used to distinguish even closely related bacteria. By comparing polymorphisms, researchers can assign a DNA sequence to the bacterium from which it originated4,5.
Many human niches contain massive numbers of bacteria. For example, the human colon contains 1011 colony forming units per gram of feces6,7. To sequence large numbers of genomes in large numbers of samples simultaneously, researchers use multiple massive parallel DNA-sequencing technologies8. Also known as Next Generation sequencing (NGS), these revolutionary technologies permit extremely high throughput at low cost per base, generating millions of sequence reads per sample for multiple samples in a single sequencing run. Sequencing technologies and techniques are advancing rapidly with increased speed, availability and reliability at decreased cost. These advances are making it possible to answer previously intractable scientific and clinical questions. Further advances are predicted to quickly move these tools into the clinic9.
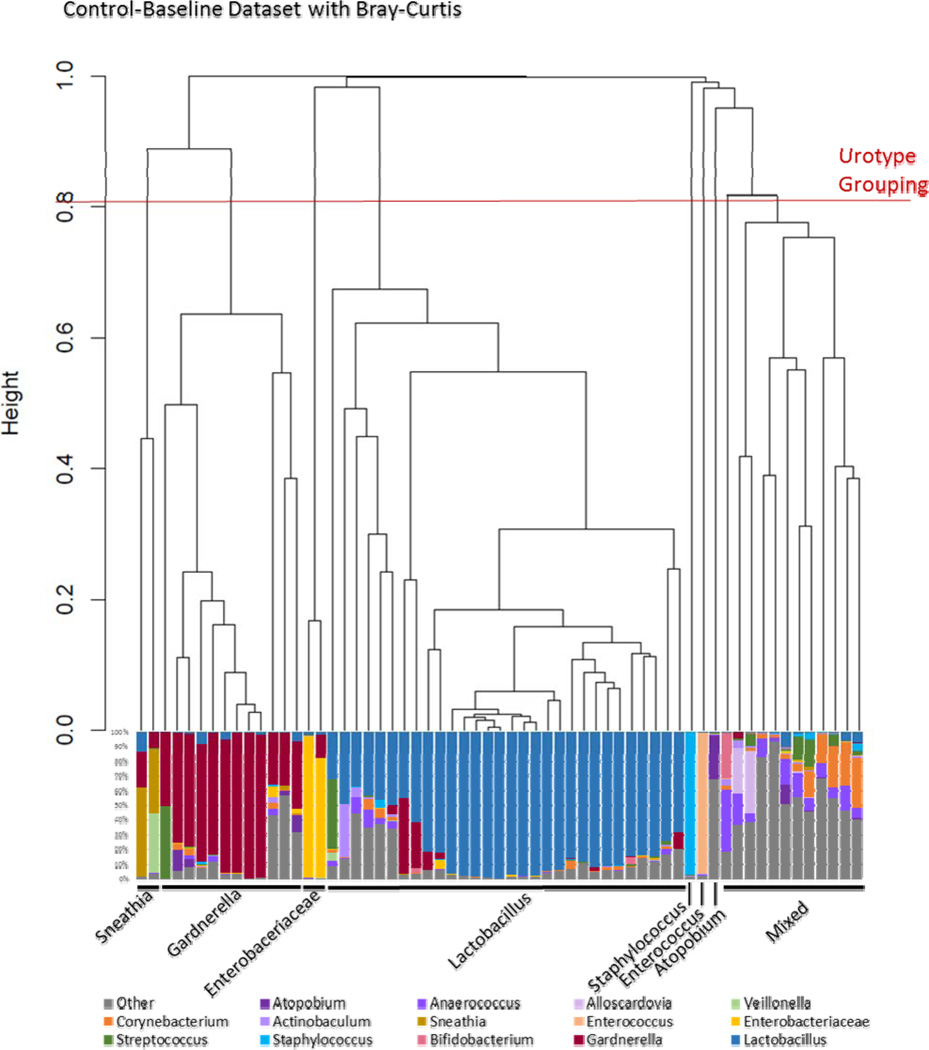
Most clinicians are unfamiliar with the attainment and presentation of DNA sequencing data. The process begins the moment the clinical sample is acquired. To halt bacterial growth and maintain DNA integrity, a preservative is added. Next, the bacteria in the sample are broken open and the DNA extracted. Using the polymerase chain reaction (PCR) and universal primers, a hypervariable region of the 16S rRNA gene is amplified. To the resultant amplicons, adaptor sequences are added. These sequences do two things. First, they contain short stretches of DNA that adapt the amplicons to the sequencing technology of choice. Second, they contain distinct DNA barcodes that permit multiplexing, i.e., simultaneous sequencing of amplicons from multiple samples (as many as 384). This library of DNA fragments, which represents the diversity of bacteria present in the original samples, is now sequenced. The output is thousands of “sequence reads,” a digitized series of As, Gs, Cs, and Ts, that must be processed. First, the adaptor sequences are removed. Second, sequences are de-multiplexed, sorted by their barcodes into computerized bins, where each bin represents the original sample from which the read originated. The barcodes are now removed and the bioinformatic analysis can begin. The intent is to assign each processed read to a unique bacterium, comparing the sequence of that read to the 16S rRNA sequences of all known bacteria. The resultant data is often displayed as a histogram, wherein each sample is represented as a bar and each bacterium by a color (Figure 1, bottom). Samples can be sorted on the basis of their bacterial composition; the resultant relationships are often represented by a dendrogram (Figure 1, top). Sophisticated bioinformatics and biostatistics approaches are then used to determine associations with demographics, symptoms and outcomes. This technology will be used in many areas of clinical medicine over the coming decade; sequencing is already being incorporated into urinary research.
Figure 1.
Relatedness of each microbiome profile as measured by Bray-Curtis method shown in a dendrogram (top), and by relative abundance of classified sequences as shown in the histogram (bottom). In a dendrogram, the length of each branch represents the relatedness between groups. The more related, the shorter the branch. We can group each branch to determine clusters. The histogram displays the bacterial taxa detected in each sample, as a percent of the total sequence reads classified. Each color represents a different family or genus. By comparing the dendrogram clustering to the classification, we can define urotypes, which are named on the basis of the dominant (most common) classified organism in each sample.
The bladder was not included in the initial HMP studies, presumably because it was considered to be sterile. Existing etiologic explanations and/or clinical treatments for many common lower urinary tract disorders are limited by this long-held belief that the healthy female urinary system contains no bacteria. However, the DNA-based evidence supporting the importance of microbiota in other anatomical sites made it increasingly implausible to think that the female bladder would be entirely free of bacteria, especially given the bladder’s anatomic location and its “life events,” which include proximity to reproductive, sexual and defecation functions. Once sequencing was incorporated into urinary research, multiple investigators quickly confirmed that there is a resident bacterial community in the urine (presumably from the bladder) of many adult women10–18. Clearly, the lower urinary tract is not sterile.
This important finding caused us to re-evaluate the reliability of the decades-old approach to the standard clinical urine culture. Most clinicians accept the standard clinical urine culture as the gold standard for bacterial testing in the urine. The current broad interpretation of the standard urine culture goes well beyond the urine culture’s very limited initial role. In the 1950s, Edward Kass, an infectious disease physician, developed the standard urine culture, using midstream urine and a cut-off of ≥105 CFU/mL to identify and prevent post-operative sepsis in patients undergoing kidney surgery19,20. Since Kass’ original work, other investigators have attempted to refine the urine culture11,21–25. Most notably, Stamm and co-workers demonstrated that 102 of a known uropathogen in the midstream urine of women was indicative of lower urinary tract infection21. In hindsight, the evolving threshold for a “positive” urine culture may have been an indication that there was more complexity to the urinary bacterial milieu in the bladder. The work of Rosalind Maskell (1981–1988) is unknown to many; yet, clearly ahead of her time, she performed scientifically rigorous studies that provided compelling evidence to disprove the dogma that urine was sterile in the absence of a clinically relevant infection25. More recently, Hooten and colleagues contributed evidence that the bladder may also include many Gram-positive bacteria, including lactobacilli, staphylococci, streptococci and Gardnerella vaginalis. They considered the possibility that the bladder may have a resident bacteria flora and suggested re-evaluation of the use of midstream urine cultures for diagnosis of lower urinary tract symptoms24. Given that the clinicians have relied on the standard urine culture test for decades, it is telling that the debate about relevant thresholds and specific organisms persists. What accounts for our inability to find consensus?
In the busy clinician’s life, a urine sample is collected, sent to a laboratory and a report is returned. What goes on behind the scenes to produce this report? While there are differences across laboratories, the typical standard urine culture protocol plates 1 microliter of urine on blood and MacConkey agar and then incubates the sample at 35°C in air for 24 hours. This protocol was designed to quickly detect a select group of known uropathogens, most notably uropathogenic Escherichia coli (UPEC), which causes the majority of urinary tract infections (UTI). This protocol assumes that we know which organisms are important to detect. While we clearly know some clinically important organisms, such as UPEC, the standard urine culture protocol was not designed to detect bacteria that require special nutrients, grow slowly, cannot tolerant oxygen, or are present in small numbers (<103 colony forming units per milliliter). Some of these organisms may be involved in urinary disorders. Moreover, the assumption that urine is sterile has led clinical microbiologists to set aside bacterial colonies that resemble those known to be part of the vaginal microbiota, because of a presumption that lactobacillus and related organisms do not live in the bladder. These limitations of standard testing, designed to detect a pre-determined list of organisms, block the ability to detect new or previously unappreciated uropathogens. Thus, clinicians get only a limited glimpse of what is in their patient’s urine specimen.
Clinicians have learned about “uropathogens” as if we have a clearly defined, complete list of such organisms. In fact, there is an emerging group of organisms that have previously gone undetected or unappreciated. These organisms often require special culture techniques, such as anaerobic conditions. Ongoing research may reveal organisms that have important interactions with known uropathogens (e.g., UPEC) or may independently cause human symptoms and/or disease. Investigations into interactions with well-studied uropathogens and their virulence factors are beyond the scope of this manuscript.
Compared to the standard urine culture, sequencing approaches provide much more information about the organisms present in the urinary microbiota. In women with and without urinary symptoms, our research group has compared the urinary microbiota using standard urine culture and sequencing approaches and found that sequencing detects many more organisms than does the standard urine culture14–16. A minority of urine samples are “sequence negative” for bacteria, although it is our belief that these should be considered sub-threshold for existing technology, rather than lacking bacteria altogether. Future studies will clarify this distinction. Nonetheless, DNA sequencing is clearly more sensitive than a standard urine culture; indeed, it may even be too sensitive for current clinical use26.
Beyond sensitivity concerns, sequencing is not ready for front line clinical care for urinary testing. In response to this clinical need, our research group developed the expanded quantitative urine culture (EQUC), which goes beyond the duration and conditions of the standard urine culture14. Using EQUC, we have shown that the standard urine culture has an astounding high false negative rate, up to 90%, depending on the clinical population of women without overt clinical UTI14,15. Khasriya and colleagues performed a similar study and came to similar conclusions11. These studies document the inadequacy of current clinical culture-based tests. To address the clinical needs, our team is working to develop a streamlined version of this technique that could be performed in most, if not all, clinical laboratory settings. However, we stress that the simple presence of bacteria in the urine should not be equated with infection nor should it immediately prompt the use of systemic antibiotics. It is quite possible that the detected bacteria contribute to urinary tract health.
This is a rapidly evolving scientific landscape, with an increasing amount of new evidence emerging as investigators bring their expertise to questions that will better inform clinical care. The clinical community should be aware of these two new tools, DNA sequencing and EQUC, for urinary assessment and be open to the possibilities that previously undetected organisms may play a role in certain women’s health conditions. Detailed descriptions of these laboratory techniques are available14,15.
What progress have we made with these new tools? Using these two complementary tools, our group and others have provided compelling evidence that most adult women have a resident urinary microbiome, regardless of current lower urinary tract symptoms10–18. We have found that these resident urinary bacteria are clearly distinct from bacteria that cause overt clinical UTI14–16,18.
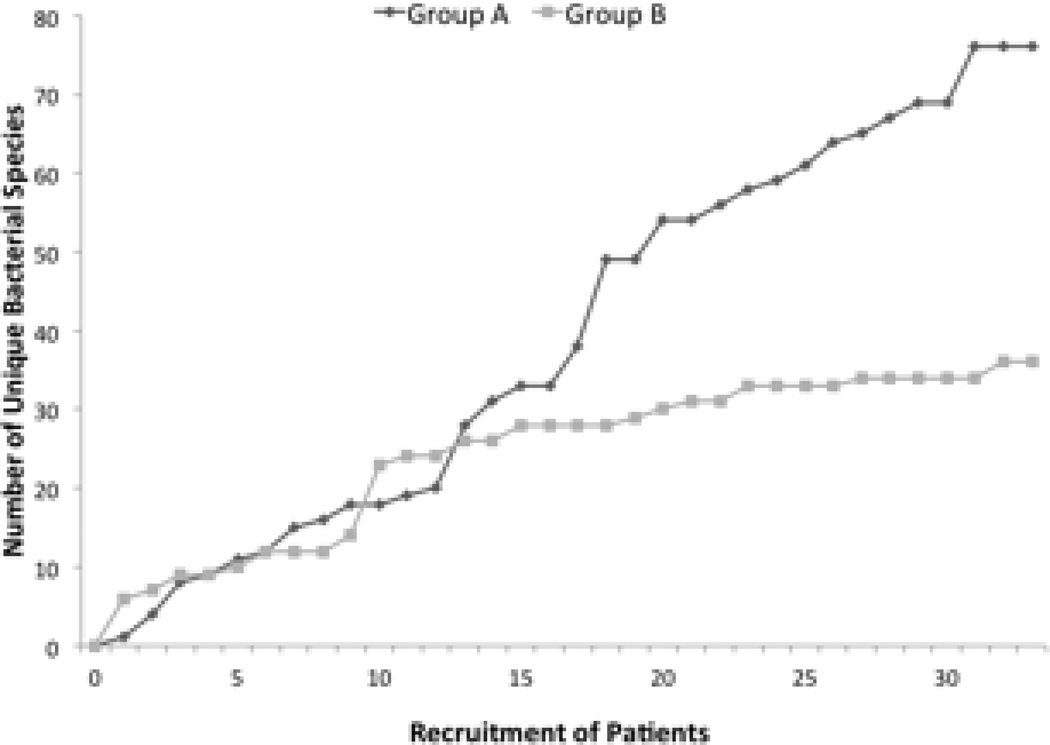
In some subpopulations of women with urinary symptoms, we have emerging evidence that the urinary bacterial community may be associated with a certain health status. For example, in women affected by urinary urgency incontinence (UUI), there is some evidence that these communities are associated with pre-treatment UUI symptoms and, perhaps, protection against UTI17. Using EQUC, we showed that the urinary microbiome of women with urgency urinary incontinence (UUI) tends to be more diverse than women without UUI15. Figure 2 presents these data as rarefaction curves, which plot the accumulation of unique species as participants are recruited and analyzed. Rarefaction curves are typically used to determine when a population has been fully sampled and further recruitment is not expected to identify new species. Two curves that plateau at different numbers of samples and/or at different numbers of unique species are evidence that the sampled populations are distinct. This difference was due mostly to a few species strongly associated with the UUI cohort, including Actinobaculum schaalii, Aerococcus urinae, 2 Corynebacterium species, Lactobacillus gasseri, Gardnerella vaginalis and Streptococcus anginosus, In contrast, Lactobacillus crispatus was associated with controls15. These are exciting findings that open previously unappreciated opportunities for scientific inquiry concerning prevention, etiology and treatment of urinary incontinence in women. While the current investigations have focused on bacteria, other organisms could be present, such as viruses or fungi.
Figure 2.
Rarefaction curves of cultured bacterial species by cohort: Women with Urgency Urinary Incontinence versus Women without Urgency Urinary Incontinence. The plot depicts the number of unique species cultured via EQUC by the number of urines assayed.
The nomenclature used to describe the bacterial status in the bladder is quite dated and unsuited for the emerging information about the female urinary tract. For example, it is likely that the dichotomous diagnosis of urinary tract infection will be insufficient for clinical purposes. In other parts of the human body, it is recognized that resident bacterial communities exist without “infection”; and that infection can occur. In the urinary tract, the threshold for infection, formerly based on the standard urine culture, will need to be reconsidered. For example, “asymptomatic bacteriuria” is a term used when the standard urine culture detects a uropathogen above the 105 CFU/ml threshold bacteria in an individual with no lower urinary tract symptoms. Obstetricians can appreciate the importance of detecting a traditional group of uropathogens, because of the association with an increased risk of preterm labor and delivery. Thus, the concept of “asymptomatic bacteriuria” is incorporated into routine urinary screening, most often with clinical dipsticks and perhaps standard urine cultures. Yet, preterm labor and delivery have proven refractory to many treatment methods. Given the current standard clinical screening protocols focused on UTI assessment during pregnancy, there is an opportunity to apply these new techniques to detect previously unappreciated pathogens or urinary microbiota characteristics of clinical importance.
“Asymptomatic bacteriuria” is an especially challenging concept in the field of Female Pelvic Medicine and Reconstructive Surgery. Using 16S rRNA sequencing and EQUC, we have learned that most women with urgency urinary incontinence have a urinary microbiota and these women are symptomatic – they typically have symptoms of urinary urgency, frequency and incontinence. Yet, their standard urine culture is typically negative. Are these women “infected” or are these traditional clinical terms inadequate to describe the clinical states that we are now able to detect?
Simply put, the term asymptomatic bacteriuria will become less and less useful over time; science will be able to better inform clinicians about the specific health condition of concern, rather than our current nomenclature aggregation of what may well be normal with abnormalities of concern. A useful concept for consideration is “dysbiosis,” essentially an unhealthy perturbation in the normal bacterial community of a particular niche, e.g. the bladder. As we expand our understanding of the female urinary microbiome, we will be able to describe the normal range of urinary microbiome states amongst groups of women (for example by age, hormonal status, race and ethnicity) and the clinically relevant variations over time within an individual woman.
We have entered a new era in our understanding of the urinary bacterial community in women. There is much to learn! For years, we have cared for our patients without this level of knowledge – we have an important opportunity to improve patient care in obstetrics and gynecology. Perhaps we have an opportunity to prevent certain conditions as well.
Acknowledgments
We wish to thank the present and former members of the Loyola Urinary Research and Education Collaboration. We would like to acknowledge the Loyola University Chicago Health Sciences Division’s Office of Informatics and Systems Development for their expertise and for the computational resources utilized in support of this research. Loyola University Chicago Stritch School of Medicine’s research computing facility was developed through grant funds awarded by the Department of Health and Human Services (1G20RR030939-01). Our research has been supported by the Falk Foundation (LU#202567), by the NIH (R21DK097435-01A1, U10-HD054136), and by Astellas Medical and Scientific Affairs (Wolfe PI, VESI-12D01).
Footnotes
Disclosures: Dr. Wolfe - Investigator Initiated Grant from Astellas Pharmaceutical.
Dr. Brubaker has nothing to disclose.
References
- 1.A framework for human microbiome research. Nature. 2012;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aagaard K, Petrosino J, Keitel W, et al. The Human Microbiome Project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB J. 2013;27:1012–1022. doi: 10.1096/fj.12-220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank DN, Zhu W, Sartor RB, Li E. Investigating the biological and clinical significance of human dysbioses. Trends Microbiol. 2011;19:427–434. doi: 10.1016/j.tim.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo PC, Lau SK, Teng JL, Tse H, Yuen KY. Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin Microbiol Infect. 2008;14:908–934. doi: 10.1111/j.1469-0691.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- 5.Chun J, Rainey FA. Integrating genomics into the taxonomy and systematics of the Bacteria and Archaea. Int J Syst Evol Microbiol. 2014;64:316–324. doi: 10.1099/ijs.0.054171-0. [DOI] [PubMed] [Google Scholar]
- 6.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 7.Tlaskalova-Hogenova H, Stepankova R, Kozakova H, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110–120. doi: 10.1038/cmi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- 9.Park JY, Kricka LJ, Fortina P. Next-generation sequencing in the clinic. Nat Biotechnol. 2013;31:990–992. doi: 10.1038/nbt.2743. [DOI] [PubMed] [Google Scholar]
- 10.Fouts DE, Pieper R, Szpakowski S Pohl, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med. 2012;10:174. doi: 10.1186/1479-5876-10-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khasriya R, Sathiananthamoorthy S, Ismail S, et al. Spectrum of bacterial colonization associated with urothelial cells from patients with chronic lower urinary tract symptoms. J Clin Microbiol. 2013;51:2054–2062. doi: 10.1128/JCM.03314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui H, Nederbragt AJ, Lagesen K, Jeansson SL, Jakobsen KS. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 2011;11:244. doi: 10.1186/1471-2180-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis DA, Brown R, Williams J, et al. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front Cell Infect Microbiol. 2013;3:41. doi: 10.3389/fcimb.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52:871–876. doi: 10.1128/JCM.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio. 2014;5:e01283–e01214. doi: 10.1128/mBio.01283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe AJ, Toh E, Shibata N, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50:1376–1383. doi: 10.1128/JCM.05852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brubaker L, Nager CW, Richter HE, et al. Urinary bacteria in adult women with urgency urinary incontinence. Int Urogynecol J. 2014;25:1179–1184. doi: 10.1007/s00192-013-2325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nienhouse V, Gao X, Dong Q, et al. Interplay between Bladder Microbiota and Urinary Antimicrobial Peptides: Mechanisms for Human Urinary Tract Infection Risk and Symptom Severity. PLoS One. 2014;9:e114185. doi: 10.1371/journal.pone.0114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kass EH. Asymptomatic infections of the urinary tract. Trans Assoc Am Physicians. 1956;69:56–64. [PubMed] [Google Scholar]
- 20.Kass EH. Pyelonephritis and bacteriuria. A major problem in preventive medicine. Ann Intern Med. 1962;56:46–53. doi: 10.7326/0003-4819-56-1-46. [DOI] [PubMed] [Google Scholar]
- 21.Stamm W, Counts G, Running K, Fihn S, Turck M, Holmes K. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307:463–468. doi: 10.1056/NEJM198208193070802. [DOI] [PubMed] [Google Scholar]
- 22.Stark RP, Maki DG. Bacteriuria in the Catheterized Patient. N Engl J Med. 1984;311:560–564. doi: 10.1056/NEJM198408303110903. [DOI] [PubMed] [Google Scholar]
- 23.Lipsky BA, Ireton RC, Fihn SD, Hackett R, Berger RE. Diagnosis of bacteriuria in men: specimen collection and culture interpretation. J Infect Dis. 1987;155:847–854. doi: 10.1093/infdis/155.5.847. [DOI] [PubMed] [Google Scholar]
- 24.Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369:1883–1891. doi: 10.1056/NEJMoa1302186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maskell RM. The natural history of urinary tract infection in women. Med Hypotheses. 2010;74:802–806. doi: 10.1016/j.mehy.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Kirkup BC. Culture-independence for surveillance and epidemiology. Pathog. 2013;2:556–570. doi: 10.3390/pathogens2030556. [DOI] [PMC free article] [PubMed] [Google Scholar]