1. INTRODUCTION
Osteoporosis is a growing major public health problem with impact that crosses medical, social, and economic lines. These guidelines have been developed by the American Association of Clinical Endocrinologists (AACE) with hopes of reducing the risk of osteoporosis-related fractures and thereby improving the quality of life for people with osteoporosis. The guidelines use the best evidence, taking into consideration the economic impact of the disease and the need for efficient and effective evaluation and treatment of postmenopausal women with osteoporosis. The intent is to provide evidence-based information about the diagnosis, evaluation, and treatment of postmenopausal osteoporosis for endocrinologists, physicians in general, regulatory bodies, health-related organizations, and interested laypersons.
2. METHODS FOR DEVELOPMENT OF AACE CLINICAL PRACTICE GUIDELINES FOR POSTMENOPAUSAL OSTEOPOROSIS
Evidence was obtained through MEDLINE searches and other designated reference sources. Expert opinion was used to evaluate the available literature and to grade references relative to evidence level (EL) (Table 1), based on the ratings of 1 through 4 from the 2010 AACE protocol for standardized production of clinical practice guidelines (1). In addition, recommendations were graded A through D, in accordance with methods established by AACE in 2004 (Table 2) (2). Information pertaining to cost-effectiveness was included when available.
Table 1.
2010 American Association of Clinical Endocrinologists Criteria for Rating of Published Evidencea
| Numerical descriptor (evidence level) |
Semantic descriptor (reference methods) |
|---|---|
| 1 | Meta-analysis of randomized controlled trials |
| 1 | Randomized controlled trial |
| 2 | Meta-analysis of nonrandomized prospective or case- controlled trials |
| 2 | Nonrandomized controlled trial |
| 2 | Prospective cohort study |
| 2 | Retrospective case-control study |
| 3 | Cross-sectional study |
| 3 | Surveillance study (registries, surveys, epidemiologic study) |
| 3 | Consecutive case series |
| 3 | Single case reports |
| 4 | No evidence (theory, opinion, consensus, or review) |
1 = strong evidence; 2 = intermediate evidence; 3 = weak evidence; 4 = no evidence. From Mechanick et al (1).
Table 2.
American Association of Clinical Endocrinologists Criteria for Grading of Recommendations
| Recommendation grade |
Description |
|---|---|
| A | Homogeneous evidence from multiple well-designed randomized controlled trials with sufficient statistical power Homogeneous evidence from multiple well-designed cohort controlled trials with sufficient statistical power ≥1 conclusive level 1 publications demonstrating benefit >> risk |
| B | Evidence from at least 1 large well-designed clinical trial, cohort or case-Controlled analytic study, or meta-analysis No conclusive level 1 publication; ≥1 conclusive level 2 publications emonstrating benefit >> risk |
| C | Evidence based on clinical experience, descriptive studies, or expert consensus opinion No conclusive level 1 or 2 publications; ≥1 conclusive level 3 publications demonstrating benefit >> risk No conclusive risk at all and no conclusive benefit demonstrated by evidence |
| D | Not rated No conclusive level 1, 2, or 3 publication demonstrating benefit >> risk Conclusive level 1, 2, or 3 publication demonstrating risk >> benefit |
From Mechanick et al (2).
3. EXECUTIVE SUMMARY OF RECOMMENDATIONS
Each recommendation is labeled “R” in this summary.
3.1. What Measures Can Be Taken to Prevent Bone Loss?
R1. Maintain adequate calcium intake; use calcium supplements, if needed, to meet minimal required intake (Grade A; “best evidence” level or BEL 1).
R2. Maintain adequate vitamin D intake; supplement vitamin D, if needed, to maintain serum levels of 25-hydroxyvitamin D [25(OH)D] between 30 and 60 ng/mL (Grade A; BEL 1).
R3. Limit alcohol intake to no more than 2 servings per day (Grade B; BEL 2).
R4. Limit caffeine intake (Grade C; BEL 3).
R5. Avoid or stop smoking (Grade B; BEL 2).
R6. Maintain an active lifestyle, including weight-bearing exercises for at least 30 minutes daily (Grade B; BEL 2).
3.2. What Nonpharmacologic Measures Can Be Recommended for Treatment of Osteoporosis?
All the foregoing measures plus the following:
R7. Maintain adequate protein intake (Grade B; BEL 3).
R8. Use proper body mechanics (Grade B; BEL 1).
R9. Consider the use of hip protectors in individuals with a high risk of falling (Grade B; BEL 1).
R10. Take measures to reduce the risk of falling (Grade B; BEL 2).
R11. Consider referral for physical therapy and occupational therapy (Grade B; BEL 1).
3.3. Who Needs to Be Screened for Osteoporosis?
R12. Women 65 years old or older (Grade B; BEL 2).
R13. Younger postmenopausal women at increased risk of fracture, based on a list of risk factors (see section 4.5) (Grade C; BEL 2).
3.4. How Is Osteoporosis Diagnosed?
R14. Use a central dual-energy x-ray absorptiometry (DXA) measurement (Grade B; BEL 3).
R15. In the absence of fracture, osteoporosis is defined as a T-score of −2.5 or below in the spine (anteroposterior), femoral neck, or total hip (Grade B; BEL 2).
R16. Osteoporosis is defined as the presence of a fracture of the hip or spine (see section 4.4.2) (in the absence of other bone conditions) (Grade B; BEL 3).
3.5. How Is Osteoporosis Evaluated?
R17. Evaluate for secondary osteoporosis (Grade B; BEL 2).
R18. Evaluate for prevalent vertebral fractures (see section 4.7.1) (Grade B; BEL 2).
3.6. Who Needs Pharmacologic Therapy?
R19. Those patients with a history of a fracture of the hip or spine (Grade A; BEL 1).
R20. Patients without a history of fractures but with a T-score of −2.5 or lower (Grade A; BEL 1).
R21. Patients with a T-score between −1.0 and −2.5 if FRAX (see section 4.5) major osteoporotic fracture probability is ≥20% or hip fracture probability is ≥3% (Grade A; BEL 2).
3.7. What Drugs Can Be Used to Treat Osteoporosis?
Use drugs with proven antifracture efficacy:
R22. Use alendronate, risedronate, zoledronic acid, and denosumab as the first line of therapy (Grade A; BEL 1).
R23. Use ibandronate as a second-line agent (Grade A; BEL 1).
R24. Use raloxifene as a second- or third-line agent (Grade A; BEL 1).
R25. Use calcitonin as the last line of therapy (Grade C; BEL 2).
R26. Use teriparatide for patients with very high fracture risk or patients in whom bisphosphonate therapy has failed (Grade A; BEL 1).
R27. Advise against the use of combination therapy (Grade B; BEL 2).
3.8. How Is Treatment Monitored?
R28. Obtain a baseline DXA, and repeat DXA every 1 to 2 years until findings are stable. Continue with follow-up DXA every 2 years or at a less frequent interval (Grade B; BEL 2).
R29. Monitor changes in spine or total hip bone mineral density (BMD) (Grade C; BEL 2).
R30. Follow-up of patients should be in the same facility, with the same machine, and, if possible, with the same technologist (Grade B; BEL 2).
R31. Bone turnover markers may be used at baseline to identify patients with high bone turnover and can be used to follow the response to therapy (Grade C; BEL 2).
3.9. What Is Successful Treatment of Osteoporosis?
R32. BMD is stable or increasing, and no fractures are present (Grade B; BEL 2).
R33. For patients taking antiresorptive agents, bone turnover markers at or below the median value for premenopausal women are achieved (see section 4.9) (Grade B; BEL 2).
R34. One fracture is not necessarily evidence of failure. Consider alternative therapy or reassessment for secondary causes of bone loss for patients who have recurrent fractures while receiving therapy (Grade B; BEL 2).
3.10. How Long Should Patients Be Treated?
R35. For treatment with bisphosphonates, if osteoporosis is mild, consider a “drug holiday” after 4 to 5 years of stability. If fracture risk is high, consider a drug holiday of 1 to 2 years after 10 years of treatment (Grade B; BEL 1).
R36. Follow BMD and bone turnover markers during a drug holiday period, and reinitiate therapy if bone density declines substantially, bone turnover markers increase, or a fracture occurs (Grade C; BEL 3).
3.11. When Should Patients Be Referred to Clinical Endocrinologists?
R37. When a patient with normal BMD sustains a fracture without major trauma (Grade C; BEL 4).
R38. When recurrent fractures or continued bone loss occurs in a patient receiving therapy without obvious treatable causes of bone loss (Grade C; BEL 4).
R39. When osteoporosis is unexpectedly severe or has unusual features (Grade C; BEL 4).
R40. When a patient has a condition that complicates management (for example, renal failure, hyperparathyroidism, or malabsorption) (Grade C; BEL 4).
4. EVIDENCE-BASED DISCUSSION OF RECOMMENDATIONS
4.1. Definition of Postmenopausal Osteoporosis
Postmenopausal osteoporosis is defined as “a (silent) skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture. Bone strength reflects the integration of 2 main features: bone density and bone quality” (3). Although the idea of “bone quality” is conceptually useful (4 [EL 4], 5 [EL 4]), except for bone turnover markers, methods are not currently available for the clinical assessment of other properties of bone that determine bone strength. Thus, for now and the near future, measurement of bone density remains the primary technique for the prefracture diagnosis of osteoporosis and for monitoring treatment.
In 1994, a Working Group of the World Health Organization (WHO) established an operational definition of postmenopausal osteoporosis based on BMD expressed as a T-score (Table 3) (4 [EL 4]). The T-score compares an individual’s BMD with the mean value for young normal persons and expresses the difference as a standard deviation (SD) score.
Table 3.
World Health Organization Criteria for Classification of Osteopenia and Osteoporosis
| Category | T-score |
|---|---|
| Normal | −1.0 or above |
| Low bone mass (osteopenia)a | Between −1.0 and −2.5 |
| Osteoporosis | −2.5 or below |
Fracture rates within this category vary widely. The category of “osteopenia” is useful for epidemiology studies and clinical research but is problematic when applied to individual patients and must be combined with clinical information to make treatment decisions.
Although the WHO criteria were not intended to serve as references for treatment decisions, they are often used for this purpose. The WHO criteria are also useful for making public health and health policy decisions. In addition, the WHO criteria are commonly accepted as standards for research purposes in terms of criteria for inclusion in clinical trials.
4.2. Background of Postmenopausal Osteoporosis
Osteoporosis is a well-defined and growing public health problem. More than 10 million Americans have osteoporosis, and more than 34 million others have low bone mass (6 [EL 4]) and are therefore at increased risk for developing osteoporosis and for fracturing. About 80% of these subjects are women, most of them postmenopausal. At age 50 years, the lifetime risk of developing fractures is about 39% for white women and 13% for white men (7 [EL 3]). Although white women are most often affected, women of all races and all ethnic origins are at risk for osteoporosis and fracture. Although osteoporosis can also develop in men and younger women, these guidelines are limited to postmenopausal women.
By age 60 years, half of the white women in the United States have osteopenia (low bone mass) or osteoporosis (8 [EL 3]). Low BMD at the femoral neck (T-score of −2.5 or below) is found in 21% of postmenopausal white women, 16% of postmenopausal Mexican American women, and 10% of postmenopausal African American women (8 [EL 3]). The mean femoral neck T-score for 75-year-old women is −2.5 (9 [EL 3]). More than 20% of postmenopausal women have prevalent vertebral fractures (10 [EL 3]).
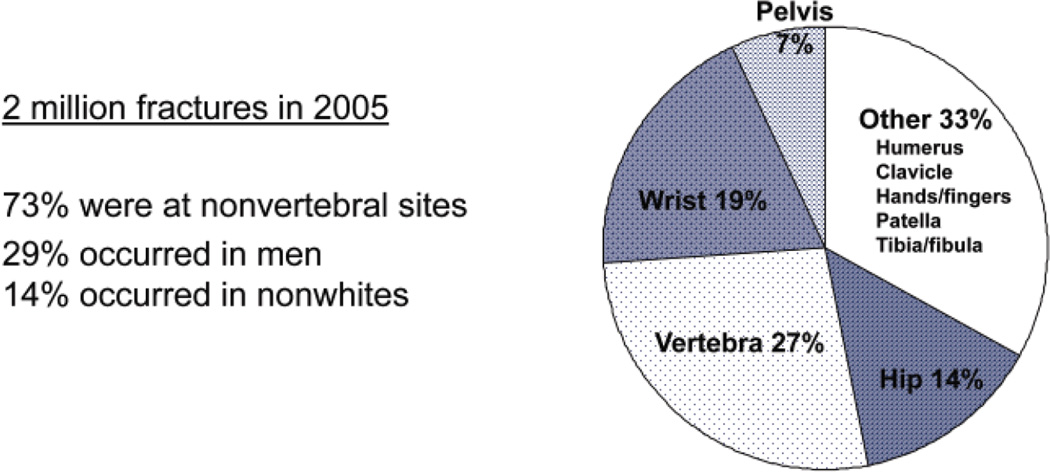
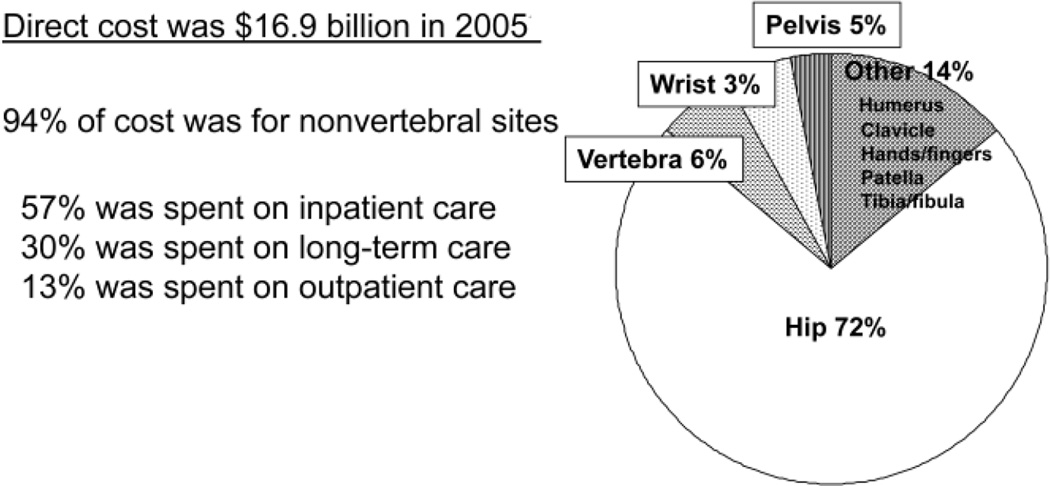
In 2005, 2 million fractures were attributed to osteoporosis (Fig. 1) (11 [EL 3]). Of these, 71% occurred in women. The direct cost was approximately $17 billion, 94% of which was attributable to fractures at nonvertebral sites (Fig. 2); 57% was spent on inpatient care, 30% was spent on long-term care, and 13% was spent on outpatient care (11 [EL 3]). This figure does not include lost productivity, unpaid caregiver time, transportation, and social services. Many more women have osteoporotic fractures (1.4 million) (11 [EL 3]) than new strokes (373,000) (12 [EL 3]), heart attacks (345,000) (12 [EL 3]), or invasive breast cancer (213,000) (13 [EL 3]) combined, according to recent statistics (2004 to 2006) (Fig. 3).
Fig. 1.
Fractures attributable to osteoporosis in the United States in 2005. Distribution by skeletal site is shown. Adapted from Burge et al (11).
Fig. 2.
Cost of osteoporosis-related fractures in the United States in 2005. The primary site of involvement was the hip, and the preponderance of cost was for inpatient care. Adapted from Burge et al (11).
Fig. 3.
Comparative incidences of osteoporosis-related fractures, new strokes, heart attacks, and invasive breast cancer in women in the United States, based on recent statistics (2004 to 2006). Data from Burge et al (11), Rosamond et al (American Heart Association Statistics Committee and Stroke Statistics Subcommittee) (12), and American Cancer Society (13).
Among all osteoporotic fractures, hip fractures are the most serious. The mortality during the first year after hip fracture is more than 30% for men and about 17% for women (14 [EL 2]). More than half of hip fracture survivors will require skilled care away from their homes, and many will have some degree of permanent disability (15 [EL 2]). Vertebral and forearm fractures are also associated with a major socioeconomic impact. Vertebral fractures cause about 70,000 hospital admissions annually (16 [EL 3]) and generate more than 66,000 office visits (17 [EL 3]). Chronic pain and deformity are common, and surgical intervention is sometimes required (7 [EL 3]). Fractures of the forearm generate about 530,000 office visits annually (17 [EL 3]) and also often result in substantial disability (18 [EL 3]).
By the year 2050, the number of people beyond age 65 years in the United States will increase from 32 million to 69 million, and more than 15 million people will exceed 85 years of age (11 [EL 3]). The incidence of hip and spine fractures increases with advancing age. From 2005 to 2025, investigators estimate that the number of osteoporosis-related fractures will increase from 2 million to 3 million, and the associated cost will increase from $17 billion to $25 billion (11 [EL 3]).
Early efforts to address osteoporosis and resulting fractures focused primarily on diagnosis, evaluation, and treatment, strategies that resulted in several major accomplishments. Important risk factors and secondary causes of osteoporosis were identified, and diagnosis and case finding were improved when Medicare abandoned its prohibition against osteoporosis screening in the late 1990s. Bisphosphonate therapy was shown to reduce fracture incidence and to have a salutary effect on bone loss. More recently, the availability of an anabolic treatment (teriparatide) has added to the therapeutic armamentarium.
Despite progress on several fronts, there is still room for improvement. Age-adjusted rates for hip fracture declined between 1980 and 2006, by 1.4% per year in women and 0.06% per year in men (19 [EL 3]). This decline seems even more significant in that the rate of fatal falls among elderly white women during the same period increased from 20.3 to 32.8 per 100,000 population—an increase of 61.6% (20 [EL 3]).
The reasons for these changes are not clear, but authors have speculated that the increase in falls was due to an increase in life expectancy (from 75.5 years in 1993 to 77.6 years in 2003) (20 [EL 3]) and therefore an increase in the susceptible population. They have further suggested that the dramatic decline in hip fracture admissions among white women is related, at least in part, to effective screening and therapy (20 [EL 3]). Similar decreases in fracture risk have been reported in Canada (21 [EL 3]), Finland (22 [EL 3]), Sweden, Australia, and Switzerland (23 [EL 3], 24 [EL 2], 25 [EL 3]).
Despite these advances, less than a third of the cases of osteoporosis have been diagnosed (26 [EL 2]), and only a seventh of the American women with osteoporosis receive treatment (27 [EL 3]).
4.3. Pathogenesis and Pathophysiology of Postmenopausal Osteoporosis
Low bone mass and skeletal fragility in adults may be the result of low peak bone mass in early adulthood, excessive bone loss in later life, or both.
The skeleton is constantly changing throughout life. During childhood and adolescence, it changes in size, shape, and constituents by a process known as modeling. Change in shape and size is complete with epiphyseal closure at the end of puberty, followed by a period of consolidation for 5 to 10 years (depending on the skeletal site) until peak adult bone mass is attained, which usually occurs in the late teens or early 20s (28 [EL 2], 29 [EL 3]).
Approximately 70% to 80% of peak bone mass is genetically determined. Several genetic markers have been identified (30 [EL 2], 31 [EL 4], 32 [EL 3], 33 [EL 2]). Many nongenetic factors contribute, including nutrition (for example, calcium, phosphate, protein, and vitamin D), load-bearing activity, and hormones involved in growth and puberty. In addition, certain genetic diseases, such as osteogenesis imperfecta, result in low peak adult bone mass and abnormal bone quality, but discussion of these uncommon disorders is outside the scope of these guidelines.
Once peak adult bone mass has been reached, a process called skeletal remodeling takes over, in which old bone is replaced by new bone. Remodeling is governed by the actions of osteoclasts that resorb old bone and osteoblasts that produce new bone. Much is known about the recruitment and activity of these cells, including the involvement of systemic hormones and local cytokines. Recently, the receptor activator of nuclear factor-κβ (RANK), its ligand RANKL, and a decoy receptor, osteoprotegerin (OPG), have emerged as major local regulators of bone remodeling (34 [EL 4]). RANKL, synthesized by osteoblasts and stromal cells and present in the bone microenvironment, binds to RANK, expressed in osteoclast progenitor cells in the bone marrow, and promotes osteoclastogenesis. OPG is also synthesized by osteoblasts and stromal cells and serves as a decoy receptor for RANKL, preventing the binding of RANKL to RANK. Regulation of osteoclast activity depends, at least in part, on the balance between RANKL and OPG. The relative amount of these 2 molecules is governed, in turn, by systemic hormones (for example, estrogen), local factors (such as interleukin-6 and tumor necrosis factor), and perhaps other factors as well. The triggering mechanisms that stimulate the cascade of activities that lead to remodeling of site-specific quantities of bone are not known. It is well documented, however, that this bone remodeling process is in balance (that is, the rate of bone formation equals the rate of bone resorption) through at least the fifth decade of life in healthy individuals. Up to this age, there is generally little net loss or gain of bone. Wnt signaling is an important pathway that influences osteoblastic bone formation. It is complex and involved in many physiologic systems beyond just the skeleton. A detailed description of this pathway is beyond the scope of these guidelines, but the key components that have thus far been most studied with respect to skeletal physiology include the frizzled family of G protein-coupled receptor proteins, low-density lipoprotein receptor-related protein 5 encoded by the LRP5 gene and associated with high bone mass in affected families, cathepsin K, Dikkopf-related protein 1, and sclerostin.
In women, the hormonal changes that occur throughout perimenopause and the immediate postmenopausal years stimulate RANKL production (both directly and indirectly), leading to accelerated bone loss. Most data suggest that the bone turnover rate (and bone loss) accelerates 3 to 5 years before the last menstrual period and slows again 3 to 5 years after the last menstrual period. With the accelerated bone turnover rate, bone balance is disturbed because there is greater net loss than gain in each of the bone remodeling units that are activated. The mean rate of bone loss during this period is about 1% per year, or about 10% during the menopausal transition.
In contrast to menopause-associated bone loss, age-related bone loss begins in the sixth decade of life in men and women and proceeds at a slower rate, about 0.5% per year. Although age-related bone loss involves the same imbalance in the bone remodeling unit as occurs in the menopause-related bone loss, the initiating process is not as clear.
In conjunction with loss of bone mass due to menopause or aging, there are also changes in bone quality. The somewhat nebulous concept of bone quality includes disruption of the microarchitectural elements of cancellous (trabecular) bone, expansion of the periosteal envelope and trabecularization of the endocortex (that is, cortical thinning), decrease in the degree of mineralization of individual skeletal elements, and likely other as yet unknown factors (5 [EL 4]). Newer technologies for monitoring these architectural changes are being introduced into the research arena but are not yet generally available. Additionally, although therapies that slow the bone remodeling process (antiresorptive drugs, also called anticatabolic drugs) appear to have a limited effect on cortical bone, anabolic therapies seem to minimize and possibly reverse these adverse effects of aging on the cortical envelope. As is the case with trabecular microarchitecture, techniques for monitoring these changes longitudinally are still limited to the realm of research. Nonetheless, the importance of this abnormality in cortical bone has been well established in cross-sectional studies.
Many factors, including nutrition, vitamin D, exercise, smoking, and the presence of other diseases and medications (Table 4), can influence the rate of bone loss and the risk of fractures in individuals. Nutrition is important during aging as well as during bone growth. In particular, vitamin D deficiency, whether isolated or associated with more generalized undernutrition, has reached almost epidemic proportions throughout the world. Although severe vitamin D deficiency impairs mineralization of the skeleton, even mild to moderate vitamin D deficiency reduces calcium absorption and can lead to parathyroid hormone (PTH)-mediated increases in bone resorption. Vitamin D deficiency also causes impairment of muscle strength and balance, leading to an increased risk of falling.
Table 4.
Some Factors That May Accelerate Bone Loss
| Endocrine disorders |
| Hyperthyroidism |
| Hypopituitarism |
| Hypogonadism |
| Cushing disease |
| Primary hyperparathyroidism |
| Gastrointestinal disorders |
| Celiac disease |
| Short bowel syndrome |
| Hematologic disorders |
| Multiple myeloma |
| Systemic mastocytosis |
| Renal disorders |
| Chronic renal failure |
| Idiopathic hypercalciuria |
| Neuromuscular disorders |
| Muscular dystrophy |
| Paraplegia, quadriplegia |
| Proximal myopathy |
| Medications |
| Corticosteroids |
| Proton pump inhibitors |
| Antiepilepsy drugs |
| Medroxyprogesterone acetate (Depo-Provera) |
| Selective serotonin reuptake inhibitors |
| Thiazolidinediones |
| Thyroxine in supraphysiologic doses |
| Excess vitamin A |
| Aromatase inhibitors |
| Androgen deprivation therapy |
| Nutritional deficiencies |
| Calcium |
| Vitamin D |
| Protein |
Most osteoporosis-related fractures are the result of falls, which probably have as important a role in the pathogenesis of osteoporosis-related fractures as many of the pathways already discussed. Risk factors for falls are summarized in Table 5. A fragility fracture is defined as a fracture that results from trauma less than or equal to that from a fall from a standing height and almost always indicates decreased skeletal strength. There is increasing evidence that patients who have low bone mass are also at increased risk for fracture after more extensive trauma (35 [EL 2]).
Table 5.
Some Factors That Increase Risk of Falling and Fracture
| Neurologic disorders |
| Parkinson disease |
| Proximal myopathy |
| Peripheral neuropathy |
| Prior stroke |
| Dementia |
| Impaired gait or balance (or both) |
| Autonomic dysfunction with orthostatic hypotension |
| Impaired vision |
| Impaired hearing |
| Frailty and deconditioning |
| Sarcopenia |
| Medications |
| Sedatives and hypnotics |
| Antihypertensive agents |
| Narcotic analgesics |
| Environmental factors |
| Poor lighting |
| Stairs |
| Slippery floors |
| Wet, icy, or uneven pavement |
| Uneven roadways |
| Electric or telephone cords |
| Pets—small or large |
| Throw rugs |
| Positioning in a wet or dry bathtub |
4.4. Clinical Features and Complications of Postmenopausal Osteoporosis
4.4.1. Low Bone Mass
Low bone mass—as assessed clinically by measurements showing low BMD—is a major characteristic of postmenopausal osteoporosis. A strong inverse relationship exists between BMD and risk of fracture. Therefore, low BMD is a major indicator of fracture risk in women without fractures, although it is important to realize that individual patients may sustain fractures at different BMD levels and that factors other than bone density influence fracture risk (Table 5). Low BMD and bone loss are not associated with symptoms before occurrence of a fracture.
4.4.2. Fracture
Fracture is the single most important manifestation of postmenopausal osteoporosis. Osteoporosis-associated fractures may occur in any bone but are most likely to occur at sites of low BMD and are usually precipitated by a fall or injury. Vertebral compression fractures, however, may occur during routine daily activities, without a specific fall or injury. In clinical practice, it may be difficult or impossible to reconstruct the mechanical force applied to bone in a particular fall.
Hip fractures are the most serious complication of osteoporosis. Half of the patients who could walk independently previously are unable to do so 1 year after a hip fracture. Women with hip fracture have an increased mortality of 12% to 20% during the subsequent 2 years, whereas men with hip fracture have an increased mortality of approximately twice that. More than 50% of the survivors are unable to return to independent living; many require long-term nursing home care (36 [EL 4]). Important secondary complications of fractures are itemized in Table 6. Potential complications as well as physical manifestations of vertebral fractures are listed in Table 7 (37 [EL 3]).
Table 6.
Important Complications of Fractures
| Pain |
| Deformity |
| Disability |
| Physical deconditioning attributable to inactivity |
| Changes in self-image |
Table 7.
Potential Complications of Vertebral Fractures
| Loss of height |
| Increased occiput-to-wall distance |
| Decreased rib-to-pelvis distance |
| Kyphosis (dowager’s hump) |
| Crowding of internal organs (especially gastrointestinal and pulmonary) |
| Back pain (acute and chronic) |
| Prolonged disability |
| Poor self-image, social isolation, depression |
| Increased mortality |
4.5. Risk Factors for Postmenopausal Osteoporosis
For years, it has been quite clear that measurement of bone density is a good assessment technique but not enough. Clinical risk factors can be used to assess fracture risk, with or without bone density results. In February 2008, a tool called FRAX was released by WHO (38 [EL 4]) and is available online at www.shef.ac.uk/FRAX. FRAX is the best effort to date to incorporate risk factors into determination of fracture risk and is more effective in conjunction with BMD than without. Important risk factors—risks that are amenable to intervention—can be determined easily. FRAX can be used for men as well as women and is validated globally, with output and utility of results adaptable to individual populations or regional/national standards, but there are also major limitations (39 [EL 4], 40 [EL 4]).
4.5.1. Risk Factors for Low Bone Mass
Age and body weight (or body mass index) correlate with BMD in older adults. Algorithms that incorporate these indices are available to predict BMD but are not sufficiently sensitive for diagnosis or exclusion of osteoporosis (41 [EL 4]). Only BMD measurements can identify patients who have low bone mass. BMD testing is the best way to identify patients at risk for fracture before the first fracture occurs, but the use of BMD can be enhanced by the addition of information about clinical risk factors.
4.5.2. Risk Factors for Fractures
Assessment of risk factors for fractures may be useful for identifying individuals at high risk of fractures, heightening clinical awareness of osteoporosis, and developing strategies for treatment of osteoporosis and prevention of fracture.
No single risk factor is sufficient for predicting total fracture risk. Only by assessing a combination of risk factors can reliable estimates of fracture risk be made (42 [EL 2]). Important risk factors for osteoporosis-related fractures are outlined in Table 8.
Table 8.
Selected Risk Factors for Osteoporosis-Related Fractures
| Prior low-trauma fracture as an adult |
| Advanced age |
| Low bone mineral density |
| Low body weight or low body mass index (not significant if adjusted for bone mineral density) |
| Family history of osteoporosis |
| Use of corticosteroids |
| Cigarette smoking |
| Excessive alcohol consumption |
| Secondary osteoporosis (for example, rheumatoid arthritis) |
A low-trauma fracture as an adult (45 years of age or older) is associated with a 1.8-fold increased risk of subsequent fracture (range, 1.6 to 1.9), after adjustment for BMD (43 [EL 2]). A prior vertebral fracture is associated with a 4-fold to 5-fold increased risk of subsequent fracture (44 [EL 4]).
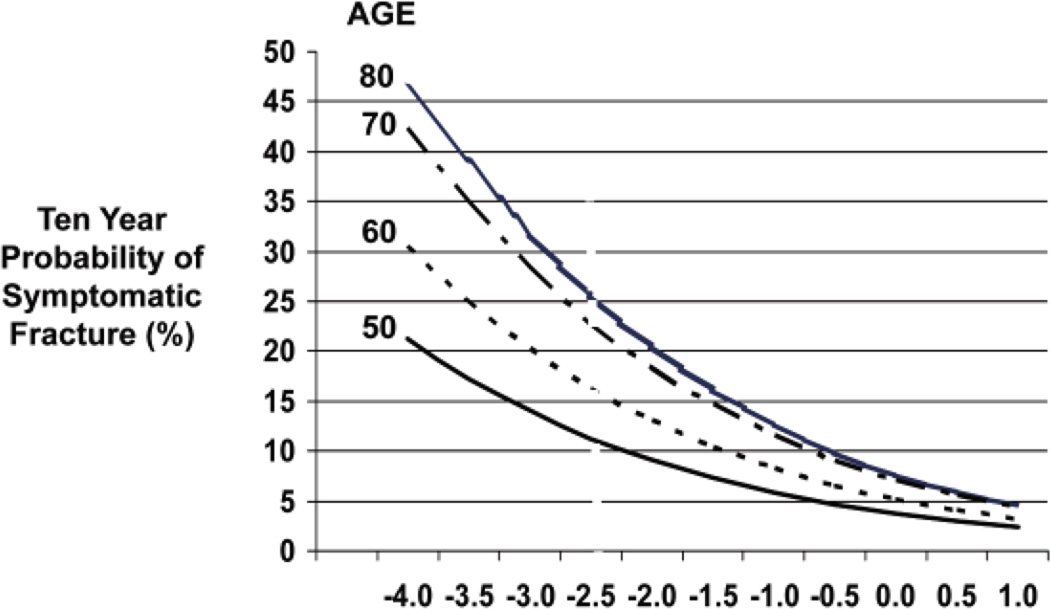
For every SD decrease in age-adjusted BMD, overall fracture risk increases by about 2-fold (range, 1.6-fold to 2.6-fold) (45–47 [EL 2]). Hip BMD predicts hip fracture better than does BMD at other sites (relative risk = 2.6/SD), but reduced bone density at any skeletal site predicts potential fracture not only at that site but also at other sites. The risk of most fragility fractures increases progressively with advancing age. The relationship between BMD and fracture risk is significantly affected by age. For any given BMD value, older adults are at higher risk of fracture than are younger adults, as shown in Figure 4 (48 [EL 1]). Many other factors have been found to correlate with an increased risk of fracture. Although the strength of these individual associations with fracture risk is small, they may be important in individual patients.
Fig. 4.
Ten-year probability of symptomatic osteoporotic fracture in adults 50 to 80 years old. The horizontal axis displays bone mineral density shown as T-scores. Adapted from Kanis et al (48).
Because many patients with osteoporosis have coexisting causes of bone loss (Table 9), the fracture risk profile must consider secondary osteoporosis (see section 4.8).
Table 9.
Some Causes of Secondary Osteoporosis in Adultsa
| Endocrine or metabolic causes |
Nutritional/ gastrointestinal conditions |
Drugs | Disorders of collagen metabolism |
Other |
|---|---|---|---|---|
| Acromegaly Diabetes mellitus Type 1 Type 2 Growth hormone deficency Hypercortisolism Hyperparathyroidism Hyperthyroidism Hypogonadism Hypophosphatasia Porphyria Pregnancy |
Alcoholism Anorexia nervosa Calcium deficency Chronic liver disease Malabsorption syndromes/ malnutrition (including celiac disease, Crohn disease, and gastric resection or bypass) Total parenteral nutrition Vitamin D deficency |
Antiepilepticsb Aromatase inhibitors Chemotheraphy/ immunosuppressants Depo-Provera Glucocorticoids Gonadotropin-releasing hormone agonists Heparin Lithium Proton pump inhibitors Selective serotonin reuptake inhibitors Thiazolidinediones Thyroid hormone (in supraphysiologic doses) Warfarin |
Ehlers-Danlos syndrome Homocystinuria due to cystathionine deficency Marfan syndrome Osteogenesis imperfecta |
AIDS/HIV Ankylosing spondylitis Chronic obstructive pulmonary disease Gaucher disease Hemophilia Hypercalciuria Immobilization Major depression Myeloma and some cancers Organ transplantation Renal insufficiency/ failure Renal tubular acidosis Rheumatoid arthritis Systemic mastocytosis Thalassemiai |
AIDS = acquired immunodeficency syndrome; HIV = human immunodeficency virus.
Phenobarbital, phenytoin, primidone, valproate, and carbamazepine have been associated with low bone mass.
4.5.3. Risk of Falling
Falls magnify the risk of fractures due to other factors and are the proximate cause of most fractures in older adults (49 [EL 2]). Factors that increase the risk of falls and fractures are shown in Table 5.
4.6. Lifestyle and Nonpharmacologic Measures for Bone Health
A “bone healthy” lifestyle (adequate dietary calcium and vitamin D, exercise, avoidance of tobacco, and so forth) is important for everyone—babies, children, teenagers, young adults, and patients with osteoporosis. Patients with osteoporosis may also benefit from physical therapy and other nonpharmacologic measures to strengthen bones and reduce fracture risk. Goals include the following:
Optimize skeletal development and maximize peak bone mass at skeletal maturity
Prevent age-related and secondary causes of bone loss
Preserve the structural integrity of the skeleton
Prevent fractures
4.6.1. Good General Nutrition
In addition to ensuring adequacy of calcium and vitamin D, a balanced diet throughout life is important for bone health. Among young adults, anorexia nervosa and intense aerobic exercise have been associated with delayed menarche and delayed or lower peak bone mass (50 [EL 4]). This outcome may also prevail among adults who are consuming restrictive diets for weight loss or who have surgically induced weight loss. Adequate protein intake (the recommended daily protein dietary allowance in the United States is 0.8 g/kg) helps minimize bone loss among patients who have sustained hip fractures (51 [EL 4]). In one study, patients with hip fracture who received supplemental protein had shorter hospital stays and better functional recovery (52 [EL 1]).
4.6.2. Calcium
Adequate calcium intake is a fundamental aspect of any osteoporosis prevention or treatment program and a lifestyle issue for healthy bones at any age (Grade A; BEL 1). The recommended daily calcium intake for various populations is outlined in Table 10 (53). For women 50 years old or older, the recommended daily calcium intake is 1,200 mg. This represents the total calcium intake (diet plus calcium supplements, if applicable). When dietary intake is insufficient, calcium supplementation may be needed. Although many of the effects of increasing calcium intake on the developing skeleton are incompletely understood, it is well recognized that supplemental calcium increases bone mass in physically active children and adolescents (54–58 [EL 1–2]).
Table 10.
Recommended Dietary Allowance for Calcium
| Age | Sex | Recommended dietary allowance (mg/d) |
|---|---|---|
| 0–6 mo | M + F | 200 |
| 6–12 mo | M + F | 260 |
| 1–3 y | M + F | 700 |
| 4–8 y | M + F | 1,000 |
| 9–18 y | M + F | 1,300 |
| 19–50 y | M + F | 1,000 |
| 51–70 y | M | 1,000 |
| 51–70 y | F | 1,200 |
| 71+ y | M + F | 1,200 |
From Ross et al (53). Reproduced with permission.
Examining a dietary history to assess calcium intake is important. The average calcium intake for adults is about half of what is recommended, with a median of approximately 600 mg/d in comparison with the goal of 1,200 mg daily (59 [EL 3]). Patients with insufficient dietary calcium intake should either change their diet or receive calcium supplements. Numerous calcium supplements are available. Calcium carbonate is generally the least expensive and necessitates use of the fewest tablets. Calcium carbonate, however, may cause gastrointestinal (GI) complaints (constipation and bloating) and, in the absence of secretion of gastric acid, must be taken with meals for adequate absorption. (All calcium preparations are generally better absorbed when taken with food.) Calcium citrate is often more expensive than calcium carbonate and necessitates the use of more tablets to achieve the desired dose; however, its absorption is not dependent on gastric acid, and it may be less likely to cause GI complaints. For optimal absorption, the amount of calcium should not exceed 500 to 600 mg per dose, irrespective of the calcium preparation. For patients requiring more than 600 mg of calcium supplement daily, the dose should be divided.
Calcium requirements increase among older persons; thus, the elderly population is particularly susceptible to calcium deficiency. Factors that lead to calcium deficiency include decreased intestinal absorption of both calcium and vitamin D and renal insufficiency that leads to decreased activation of vitamin D. Patients with GI malabsorption, those who are taking high-dose glucocorticoids, those who have diminished gastric acid production (for example, with a history of gastric bypass, with pernicious anemia, or with use of proton pump inhibitors), those receiving antiepilepsy drugs, and even those with asymptomatic celiac disease are particularly predisposed to calcium and vitamin D deficiency. Consideration should be given to laboratory assessment of adequacy of calcium and vitamin D in patients who are candidates for pharmacologic therapy.
Calcium supplementation has been shown to increase BMD slightly, but no scientific evidence supports its ability to reduce fracture risk, independent of vitamin D. The lack of evidence of an independent effect of calcium on fracture risk reduction is likely due, in part, to problems with study design and patient compliance (60–63 [EL 1]). A large study raised concerns about the risk of nephrolithiasis from calcium supplementation (62 [EL 1]); however, hypercalciuria may worsen with calcium supplementation, and participants in the study were not evaluated for renal calcium wasting. Moreover, the absolute risk of kidney stones was small (2.5% in the calcium-supplemented group versus 2.1% in the control group). In addition, in these study subjects, the mean total calcium intake from diet and supplements was higher than currently recommended. Generally, healthy persons should not require more than 1,000 mg of calcium supplements daily. Patients with a history of nephrolithiasis should be evaluated for the cause of renal stone formation or hypercalciuria before a decision is made about calcium supplementation.
4.6.3. Vitamin D
It is important to ensure sufficiency of vitamin D among children and adults to prevent osteoporosis (Grade A; BEL 1). Most “healthy” adults have serum 25(OH)D levels that are lower than desirable (64 [EL 2]). Vitamin D is not widely available in natural food sources. It is primarily found in fish oils (including cod liver oil), fortified milk, cereals, and breads. Vitamin D is produced in the skin by exposure to sunlight that is not blocked by sunblock agents, but not in northern or southern latitudes during winter. The National Academy of Sciences recommends 400 IU of vitamin D per day for normal adults 51 to 70 years old and 600 IU/d for those above 70 years old. Many experts now believe that these recommendations are too low (65 [EL 4]). For adults 50 years old or older, the National Osteoporosis Foundation recommends 800 to 1,000 IU of vitamin D per day, but many experts recommend more–1,000 to 2,000 IU per day (see http://www.aace.com/alert/alert11302010.php) [4,000 IU per day is the “safe upper limit” (53)]—and some patients require considerably more supplementation to achieve desirable levels. Home-bound individuals with limited mobility, patients who have intestinal malabsorption, or those who are receiving long-term anticonvulsant or glucocorticoid therapy are particularly at risk for vitamin D deficiency. The currently accepted minimal level for 25(OH)D adequacy is 30 to 32 ng/mL, on the basis of a growing body of evidence indicating that secondary hyperparathyroidism is increasingly common as 25(OH)D levels decline below 30 ng/mL (60 [EL 1]) and that fractional calcium absorption improves with vitamin D supplementation in patients with levels below 30 ng/mL but not in patients with levels above 30 ng/mL. A reasonable upper limit, based on levels in sun-exposed healthy young adults, is 60 ng/mL (66 [EL 3]).
A meta-analysis of studies in postmenopausal women found a significant reduction in hip and nonvertebral fractures with vitamin D supplementation at doses of 700 to 800 IU/d or more (67 [EL 2]). The Women’s Health Initiative (WHI) study showed a small but significant increase in hip BMD (1%) in the group of patients who received 1,000 mg of calcium and 400 IU of vitamin D per day (62 [EL 1]). In addition to the skeletal effects of vitamin D, studies have also shown improvement in muscle strength, balance, and risk of falling (68–70 [EL 2]) as well as improvement in survival (71 [EL 2]).
Vitamin D supplements are available as ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) in strengths up to 50,000 IU per tablet. With daily dosing, vitamin D2 and D3 appear to be equally potent (72 [EL 1]), but with intermittent (weekly or monthly) dosing, vitamin D3 appears to be about 3 times more potent than vitamin D2 (73 [EL 2]). Blood levels of 25(OH)D provide the best index of vitamin D stores. A desirable range is between 30 and 60 ng/mL, although levels up to 100 ng/mL are unlikely to result in vitamin D toxicity. Many people require vitamin D supplements of 2,000 IU per day or more to achieve desirable levels. (Cholecalciferol, 1,000 IU daily, will raise blood levels, on average, by approximately 10 ng/mL.)
4.6.4. Other Dietary Supplements
Patients frequently inquire about the need for magnesium supplementation. No randomized controlled study has been done to show that the intake of magnesium decreases fracture risk or increases BMD. One study showed that adding 789 to 826 mg of magnesium per day did not increase the rates of calcium absorption (74 [EL 1]). Individuals who are at risk for hypomagnesemia (for example, those who have GI malabsorption or chronic liver disease [alcoholics]), however, may benefit from magnesium supplementation. Magnesium may help prevent constipation, which is sometimes associated with calcium supplementation.
Excessive intake of vitamin A (more than 10,000 IU daily) should be avoided because this has been shown to have detrimental effects on bone (75 [EL 4]). In contrast, published data have shown that vitamin K (1 mg/d) may reduce bone turnover and bone loss in postmenopausal women (76 [EL 1]). Further studies need to confirm this finding before this strategy can be part of the standard recommendations for prevention of osteoporosis.
“Natural” estrogens (isoflavones) are promoted to prevent bone loss. No conclusive data, however, support the use of these agents for increasing bone density or decreasing fracture risk (77 [EL 1], 78 [EL 1], 79 [EL 2]).
4.6.5. Alcohol
Excessive intake of alcohol should be avoided because investigators have proved that alcohol has detrimental effects on fracture risk (Grade B; BEL 2) (80 [EL 2]). The mechanisms are multifactorial and include predisposition to falls, calcium deficiency, and chronic liver disease. Chronic liver disease, in turn, predisposes to vitamin D deficiency. Postmenopausal women at risk for osteoporosis should be advised against consuming more than 7 drinks/wk, with 1 drink equivalent to 120 mL of wine, 30 mL of liquor, or 260 mL of beer.
4.6.6. Caffeine
Patients should be advised to limit their caffeine intake to less than 1 to 2 servings (8 to 12 ounces in each serving) of caffeinated drinks per day (Grade C; BEL 3). Several observational studies have shown an association between consumption of caffeinated beverages and fractures (81 [EL 3], 82 [EL 3]). Caffeine intake leads to a slight decrease in intestinal calcium absorption and an increase in urinary calcium excretion, suggesting that a moderate intake of caffeine would not be harmful to bone health. The most important effect of caffeinated beverages is that, by replacing milk in the diet, they contribute to overall inadequate calcium intake in the United States.
4.6.7. Smoking
Cigarette smoking is a risk factor that has been validated by multiple studies to increase osteoporotic fracture risk and thus should be avoided (Grade B; BEL 2). The exact mechanism is unclear but may be related to increased metabolism of endogenous estrogen or direct effects of cadmium on bone metabolism. No prospective studies have been done to determine whether smoking cessation reduces fracture risk, but a meta-analysis showed a higher risk of fractures in current smokers than in previous smokers (83 [EL 2]). Smokers should be advised on smoking cessation.
4.6.8. Exercise
Regular weight-bearing exercise (for example, walking for 30 to 40 minutes per session), and back and posture exercises for a few minutes on most days of the week (see http://www.nof.org/aboutosteoporosis/prevention/exercise) should be advocated throughout life (Grade B; BEL 2). Children and young adults who are active reach a higher peak bone mass than those who are not. In studies of young women, muscle strength appeared to correlate with BMD (84 [EL 2], 85 [EL 1]). Studies involving early postmenopausal women have shown that strength training leads to small yet significant changes in BMD. A meta-analysis of 16 trials and 699 subjects showed a 2% improvement in lumbar spine BMD in the group that exercised in comparison with the group that did not (86 [EL 2]). Among elderly patients, these exercises help slow bone loss attributable to disuse, improve balance, increase muscle strength, and ultimately reduce the risk of falls (87 [EL 2], 88 [EL 2]). These outcomes may be as important as—or even more important than—the effects of exercise on BMD.
Patients with severe osteoporosis should avoid engaging in motions such as forward flexion exercises, using heavy weights, or even performing side-bending exercises because pushing, pulling, lifting, and bending exert compressive forces on the spine that may lead to fracture. An initial visit with a physical therapist may help clarify what exercises are safe and unsafe to do.
4.6.9. Prevention of Falls
Falls are the precipitating cause of the majority of osteoporotic fractures, and an effective osteoporosis treatment regimen must include a program for fall prevention (Grade B; BEL 2). All patients should be counseled on fall prevention. Some measures that can be taken to avoid falls at home are outlined in Table 11. Particularly predisposed are persons who are older, are frail, have had a stroke, or are taking medications that decrease mental alertness. Although several interventions have been shown to reduce the risk of falling, none has been shown to reduce the risk of fractures, although it is logical that they would.
Table 11.
Some Measures for Prevention of Falls
| Anchor rugs |
| Minimize clutter |
| Remove loose wires |
| Use nonskid mats |
| Install handrails in bathrooms, halls, and long stairways |
| Light hallways, stairwells, and entrances |
| Encourage patient to wear sturdy, low-heeled shoes |
| Recommend hip protectors for patients who are predisposed to falling |
Hip protectors do not reduce the risk of falling. Intuitively, hip protectors should reduce the risk of fracture. Positive results have been seen in some trials, but not in all, and compliance is poor (89–94 [EL 1–3]). Hip protectors may be considered for patients who have sustained a prior hip fracture, for slender or frail patients who have fallen in the past, and for patients who have major risk factors for falling because of postural hypotension or difficulty with balance, whether they have osteoporosis or not (Grade B; BEL 1).
Elderly patients with severe kyphosis, back discomfort, and gait instability could benefit from physical and occupational therapy referral. A treatment plan that focuses on weight-bearing exercises, back strengthening and balance training, and selective use of orthotics could help reduce discomfort, prevent falls and fractures, and improve quality of life (95 [EL 1]). Lifestyle issues that could help prevent osteoporosis are summarized in Table 12.
Table 12.
Recommendations Regarding Lifestyle Issues
| Ensure adequate intake of calcium throughout life |
| Ensure adequacy of vitamin D intake |
| Consume a balanced diet |
| Regularly perform weight-bearing exercises |
| Avoid use of tobacco |
| Limit alcohol consumption |
| Take measures to avoid falls |
| Consider use of hip protectors |
4.7. Evaluation for Risk Factors for Postmenopausal Osteoporosis
Because the risk for osteoporosis-related fractures rises steeply in women beyond age 50 years, all postmenopausal women should undergo clinical assessment to identify risk factors for osteoporosis and fractures. This assessment should include the measures outlined in Table 13.
Table 13.
Measures for Risk Assessment in Postmenopausal Womena
| •Medical history and physical examination to identify: |
| Prior fracture without major trauma (other than fingers, toes, skull) after age 50 y |
| Clinical risk factors for osteoporosis |
| Age ≥65 y |
| Low body weight (<57.6 kg [127 lb]) |
| Family history of osteoporosis or fractures |
| Smoking |
| Early menopause |
| Excessive alcohol intake (3 or more drinks daily) |
| Secondary osteoporosis (see Table 9) |
| Height loss or kyphosis |
| Risk factors for falling |
| Patient’s reliability, understanding, and willingness to accept interventions |
| •Lateral spine imaging with x-ray studies or vertebral fracture assessment in patients with unexplained height loss, kyphosis, or suspected spine fractures (Grade B; BEL 2) |
| •Bone mineral density measurements in those at increased risk for osteoporosis and fractures and willing to consider pharmacologic treatment if low bone mass is documented: |
| All women 65 y of age or older (Grade B; BEL 2) |
| Younger postmenopausal women |
| With a history of fracture(s) without major trauma (Grade B; BEL 2) |
| Starting or taking long-term systemic glucocorticoid therapy (Grade C; BEL 2) |
| With radiographic osteopenia (Grade C; BEL 3) |
| With clinical risk factors for osteoporosis (low body weight, cigarette smoking, family history of spine or hip fractures, early menopause, or secondary osteoporosis) (Grade C; BEL 3) |
| •In women who are candidates for pharmacologic therapy, laboratory evaluation to identify coexisting |
| conditions that may contribute to bone loss or interfere with therapy (or both) (Grade C; BEL 3) |
BEL = “best evidence” level.
4.7.1. Spine Imaging
Vertebral fracture is the most common osteoporotic fracture and indicates a high risk for future fractures, even in patients whose bone density does not meet the −2.5 T-score threshold for the densitometric diagnosis of osteoporosis. Knowledge of vertebral fractures, therefore, may change an individual patient’s diagnostic classification, estimated risk of future fractures, and clinical management. Most vertebral fractures, however, remain undetected unless specifically sought by radiologic or densitometric techniques (96 [EL 4]).
In patients with unexplained height loss, thoracic and lumbar spine radiography or vertebral fracture assessment by DXA is indicated if knowledge of vertebral fractures would alter clinical management (Grade B; BEL 2). Although height loss may occur for reasons other than vertebral fracture (97 [EL 2]), there is evidence to support radiography for measured height loss >2 cm (>0.8 inch) (98 [EL 2]) or historical height loss (loss from patient’s recalled maximal height) >4 to 6 cm (>1.5 to 2.4 inches) (99 [EL 2]). Although these thresholds of height loss have >90% specificity, the sensitivity for detecting prevalent vertebral fractures is low. Other indications for vertebral imaging include kyphosis and systemic glucocorticoid therapy, both of which are associated with increased vertebral fracture risk. Radiographic studies are also indicated for the evaluation of acute back pain suggestive of compression fracture. The sensitivity and reliability of standard radiography to assess BMD are poor, and in the absence of vertebral fractures, this technique cannot be used to diagnose osteoporosis.
4.7.2. BMD Measurement
In women who are at risk for postmenopausal osteoporosis, there are several potential uses of BMD measurements, as shown in Table 14.
Table 14.
Bone Mineral Density Measurements: Potential Uses in Postmenopausal Women
| Screening for osteoporosis |
| Establishing the severity of osteoporosis or bone loss in patients with suspected osteoporosis (for example, patients with fractures or radiographic evidence of osteopenia) |
| Determining fracture risk—especially when combined with other risk factors for fractures (see Table 8) |
| Identifying candidates for pharmacologic intervention |
| Assessing changes in bone mass over time in treated and untreated patients |
| Enhancing acceptance of, and perhaps adherence with, treatment |
| Assessing skeletal consequences of diseases, conditions, or medications known to cause bone loss |
4.7.2.1. Measurement techniques
DXA of the lumbar spine and proximal femur (hip) provides accurate and reproducible BMD measurements at important sites of osteoporosis-associated fracture (Grade B; BEL 1). Optimally, both hips should be measured during the initial visit to prevent misclassification that may result if only one hip is measured and to have a baseline for both hips in case a fracture or replacement occurs in one hip. These central sites are also more likely than peripheral sites to show a response to treatment and are preferred for baseline and serial measurements. The most reliable comparative results are obtained when the same instrument and, ideally, the same technologist are used for serial measurements.
For BMD measurement, several other techniques are available, including quantitative computed tomography for measurement of both central and peripheral sites, quantitative ultrasonometry, radiographic absorptiometry, and single-energy x-ray absorptiometry. Of note, the diagnostic criteria established by WHO and recommended by AACE apply only to central DXA measurements (specifically, lumbar spine, femoral neck, and total hip) and to DXA of the 1/3 (33%) radius site. Thus, other technologies cannot be used to diagnose osteoporosis but may be used to assess fracture risk.
4.7.2.2. Bone density reports
Bone density results are reported as grams of mineral per square centimeter of projected bone area but are also expressed as T-scores and Z-scores. The T-score represents the number of SDs from the normal young adult mean values, whereas the Z-score represents the number of SDs from the normal mean value for age-, race-, and sex-matched control subjects. Only T-scores are used for diagnosis. Low Z-scores may suggest a secondary cause of osteoporosis, but normal Z-scores do not rule out the possibility of underlying disorders.
4.7.2.3. Measurement sites
Diagnostic criteria, therapeutic studies, cost analyses, and cost-effectiveness data have been based primarily on DXA measurements of the total hip, femoral neck, or total lumbar spine (or some combination of these sites), which are the preferred measurement sites (Grade B; BEL 3). Use of other subregions within the proximal femur or of an individual vertebra has not been validated and is not recommended.
Peripheral measurements can identify patients at increased risk for fracture; however, the WHO criteria for the densitometric diagnosis of low BMD (osteopenia) and osteoporosis do not apply to T-scores from peripheral devices. Currently, work is under way to define the appropriate diagnostic thresholds for peripheral measurement devices. In the interim, peripheral measurements should be limited to the assessment of fracture risk.
4.7.2.4. Role in diagnosis and clinical decision making
A clinical diagnosis of osteoporosis and decision to initiate pharmacologic therapy can be made without BMD testing in postmenopausal women who have fragility fractures of the hip or spine. Nevertheless, BMD measurement is useful in these patients to quantify fracture risk and to establish a baseline for monitoring the response to treatment.
For women without prior fractures (in the absence of major trauma), BMD is the single predictor of future fracture risk (for every 1-SD decrease in age-adjusted BMD, the relative risk of fracture increases 1.6-fold to 2.6-fold) (47 [EL 2]). The relationship between bone density and fracture risk, however, is a continuum, without a clear “fracture threshold.” WHO has defined T-score criteria for the classification of osteoporosis and low bone mass (osteopenia) (Table 3) on the basis of DXA measurements (Grade C; BEL 2). These criteria are useful for classification and risk stratification in individual patients, epidemiologic studies, and therapeutic trial design, but they are not intended as treatment thresholds. Although there is good evidence that the risk for fractures is sufficiently high in most postmenopausal women with osteoporosis (T-scores ≤−2.5) to merit pharmacologic intervention, cost-effective management of women with osteopenia (T-scores between −1.0 and −2.5) is less clear-cut. Although their overall rate of fractures is lower than that of patients with osteoporosis, more than 50% of fragility fractures occur in these patients with low bone mass. In order to identify those patients who are most likely to sustain a fracture, BMD results must be used in combination with other clinical risk factors for osteoporosis-related fractures (Table 8) for accurate assessment of fracture risk and appropriate treatment decisions. The FRAX tool integrates the contribution of BMD and other clinical risk factors and calculates an individual patient’s absolute probability of fracture during a period of 10 years. It is now recommended that treatment decisions include consideration of fracture probability.
4.7.2.5. Indications
BMD testing is useful for screening people at high risk for osteoporosis (for example, postmenopausal women), for disease management in patients with hyperparathyroidism and other disorders or those taking medications (such as glucocorticoids) associated with bone loss (Table 4), if evidence of bone loss would result in modification of therapy, and for monitoring of pharmacologic therapy with bone-active agents. A list of indications for BMD testing is shown in Table 15.
Table 15.
Indications for Bone Mineral Density Testing
| All women 65 y of age or older |
| All postmenopausal women |
| With a history of fracture(s) without major trauma after age 40 to 45 y |
| With osteopenia identified radiographically |
| Starting or taking long-term systemic glucocorticoid therapy (≥3 mo) |
| Other perimenopausal or postmenopausal women with risk factors for osteoporosis if willing to consider pharmacologic interventions |
| Low body weight (<127 lb or body mass index <20 kg/m2) |
| Ever use of long-term systemic glucocorticoid therapy (≥3 mo) |
| Family history of osteoporotic fracture |
| Early menopause |
| Current smoking |
| Excessive consumption of alcohol |
| Secondary osteoporosis (see Table 9) |
The cost-effectiveness of BMD testing and its benefits to society are controversial (100 [EL 1]). Clinicians, politicians, patients, industry, and third-party payers all have different perspectives on the indications for and timing of BMD measurements. The current recommendations are intended to outline reasonable use of this technology within the context of the endocrine specialty practice. Because universal BMD testing is not cost-effective, AACE recommendations for screening include women 65 years of age or older (Grade B; BEL 3) and younger postmenopausal women at increased risk based on fracture risk analysis (Grade C; BEL 2). Indications for screening men for osteoporosis are outside the scope of these guidelines.
4.8. Medical Evaluation for Postmenopausal Osteoporosis
A comprehensive medical evaluation is indicated in all women with postmenopausal osteoporosis to identify coexisting medical conditions that cause or contribute to bone loss (Grade B; BEL 2). Some of these disorders may be asymptomatic and require laboratory testing for detection. Some causes of osteoporosis in adults are summarized in Table 9.
Because of the high prevalence of secondary osteoporosis, even in apparently healthy postmenopausal women, some laboratory testing should be considered for all women with osteoporosis. In a retrospective study, the laboratory tests itemized in Table 16 were found to detect more than 90% of disorders in postmenopausal women with osteoporosis who were otherwise asymptomatic (101 [EL 2], 102 [EL 4]). If the medical history, physical findings, or laboratory test results suggest the presence of secondary osteoporosis, additional laboratory evaluation is warranted and may include (but is not limited to) the tests listed in Table 17.
Table 16.
Laboratory Tests to Consider in Screening for Secondary Osteoporosis
| Complete blood cell count |
| Serum chemistry, including calcium, phosphorus, total protein, albumin, liver enzymes, alkaline phosphatase, creatinine, and electrolytes |
| Urinalysis (24-h collection) for calcium, sodium, and creatinine excretion (to identify calcium malabsorption or hypercalciuria) |
| Serum 25-hydroxyvitamin D |
Table 17.
Tests for Secondary Osteoporosis to Be Considered If There Is Clinical Suspicion
| Serum thyrotropin |
| Erythrocyte sedimentation rate |
| Serum parathyroid hormone concentration for possible primary or secondary hyperparathyroidism |
| Tissue transglutaminase antibodies for suspected celiac disease |
| Urinary free cortisol or other tests for suspected adrenal hypersecretion |
| Acid-base studies |
| Serum tryptase, urine N-methylhistamine, or other tests for mastocytosis |
| Serum protein electrophoresis and free kappa and lambda light chains for suspected myeloma |
| Bone marrow aspiration and biopsy to look for marrow-based diseases |
| Undecalcified iliac crest bone biopsy with double tetracycline labeling |
| Recommended for patients with bone disease and renal failure to establish the correct diagnosis and direct management |
| May be helpful in the assessment of patients with the following: |
| Suspected osteomalacia or mastocytosis when laboratory test results are inconclusive |
| Fracture without major trauma despite normal or high bone density |
| Vitamin D-resistant osteomalacia and similar disorders to assess response to treatment |
| Unusual features that suggest a rare metabolic bone disease |
4.9. Biochemical Markers of Bone Turnover
Biochemical markers of bone turnover provide a dynamic assessment of skeletal activity and are useful modalities for skeletal assessment. Although they cannot be used to diagnose osteoporosis, elevated levels have been shown to predict more rapid rates of bone loss in groups of patients (103 [EL 1]) and to be associated with increased fracture risk independent of BMD at menopause and in elderly women (104 [EL 2]). In addition, these markers respond quickly to therapeutic intervention, and changes in markers have been associated with bone response to therapy and fracture risk reduction (105 [EL 1]). Their use in clinical practice, however, is limited by high in vivo and assay variability (resorption markers), poor predictive ability in individual patients, and lack of evidence-based thresholds for clinical decision making.
Nevertheless, biochemical markers of bone turnover may be useful in certain situations, including for assessment of fracture risk in elderly patients when the finding of elevated levels would influence the decision to begin pharmacotherapy, as an early indicator of therapeutic response to anabolic or antiresorptive therapy or in the laboratory evaluation of patients losing BMD despite antiresorptive therapy, and for assessment of medication compliance, drug absorption, or therapeutic efficacy (Grade C; BEL 1).
4.10. Treatment of Osteoporosis
4.10.1. Goals of Treatment
The therapeutic goals in patients with osteoporosis are as follows:
To prevent fractures by improving bone strength and reducing the risk of falling and injury
To relieve symptoms of fractures and skeletal deformity
To maximize physical function
Achieving these goals depends on commitment to therapy from the patient and the health-care provider and the potential for the chosen therapy to yield results. Measures to achieve these goals are shown in Table 18.
Table 18.
Measures for Decreasing the Risk of Osteoporosis and Fractures in High-Risk Women
| Identify and treat women with osteoporosis-related fractures, and consider pharmacologic therapy for women with low bone mass |
| Identify and treat sensory defects, neurologic disease, and arthritis, which can contribute to frequency of falls |
| Adjust dosage of drugs with sedative effects, which could slow reflexes or decrease coordination and impair patient’s ability to break impact of a fall |
| Recommend appropriate lifestyle changes, including smoking cessation, increased weight-bearing activities, and dietary improvements |
| Minimize risk of falls and injuries with gait and balance training |
4.10.2. Candidates for Pharmacologic Treatment
AACE has endorsed the 2008 National Osteoporosis Foundation Clinician’s Guide to Prevention and Treatment of Osteoporosis (106 [EL 4]). The Guide recommends pharmacologic treatment for postmenopausal women with the following:
A hip or spine fracture (either clinical spine fracture or radiographic fracture) (Grade A; BEL 1)
A T-score of −2.5 or below at the spine, femoral neck, or total hip (Grade A; BEL 1)
A T-score between −1.0 and −2.5 at high 10-year risk of fracture with use of the US-adapted FRAX tool provided by WHO at www.shef.ac.uk/FRAX, where treatment is considered cost-effective if the 10-year risk is 3% or more for hip fracture or 20% or more for “major” osteoporosis-related fracture (humerus, forearm, hip, or clinical vertebral fracture) (Grade A; BEL 2). FRAX has been described more completely earlier (see section 4.5).
4.11. Pharmacologic Agents for Treatment of Osteoporosis
AACE and the American College of Endocrinology recommend the following pharmacologic agents when pharmacotherapy is indicated:
First priority: agents approved by the US Food and Drug Administration (FDA) for the prevention or treatment (or both) of osteoporosis
Second priority: agents not approved by the FDA but for which level 1 or level 2 evidence for efficacy and safety is available. These agents may be appropriate for patients who are unable to take approved agents or who have complex and extenuating medical problems that preclude the effective use of approved agents.
The manufacturer’s prescribing information should be consulted for risks and benefits of any medication that is prescribed.
Adherence and persistence are poor for osteoporosis therapies (107 [EL 2], 108 [EL 1]), as is the case for other “silent” conditions such as hypertension and hyperlipidemia. Special efforts should be made to explain to the patient about the need for therapy and the expectations, as well as to schedule periodic follow-up to ensure that the medication is still being used correctly and appropriately.
Agents approved by the FDA for prevention or treatment of osteoporosis are shown in Table 19. They include (in alphabetical order) bisphosphonates (alendronate, ibandronate, risedronate, and zoledronic acid), calcitonin, denosumab, estrogen, raloxifene, and teriparatide. All these drugs act by reducing bone resorption, except for teriparatide, which has anabolic effects on bone. Because changes in intermediate end points, such as BMD and bone turnover markers, do not correlate strongly with fracture risk reduction, agents should be chosen on the basis of their proven efficacy in reducing fracture risk.
Table 19.
Drugs Approved by the US Food and Drug Administration for Prevention and Treatment of Osteoporosisa
| Postmenopausal osteoporosis |
Glucocorticoid-induced osteoporosis |
||||
|---|---|---|---|---|---|
| Drug | Prevention | Treatment | Prevention | Treatment | In men |
| Estrogen (multiple formulations) |
Multiple regimens | … | … | … | … |
| Calcitonin (Miacalcin, Fortical) |
… | 200 IU intransally once daily, or 100 IU SQ qod |
… | … | … |
| Denosumab (Prolia) | … | 60 mg SQ every 6 mo |
… | … | … |
| Raloxifene (Evista) | 60 mg PO daily | 60 mg PO daily | … | … | … |
| Ibandronate (Boniva) | 2.5 mg PO daily | 2.5 mg PO daily | … | … | … |
| 150 mg PO monthly | 150 mg PO monthly | ||||
| 3 mg IV every 3 mo | |||||
| Alendronate (Fosamax) | 5 mg PO daily | 10 mg PO daily | … | 5 mg PO dailyd | 10 mg PO daily |
| 35 mg PO weekly | 70 mg PO weeklyb | 10 mg PO dailye | |||
| 70 mg + Dc | 70 mg PO weekly |
||||
| Risedronate (Actonel) | 5 mg PO daily | 5 mg PO daily | 5 mg PO daily | 5 mg PO daily | 35 mg PO weekly |
| 35 mg PO weekly | 35 mg PO weekly | ||||
| 150 mg PO monthly | 150 mg PO monthly |
150 mg PO monthly |
|||
| Zoledronic acid (Reclast) | 5 mg IV every 2nd y | 5 mg IV once yearly |
5 mg IV once yearly |
5 mg IV once yearly |
5 mg IV once yearly |
| Teriparatide (Forteo) | … | 20 µg SQ daily | … | 20 µg SQ daily | 20 µg SQ daily |
Please review the package inserts for specific prescribing information. IV = intravenously; PO = orally; qod = every other day; SQ = subcutaneously.
Fosamax 70 mg is available as both a tablet and a unit dose liquid. Alendronate (generic Fosamax) is available.
Fosamax Plus D is a tablet containing 70 mg of alendronate and 2,800 IU or 5,600 IU of vitamin D for weekly administration.
The approved dosage of alendronate for treatment of glucocorticoid-induced osteroporosis in men and estrogen-replete women is 5 mg daily.
The approved dosage of alendronate for treatment of glucocorticoid-induced osteoporosis in estrogen-deficent women is 10 mg daily.
Level 1 evidence for efficacy in reducing the risk of new vertebral fractures is available for all the agents approved for treatment of osteoporosis (alendronate, ibandronate, risedronate, zoledronic acid, calcitonin, denosumab, raloxifene, and teriparatide). Prospective trials have demonstrated the effectiveness of alendronate, risedronate, zoledronic acid, denosumab, and teriparatide in reducing the risk of nonvertebral fractures (EL 1); only alendronate, risedronate, zoledronic acid, and denosumab have been shown to reduce the risk of hip fractures in prospective controlled osteoporosis trials (EL 1). AACE recommends alendronate, risedronate, zoledronic acid, or denosumab as first-line agents (Grade A; BEL 1), ibandronate as a second-line agent (Grade A; BEL 1), raloxifene as a second- or third-line agent (Grade A; BEL 1), and calcitonin as the last-line agent (Grade C; BEL 2). Teriparatide has been shown to reduce the risk of vertebral and nonvertebral fractures. It is recommended for patients with very high fracture risk or those in whom bisphosphonate therapy has been ineffective (Grade A; BEL 1). The evidence for fracture risk reduction at categorical sites is summarized in Table 20.
Table 20.
Summary of Evidence for Fracture Risk Reduction
| Fracture risk reduction | |||
|---|---|---|---|
| Drug | Vertebral | Nonvertebral | Hip |
| Calcitonin (Miacalcin, Fortical) | Yes | No effect demonstrateda | No effect demonstrateda |
| Raloxifene (Evista) | Yes | No effect demonstrateda | No effect demonstrateda |
| Ibandronate (Boniva) | Yes | No effect demonstrateda | No effect demonstrateda |
| Alendronate (Fosamax) | Yes | Yes | Yes |
| Risedronate (Actonel) | Yes | Yes | Yes |
| Zoledronic acid (Reclast) | Yes | Yes | Yes |
| Denosumab (Prolia) | Yes | Yes | Yes |
| Teriparatide (Forteo) | Yes | Yes | No effect demonstrateda |
The lack of demonstrable effect at these sites should be considered in the context that the studies may not have been adequately powered.
4.12. Bisphosphonates
Bisphosphonates are the most widely used drugs for treatment of osteoporosis. Orally administered bisphosphonates must be taken after a prolonged fast (usually the first thing in the morning) and washed down with a full glass of water, not just a sip (to minimize the chance that the tablet will stick in the esophagus). Nothing other than water should be taken for 30 minutes (for alendronate and risedronate) or 60 minutes (for ibandronate). Under ideal conditions, the absorption of orally administered bisphosphonates is less than 1%. Taking any bisphosphonate in conjunction with food, any beverage other than plain water, or certain other medications or ingesting it within 2 hours after a meal may substantially reduce or abolish the absorption of the drug.
Contraindications to bisphosphonate therapy include hypersensitivity or hypocalcemia. Bisphosphonates should be used with caution, if at all, in patients with reduced kidney function (glomerular filtration rate below 30 mL/min for risedronate and ibandronate or below 35 mL/min for alendronate and zoledronate) (109). There is some evidence that alendronate and risedronate are safe and effective in patients with moderate reduction of renal function (107 [EL 2], 108 [EL 1]).
Orally administered bisphosphonates should be used with caution in patients with active upper GI disease, inability to follow the dosing regimen for oral use (that is, inability to remain upright for 30 to 60 minutes), or presence of anatomic or functional esophageal abnormalities that might delay transit of the tablet (for example, achalasia or stricture).
Intravenous administration of nitrogen-containing bisphosphonates, such as ibandronate and zoledronate, causes acute phase reactions in up to 30% to 40% of patients receiving their first dose (110 [EL 3]). These reactions are characterized by fever and muscle aches lasting several days. Acetaminophen given at the time of treatment may reduce the likelihood of these reactions and can also be given to treat the symptoms.
Although rapid administration of nitrogen-containing bisphosphonates may interfere with kidney function (111–113 [EL 3]), this adverse effect has not been observed with intravenously administered ibandronate or zoledronic acid given to patients with normal renal function in accordance with appropriate dosing instructions (114 [EL 3]).
Some patients treated with an orally or intravenously administered bisphosphonate experience bone, joint, or muscle complaints that may be severe (115 [EL 3]) but that usually resolve when use of the drug is discontinued. Osteonecrosis of the jaw (ONJ) has been associated rarely with bisphosphonate therapy for osteoporosis (116–118 [EL 4]); risk factors include dental pathologic conditions, invasive dental procedures, or poor dental hygiene.
Another rare event that may be associated with alendronate is a subtrochanteric fracture (119 [EL 1], 120 [EL 2]). Occasionally, such fractures are described as “chalk stick” because of their radiologic appearance. They occur after minimal or no trauma. Sometimes the patient complains of leg pain preceding the event. A sclerotic appearance to the subtrochanteric region may be seen radiologically. It has been claimed that these patients may have very low bone turnover, although this point has not been rigorously substantiated. Whether a direct etiologic relationship exists between ONJ or these femoral fractures and the use of bisphosphonates is not clear (121 [EL 4], 122 [EL 3]). Evidence for atypical femoral shaft fractures has recently been reviewed by a task force of the American Society for Bone and Mineral Research (123).
The possible association between orally administered bisphosphonates and esophageal cancer has been explored. One study suggested no increased risk (124 [EL 2]), and one suggested that risk was increased with long-term use but small in absolute terms—from 1 case per 1,000 in untreated subjects to 2 cases per 1,000 with bisphosphonate use of 5 years or more (125 [EL 2]).
Atrial fibrillation as a serious adverse event was noted in the zoledronic acid Pivotal Fracture Trial (126 [EL 1]) but was not seen in other trials of zoledronic acid or other bisphosphonates and is thought by the FDA to be a chance finding (see http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm101551.htm).
4.12.1. Alendronate
4.12.1.1. Role in clinical practice
Alendronate is approved by the FDA for prevention and treatment of postmenopausal osteoporosis, treatment of glucocorticoid-induced osteoporosis, and treatment of osteoporosis in men.
4.12.1.2. Available forms and recommended dosing
Initially, 10 mg daily of alendronate was approved for treatment of postmenopausal osteoporosis, and 5 mg daily was approved for prevention of bone loss in recently menopausal women. Subsequently, 70 mg weekly of alendronate was approved for treatment of postmenopausal osteoporosis, and 35 mg weekly was approved for prevention of bone loss. Alendronate 5 mg daily is the approved dosage for treatment of corticosteroid-induced osteoporosis in men and estrogen-replete women, and 10 mg daily is the approved dosage for treatment of corticosteroid-induced osteoporosis in estrogen-deficient women. Alendronate dosages of 10 mg daily and 70 mg weekly are approved for treatment of osteoporosis in men (127).
Alendronate is supplied in 5-mg and 10-mg tablets for daily administration and as 35-mg and 70-mg tablets, as 70-mg liquid unit dose, and as Fosamax Plus D (70 mg of alendronate plus 2,800 IU or 5,600 IU of vitamin D), all for once-weekly oral administration. Alendronate is also now available in generic tablets for both daily and weekly dosing. Many physicians are unsure about the tolerability and efficacy of generic alendronate, but to the time of this writing, there have been no publications to alleviate or address these concerns for generic preparations available in the US.
4.12.1.3. Efficacy
Alendronate has been shown to reduce the risk of fractures of the spine (128 [EL 1], 129 [EL 1]), hip (128[EL 1]), and nonvertebral sites (130 [EL 2], 131 [EL 1]) in women with postmenopausal osteoporosis. Alendronate increases BMD at the spine and hip and prevents bone loss at the forearm (128 [EL 1], 129 [EL 1], 132 [EL 1]). Studies of up to 10 years’ duration suggest continued efficacy (133 [EL 4], 134 [EL 1]).
4.12.1.4. Side effects
Studies of up to 13 years’ duration indicate a good safety profile. In clinical trials, adverse events with alendronate did not differ from those with placebo (119 [EL 1], 121 [EL 4]). In clinical practice, however, upper GI symptoms such as heartburn, indigestion, substernal discomfort, and pain with swallowing may occur, and rare instances of esophageal erosion, ulceration, or bleeding have been described (122 [EL 3], 135 [EL 3], 136 [EL 3]). Most GI side effects are mild, but serious problems are seen in approximately 1 of 10,000 alendronate users (137 [EL 2]), often attributable to errors in patient selection or dosing. Weekly dosing is at least as well tolerated as daily dosing (138 [EL 1]) and may actually be better tolerated. If GI side effects occur, alendronate should be discontinued until symptoms are resolved, after which a bisphosphonate rechallenge could be considered with either alendronate or another orally administered bisphosphonate.
4.12.1.5. Duration of treatment
Alendronate has been studied in trials of up to 10 years’ duration (133 [EL 4], 134 [EL 1]). Efficacy and safety beyond 10 years have not yet been established, but observational tracking is now up to 13 years. When alendronate is discontinued, no acceleration of bone loss relative to placebo has been noted, although slow but significant bone loss at the hip has been reported (135 [EL 3]). There is some suggestion that, after 4 to 5 years of therapy (and longer for those with severe osteoporosis), a drug holiday of 1 or 2 years could be offered without substantial loss of antifracture efficacy (134 [EL 1], 139 [EL 4]) (Grade B; BEL 1).
The concept of the drug holiday is based on persistent effects without active drug administration for a year or longer and the idea that some of the aforementioned adverse events may be related to bone turnover. When use of alendronate is discontinued for a year, bone turnover markers typically increase to about 25% to 30% higher than their values at the time the drug was stopped. When bone loss ensues, resumption of drug therapy is recommended.
Alendronate is also subject to class effect warnings discussed in section 4.12.
4.12.2. Risedronate
4.12.2.1. Role in clinical practice
Risedronate is approved by the FDA for prevention and treatment of postmenopausal osteoporosis, prevention and treatment of glucocorticoid-induced osteoporosis in men and women, and treatment of osteoporosis in men (140).
4.12.2.2. Available forms and recommended dosing
Risedronate was initially approved as a 5-mg daily dose for prevention of bone loss in recently menopausal women, treatment of postmenopausal osteoporosis, and prevention and treatment of corticosteroid-induced osteoporosis in men and women. Risedronate in doses of 35 mg once weekly and 150 mg once monthly is approved for treatment of postmenopausal osteoporosis. Risedronate is supplied as 5-, 35-, and 150-mg tablets and as Actonel with calcium, which consists of a blister pack containing 1 Actonel 35-mg tablet for weekly administration plus 6 calcium tablets to be taken daily (on the other days of the week) (140). The 75-mg tablets have been discontinued in the United States but may be available in other markets.
4.12.2.3. Efficacy
Risedronate has been shown to reduce the risk of fractures of the spine (141 [EL 1], 142 [EL 1]), hip (143 [EL 1]), and nonvertebral sites (141 [EL 1]) in women with postmenopausal osteoporosis. Risedronate increases BMD at the spine and hip and prevents bone loss at the forearm (141 [EL 1], 143 [EL 1]). Studies of up to 7 years’ duration suggest continued efficacy (144 [EL 1], 145 [EL 2]).
4.12.2.4. Side effects
Studies of up to 9 years’ duration indicate a good safety profile (145 [EL 2]). In clinical trials, adverse events with risedronate did not differ from those with placebo (141–143 [EL 1]). Side effects are generally mild and primarily affect the upper GI system. If GI side effects occur, risedronate should be discontinued until symptoms are resolved, after which a rechallenge with risedronate or other bisphosphonate should be considered.
Risedronate is also subject to class effect warnings discussed in section 4.12.
4.12.2.5. Duration of treatment
Risedronate has been studied in trials of up to 7 years’ duration (145 [EL 2]). Efficacy and safety beyond 7 years have not yet been established, although clinical experience now extends to 9 years. When risedronate is discontinued, no acceleration of bone loss relative to placebo has been noted, although slow bone loss may occur (145 [EL 2], 146 [EL 1]). There is some suggestion that, after 3 years of therapy, a drug holiday of up to 1 year can be offered without significant loss of antifracture efficacy (146 [EL 1]). After 1 year of discontinuation, bone turnover markers essentially returned to baseline pretreatment levels (146 [EL 1]). Resuming risedronate therapy after 1 year is generally recommended (139 [EL 4]).
4.12.3. Ibandronate
4.12.3.1. Role in clinical practice
Ibandronate is approved by the FDA for prevention and treatment of postmenopausal osteoporosis (147).
4.12.3.2. Available forms and recommended dosing
Ibandronate 2.5 mg daily is the approved dose for prevention of bone loss in recently menopausal women and treatment of postmenopausal osteoporosis. Ibandronate 150 mg once monthly is also approved for prevention and treatment of postmenopausal osteoporosis, and ibandronate 3 mg given intravenously every third month is approved for treatment of postmenopausal osteoporosis (147).
Ibandronate is supplied as 2.5-mg and 150-mg tablets and as a 3-mg sterile solution for intravenous administration (147). Intravenous administration of ibandronate is by injection given during 15 to 30 seconds (147).
4.12.3.3. Efficacy
Ibandronate has been shown to reduce the risk of fractures of the spine in women with postmenopausal osteoporosis (148 [EL 1]). It has not been shown to reduce nonvertebral or hip fractures in prospective analysis. Ibandronate increases BMD at the spine and hip and prevents bone loss at the forearm (148 [EL 1], 149 [EL 1]).
4.12.3.4. Side effects
Studies of up to 3 years’ duration indicate a good safety profile (148 [EL 1]). In clinical trials, adverse events with ibandronate did not differ from those with placebo (148 [EL 1]). Side effects are generally mild and primarily affect the upper GI system. As with the other bisphosphonates, upper GI side effects can occur with use of ibandronate. If they do occur, ibandronate should be discontinued until symptoms are resolved, after which a rechallenge should be considered.
Ibandronate is also subject to class effect warnings discussed in section 4.12.
4.12.3.5. Duration of treatment
Ibandronate has been studied in trials of up to 3 years’ duration (148 [EL 1]). Efficacy and safety beyond 3 years have not yet been established. No published studies have addressed the discontinuation of ibandronate therapy.
4.12.4. Zoledronic Acid
4.12.4.1. Role in clinical practice
Zoledronic acid is approved by the FDA for prevention and treatment of postmenopausal osteoporosis, for men with osteoporosis, for patients after surgical repair of hip fracture, and for prevention and treatment of glucocorticoid-induced osteoporosis (150).
4.12.4.2. Available forms and recommended dosing
The approved dosage of zoledronic acid (Reclast) for all indications is 5 mg given by intravenous infusion during a 15-minute period once yearly. It is important to distinguish this indication for zoledronic acid for osteoporosis from another dosing regimen of the same drug (that is, Zometa, 4 mg administered intravenously each month) for patients with skeletal complications of malignancy. The branded product for osteoporosis is Reclast; the branded product for patients with a malignant condition is Zometa. Reclast and Zometa should not be used in the same patient.
Zoledronic acid is administered intravenously as a 5-mg infusion over a minimum of 15 minutes. For treatment of postmenopausal osteoporosis and osteoporosis in men as well as prevention and treatment of glucocorticoid-induced osteoporosis, it is given once yearly. For prevention of postmenopausal osteoporosis, the 5-mg dose is given once every 24 months. Before administration, patients should be appropriately hydrated, especially those receiving diuretic therapy. Serum calcium and creatinine concentrations should be monitored before administration of each dose (151 [EL 1]).
4.12.4.3. Efficacy
Zoledronic acid has been shown to reduce the risk of spine, nonvertebral, and hip fractures in women with postmenopausal osteoporosis (150), to reduce the rate of new clinical fractures in patients treated after surgical repair of hip fracture, and to reduce mortality in these patients. Zoledronic acid increases BMD at the spine and hip and prevents bone loss in men, postmenopausal women, and patients treated with glucocorticoids (126 [EL 1], 152 [EL 1]).
4.12.4.4. Side effects
Intravenous administration of zoledronic acid can cause acute phase reactions in up to 30% of patients receiving their first dose (126 [EL 1]). Subsequent doses or administration in patients who have previously been treated with alendronate is associated with a much smaller incidence (less than 2%) (151 [EL 1]). These reactions are characterized by fever and muscle aches lasting several days. Acetaminophen given at the time of treatment may reduce the likelihood of this reaction, and it can also be given to treat the symptoms.
With zoledronic acid, most of the published literature has associated ONJ with the much higher dose that is used in patients with a malignant condition (132 [EL 1], 134 [EL 1], 153 [EL 1]). No published information suggests that ONJ is more common with intravenously administered zoledronic acid in the dose used to treat osteoporosis in comparison with orally administered bisphosphonates.
Zoledronic acid is also subject to class effect warnings discussed in section 4.12.
4.12.4.5. Duration of treatment
Zoledronic acid has been studied in trials of up to 3 years’ duration (126 [EL 1]). Studies of efficacy and safety through 6 years have been completed but are not yet published. No published studies have addressed the discontinuation of zoledronic acid therapy.
4.13. Raloxifene
4.13.1. Role in Clinical Practice
Raloxifene is approved by the FDA for prevention and treatment of postmenopausal osteoporosis as well as for the reduction of risk of breast cancer in women with postmenopausal osteoporosis or at high risk of breast cancer (154).
4.13.2. Available Forms and Recommended Dosing
The approved dosage of raloxifene for all indications is 60 mg daily. It can be taken at any time of day, without regard for meals. Raloxifene is supplied as a 60-mg tablet (154).
4.13.3. Contraindications
Raloxifene is contraindicated in women of childbearing potential, those who have had venous thromboembolic disease, or those who are known to be hypersensitive to any component of raloxifene tablets (154).
4.13.4. Efficacy
Raloxifene has been shown to reduce the risk of fractures of the spine in women with postmenopausal osteoporosis (155 [EL 1]). Nonvertebral or hip fracture efficacy has not been demonstrated. It increases BMD in the spine and hip (153 [EL 1], 155 [EL 1]). Studies of up to 4 years’ duration (156 [EL 1]) suggest continued efficacy.
4.13.5. Extraskeletal Effects
In studies in postmenopausal women, raloxifene reduced total cholesterol and low-density lipoprotein cholesterol fractions, but it had no apparent effect on high-density lipoprotein cholesterol (153 [EL 1], 155 [EL 1]). A large placebo-controlled study of raloxifene showed no beneficial or adverse cardiovascular effects or cerebrovascular events (that is, a neutral effect), but there was an overall increase in fatal strokes (157 [EL 1]).
In an osteoporosis trial with raloxifene, a significant reduction in breast cancer was seen (155 [EL 1]). This finding was confirmed in a larger trial of women at high risk of breast cancer (158 [EL 1]). Of note, raloxifene is not indicated for the treatment of invasive breast cancer, for reduction of the risk of recurrence of breast cancer, or for reduction of the risk of noninvasive breast cancer.
4.13.6. Side Effects
Raloxifene is associated with an approximate threefold increase in occurrence of venous thromboembolic diseases (similar to estrogen), although the absolute risk is low (157 [EL 1]). Other side effects include menopausal symptoms (for example, hot flashes and night sweats) and leg cramps (154).
4.13.7. Duration of Treatment
Efficacy has been determined for up to 4 years (156 [EL 1]), and safety has been determined for up to 8 years (159 [EL 1]). When use of raloxifene is stopped, the skeletal benefits appear to be lost fairly quickly (during the following 1 or 2 years).
4.14. Teriparatide
4.14.1. Role in Clinical Practice
Teriparatide—recombinant human PTH(1–34)—is approved by the FDA for treatment of women with postmenopausal osteoporosis who are at high risk of fracture or who have failed or been intolerant of previous osteoporosis therapy and to increase bone mass in men with idiopathic or hypogonadal osteoporosis who are at high risk of fracture or who have failed or been intolerant of previous osteoporosis therapy (160). Teriparatide is also approved for treatment of men and women with glucocorticoid-induced osteoporosis. It is prudent to measure the serum calcium, PTH, and 25(OH)D levels before treatment with teriparatide.
4.14.2. Available Forms and Recommended Dosing
The approved dosage of teriparatide is 20 µg once daily injected subcutaneously. Teriparatide is dispensed in a glass cartridge that is preassembled into a disposable multiple-dose pen syringe device designed to provide 28 doses (160).
4.14.3. Efficacy
Teriparatide has been shown to reduce the risk of vertebral and nonvertebral fractures in women with postmenopausal osteoporosis (161 [EL 1]). Teriparatide increases BMD in the spine dramatically but has little effect on BMD in the hip or forearm (161 [EL 1]). Patients who lose BMD in the hip with teriparatide treatment are still protected against vertebral fracture (162 [EL 2]).
4.14.4. Contraindications
Teriparatide has a “black box” warning because of the occurrence of osteosarcomas in rats treated with very high doses of teriparatide (3 to 58 times higher than the human equivalent dose) starting at 2 weeks of age and continued for their lifetimes (approximately 75 human-year equivalents) (163 [EL 1]). Subsequent studies in the same strain of rats showed no development of malignant bone tumors with use of doses of teriparatide up to 3 times higher than the human equivalent dose (164 [EL 1]). Because teriparatide caused an increased incidence of osteosarcomas in rats, it is contraindicated in patients at increased risk of osteosarcoma (those with Paget disease of bone, open epiphyses, a history of irradiation involving the skeleton, or an unexplained elevation of alkaline phosphatase level of skeletal origin) (160). The incidence of osteosarcomas in women 50 years old or older is approximately 1 in 250,000. The actual incidence of osteosarcoma in users of teriparatide is unknown; there are rare reports, consistent with the background incidence (165 [EL 4], 166 [EL 3]). Teriparatide should also not be administered to patients with primary or any form of secondary untreated or unresolved hyperparathyroidism (160).
4.14.5. Side Effects
Side effects of teriparatide have been mild and transient and include nausea, orthostatic hypotension (which usually does not necessitate discontinuation of the drug, occurs in association with the first few doses, and responds to assumption of a recumbent posture), and leg cramps. Hypercalcemia, usually mild, asymptomatic, and transient, has been observed but is not common (160).
4.14.6. Duration of Treatment
Efficacy and safety of teriparatide have been assessed for a period of 2 years and are currently unknown thereafter. Treatment with teriparatide is not recommended to exceed 2 years (160). When use of teriparatide is stopped, bone density declines quickly during the following year, although fracture reduction may persist for 1 or 2 years (167 [EL 2]). Use of alendronate after teriparatide therapy prevents this loss and in some cases will be associated with a further increase in BMD (168 [EL 1]).
4.15. Calcitonin
4.15.1. Role in Clinical Practice
Injectable and nasal spray salmon calcitonin are approved by the FDA for treatment of postmenopausal osteoporosis (169,170).
4.15.2. Available Forms and Recommended Dosing
The approved dosage of injectable calcitonin for treatment of postmenopausal osteoporosis is 100 IU daily given subcutaneously or intramuscularly. The approved dose of nasal spray calcitonin is 200 IU (1 spray) daily. Injectable salmon calcitonin is available in a sterile solution. Intranasally administered calcitonin is available in a spray bottle that delivers 200 IU per spray (169,170).
4.15.3. Contraindications
The main contraindication to use of calcitonin is hypersensitivity (169,170). For patients with suspected sensitivity to the drug, skin testing is recommended before treatment.
4.15.4. Efficacy
There are no published studies with injectable calcitonin that show antifracture efficacy. Nasal spray salmon calcitonin (200 IU daily) has been shown to reduce the risk of new vertebral fractures in women with postmenopausal osteoporosis, but neither a lower dose (100 IU daily) nor a higher dose (400 IU daily) was effective (171 [EL 1]). Nonvertebral and hip fracture efficacy has not been demonstrated. Calcitonin produces a minimal increase in BMD in the spine in women >5 years after onset of menopause but does not increase BMD at sites other than the spine (171 [EL 1]).
4.15.5. Side Effects
Studies of up to 5 years’ duration indicate a good safety profile (171 [EL 1]). Common side effects of parenterally administered calcitonin include nausea, local inflammatory reactions at the injection site, and vasomotor symptoms including sweating and flushing. The most common side effect of nasally administered calcitonin is nasal discomfort, including rhinitis, irritation of the nasal mucosa, and occasional epistaxis. Use of calcitonin with either route of administration is well tolerated (169,170).
4.15.6. Duration of Treatment
The optimal duration of treatment with calcitonin is unknown. Safety and efficacy data are available through 5 years (171 [EL 1]). When use of calcitonin is stopped, the skeletal benefits are lost fairly quickly (during the subsequent 1 or 2 years).
4.16. Denosumab
4.16.1. Role in Clinical Practice
Denosumab is a fully human monoclonal antibody against RANKL that reduces the amount of RANKL in the bone microenvironment, reduces the differentiation of precursor cells into mature osteoclasts, and decreases the function and survival of activated osteoclasts. Denosumab is approved by the FDA for treatment of postmenopausal women at high risk of fracture, defined as having a history of osteoporotic fracture or multiple risk factors for fracture, or patients who have failed or are intolerant of other available osteoporosis therapy.
4.16.2. Available Forms and Recommended Dosing
The approved dosage of denosumab is 60 mg by subcutaneous injection given once every 6 months. It is available in prefilled syringes or single-dose vials.
4.16.3. Efficacy
Denosumab has been shown to reduce the risk of fractures of the spine, hip, and nonvertebral sites (172 [EL 1]) in women with postmenopausal osteoporosis. Denosumab increases BMD at the spine, hip, and forearm (172 [EL 1]). Studies of up to 3 years’ duration suggest continued efficacy (172 [EL 1]).
4.16.4. Side Effects
Studies of up to 6 years’ duration indicate a good safety profile (172–174 [EL 1]). Hypocalcemia must be corrected before initiation of therapy. Serious infections, including skin infections, may occur. Patients should be advised to seek prompt medical attention if signs or symptoms of infection, including cellulitis, develop. Dermatitis, rashes, and eczema have been reported; consider discontinuing the use of denosumab if severe symptoms develop. In patients treated with denosumab, ONJ has been reported. Suppression of bone turnover of uncertain clinical significance has been demonstrated.
4.16.5. Duration of Treatment
Denosumab has been studied in trials of up to 6 years’ duration (174 [EL 1]). Efficacy and safety beyond 6 years have not yet been established, but clinical trials are likely to be extended through 10 years. When treatment with denosumab was stopped after 2 years, BMD decreased to baseline values and bone turnover markers increased to values above baseline by 12 months after discontinuation (174 [EL 1]).
4.17. Estrogen and Menopausal Hormone Therapy
4.17.1. Role in Clinical Practice
Although once considered the “treatment of choice” for postmenopausal osteoporosis, estrogen was never specifically approved for treatment of osteoporosis. It is approved by the FDA for prevention of postmenopausal osteoporosis with the added caveat, “when prescribing solely for the prevention of postmenopausal osteoporosis, therapy should only be considered for women at significant risk of osteoporosis and for whom non-estrogen medications are not considered to be appropriate” (175).
4.17.2. Available Forms and Recommended Dosing
Several different formulations of estrogen are available (for example, estradiol, conjugated equine estrogens, esterified estrogens) for administration by oral and transdermal routes. The optimal dose and route of administration for skeletal effects are not known. When estrogen is prescribed for a patient who still has her uterus, a progestin should also be used, either daily or cyclically, to protect against endometrial stimulation.
4.17.3. Efficacy
Conjugated equine estrogen (0.625 mg daily), with or without medroxyprogesterone acetate, has been shown to reduce the risk of fractures of the spine, hip, and nonvertebral sites in postmenopausal women (176 [EL 1], 177 [EL 1]). Estrogen increases BMD in the spine, hip, and forearm (178–180 [EL 1]).
4.17.4. Extraskeletal Effects
There has been considerable controversy regarding the extraskeletal effects of estrogen, particularly with regard to cardiovascular disease and breast cancer. The WHI trial of combination estrogen plus progestin therapy suggested an increased risk of thromboembolic, cerebrovascular, and cardiovascular events, as well as breast cancer, although the risk-to-benefit ratio was close to neutral (181 [EL 1]). In the WHI estrogen-only trial, there was no increased risk of cardiovascular events or breast cancer but no overall benefit either (182 [EL 1]).
4.17.5. Side Effects
In women with an intact uterus, unopposed estrogen therapy is associated with an increased risk of endometrial hyperplasia and carcinoma. When appropriate dosages of progestin are used along with estrogen, this added risk is eliminated. When estrogen therapy is initiated, particularly continuous estrogen-progestin regimens, irregular vaginal bleeding can occur in women with an intact uterus. Vaginal spotting may diminish with time. Estrogen increases the risk of cholelithiasis twofold. Fluid retention, mastalgia, abdominal pain, and headache may occur but may be ameliorated with use of a lower dose. There is an approximate 3-fold increased risk of venous thromboembolism in women who use estrogen in comparison with nonusers. The absolute risk is small (approximately 3 in 1,000 to 3 in 10,000). In some women with sensitivity, estrogen therapy can be associated with dramatic increases in serum triglyceride levels.
4.17.6. Contraindications
The following are contraindications to estrogen or combination estrogen-progestin therapy (175):
Known or suspected pregnancy
Known or suspected cancer of the breast
Known or suspected estrogen-dependent neoplasm
Undiagnosed abnormal genital bleeding
Active thrombophlebitis or thromboembolic disorders or a history of thromboembolic disease
Hypersensitivity to the hormones
4.17.7. Duration of Treatment
The main indication for the use of estrogen is for relief of menopausal symptoms. When given for this indication, estrogen should be administered in the lowest dose necessary to relieve symptoms and for the shortest duration possible. When use of estrogen is stopped, the antifracture benefits are lost fairly quickly (during the subsequent 1 or 2 years). Bone density may decrease as much as 5% during the first year after discontinuation of estrogen therapy (183 [EL 1]).
4.18. Concomitant Use of Therapeutic Agents
There are no studies showing that combination treatment with 2 or more osteoporosis drugs has a greater effect on fracture reduction than treatment with a single agent (184 [EL 4]). Modest additive effects on BMD and bone turnover have been observed with combinations of 2 antiresorptive agents. The combined use of an antiresorptive drug and teriparatide or PTH may alter the BMD and bone turnover response, depending on which antiresorptive agent is used (185 [EL 1]). Combination therapy substantially increases the cost and probably increases the potential for side effects. Until the effect of combination therapy on fracture risk is better understood, however, AACE does not recommend concomitant use of these agents for prevention or treatment of postmenopausal osteoporosis (Grade B; BEL 2).
4.19. Sequential Use of Therapeutic Agents
Sequential use of therapeutic interventions can be considered in the context of the 2 major classes of drugs that are available—the antiresorptive and the anabolic agents. In patients who are being considered for anabolic therapy after antiresorptive treatment, experimental support exists for the idea that the more potent the antiresorptive agent in suppressing bone turnover, the more sluggish the initial response to anabolic therapy (186 [EL 1], 187). The rationale for using an antiresorptive agent after anabolic therapy is based, in part, on the limited period that anabolic therapy with teriparatide is used and, in part, on observations that if antiresorptive therapy is not used after treatment with teriparatide is discontinued, bone loss is rapid (170).
4.20. Nonapproved Therapies
Etidronate and pamidronate are bisphosphonates that are available in the United States for specific indications but are not approved for prevention or treatment of osteoporosis. Because they are available, these agents can be used “off label” for patients with osteoporosis.
Etidronate is approved in the United States for treatment of Paget disease of bone (188). It has antifracture efficacy in women with postmenopausal osteoporosis (186 [EL 1]) and is approved for treatment of osteoporosis in several countries but not in the United States. It is an alternative for patients who have GI intolerance of approved orally administered bisphosphonates and who are not candidates for intravenous bisphosphonate treatment. Etidronate for osteoporosis is given in an intermittent cyclic regimen, 400 mg daily for 14 days, with cycles repeated every 3 months (186 [EL 1]). Because it is not a nitrogen-containing bisphosphonate, etidronate does not irritate the esophageal mucosa.
Pamidronate is approved in the United States for treatment of Paget disease of bone and treatment of skeletal complications of malignant disease (187). Given by intravenous infusion, it may be useful for patients who cannot tolerate orally administered bisphosphonates or who may not absorb orally taken bisphosphonates because of GI disease. It has been shown to increase bone density in the spine and hip (EL 2), but there is no evidence for antifracture efficacy. A typical treatment schedule for pamidronate is a loading dose of 90 mg followed by 30 mg every third month (189 [EL 2]) given by intravenous infusion in dextrose or saline during a 2-hour period.
Agents not available in the United States but used in some countries include strontium ranelate, clodronate, tibolone, and the full-length molecule of PTH(1–84).
4.21. BMD and Fracture Assessment for Monitoring Skeletal Status
BMD testing may be done to determine whether or when to initiate treatment or to monitor the response to treatment. In untreated patients, the frequency of testing depends on the results of the initial test (for example, how close the patient is to an intervention threshold) and the likelihood of clinically significant bone loss. Age-related bone loss, which begins in the fifth decade of life, occurs at the rate of 0.5% to 1.0% per year (190 [EL 2]). Menopause-related bone loss, which begins 3 to 5 years before the last menstrual period and continues for 3 to 5 years afterward, occurs at the rate of 1% to 2% per year (191 [EL 2]). Rapid bone loss (3% to 5% in a year) may occur with the initiation of glucocorticoid therapy (192 [EL 4]) or after discontinuing postmenopausal estrogen therapy. A “Bone Loss Calculator” is available through the International Society for Clinical Densitometry (ISCD) (www.iscd.org). One SD is about 10% of the young adult mean; thus, loss of 10% (which typically takes 10 to 20 years of age-related bone loss or 5 to 10 years of menopause-related bone loss) will result in a decrease of about 1 T-score unit. The baseline result is also important. “Normal” and “osteopenia” are ranges, not points. Someone who has normal BMD with a T-score of +1.0 can afford to lose more bone than someone else who has normal BMD with a T-score of −0.9. For patients receiving treatment or approaching an intervention threshold, retesting every 1 to 2 years is often appropriate. For those who have borderline low results of BMD, retesting every 3 to 5 years is usually sufficient. Patients who are comfortably above an intervention threshold may not need to undergo reassessment for 5 or 10 years, or ever, unless there is some new indication.
Among patients receiving treatment, the goal of monitoring is to identify those who have substantial bone loss at clinically relevant sites: the posteroanterior (PA) spine or the hip (total hip or femoral neck). Stable or increasing BMD at these sites indicates a satisfactory response to treatment (193 [EL 4]). If BMD decreases considerably, patients should undergo assessment for noncompliance, secondary osteoporosis, or use of new medications that might cause bone loss.
To determine whether a difference in BMD is real or simply within the inherent variability of the measurement, testing facilities must calculate the “least significant change” (LSC) for relevant measurement sites to ascertain the magnitude of difference that represents a real change. This is determined by using a facility’s regular technologist, patients, and device (194 [EL 4], 195 [EL 4]). The ISCD has established guidelines for determining the number of patients and repetitive scans needed to calculate the LSC (30 patients in duplicate or 15 patients in triplicate) (194 [EL 4], 195 [EL 4]). The LSC is usually set at the 95% confidence limit for the change. The manufacturer’s LSC should not be used because it does not account for differences in actual patients who will be tested and the performance and skill of the technologist. If serial studies show a difference in BMD that exceeds the LSC, the probability that the difference is real is greater than 95%. Small changes, within the bounds of the LSC, should not be reported.
In addition to knowing the LSC, it is important to compare “apples with apples.” Differences in regions of interest, local structural change, or artifacts may result in a “change” that does not reflect actual progression of bone loss or response to osteoporosis treatment. Before acceptance of a report of significant bone loss, the images and numeric results of the studies should be reviewed to assess comparability.
Changes in BMD are usually small (0.5% to 2.0% per year) and often close to the precision error of the measurement (1.0% to 1.2% for the PA spine and 0.8% to 1.7% for the total hip). The definition of a “nonresponder” to therapy is complex, and the proportion of nonresponders for different therapies will vary depending on the definition. Furthermore, studies have shown that the change in BMD accounts for less than 20% of the fracture risk reduction after antiresorptive therapy (196 [EL 4], 197 [EL 1]). Finally, although it has been suggested that monitoring might improve patient compliance, nonadherence to therapy usually occurs early (after 6 to 7 months), before the second BMD measurement would be performed (198 [EL 3]).
Ideally, monitoring should occur at the same facility and with use of the same machine as for the previous DXA, and if possible, it should be performed by the same technologist and should involve the same regions of interest for both the spine and the hip (Grade B; BEL 2). The 1/3 (33%) radius site is also acceptable. Other peripheral sites (for example, heel, finger, and tibia) cannot be used for monitoring. Most third-party payers and some Medicare carriers cover BMD testing repeated yearly; all Medicare carriers cover testing every 2 years. AACE recommends a repeated DXA at 1 to 2 years after initiation of therapy until bone density is stable. This testing pattern can be continued at every 2-year interval and reduced with evidence of persistent BMD stability (Grade B; BEL 2). Because sites rich in trabecular bone, such as the PA spine, are more metabolically active and likely to respond to therapy, an appreciable change is likely to occur earlier at the spine than at the hip.
Treatment failure may be defined as a substantial decrease in BMD or, alternatively, the occurrence of a fracture (Grade B; BEL 2). Some of the treated patients in clinical trials showed bone loss or sustained fractures (or both). It may be, however, that all patients benefit from treatment (if they lose bone with treatment, they may have lost more without it; if they sustain a fracture despite treatment, they may have had a fracture sooner or had multiple fractures without it) (193 [EL 4]). Nevertheless, it is reasonable that a patient with considerable bone loss or a new fragility fracture undergo assessment for compliance with medication, secondary causes of bone loss, or the addition of new medications or diseases that can cause bone loss (Grade B; BEL 2).
The skeletal status can also be examined by assessing the development or progression of asymptomatic vertebral fractures. This can be done by lateral radiography of the thoracic and lumbar spine. Alternatively, vertebral fracture assessment, a technique that can assess vertebral fractures with DXA technology, can often be done at the same time as DXA (199 [EL 2], 200 [EL 3], 201 [EL 2]). Both historical height loss and prospective height loss have been associated with a new vertebral fracture (98 [EL 2], 99 [EL 2]). The ISCD recommends screening for vertebral fractures in older patients with historical height loss >1.6 inches (>4 cm) in women and >2.4 inches (>6 cm) in men, prospective height loss of 0.8 inch (2 cm) in women and 1.2 inches (3 cm) in men, or a self-reported nonvertebral fracture (202 [EL 4]).
Limited published data are available on the use of biochemical markers of bone turnover for follow-up in individual patients. Clinical trials have shown that early changes in bone turnover markers are associated with long-term changes in bone density in women taking antiresorptive (203 [EL 1]) or anabolic (204 [EL 1]) drugs. Significant reductions in bone turnover markers have also been associated with fracture reduction (197 [EL 1]). Antiresorptive therapy is likely effective if bone turnover markers are at or below the median value for premenopausal women (Grade B; BEL 2). Use of a resorption marker, such as a fasting, second-voided urinary N-telopeptide cross-linked collagen type 1 or a fasting morning serum C-telopeptide value, may be helpful in the evaluation of a nonresponder who has bone loss or fractures while receiving therapy or the identification of patients who have high bone turnover. An elevated level associated with high bone turnover in patients receiving therapy could represent poor compliance or the need for evaluation of a secondary cause of bone loss (Grade C; BEL 2).
4.22. Surgical Treatment of Osteoporotic Fractures
Fracture care is usually provided by orthopedic surgeons and is unlikely to be influenced by nonorthopedists. For vertebral fractures, vertebroplasty and kyphoplasty are in the purview of radiologists and neurosurgeons. These procedures are indicated for relief of pain; kyphoplasty has been suggested to provide at least partial reversal of the vertebral deformity. Two recently published comprehensive reviews discussed these treatments (205 [EL 4], 206 [EL 4]). Two sham-controlled studies concluded that vertebroplasty was without benefits (207 [EL 1], 208 [EL 1]), and a controlled study suggested that kyphoplasty was beneficial in restoring vertebral height (209 [EL 2]). Both procedures have been suggested to increase the risk of vertebral fractures in the adjacent vertebrae. Because of limitations to the published studies, the role for these surgical procedures is still uncertain.
Abbreviations
- AACE
American Association of Clinical Endocrinologists
- BEL
“best evidence” level
- BMD
bone mineral density
- DXA
dual-energy x-ray absorptiometry
- EL
evidence level
- FDA
US Food and Drug Administration
- GI
gastrointestinal
- ISCD
International Society for Clinical Densitometry
- LSC
least significant change
- 25(OH)D
25-hydroxyvitamin D
- ONJ
osteonecrosis of the jaw
- OPG
osteoprotegerin
- PA
posteroanterior
- PTH
parathyroid hormone
- R
recommendation
- RANK
receptor activator of nuclear factor-κβ
- RANKL
RANK ligand
- SD
standard deviation
- WHI
Women’s Health Initiative
- WHO
World Health Organization
AACE Osteoporosis Task Force
Chair
Nelson B. Watts, MD, FACP, MACE
Task Force Members
John P. Bilezikian, MD, MACE
Pauline M. Camacho, MD, FACE
Susan L. Greenspan, MD, FACP, FACE
Steven T. Harris, MD, FACE
Stephen F. Hodgson, MD, FACP, MACE
Michael Kleerekoper, MD, MACE
Marjorie M. Luckey, MD, FACE
Michael R. McClung, MD, FACP, FACE
Rachel Pessah Pollack, MD
Steven M. Petak, MD, JD, FACE, FCLM
Reviewers
Donald A. Bergman, MD, FACP, FACE
Neil Binkley, MD
Paul D. Miller, MD, FACP
DISCLOSURE
Chair
Dr. Nelson B. Watts reports that he has received speaker honoraria from Amgen Inc., the International Society for Clinical Densitometry, Novartis AG, and Warner Chilcott; consultant honoraria from Amgen Inc., Arena Pharmaceuticals, Inc., Baxter, InteKrin Therapeutics Inc., Johnson & Johnson Services, Inc., Medpace, Merck & Co., Inc., NPS Pharmaceuticals, Orexigen Therapeutics, Inc., Pfizer Inc, sanofi-aventis U.S. LLC, Takeda, VIVUS, Inc., and Warner Chilcott; and research grant support through the University of Cincinnati Osteoporosis Center from Amgen Inc., Eli Lilly and Company, Merck & Co., Inc., Novartis AG, and NPS Pharmaceuticals.
Task Force Members
Dr. John P. Bilezikian reports that he has received speaker honoraria from Amgen Inc., Eli Lilly and Company, and Novartis AG and consultant honoraria from Amgen Inc., Eli Lilly and Company, Merck & Co., Inc., and Warner Chilcott.
Dr. Pauline M. Camacho reports that she has received research grant support for her role as principal investigator from Eli Lilly and Company and Procter & Gamble.
Dr. Susan L. Greenspan reports that she has received consultant honoraria from Amgen Inc. and Merck & Co., Inc. and research grant support for her role as principal investigator from the Alliance for Better Bone Health (Procter & Gamble/sanofi-aventis U.S. LLC), Eli Lilly and Company, and Warner Chilcott.
Dr. Steven T. Harris reports that he has received speaker honoraria from Amgen Inc., Genentech, Inc., Gilead, GlaxoSmithKline plc, F. Hoffmann-La Roche Ltd, Eli Lilly and Company, Novartis AG, Procter & Gamble, sanofi-aventis U.S. LLC, and Warner Chilcott and consultant honoraria from Amgen Inc., Gilead, GlaxoSmithKline plc, F. Hoffmann-La Roche Ltd, Eli Lilly and Company, Merck & Co., Inc., and Novartis AG.
Dr. Stephen F. Hodgson reports that he does not have any relevant financial relationships with any commercial interests.
Dr. Michael Kleerekoper reports that he has received speaker honoraria from Amgen Inc. and Eli Lilly and Company and speaker and consultant honoraria from F. Hoffmann-La Roche Ltd Diagnostics.
Dr. Marjorie M. Luckey reports that she has received speaker honoraria and consultant fees from Amgen Inc. and Novartis AG.
Dr. Michael R. McClung reports that he has received research grant support, consulting fees, and/or speakers’ bureau honoraria from Amgen Inc., Eli Lilly and Company, Merck & Co., Inc., Novartis AG, and Warner Chilcott.
Dr. Rachel Pessah Pollack reports that she does not have any relevant financial relationships with any commercial interests.
Dr. Steven M. Petak reports that he has received speaker honoraria from Amgen Inc. and the International Society for Clinical Densitometry.
Reviewers
Dr. Donald A. Bergman reports that he does not have any relevant financial relationships with any commercial interests.
Dr. Neil Binkley reports that he has received consultant honoraria from Amgen Inc., Merck & Co. Inc., and Tarsa Therapeutics, Inc. and research grant support from Amgen Inc., Eli Lilly and Company, Merck & Co., Inc., and Tarsa Therapeutics, Inc.
Dr. Paul D. Miller reports that he has received speaker, consultant, and advisory board honoraria from Amgen Inc., Genentech, Inc., GlaxoSmithKline plc, Eli Lilly and Company, Merck & Co., Inc., Novartis AG, NPS Pharmaceuticals, and Warner Chilcott. He also reports that he has received scientific grants from Amgen Inc., F. Hoffmann-La Roche Ltd, Eli Lilly and Company, Merck & Co., Inc., Novartis AG, and Warner Chilcott.
REFERENCES
Note: Reference sources are followed by an evidence level [EL] rating of 1, 2, 3, or 4. The strongest evidence levels (EL 1 and EL 2) appear in red for easier recognition.
- 1.Mechanick JI, Camacho PM, Cobin RH, et al. American Association of Clinical Endocrinologists Clinical Practice Guidelines Subcommittee. American Association of Clinical Endocrinologists protocol for standardized production of clinical practice guidelines—2010 update. Endocr Pract. 2010;16:270–283. doi: 10.4158/EP.16.2.270. [DOI] [PubMed] [Google Scholar]
- 2.Mechanick JI, Bergman DA, Braithwaite SS, Palumbo PJ American Association of Clinical Endocrinologists Ad Hoc Task Force for Standardized Production of Clinical Practice Guidelines. American Association of Clinical Endocrinologists protocol for standardized production of clinical practice guidelines. Endocr Pract. 2004;10:353–361. doi: 10.4158/EP.10.4.353. [published correction appears in Endocr Pract. 2008;14:802–803] [DOI] [PubMed] [Google Scholar]
- 3.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 4.Kanis JA, Melton LJ, III, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [EL 4] [DOI] [PubMed] [Google Scholar]
- 5.Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [EL 4] [DOI] [PubMed] [Google Scholar]
- 6.National Osteoporosis Foundation. America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. Washington, DC: National Osteoporosis Foundation; 2002. [EL 4] [Google Scholar]
- 7.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [EL 3] [DOI] [PubMed] [Google Scholar]
- 8.Looker AC, Orwoll ES, Johnston CC, Jr, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [EL 3] [DOI] [PubMed] [Google Scholar]
- 9.Faulkner KG, von Stetten E, Miller P. Discordance in patient classification using T-scores. J Clin Densitom. 1999;2:343–350. doi: 10.1385/jcd:2:3:343. [EL 3] [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ, III, Lane AW, Cooper C, Eastell R, O’Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993;3:113–119. doi: 10.1007/BF01623271. [EL 3] [DOI] [PubMed] [Google Scholar]
- 11.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [EL 3] [DOI] [PubMed] [Google Scholar]
- 12.Rosamond W, Flegal K, Friday G, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [published corrections appear in Circulation. 2007;115:e172 and 2010;122:e9]. [EL 3] [DOI] [PubMed] [Google Scholar]
- 13.American Cancer Society. Cancer Facts and Figures 2006. Atlanta, GA: American Cancer Society; 2006. [Accessed for verification November 7, 2010]. http://www.cancer.org/acs/groups/content/@nho/documents/document/caff2006pwsecuredpdf.pdf. [EL 3] [Google Scholar]
- 14.Forsén L, Sogaard AJ, Meyer HE, Edna T, Kopjar B. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10:73–78. doi: 10.1007/s001980050197. [EL 2] [DOI] [PubMed] [Google Scholar]
- 15.Poór G, Atkinson EJ, Lewallen DG, O’Fallon WM, Melton LJ., III Age-related hip fractures in men: clinical spectrum and short-term outcomes. Osteoporos Int. 1995;5:419–426. doi: 10.1007/BF01626602. [EL 2] [DOI] [PubMed] [Google Scholar]
- 16.Gehlbach SH, Burge RT, Puleo E, Klar J. Hospital care of osteoporosis-related vertebral fractures. Osteoporos Int. 2003;14:53–60. doi: 10.1007/s00198-002-1313-z. [EL 3] [DOI] [PubMed] [Google Scholar]
- 17.Ray NF, Chan JK, Thamer M, Melton LJ., III Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [EL 3] [DOI] [PubMed] [Google Scholar]
- 18.Greendale GA, Barrett-Connor E. Outcomes of osteoporotic fracture. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego, CA: Academic Press; 2001. pp. 819–829. [EL 3] [Google Scholar]
- 19.Melton LJ, III, Kearns AE, Atkinson EJ, et al. Secular trends in hip fracture incidence and recurrence. Osteoporos Int. 2009;20:687–694. doi: 10.1007/s00198-008-0742-8. [EL 3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Fatalities and injuries from falls among older adults—United States, 1993–2003 and 2001–2005. MMWR Morb Mortal Weekly Rep. 2006;55:1221–1224. [published correction appears in MMWR Morb Mortal Weekly Rep. 2006;55:1303]. [EL 3] [PubMed] [Google Scholar]
- 21.Leslie WD, O’Donnell S, Jean S, et al. Osteoporosis Surveillance Expert Working Group. Trends in hip fracture rates in Canada. JAMA. 2009;302:883–889. doi: 10.1001/jama.2009.1231. [EL 3] [DOI] [PubMed] [Google Scholar]
- 22.Huusko TM, Karppi P, Avikainen V, Kautiainen H, Sulkava R. The changing picture of hip fractures: dramatic change in age distribution and no change in age-adjusted incidence within 10 years in Central Finland. Bone. 1999;24:257–259. doi: 10.1016/s8756-3282(98)00182-3. [EL 3] [DOI] [PubMed] [Google Scholar]
- 23.Newman ED, Ayoub WT, Starkey RH, Diehl JM, Wood GC. Osteoporosis disease management in a rural health care population: hip fracture reduction and reduced costs in postmenopausal women after 5 years. Osteoporos Int. 2003;14:146–151. doi: 10.1007/s00198-002-1336-5. [EL 3] [DOI] [PubMed] [Google Scholar]
- 24.Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am. 2008;90(suppl 4):188–194. doi: 10.2106/JBJS.H.00628. [EL 2] [DOI] [PubMed] [Google Scholar]
- 25.Chevalley T, Guilley E, Herrmann FR, Hoffmeyer P, Rapin CH, Rizzoli R. Incidence of hip fracture over a 10-year period (1991–2000): reversal of a secular trend. Bone. 2007;40:1284–1289. doi: 10.1016/j.bone.2006.12.063. [EL 3] [DOI] [PubMed] [Google Scholar]
- 26.Kilgore ML, Morrisey MA, Becker DJ, et al. Health care expenditures associated with skeletal fractures among Medicare beneficiaries, 1999–2005. J Bone Miner Res. 2009;24:2050–2055. doi: 10.1359/jbmr.090523. [EL 2] [DOI] [PubMed] [Google Scholar]
- 27.Cadarette SM, Katz JN, Brookhart MA, et al. Trends in drug prescribing for osteoporosis after hip fracture, 1995–2004. J Rheumatol. 2008;35:319–326. [EL 3] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YC, Lyle RM, Weaver CM, et al. Peak spine and femoral neck bone mass in young women. Bone. 2003;32:546–553. doi: 10.1016/s8756-3282(03)00062-0. [EL 2] [DOI] [PubMed] [Google Scholar]
- 29.Matkovic V, Jelic T, Wardlaw GM, et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis: inference from a cross-sectional model. J Clin Invest. 1994;93:799–808. doi: 10.1172/JCI117034. [EL 3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown LB, Streeten EA, Shapiro JR, et al. Genetic and environmental influences on bone mineral density in pre- and post-menopausal women. Osteoporos Int. 2005;16:1849–1856. doi: 10.1007/s00198-005-1948-7. [EL 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recker RR, Deng HW. Role of genetics in osteoporosis. Endocrine. 2002;17:55–66. doi: 10.1385/ENDO:17:1:55. [EL 4] [DOI] [PubMed] [Google Scholar]
- 32.Williams F. Genetic regulation of bone mass and susceptibility to osteoporosis. J Musculoskel Neuron Interact. 2006;6:27–35. [EL 3] [Google Scholar]
- 33.Richards JB, Kavvoura FK, Rivadeneira F, et al. Genetic Factors for Osteoporosis Consortium. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med. 2009;151:528–537. doi: 10.7326/0003-4819-151-8-200910200-00006. [EL 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vega D, Maalouf NM, Sakhaee K. The role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: clinical implications. J Clin Endocrinol Metab. 2007;92:4514–4521. doi: 10.1210/jc.2007-0646. [EL 4] [DOI] [PubMed] [Google Scholar]
- 35.Mackey DC, Lui LY, Cawthon PM, et al. Study of Osteoporotic Fractures [SOF] and Osteoporotic Fractures in Men Study [MrOS] Research Groups. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–2388. doi: 10.1001/jama.298.20.2381. [EL 2] [DOI] [PubMed] [Google Scholar]
- 36.Orwig DL, Chan J, Magaziner J. Hip fracture and its consequences: differences between men and women. Orthop Clin North Am. 2006;37:611–622. doi: 10.1016/j.ocl.2006.08.003. [EL 4] [DOI] [PubMed] [Google Scholar]
- 37.Green AD, Colón-Emeric CS, Bastian L, Drake MT, Lyles KW. Does this woman have osteoporosis? JAMA. 2004;292:2890–2900. doi: 10.1001/jama.292.23.2890. [EL 3] [DOI] [PubMed] [Google Scholar]
- 38.Kanis JA World Health Organisation Scientific Group. Assessment of Osteoporosis at the Primary Health Care Level: WHO Scientific Technical Report. Sheffield, UK: WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield; 2008. [EL 4] [Google Scholar]
- 39.Watts NB, Ettinger B, LeBoff MS. FRAX facts. J Bone Miner Res. 2009;24:975–979. doi: 10.1359/jbmr.090402. [EL 4] [DOI] [PubMed] [Google Scholar]
- 40.Watts NB, Lewiecki EM, Miller PD, Baim S. National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom. 2008;11:473–477. doi: 10.1016/j.jocd.2008.04.003. [EL 4] [DOI] [PubMed] [Google Scholar]
- 41.Rud B, Hilden J, Hyldstrup L, Hróbjartsson A. Performance of the Osteoporosis Self-Assessment Tool in ruling out low bone mineral density in postmenopausal women: a systematic review. Osteoporos Int. 2007;18:1177–1187. doi: 10.1007/s00198-006-0319-3. [published correction appears in Osteoporos Int. 2007;18:1307]. [EL 4] [DOI] [PubMed] [Google Scholar]
- 42.Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [EL 2] [DOI] [PubMed] [Google Scholar]
- 43.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [EL 2] [DOI] [PubMed] [Google Scholar]
- 44.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, III, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [EL 4] [DOI] [PubMed] [Google Scholar]
- 45.Cummings SR, Cawthon PM, Ensrud KE, Cauley JA, Fink HA, Orwoll ES Osteoporotic Fractures in Men [MrOS] Research Groups; Study of Osteoporotic Fractures Research Groups. BMD and risk of hip and nonvertebral fractures in older men: a prospective study and comparison with older women. J Bone Miner Res. 2006;21:1550–1556. doi: 10.1359/jbmr.060708. [EL 2] [DOI] [PubMed] [Google Scholar]
- 46.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [published correction appears in J Bone Miner Res. 2007;22:774]. [EL 2] [DOI] [PubMed] [Google Scholar]
- 47.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [EL 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–995. doi: 10.1007/s001980170006. [EL 1] [DOI] [PubMed] [Google Scholar]
- 49.Sambrook PN, Cameron ID, Chen JS, et al. Influence of fall related factors and bone strength on fracture risk in the frail elderly. Osteoporos Int. 2007;18:603–610. doi: 10.1007/s00198-006-0290-z. [EL 2] [DOI] [PubMed] [Google Scholar]
- 50.Misra M, Klibanski A. Anorexia nervosa and osteoporosis. Rev Endocr Metab Disord. 2006;7:91–99. doi: 10.1007/s11154-006-9005-1. [EL 4] [DOI] [PubMed] [Google Scholar]
- 51.Rizzoli R, Ammann P, Chevalley T, Bonjour JP. Protein intake and bone disorders in the elderly. Joint Bone Spine. 2001;68:383–392. doi: 10.1016/s1297-319x(01)00295-0. [EL 4] [DOI] [PubMed] [Google Scholar]
- 52.Schürch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:801–809. doi: 10.7326/0003-4819-128-10-199805150-00002. [EL 1] [DOI] [PubMed] [Google Scholar]
- 53.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. doi: 10.1210/jc.2010-2704. [published online ahead of print November 29, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan GM, Hoffman K, McMurry M. Effects of dairy products on bone and body composition in pubertal girls. J Pediatr. 1995;126:551–556. doi: 10.1016/s0022-3476(95)70348-9. [EL 1] [DOI] [PubMed] [Google Scholar]
- 55.Gibbons MJ, Gilchrist NL, Frampton C, et al. The effects of a high calcium dairy food on bone health in pre-pubertal children in New Zealand. Asia Pac J Clin Nutr. 2004;13:341–347. [EL 1] [PubMed] [Google Scholar]
- 56.Ho SC, Guldan GS, Woo J, et al. A prospective study of the effects of 1-year calcium-fortified soy milk supplementation on dietary calcium intake and bone health in Chinese adolescent girls aged 14 to 16. Osteoporos Int. 2005;16:1907–1916. doi: 10.1007/s00198-005-1963-8. [EL 2] [DOI] [PubMed] [Google Scholar]
- 57.Merrilees MJ, Smart EJ, Gilchrist NL, et al. Effects of dairy food supplements on bone mineral density in teenage girls. Eur J Nutr. 2000;39:256–262. doi: 10.1007/s003940070004. [EL 2] [DOI] [PubMed] [Google Scholar]
- 58.Winzenberg T, Shaw K, Fryer J, Jones G. Effects of calcium supplementation on bone density in healthy children: meta-analysis of randomised controlled trials. BMJ. 2006;333:775. doi: 10.1136/bmj.38950.561400.55. [EL 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alaimo K, McDowell MA, Briefel RR, et al. Advance data from vital and health statistics, No. 258. Hyattsville, MD: National Center for Health Statistics; 1994. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, phase 1, 1988–91; pp. 1–28. [EL 3] [PubMed] [Google Scholar]
- 60.Porthouse J, Cockayne S, King C, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330:1003. doi: 10.1136/bmj.330.7498.1003. [EL 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shea B, Wells G, Cranney A, et al. Osteoporosis Methodology Group and the Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis, VII: meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev. 2002;23:552–559. doi: 10.1210/er.2001-7002. [EL 1] [DOI] [PubMed] [Google Scholar]
- 62.Jackson RD, LaCroix AZ, Gass M, et al. Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [EL 1] [DOI] [PubMed] [Google Scholar]
- 63.Grant AM, Avenell A, Campbell MK, et al. RECORD Trial Group. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium OR vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365:1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [EL 1] [DOI] [PubMed] [Google Scholar]
- 64.Holick MF, Siris ES, Binkley N, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [EL 2] [DOI] [PubMed] [Google Scholar]
- 65.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [EL 4] [DOI] [PubMed] [Google Scholar]
- 66.Binkley N, Novotny R, Krueger D, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–2135. doi: 10.1210/jc.2006-2250. [EL 3] [DOI] [PubMed] [Google Scholar]
- 67.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [EL 2] [DOI] [PubMed] [Google Scholar]
- 68.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [EL 2] [DOI] [PubMed] [Google Scholar]
- 69.Bischoff-Ferrari HA, Conzelmann M, Stähelin HB, et al. Is fall prevention by vitamin D mediated by a change in postural or dynamic balance? Osteoporos Int. 2006;17:656–663. doi: 10.1007/s00198-005-0030-9. [EL 2] [DOI] [PubMed] [Google Scholar]
- 70.Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–1118. doi: 10.1359/jbmr.2000.15.6.1113. [published corrections appear in J Bone Miner Res. 2001;16:1735, 1935]. [EL 2] [DOI] [PubMed] [Google Scholar]
- 71.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [EL 2] [DOI] [PubMed] [Google Scholar]
- 72.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–681. doi: 10.1210/jc.2007-2308. [EL 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–5391. doi: 10.1210/jc.2004-0360. [EL 2] [DOI] [PubMed] [Google Scholar]
- 74.Spencer H, Fuller H, Norris C, Williams D. Effect of magnesium on the intestinal absorption of calcium in man. J Am Coll Nutr. 1994;13:485–492. doi: 10.1080/07315724.1994.10718439. [EL 1] [DOI] [PubMed] [Google Scholar]
- 75.Jackson HA, Sheehan AH. Effect of vitamin A on fracture risk. Ann Pharmacother. 2005;39:2086–2090. doi: 10.1345/aph.1G028. [EL 4] [DOI] [PubMed] [Google Scholar]
- 76.Braam LA, Knapen MH, Geusens P, et al. Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age. Calcif Tissue Int. 2003;73:21–26. doi: 10.1007/s00223-002-2084-4. [EL 1] [DOI] [PubMed] [Google Scholar]
- 77.Alexandersen P, Toussaint A, Christiansen C, et al. Ipraflavone Multicenter European Fracture Study. Ipriflavone in the treatment of postmenopausal osteoporosis: a randomized controlled trial. JAMA. 2001;285:1482–1488. doi: 10.1001/jama.285.11.1482. [EL 1] [DOI] [PubMed] [Google Scholar]
- 78.Atkinson C, Compston JE, Day NE, Dowsett M, Bingham SA. The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2004;79:326–333. doi: 10.1093/ajcn/79.2.326. [EL 1] [DOI] [PubMed] [Google Scholar]
- 79.Gallagher JC, Satpathy R, Rafferty K, Haynatzka V. The effect of soy protein isolate on bone metabolism. Menopause. 2004;11:290–298. doi: 10.1097/01.gme.0000097845.95550.71. [EL 2] [DOI] [PubMed] [Google Scholar]
- 80.Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–742. doi: 10.1007/s00198-004-1734-y. [EL 2] [DOI] [PubMed] [Google Scholar]
- 81.Hallström H, Wolk A, Glynn A, Michaëlsson K. Coffee, tea and caffeine consumption in relation to osteoporotic fracture risk in a cohort of Swedish women. Osteoporos Int. 2006;17:1055–1064. doi: 10.1007/s00198-006-0109-y. [EL 3] [DOI] [PubMed] [Google Scholar]
- 82.Kiel DP, Felson DT, Hannan MT, Anderson JJ, Wilson PW. Caffeine and the risk of hip fracture: the Framingham Study. Am J Epidemiol. 1990;132:675–684. doi: 10.1093/oxfordjournals.aje.a115709. [EL 3] [DOI] [PubMed] [Google Scholar]
- 83.Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [EL 2] [DOI] [PubMed] [Google Scholar]
- 84.Snow-Harter C, Bouxsein M, Lewis B, Charette S, Weinstein P, Marcus R. Muscle strength as a predictor of bone mineral density in young women. J Bone Miner Res. 1990;5:589–595. doi: 10.1002/jbmr.5650050608. [EL 2] [DOI] [PubMed] [Google Scholar]
- 85.Snow-Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R. Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res. 1992;7:761–769. doi: 10.1002/jbmr.5650070706. [EL 1] [DOI] [PubMed] [Google Scholar]
- 86.Kelley GA, Kelley KS, Tran ZV. Exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis of individual patient data. J Gerontol A Biol Sci Med Sci. 2002;57:M599–M604. doi: 10.1093/gerona/57.9.m599. [EL 2] [DOI] [PubMed] [Google Scholar]
- 87.Gardner MM, Phty M, Robertson MC, McGee R, Campbell AJ. Application of a falls prevention program for older people to primary health care practice. Prev Med. 2002;34:546–553. doi: 10.1006/pmed.2002.1017. [EL 2] [DOI] [PubMed] [Google Scholar]
- 88.Robertson MC, Campbell AJ, Gardner MM, Devlin N. Preventing injuries in older people by preventing falls: a meta-analysis of individual-level data. J Am Geriatr Soc. 2002;50:905–911. doi: 10.1046/j.1532-5415.2002.50218.x. [EL 2] [DOI] [PubMed] [Google Scholar]
- 89.Parker MJ, Gillespie WJ, Gillespie LD. Effectiveness of hip protectors for preventing hip fractures in elderly people: systematic review. BMJ. 2006;332:571–574. doi: 10.1136/bmj.38753.375324.7C. [EL 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sawka AM, Boulos P, Beattie K, et al. Do hip protectors decrease the risk of hip fracture in institutional and community-dwelling elderly? A systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2005;16:1461–1474. doi: 10.1007/s00198-005-1932-2. [EL 1] [DOI] [PubMed] [Google Scholar]
- 91.Singh S, Sun H, Anis AH. Cost-effectiveness of hip protectors in the prevention of osteoporosis related hip fractures in elderly nursing home residents. J Rheumatol. 2004;31:1607–1613. [EL 3] [PubMed] [Google Scholar]
- 92.van Schoor NM, Asma G, Smit JH, Bouter LM, Lips P. The Amsterdam Hip Protector Study: compliance and determinants of compliance. Osteoporos Int. 2003;14:353–359. doi: 10.1007/s00198-003-1382-7. [EL 2] [DOI] [PubMed] [Google Scholar]
- 93.Warnke A, Meyer G, Bender R, Mühlhauser I. Predictors of adherence to the use of hip protectors in nursing home residents. J Am Geriatr Soc. 2004;52:340–345. doi: 10.1111/j.1532-5415.2004.52103.x. [EL 2] [DOI] [PubMed] [Google Scholar]
- 94.Kiel DP, Magaziner J, Zimmerman S, et al. Efficacy of a hip protector to prevent hip fracture in nursing home residents: the HIP PRO randomized controlled trial. JAMA. 2007;298:413–422. doi: 10.1001/jama.298.4.413. [EL 1] [DOI] [PubMed] [Google Scholar]
- 95.Li WC, Chen YC, Yang RS, Tsauo JY. Effects of exercise programmes on quality of life in osteoporotic and osteopenic postmenopausal women: a systematic review and meta-analysis. Clin Rehabil. 2009;23:888–896. doi: 10.1177/0269215509339002. [EL 1] [DOI] [PubMed] [Google Scholar]
- 96.Lewiecki EM, Laster AJ. Clinical review: clinical applications of vertebral fracture assessment by dual-energy x-ray absorptiometry. J Clin Endocrinol Metab. 2006;91:4215–4222. doi: 10.1210/jc.2006-1178. [EL 4] [DOI] [PubMed] [Google Scholar]
- 97.Schneider DL, von Mühlen D, Barrett-Connor E, Sartoris DJ. Kyphosis does not equal vertebral fractures: the Rancho Bernardo study. J Rheumatol. 2004;31:747–752. [EL 2] [PubMed] [Google Scholar]
- 98.Siminoski K, Jiang G, Adachi JD, et al. Accuracy of height loss during prospective monitoring for detection of incident vertebral fractures. Osteoporos Int. 2005;16:403–410. doi: 10.1007/s00198-004-1709-z. [EL 2] [DOI] [PubMed] [Google Scholar]
- 99.Siminoski K, Warshawski RS, Jen H, Lee K. The accuracy of historical height loss for the detection of vertebral fractures in postmenopausal women. Osteoporos Int. 2006;17:290–296. doi: 10.1007/s00198-005-2017-y. [EL 2] [DOI] [PubMed] [Google Scholar]
- 100.Barr RJ, Stewart A, Torgerson DJ, Reid DM. Population screening for osteoporosis risk: a randomised control trial of medication use and fracture risk. Osteoporos Int. 2009;21:561–568. doi: 10.1007/s00198-009-1007-x. [EL 1] [DOI] [PubMed] [Google Scholar]
- 101.Tannenbaum C, Clark J, Schwartzman K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2002;87:4431–4437. doi: 10.1210/jc.2002-020275. [EL 2] [DOI] [PubMed] [Google Scholar]
- 102.Barzel US. Recommended testing in patients with low bone density [with author reply] J Clin Endocrinol Metab. 2003;88:1404–1405. doi: 10.1210/jc.2002-021951. [EL 4] [DOI] [PubMed] [Google Scholar]
- 103.Chesnut CH, III, Bell NH, Clark GS, et al. Hormone replacement therapy in postmenopausal women: urinary N-telopeptide of type I collagen monitors therapeutic effect and predicts response of bone mineral density. Am J Med. 1997;102:29–37. doi: 10.1016/s0002-9343(96)00387-7. [EL 1] [DOI] [PubMed] [Google Scholar]
- 104.Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11:1531–1538. doi: 10.1002/jbmr.5650111021. [EL 2] [DOI] [PubMed] [Google Scholar]
- 105.Greenspan SL, Rosen HN, Parker RA. Early changes in serum N-telopeptide and C-telopeptide cross-linked collagen type 1 predict long-term response to alendronate therapy in elderly women. J Clin Endocrinol Metab. 2000;85:3537–3540. doi: 10.1210/jcem.85.10.6911. [EL 1] [DOI] [PubMed] [Google Scholar]
- 106.National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008. [Accessed for verification November 7, 2010]. http://www.nof.org/sites/default/files/pdfs/NOF_Clinicians_Guide2008.pdf. [EL 4] [Google Scholar]
- 107.Jamal SA, Bauer DC, Ensrud KE, et al. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the Fracture Intervention Trial. J Bone Miner Res. 2007;22:503–508. doi: 10.1359/jbmr.070112. [EL 2] [DOI] [PubMed] [Google Scholar]
- 108.Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20:2105–2115. doi: 10.1359/JBMR.050817. [EL 1] [DOI] [PubMed] [Google Scholar]
- 109.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorders (CKD-MBD) Kidney Int Suppl. 2009 Aug;76(suppl 113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 110.Adami S, Bhalla AK, Dorizzi R, et al. The acute-phase response after bisphosphonate administration. Calcif Tissue Int. 1987;41:326–331. doi: 10.1007/BF02556671. [EL 3] [DOI] [PubMed] [Google Scholar]
- 111.Jones SG, Dolan G, Lengyel K, Myers B. Severe increase in creatinine with hypocalcaemia in thalidomide-treated myeloma patients receiving zoledronic acid infusions. Br J Haematol. 2002;119:576–577. doi: 10.1046/j.1365-2141.2002.03835_4.x. [EL 3] [DOI] [PubMed] [Google Scholar]
- 112.Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid [letter] N Engl J Med. 2003;349:1676–1678. doi: 10.1056/NEJM200310233491721. [EL 3] [DOI] [PubMed] [Google Scholar]
- 113.Tarassoff P, Hei YJ, Maladorno D. Renal failure with the use of zoledronic acid [reply to letter] N Engl J Med. 2003;349:1678–1679. [EL 3] [Google Scholar]
- 114.FDA U.S. Food and Drug Administration. Zoledronic acid for osteoporosis (marketed as Reclast): renal impairment and acute renal failure. [Accessed for verification November 7, 2010];Drug Safety Newsletter. 2009 2:13–15. http://www.fda.gov/Drugs/DrugSafety/DrugSafetyNewsletter/default.htm. [EL 3] [Google Scholar]
- 115.Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med. 2005;165:346–347. doi: 10.1001/archinte.165.3.346-b. [EL 3] [DOI] [PubMed] [Google Scholar]
- 116.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [published correction appears in Ann Intern Med. 2006;145:235]. [EL 4] [DOI] [PubMed] [Google Scholar]
- 117.Bilezikian J. Osteonecrosis of the jaw—do bisphosphonates pose a risk? N Engl J Med. 2006;355:2278–2282. doi: 10.1056/NEJMp068157. [EL 4] [DOI] [PubMed] [Google Scholar]
- 118.Khosla S, Burr D, Cauley J, et al. American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [EL 4] [DOI] [PubMed] [Google Scholar]
- 119.Bauer DC, Black D, Ensrud K, et al. Upper gastrointestinal tract safety profile of alendronate: the Fracture Intervention Trial. Arch Intern Med. 2000;160:517–525. doi: 10.1001/archinte.160.4.517. [EL 1] [DOI] [PubMed] [Google Scholar]
- 120.Lenart BA, Neviaser AS, Lyman S, et al. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;20:1353–1362. doi: 10.1007/s00198-008-0805-x. [EL 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Watts N, Freedholm D, Daifotis A. The clinical tolerability profile of alendronate. Int J Clin Pract Suppl. 1999;101:51–61. [EL 4] [PubMed] [Google Scholar]
- 122.de Groen PC, Lubbe DF, Hirsch LJ, et al. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335:1016–1021. doi: 10.1056/NEJM199610033351403. [EL 3] [DOI] [PubMed] [Google Scholar]
- 123.Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 124.Cardwell CR, Abnet CC, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA. 2010;304:657–663. doi: 10.1001/jama.2010.1098. [EL 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341:c4444. doi: 10.1136/bmj.c4444. [EL 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Black DM, Delmas PD, Eastell R, et al. HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [EL 1] [DOI] [PubMed] [Google Scholar]
- 127.Fosamax [package insert] Whitehouse Station, NJ: Merck & Co., Inc.; 2010. [Google Scholar]
- 128.Black DM, Cummings SR, Karpf DB, et al. Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [EL 1] [DOI] [PubMed] [Google Scholar]
- 129.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–2082. doi: 10.1001/jama.280.24.2077. [EL 1] [DOI] [PubMed] [Google Scholar]
- 130.Black DM, Thompson DE, Bauer DC, et al. Fracture Intervention Trial [FIT] Research Group. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. J Clin Endocrinol Metab. 2000;85:4118–4124. doi: 10.1210/jcem.85.11.6953. [published correction appears in J Clin Endocrinol Metab. 2001;86:938]. [EL 2] [DOI] [PubMed] [Google Scholar]
- 131.Pols HA, Felsenberg D, Hanley DA, et al. Fosamax International Trial Study Group. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Osteoporos Int. 1999;9:461–468. doi: 10.1007/pl00004171. [EL 1] [DOI] [PubMed] [Google Scholar]
- 132.Liberman UA, Weiss SR, Bröll J, et al. Alendronate Phase III Osteoporosis Treatment Study Group. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [EL 1] [DOI] [PubMed] [Google Scholar]
- 133.Bone HG, Hosking D, Devogelaer JP, et al. Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [EL 4] [DOI] [PubMed] [Google Scholar]
- 134.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX); a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [EL 1] [DOI] [PubMed] [Google Scholar]
- 135.Castell DO. “Pill esophagitis”—the case of alendronate. N Engl J Med. 1996;335:1058–1059. doi: 10.1056/NEJM199610033351412. [EL 3] [DOI] [PubMed] [Google Scholar]
- 136.Famularo G, De Simone C. Fatal esophageal perforation with alendronate. Am J Gastroenterol. 2001;96:3212–3213. doi: 10.1111/j.1572-0241.2001.05291.x. [EL 3] [DOI] [PubMed] [Google Scholar]
- 137.Donahue JG, Chan KA, Andrade SE, et al. Gastric and duodenal safety of daily alendronate. Arch Intern Med. 2002;162:936–942. doi: 10.1001/archinte.162.8.936. [EL 2] [DOI] [PubMed] [Google Scholar]
- 138.Greenspan S, Field-Munves E, Tonino R, et al. Tolerability of once-weekly alendronate in patients with osteoporosis: a randomized, double-blind, placebo-controlled study. Mayo Clin Proc. 2002;77:1044–1052. doi: 10.4065/77.10.1044. [EL 1] [DOI] [PubMed] [Google Scholar]
- 139.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–1565. doi: 10.1210/jc.2009-1947. [EL 4] [DOI] [PubMed] [Google Scholar]
- 140.Actonel [package insert] Cincinnati, OH: Procter & Gamble Pharmaceuticals, Inc.; 2010. [Google Scholar]
- 141.Harris ST, Watts NB, Genant HK, et al. Vertebral Efficacy With Risedronate Therapy [VERT] Study Group. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [EL 1] [DOI] [PubMed] [Google Scholar]
- 142.Reginster J, Minne HW, Sorensen OH, et al. Vertebral Efficacy With Risedronate Therapy [VERT] Study Group. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [EL 1] [DOI] [PubMed] [Google Scholar]
- 143.McClung MR, Geusens P, Miller PD, et al. Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [EL 1] [DOI] [PubMed] [Google Scholar]
- 144.Sorensen OH, Crawford GM, Mulder H, et al. Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience. Bone. 2003;32:120–126. doi: 10.1016/s8756-3282(02)00946-8. [EL 1] [DOI] [PubMed] [Google Scholar]
- 145.Mellström DD, Sörensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75:462–468. doi: 10.1007/s00223-004-0286-7. [EL 2] [DOI] [PubMed] [Google Scholar]
- 146.Watts NB, Chines A, Olszynski WP, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008;19:365–372. doi: 10.1007/s00198-007-0460-7. [EL 1] [DOI] [PubMed] [Google Scholar]
- 147.Boniva [package insert] Nutley, NJ: Roche Therapeutics Inc.; 2010. [Google Scholar]
- 148.Chesnut CH, III, Skag A, Christiansen C, et al. Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe [BONE] Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249. doi: 10.1359/JBMR.040325. [EL 1] [DOI] [PubMed] [Google Scholar]
- 149.McClung MR, Wasnich RD, Recker R, et al. Oral Ibandronate Study Group. Oral daily ibandronate prevents bone loss in early postmenopausal women without osteoporosis. J Bone Miner Res. 2004;19:11–18. doi: 10.1359/JBMR.0301202. [EL 1] [DOI] [PubMed] [Google Scholar]
- 150.Reclast [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2010. [Google Scholar]
- 151.McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41:122–128. doi: 10.1016/j.bone.2007.03.011. [EL 1] [DOI] [PubMed] [Google Scholar]
- 152.Reid DM, Devogelaer JP, Saag K, et al. HORIZON Investigators. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2009;373:1253–1263. doi: 10.1016/S0140-6736(09)60250-6. [EL 1] [DOI] [PubMed] [Google Scholar]
- 153.Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [EL 1] [DOI] [PubMed] [Google Scholar]
- 154.Evista [package insert] Indianapolis, IN: Eli Lilly and Company; 2008. [Google Scholar]
- 155.Ettinger B, Black DM, Mitlak BH, et al. Multiple Outcomes of Raloxifene Evaluation [MORE] Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [EL 1] [DOI] [PubMed] [Google Scholar]
- 156.Delmas PD, Ensrud KE, Adachi JD, et al. Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617. doi: 10.1210/jcem.87.8.8750. [EL 1] [DOI] [PubMed] [Google Scholar]
- 157.Barrett-Connor E, Mosca L, Collins P, et al. Raloxifene Use for the Heart [RUTH] Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [EL 1] [DOI] [PubMed] [Google Scholar]
- 158.Vogel VG, Costantino JP, Wickerham DL, et al. National Surgical Adjuvant Breast and Bowel Project [NSABP] Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [published corrections appear in JAMA 2006;296:2926 and JAMA. 2007;298:973]. [EL 1] [DOI] [PubMed] [Google Scholar]
- 159.Siris ES, Harris ST, Eastell R, et al. Continuing Outcomes Relevant to Evista [CORE] Investigators. Skeletal effects of raloxifene after 8 years: results from the Continuing Outcomes Relevant to Evista (CORE) study. J Bone Miner Res. 2005;20:1514–1524. doi: 10.1359/JBMR.050509. [EL 1] [DOI] [PubMed] [Google Scholar]
- 160.Forteo [package insert] Indianapolis, IN: Eli Lilly and Company; 2010. [Google Scholar]
- 161.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [EL 1] [DOI] [PubMed] [Google Scholar]
- 162.Watts NB, Miller PD, Kohlmeier LA, et al. Vertebral fracture risk is reduced in women who lose femoral neck BMD with teriparatide treatment. J Bone Miner Res. 2009;24:1125–1131. doi: 10.1359/jbmr.081256. [EL 2] [DOI] [PubMed] [Google Scholar]
- 163.Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–321. doi: 10.1080/01926230252929882. [EL 1] [DOI] [PubMed] [Google Scholar]
- 164.Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1–34)] are dependent on duration of treatment and dose. Toxicol Pathol. 2004;32:426–438. doi: 10.1080/01926230490462138. [EL 1] [DOI] [PubMed] [Google Scholar]
- 165.Harper KD, Krege JH, Marcus R, Mitlak BH. Osteosarcoma and teriparatide? J Bone Miner Res. 2007;22:334. doi: 10.1359/jbmr.061111. [EL 4] [DOI] [PubMed] [Google Scholar]
- 166.Subbiah V, Madsen VS, Raymond AK, Benjamin RS, Ludwig JA. Of mice and men: divergent risks of teriparatide-induced osteosarcoma. Osteoporos Int. 2010;21:1041–1045. doi: 10.1007/s00198-009-1004-0. [EL 3] [DOI] [PubMed] [Google Scholar]
- 167.Lindsay R, Scheele WH, Neer R, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med. 2004;164:2024–2030. doi: 10.1001/archinte.164.18.2024. [EL 2] [DOI] [PubMed] [Google Scholar]
- 168.Black DM, Bilezikian JP, Ensrud KE, et al. PaTH Study Investigators. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med. 2005;353:555–565. doi: 10.1056/NEJMoa050336. [EL 1] [DOI] [PubMed] [Google Scholar]
- 169.Fortical [package insert] Minneapolis, MN: Upsher-Smith Laboratories, Inc.; 2009. [Google Scholar]
- 170.Miacalcin [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2009. [Google Scholar]
- 171.Chesnut CH, III, Silverman S, Andriano K, et al. PROOF Study Group. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the Prevent Recurrence Of Osteoporotic Fractures study. Am J Med. 2000;109:267–276. doi: 10.1016/s0002-9343(00)00490-3. [EL 1] [DOI] [PubMed] [Google Scholar]
- 172.Cummings SR, San Martin J, McClung MR, et al. FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [published correction appears in N Engl J Med. 2009;361:1914]. [EL 1] [DOI] [PubMed] [Google Scholar]
- 173.Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2008;93:2149–2157. doi: 10.1210/jc.2007-2814. [EL 1] [DOI] [PubMed] [Google Scholar]
- 174.Miller PD, Bolognese MA, Lewiecki EM, et al. AMG Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229. doi: 10.1016/j.bone.2008.04.007. [EL 1] [DOI] [PubMed] [Google Scholar]
- 175.Premarin [package insert] Philadelphia, PA: Ayerst Laboratories; 2010. [Google Scholar]
- 176.Cauley JA, Robbins J, Chen Z, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [EL 1] [DOI] [PubMed] [Google Scholar]
- 177.Jackson RD, Wactawski-Wende J, LaCroix AZ, et al. Women’s Health Initiative Investigators. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the Women’s Health Initiative randomized trial. J Bone Miner Res. 2006;21:817–828. doi: 10.1359/jbmr.060312. [EL 1] [DOI] [PubMed] [Google Scholar]
- 178.Writing Group for the PEPI. Effects of hormone therapy on bone mineral density: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. JAMA. 1996;276:1389–1396. [EL 1] [PubMed] [Google Scholar]
- 179.Genant HK, Lucas J, Weiss S, et al. Estratab/Osteoporosis Study Group. Low-dose esterified estrogen therapy: effects on bone, plasma estradiol concentrations, endometrium, and lipid levels. Arch Intern Med. 1997;157:2609–2615. doi: 10.1001/archinte.157.22.2609. [EL 1] [DOI] [PubMed] [Google Scholar]
- 180.Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668–2676. doi: 10.1001/jama.287.20.2668. [EL 1] [DOI] [PubMed] [Google Scholar]
- 181.Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [EL 1] [DOI] [PubMed] [Google Scholar]
- 182.Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [EL 1] [DOI] [PubMed] [Google Scholar]
- 183.Gallagher JC, Rapuri PB, Haynatzki G, Detter JR. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab. 2002;87:4914–4923. doi: 10.1210/jc.2002-020727. [EL 1] [DOI] [PubMed] [Google Scholar]
- 184.Compston JE, Watts NB. Combination therapy for postmenopausal osteoporosis. Clin Endocrinol (Oxf) 2002;56:565–569. doi: 10.1046/j.1365-2265.2002.01536.x. [EL 4] [DOI] [PubMed] [Google Scholar]
- 185.Black DM, Greenspan SL, Ensrud KE, et al. PaTH Study Investigators. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [EL 1] [DOI] [PubMed] [Google Scholar]
- 186.Watts NB, Harris ST, Genant HK, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med. 1990;323:73–79. doi: 10.1056/NEJM199007123230201. [EL 1] [DOI] [PubMed] [Google Scholar]
- 187.Aredia [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2008. [Google Scholar]
- 188.Didronel [package insert] Cincinnati, OH: Procter & Gamble Pharmaceuticals, Inc.; 2010. [Google Scholar]
- 189.Peretz A, Body JJ, Dumon JC, et al. Cyclical pamidronate infusions in postmenopausal osteoporosis. Maturitas. 1996;25:69–75. doi: 10.1016/0378-5122(96)01118-8. [EL 2] [DOI] [PubMed] [Google Scholar]
- 190.Greenspan SL, Maitland LA, Myers ER, Krasnow MB, Kido TH. Femoral bone loss progresses with age: a longitudinal study in women over age 65. J Bone Miner Res. 1994;9:1959–1965. doi: 10.1002/jbmr.5650091216. [EL 2] [DOI] [PubMed] [Google Scholar]
- 191.Ooms ME, Lips P, Roos JC, et al. Vitamin D status and sex hormone binding globulin: determinants of bone turnover and bone mineral density in elderly women. J Bone Miner Res. 1995;10:1177–1184. doi: 10.1002/jbmr.5650100806. [EL 2] [DOI] [PubMed] [Google Scholar]
- 192.Lane NE. An update on glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 2001;27:235–253. doi: 10.1016/s0889-857x(05)70196-4. [EL 4] [DOI] [PubMed] [Google Scholar]
- 193.Lewiecki EM, Watts NB. Assessing response to osteoporosis therapy. Osteoporos Int. 2008;19:1363–1368. doi: 10.1007/s00198-008-0661-8. [EL 4] [DOI] [PubMed] [Google Scholar]
- 194.Lenchik L, Kiebzak GM, Blunt BA International Society for Clinical Densitometry Position Development Panel and Scientific Advisory Committee. What is the role of serial bone mineral density measurements in patient management? J Clin Densitom. 2002;5(suppl):S29–S38. doi: 10.1385/jcd:5:3s:s29. [EL 4] [DOI] [PubMed] [Google Scholar]
- 195.Baim S, Wilson CR, Lewiecki EM, Luckey MM, Downs RW, Jr, Lentle BC. Precision assessment and radiation safety for dual-energy x-ray absorptiometry: position paper of the International Society for Clinical Densitometry. J Clin Densitom. 2005;8:371–378. doi: 10.1385/jcd:8:4:371. [EL 4] [DOI] [PubMed] [Google Scholar]
- 196.Bouxsein ML. Mechanisms of osteoporosis therapy: a bone strength perspective. Clin Cornerstone. 2003;5(suppl 2):S13–S21. doi: 10.1016/s1098-3597(03)90043-3. [EL 4] [DOI] [PubMed] [Google Scholar]
- 197.Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002;87:1586–1592. doi: 10.1210/jcem.87.4.8415. [EL 1] [DOI] [PubMed] [Google Scholar]
- 198.Tosteson AN, Grove MR, Hammond CS, et al. Early discontinuation of treatment for osteoporosis. Am J Med. 2003;115:209–216. doi: 10.1016/s0002-9343(03)00362-0. [EL 3] [DOI] [PubMed] [Google Scholar]
- 199.Greenspan SL, von Stetten E, Emond SK, Jones L, Parker RA. Instant vertebral assessment: a noninvasive dual x-ray absorptiometry technique to avoid misclassification and clinical mismanagement of osteoporosis. J Clin Densitom. 2001;4:373–380. doi: 10.1385/jcd:4:4:373. [EL 2] [DOI] [PubMed] [Google Scholar]
- 200.Schousboe JT, Debold CR. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int. 2006;17:281–289. doi: 10.1007/s00198-005-2010-5. [EL 3] [DOI] [PubMed] [Google Scholar]
- 201.Binkley N, Krueger D, Gangnon R, Genant HK, Drezner MK. Lateral vertebral assessment: a valuable technique to detect clinically significant vertebral fractures. Osteoporos Int. 2005;16:1513–1518. doi: 10.1007/s00198-005-1891-7. [EL 2] [DOI] [PubMed] [Google Scholar]
- 202.Schousboe JT, Vokes T, Broy SB, et al. Vertebral Fracture Assessment: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:92–108. doi: 10.1016/j.jocd.2007.12.008. [EL 4] [DOI] [PubMed] [Google Scholar]
- 203.Greenspan SL, Resnick NM, Parker RA. Early changes in biochemical markers of bone turnover are associated with long-term changes in bone mineral density in elderly women on alendronate, hormone replacement therapy, or combination therapy: a three-year, double-blind, placebo-controlled, randomized clinical trial. J Clin Endocrinol Metab. 2005;90:2762–2767. doi: 10.1210/jc.2004-1091. [EL 1] [DOI] [PubMed] [Google Scholar]
- 204.Bauer DC, Garnero P, Bilezikian JP, et al. Short-term changes in bone turnover markers and bone mineral density response to parathyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2006;91:1370–1375. doi: 10.1210/jc.2005-1712. [EL 1] [DOI] [PubMed] [Google Scholar]
- 205.Anselmetti GC, Muto M, Guglielmi G, Masala S. Percutaneous vertebroplasty or kyphoplasty. Radiol Clin North Am. 2010;48:641–649. doi: 10.1016/j.rcl.2010.02.020. [EL 4] [DOI] [PubMed] [Google Scholar]
- 206.Röllinghoff M, Zarghooni K, Dargel J, et al. The present role of vertebroplasty and kyphoplasty in the treatment of fresh vertebral compression fractures. Minerva Chir. 2010;65:429–437. [EL 4] [PubMed] [Google Scholar]
- 207.Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557–568. doi: 10.1056/NEJMoa0900429. [EL 1] [DOI] [PubMed] [Google Scholar]
- 208.Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569–579. doi: 10.1056/NEJMoa0900563. [EL 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shindle MK, Gardner MJ, Koob J, Bukata S, Cabin JA, Lane JM. Vertebral height restoration in osteoporotic compression fractures: kyphoplasty balloon tamp is superior to postural correction alone. Osteoporos Int. 2006;17:1815–1819. doi: 10.1007/s00198-006-0195-x. [EL 2] [DOI] [PubMed] [Google Scholar]