Abstract
X-linked agammaglobulinemia (XLA) is a primary immunodeficiency characterized by marked reduction in all classes of serum immunoglobulins and the near absence of mature CD19+ B-cells. Although malignancy has been observed in patients with XLA, we present the first reported case of acute myeloid leukemia (AML) in a patient with XLA. We also demonstrate the complete correction of the XLA phenotype following allogeneic hematopoietic cell transplantation for treatment of the patient’s leukemia.
Keywords: acute myeloid leukemia, stem cell transplantation, X-linked agammaglobulinemia
INTRODUCTION
X-linked agammaglobulinemia (XLA) is characterized by early-onset bacterial infections, marked reduction in all classes of serum immunoglobulins, and the near absence of mature CD19+ B-cells.[1] XLA is caused by mutations in the Bruton’s tyrosine kinase (BTK) gene, which is essential for B-cell development.[2–4] Although an increased risk of malignancy has been previously described in patients with XLA,[5,6] acute myeloid leukemia (AML) and the use of unrelated hematopoietic cell transplantation (HCT) has not been reported. We present the first reported case of AML in a pediatric patient with XLA. Although HCT was initiated to treat the patient’s relapsed AML, it also completely corrected his XLA phenotype.
CASE PRESENTATION
The male patient initially presented at age 5 years with a history of recurrent sino-pulmonary infections, intermittent fevers, splenomegaly, and pancytopenia. Bone marrow aspiration/biopsy (BMA/Bx) revealed B-cell hypoplasia with no evidence of malignancy. BM immunophenotyping demonstrated complete absence of CD19+ B-cells. Immunoglobulin levels were very low. Genomic DNA analysis revealed a novel point mutation in BTK, 192 nucleotides upstream of the initiation (ATG) codon (c.1-192A>G), affecting the promoter region of BTK and confirming the diagnosis of XLA. The patient was started on monthly intravenous immunoglobulin (IVIG) infusions. His splenomegaly resolved, and he had no further infections after the initiation of IVIG therapy.
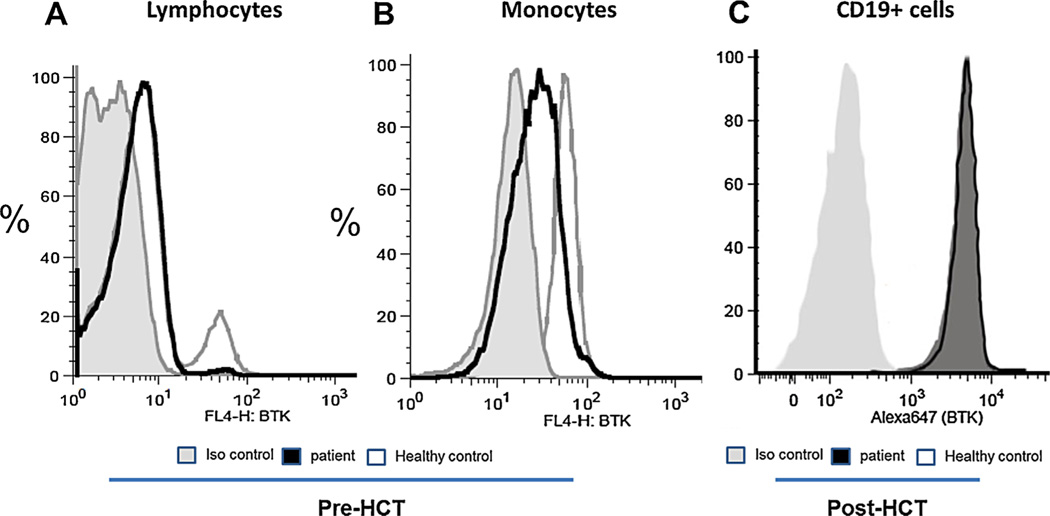
At age 13 years, a routine laboratory evaluation revealed pancytopenia. BMA/Bx showed 20% blast partially myeloperoxidase positive and containing Auer rods with an immunophenotype profile (CD13+, CD15 dim, CD19−, CD33+, CD34+, and CD117+) consistent with the diagnosis of AML. No cytogenetic abnormalities were detected and FLT3 mutation was negative. Because AML has not been previously reported in XLA, flow analysis of peripheral blood mononuclear cells was performed to assess BTK expression and to confirm that the patient’s B-cells lacked BTK. Consistent with the BTK mutation identified at original presentation, the patient was found to have markedly reduced numbers of lymphocytes expressing BTK (Fig. 1A) and reduced expression in his monocytes as well (Fig. 1B). Because haploinsufficiency of the hematopoietic transcription factor GATA2 has been associated with monocytopenia, B-cell deficiency, and AML,[7] we sequenced the GATA2 gene, which was wild type. Resequencing of genomic DNA confirmed the c.1-192A>G mutation (AAAGGGAACTG to AAAGGGAGCTG) affecting the invariable core sequence (GGAA) located within the critical Pu.1 transcription factor binding site of the BTK-promoter region, which is required for normal BTK expression.[8] Reduced BTK expression in the patient’s mononuclear cells seen by flow cytometry is compatible with a mutation in the Pu.1 transcription factor binding site.[9] Furthermore, the small amount of BTK still produced is expected to be functionally normal as the coding region is unaffected, thus allowing for the production of a minute number of B-cells (Fig. 1A). There has been one previously reported XLA patient with decreased BTK expression due to a BTK mutation in the promoter region within the Pu.1 site.[10] This patient’s mutation was reported to be an A to G mutation located at 193 base pairs upstream of the initiation codon (c.1-193A>G).
Fig. 1.
BTK expression by peripheral blood mononuclear cells (PBMC) before and after HCT:PBMC were fixed, permeabilzed and stained with isotype control or a fluorescent labeled anti-BTK mAb. (A) A proportion of lymphocytes from a normal control express BTK, representing B cells; patient PBMC reveal a minute number of B cells expressing BTK at normal fluorescence intensity. (B) when gated for monocytes, BTK is absent or markedly reduced. (C) one year post HCT, all CD19+ cells express BTK.
Treatment for his newly diagnosed AML included chemotherapy per the standard arm of Children’s Oncology Group study AAML0531. His AML was in complete remission at the end of induction II. Unfortunately, the patient suffered an isolated medullary relapse 7 months after completion of therapy. After two re-induction attempts, he continued have persistent disease manifested by significant myelodyplasia by morphology and 3% atypical myeloblasts by flow cytometry. In this context, a 10/10 HLA allelic-matched unrelated donor was identified. The patient received myeloablative conditioning with fractionated total body irradiation (1,200 cGy), cyclophosphamide (60 mg/kg/day for 2 days), and etoposide (40 mg/kg for 1 day). The patient received peripheral blood stem cells on day 0 (D0). His graft-versus-host disease (GvHD) prophylaxis included methotrexate and tacrolimus. D30 BMA/Bx demonstrated complete remission. Peripheral blood chimerism on D30, D60, D100 and 1 year post-HSCT showed >95% donor DNA at each post-transplant time point. Transplant-related complications included grade 4 mucositis, culture-negative neutropenic fevers, grade 1 (stage 1 skin) and grade 2 (stage 1) upper GI acute GvHD.
Peripheral lymphocyte phenotyping performed 1 year post-transplant showed normal range CD19 percentage and protective levels of antibodies to pneumococcal polysaccharide were measured post-vaccination (Tables I and II). The patient received IVIG every 3 weeks until 6 months post-transplant. He maintained normal immunoglobulin levels thereafter. BTK gene analysis performed at 1 year post-transplant showed the absence of his previous BTK mutation, and restoration of BTK expression was demonstrated in CD19+ B cells (Fig. 1C) and monocytes comparable to a healthy control. Two years post-transplant, the patient’s AML remains in second complete remission, and he has normal CD19 and immunoglobulin levels.
TABLE I.
Absolute CD19 Numbers and Percentages Analyzed Pre and Periodically Post-HCT
| Pre-transplant | D +30 | D +60 | D +100 | D +6 months | D +12 months | D +15 months | |
|---|---|---|---|---|---|---|---|
| CD 19% | 0% | 1% | 2% | 7% | 8% | 19% | 21% |
| CD 19 Number | 0.013 × 10e9/L | 0.029 × 10e9/L | 0.114 × 10e9/L | 0.123 × 123e9/L | 0.281 × 10e9/L | 0.286 × 10e9/L |
TABLE II.
Pre and Post Pneumococcal Vaccine Titers
| Test | Pre-Pneumococcal vaccine Titers (PPV 23-Valent) |
Post-Pneumococcal vaccine Titers (PPV 23-Valent) |
Ref. Range |
|---|---|---|---|
| Type 1 | 0.2 µg/ml | 4.8 µg/ml | >1.3 |
| Type 3 | 1.4 µg/ml | 4.1 µg/ml | >1.3 |
| Type 4 | 0.1 µg/ml | 9.8 µg/ml | >1.3 |
| Type 8 | 0.1 µg/ml | 2.2 µg/ml | >1.3 |
| Type 9 (9N) | 0.2 µg/ml | 5.0 µg/ml | >1.3 |
| Type 12 (12F) | <0.1 µg/ml | 0.4 µg/ml | >1.3 |
| Type 14 | 0.3 µg/ml | 8.4 µg/ml | >1.3 |
| Type 19 (19F) | 0.2 µg/ml | 18.7 µg/ml | >1.3 |
| Type 23 (23F) | <0.1 µg/ml | 6.8 µg/ml | >1.3 |
| Type 26 (6B) | <0.1 µg/ml | 3.7 µg/ml | >1.3 |
| Type 51 (7F) | <0.1 µg/ml | 4.4 µg/ml | >1.3 |
| Type 56 (18C) | <0.1 µg/ml | 2.4 µg/ml | >1.3 |
DISCUSSION
The overall prognosis for XLA has improved dramatically in the last 25 years due in large part to earlier diagnosis, the judicious use of antibiotics, and the development of gammaglobulin preparations that allow the achievement of normal concentrations of serum IgG.[11] As a result, children with XLA are now surviving into adulthood. While the lives of many survivors return toward normalcy, approximately 10% of individuals develop significant infections, chronic lung disease, or neurodegenerative disease despite appropriate therapy [12,13] in addition to the side effects of monthly immunoglobulin infusions.[14] Therefore, a significant proportion of XLA patients have to cope with chronic disease during their childhood and adolescence.[15,16] Malignancy has also been reported in long-term survivors with XLA [5,6] and has an estimated 30-fold increase in the incidence of colorectal cancer. However, to our knowledge this is the first reported case of AML in a patient with XLA.
Unlike other primary immune deficiencies,[17] the use of HCT in patients with XLA has not been explored, likely due to the impact advancements in diagnosis and supportive care have had on improving survival in XLA patients.[18] In 2003 Porpiglia et al. [19] reported successful long lasting donor B-cell engraftment using HCT in two murine models of B-cell deficiency without administration of irradiation or myelotoxic drugs. These findings suggest that it may not be necessary to make “space” in the bone marrow to achieve sufficient engraftment. Moreover, studies completed in human and murine heterozygous carriers of BTK mutations have shown that B-cell precursors with a normal BTK gene have a strong selective advantage in proliferation, differentiation, and/or survival compared to precursors with mutant BTK.
These pre-clinical observations formed the basis of a trial reported by Howard et al. [20] wherein six patients with XLA underwent MSD HCT using un-manipulated BM without the use of conditioning or immune suppression (n = 3 patients) or using post-transplant immune suppression alone (n = 3 patients). Unfortunately, none of the patients showed any signs of engraftment or B-cell reconstitution.
Although the primary reason for our decision to perform myeloablative HCT in our patient was to treat his underlying refractory AML, complete B-cell engraftment and full recovery can occur in this setting. Importantly, allogeneic HCT using a suitable MUD and full intensity conditioning resulted in complete resolution of the underlying B-cell defect. It is possible that the use of reduced intensity conditioning (RIC) regimens that foster full or stable mixed donor chimerism and are associated with fewer early and long term post-transplant complications may be sufficient to achieve B-cell reconstitution and correct the underlying immunodeficiency in XLA patients as has been recently shown for other immune disorders.[21] To this end, prospective clinical trials incorporating RIC regimens should be initiated for a subset of patients who experience significant complications despite receiving advanced supportive care and IVIG replacement before significant end organ damage or malignancy has developed.
Footnotes
AUTHORS CONTRIBUTION
RAA was responsible for patient care, data collection and analysis, and writing of this article. LRC and RE were responsible for patient care, data collection and analysis; they also contributed to writing of this article. GA contributed to writing of this article. JA and LC were responsible for patient care and data collection. HDO was responsible for data collection and analysis and contributed to writing of this article. TRT and JLG were responsible for data collection and analysis. RWH was responsible for patient care, data collection and analysis. HT was responsible for data collection and analysis and contributed to writing of this article. KRC was responsible for patient care, data collection and analysis and contributed to writing of this article; he was also of in charge of overall supervision of article.
REFERENCES
- 1.Conley ME, Howard V. Clinical findings leading to the diagnosis of X-linked agammaglobulinemia. J Pediatr. 2002;141:566–571. doi: 10.1067/mpd.2002.127711. [DOI] [PubMed] [Google Scholar]
- 2.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarström L, Kinnon C, Levinsky R, Bobrow M, Smith CIE, Bentley D. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 3.Ochs HD, Smith CI. X-linked agammaglobulinemia A clinical and molecular analysis. Medicine. 1996;75:287–299. doi: 10.1097/00005792-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kerns HM, Ryu BY, Stirling BV, Sather BD, Astrakhan A, Humblet-Baron S, Liggitt D, Rawlings DJ. B cell-specific lentiviral gene therapy leads to sustained B-cell functional recovery in a murine model of X-linked agammaglobulinemia. Blood. 2010;115:2146–2155. doi: 10.1182/blood-2009-09-241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, Conley ME, Cunningham-Rundles C, Ochs HD. X-linked agammaglobulinemia: Report on a United States registry of 201 patients. Medicine (Baltimore) 2006;85:193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino A, Okuno Y, Migita M, Ban H, Yang X, Kiyokawa N, Adachi Y, Kojima S, Ohara O, Kanegane H. X-Linked Agammaglobulinemia Associated with B-Precursor Acute Lymphoblastic Leukemia. J Clin Immunol. 2015;35:101–111. doi: 10.1007/s10875-015-0127-7. [DOI] [PubMed] [Google Scholar]
- 7.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, Arthur DC, Gu W, Gould CM, Brewer CC, Cowen EW, Freeman AF, Olivier KN, Uzel G, Zelazny AM, Daub JR, Spalding CD, Claypool RJ, Giri NK, Alter BP, Mace EM, Orange JS, Cuellar-Rodriguez J, Hickstein DD, Holland SM. GATA2 deficiency: A protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Himmelmann A, Thevenin C, Harrison K, Kehrl JH. Analysis of the Bruton’s tyrosine kinase gene promoter reveals critical PU.1 and SP1 sites. Blood. 1996;87:1036–1044. [PubMed] [Google Scholar]
- 9.Muller S, Sideras P, Smith CI, Xanthopoulos KG. Cell specific expression of human Bruton’s agammaglobulinemia tyrosine kinase gene (Btk) is regulated by Sp1- and Spi-1/PU.1-family members. Oncogene. 1996;13:1955–1964. [PubMed] [Google Scholar]
- 10.Holinski-Feder E, Weiss M, Brandau O, Jedele KB, Nore B, Bäckesjö CM, Vihinen M, Hubbard SR, Belohradsky BH, Smith CI, Meindl A. Mutation screening of the BTK gene in 56 families with X-linked agammaglobulinemia (XLA): 47 unique mutations without correlation to clinical course. Pediatrics. 1998;101:276–284. doi: 10.1542/peds.101.2.276. [DOI] [PubMed] [Google Scholar]
- 11.Howard V, Greene JM, Pahwa S, Winkelstein JA, Boyle JM, Kocak M, Conley ME. The health status and quality of life of adults with X-linked agammaglobulinemia. Clin Immunol. 2006;118:201–208. doi: 10.1016/j.clim.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Quartier P, Debre M, De Blic J, de Sauverzac R, Sayegh N, Jabado N, Haddad E, Blanche S, Casanova JL, Smith CI, Le Deist F, de Saint Basile G, Fischer A. Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: A retrospective survey of 31 patients. J Pediatr. 1999;134:589–596. doi: 10.1016/s0022-3476(99)70246-5. [DOI] [PubMed] [Google Scholar]
- 13.Plebani A, Soresina A, Rondelli R, Amato GM, Azzari C, Cardinale F, Cazzola G, Consolini R, De Mattia D, Dell’Erba G, Duse M, Fiorini M, Martino S, Martire B, Masi M, Monafo V, Moschese V, Notarangelo LD, Orlandi P, Panei P, Pession A, Pietrogrande MC, Pignata C, Quinti I, Ragno V, Rossi P, Sciotto A, Stabile A. Italian Pediatric Group for XLA-AIEOP. Clinical, immunological, and molecular analysis in a large cohort of patients with X-linked agammaglobulinemia: An Italian multicenter study. Clin Immunol. 2002;104:221–230. doi: 10.1006/clim.2002.5241. [DOI] [PubMed] [Google Scholar]
- 14.Chun JK, Lee TJ, Song JW, Linton JA, Kim DS. Analysis of clinical presentations of Bruton disease: A review of 20 years of accumulated data from pediatric patients at Severance Hospital. Yonsei Med J. 2008;49:28–36. doi: 10.3349/ymj.2008.49.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegner UH, Kobayashi RH, Cunningham-Rundles C, Español T, Fasth A, Huttenlocher A, Krogstad P, Marthinsen L, Notarangelo LD, Pasic S, Rieger CH, Rudge P, Sankar R, Shigeoka AO, Stiehm ER, Sullivan KE, Webster AD, Ochs HD. Progressive neurodegeneration in patients with primary immunodeficiency disease on IVIG treatment. Clin Immunol. 2002;102:19–24. doi: 10.1006/clim.2001.5140. [DOI] [PubMed] [Google Scholar]
- 16.Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27:171–178. doi: 10.1016/j.tmrv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Burroughs L, Woolfrey A. Hematopoietic cell transplantation for treatment of primary immune deficiencies. Cell Ther Transplant. 2010;2:01. doi: 10.3205/ctt-2010-en-000077.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soresina A, Nacinovich R, Bomba M, Cassani M, Molinaro A, Sciotto A, Martino S, Cardinale F, De Mattia D, Putti C, Dellepiane RM, Felici L, Parrinello G, Neri F, Plebani A. Italian Network for Primary Immunodeficiencies. The quality of life of children and adolescents with X-linked agammaglobulinemia. J Clin Immunol. 2009;29:501–507. doi: 10.1007/s10875-008-9270-8. [DOI] [PubMed] [Google Scholar]
- 19.Porpiglia AS, Rohrer J, Conley ME. Reconstitution of B cell function in murine models of immunodeficiency. Clin Immunol. 2003;107:90–97. doi: 10.1016/s1521-6616(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 20.Howard V, Myers LA, Williams DA, Wheeler G, Turner EV, Cunningham JM, Conley ME. Stem cell transplants for patients with X-linked agammaglobulinemia. Clin Immunol. 2003;107:98–102. doi: 10.1016/s1521-6616(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 21.Burroughs LM, Torgerson TR, Storb R, Carpenter PA, Rawlings DJ, Sanders J, Scharenberg AM, Skoda-Smith S, Englund J, Ochs HD, Woolfrey AE. Stable hematopoietic cell engraftment after low-intensity nonmyeloablative conditioning in patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. J Allergy Clin Immunol. 2010;126:1000–1005. doi: 10.1016/j.jaci.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]