Abstract
Burst suppression is actively studied as a control signal to guide anesthetic dosing in patients undergoing medically induced coma. The ability to automatically identify periods of EEG suppression and compactly summarize the depth of coma using the burst suppression probability (BSP) is crucial to effective and safe monitoring and control of medical coma. Current literature however does not explicitly account for the potential variation in burst suppression parameters across different scalp locations. In this study we analyzed standard 19-channel EEG recordings from 8 patients with refractory status epilepticus who underwent pharmacologically induced burst suppression as medical treatment for refractory seizures. We found that although burst suppression is generally considered a global phenomenon, BSP obtained using a previously validated algorithm varies systematically across different channels. A global representation of information from individual channels is proposed that takes into account the burst suppression characteristics recorded at multiple electrodes. BSP computed from this representative burst suppression pattern may be more resilient to noise and a better representation of the brain state of patients. Multichannel data integration may enhance the reliability of estimates of the depth of medical coma.
I. Introduction
Burst suppression is a stereotypical time domain EEG pattern characterized by high voltage activity alternating with periods of low-voltage activity (‘suppressions’). It generally reflects a state of profound brain inactivation and unconsciousness, associated with various normal developmental (early development), pathological (hypothermia, diffuse anoxic brain injury) and therapeutic (deep anesthesia) scenarios. While the neurophysiology of burst suppression remains an area of active investigation, current theories suggest that it arises from a nonlinear interaction. This consists of a ‘fast’ dynamical process that generates background EEG activity, and a ‘slow’ process that periodically interrupts the fast process, leading to suppression of background activity. The slow process is thought to be a depletion-recovery cycle in which some energy resource (such as ATP stores) necessary for maintenance of background activity is periodically depleted during high-voltage EEG ‘bursts’, and regenerated during suppressions [1]. Significantly for medical engineers, this process exhibits robust parametric sensitivity to the depth of anesthesia. That is, the duration of suppressions becomes progressively longer and bursts become progressively briefer as the concentration of anesthetic in the brain increases. This makes burst suppression a neurophysiology-based EEG signature that can be used to non-invasively monitor the depth of pharmacologically induced coma in real-time.
Pharmacologically induced coma is currently used in clinical settings as treatment for patients with high risk of brain injury either from physical trauma, drug overdose or disease such as intracranial hypertension and status epilepticus. In the case of refractory status epilepticus, defined as ongoing seizure activity resistant to first line and second line anti-convulsant agents and lasting more than 30 min, burst suppression-targeting pharmacologically induced coma over extended periods of time is the standard of care [2]. It is thought to stop seizure activity and thereby achieve neuroprotection [3].
A standard medical goal in such pharmacologically induced coma is to maintain the brain in a burst-suppressed state with less than 1 burst per 10 seconds for 12–24 hours or more. This duration is significantly longer than any human operator can maintain tight control over. Therefore, defining a precise quantitative target level of burst suppression and maintaining the target automatically using a closed-loop feedback system would be a much more efficient and pragmatic approach.
Over the past years, there has been tangible effort by researchers to advance towards this goal. A statistically-rigorous algorithm based on Bayesian estimation and pharmacokinetic and pharmacodynamic models to compactly quantify the state of burst suppression as the burst suppression probability (BSP) has been developed for real time quantification, and models have been developed to relate BSP to the underlying anesthetic states [4]. This has enabled real-time monitoring of coma depth and has allowed rapid advances in design of closed-loop anesthetic delivery systems (CLAD) to control burst suppression. Such systems have been built and shown to work in rodent experiments with high reliability and precision [5]–[7].
Furthering the work to translate these research devices into a clinical tool requires considerable effort to adapt and account for the difference between laboratory rodent experiments and real clinical applications. Two such differences in terms of data collection are 1) rodent experiments use a single intradural electrode for recording while multichannel scalp EEG is often collected in the clinical setting; and 2) rodent experiments are conducted in a controlled environment with the electrode affixed to the scalp for a short duration of 1–4 hours. With rodent recordings, there is thus less concern for movement artifacts, dislodged electrodes and other disruptions in recording; these issues can be significant in a clinical setting with extended recording periods and can affect detection of burst suppression patterns.
Many groups have discussed methods of automated burst suppression detection but no work to our knowledge has explicitly addressed how multi-channel EEG data affects burst suppression detection and monitoring [8]–[12]. Research effort has been directed at describing how suppression patterns can be successfully extracted automatically, usually by first identifying one or more features that distinguish bursts from suppressions (e.g. instantaneous variance, amplitude, median, standard deviation, entropy, 95% edge frequency, non-linear energy operator, etc.) followed by some form of classification (e.g. segmentation using hard and soft thresholds, classification by artificial neural networks, etc.). None of these studies explicitly addressed how data from multiple channels are optimally combined. In work that did utilize EEG signals across multiple channels, the authors described integrating instantaneous amplitude across all channels, stating that a priori, this should help to overcome contamination of channels by artifacts, but no further systematic analysis of the impact on detector behavior was performed [8], [12].
In the following study we explore the spatial characteristics of single channel BSP recordings and explore the impact and potential value of using multi-channel data in burst suppression monitoring. We hypothesized that different channels may capture different local information while having multiple channels may facilitate artifact rejection.
II. Method
A. EEG Recording and Patient Profile
EEG data was recorded from 8 patients with refractory status epilepticus (RSE) who were placed under pharmacologically induced burst suppression with either propofol or midazolam or both in the Neurosciences Intensive Care Unit of Massachusetts General Hospital (MGH). The retrospective analysis in this paper was performed with the approval of the Institutional Review Board. Three of the 8 patients had a period of cardiac arrest before the onset of RSE (post-anoxic RSE, pRSE). Retrospective data collection was done under an MGH IRB approved protocol. All EEGs were recorded using 19 silver/silver chloride electrodes, affixed to the scalp according to the international 10–20 system. Data were recorded at 512 or 256 Hz, using XLTEK clinical EEG equipment (Natus Medical Inc., Oakville, Canada), and subsequently down-sampled to 200 Hz.
B. Preprocessing and Burst Suppression Detection
To precondition the data for analysis, a sliding window of 1 sec is used to remove regions where the average power is >40dB, to remove high voltage artifacts with frequent zero-crossing such as due to loose electrodes. Further artifact removal is done by rejecting instantaneous high amplitude data (>500uV) and electromyography artifacts (>5 SD in the 15 – 30 Hz band). Finally the data is put into average montage and band-pass filtered at 3 – 35Hz. The lower bound of this filter is higher than conventionally used for EEG analysis but is well suited for burst suppression detection.
Next, a previously validated algorithm for burst suppression segmentation is used to generate the binary signals representing suppressions [8]. This algorithm detects suppression by thresholding a recursive estimate of the local signal variance as expressed in the following equations:
| (1) |
| (2) |
| (3) |
where xt is the EEG signal at time t, μt is the mean, σt is the variance, zt is the current value of the binary signal produced, β is a parameter called the “forgetting factor”, δ[․] is the indicator function (equal to 1 if the inequality is satisfied and 0 otherwise) and θ is the classification threshold. We set the forgetting factor to the globally optimal value reported in the referenced paper. The threshold θ is set to 1.75, which was determined by visually scoring the performance of 6 candidate thresholds (1.4 – 3.5) in 93 30-sec single channel test segments randomly extracted from the recording.
C. Burst Suppression Probability (BSP)
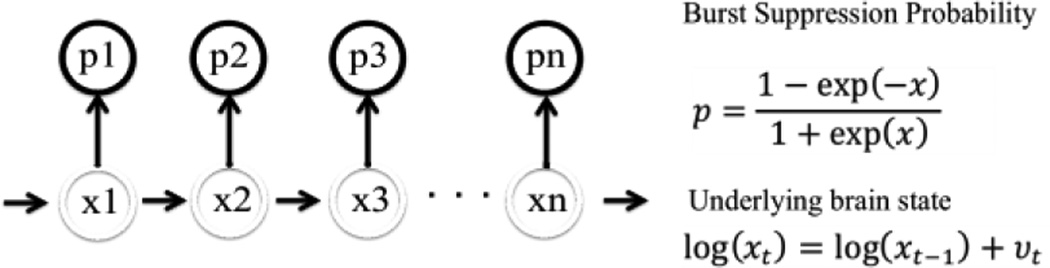
The BSP is a compact representation of the burst suppression pattern that allows for second-to-second analysis and across-time comparison. It defines the brain’s instantaneous propensity for being in the suppressed state, using a link function to map the amount of anesthetic in the brain onto a well-defined probability as shown in Fig 1 [4]. Here we used a real-time binary filter to calculate the BSP from the binary signal obtained in the previous step [5].
Figure 1.
Burst Suppression Probability Model Used in Analysis
D. Global Representation of Binary Data
To provide a basis for comparing BSP values between channels, we designed a simple global representation of binary data. This is formulated by implementing a voting system among the binary data obtained from individual channels whereby for any instance in time at least 60% of the valid (i.e. artifact free) channels have to agree on the observation of suppression for it to be considered a ‘true’ suppression. This scheme is motivated by the observation in previous studies that burst suppression is a global phenomenon [13]. The new binary signal summarizes the data from all channels considered. From it, a global representation of the burst suppression probability (BSP) is found using the binary filter algorithm described earlier. To distinguish this BSP from the BSP calculated from data from just one channel, hereafter we refer to the former as the global BSP and the latter as single channel BSP.
III. Results
A total of 210 hours of recording were analyzed, with 20–25 hours of data coming from each patient. Overall, patients had BSP>0.1 for 91 hours or 43% of the total recording time. Patients with post anoxic refractory status epilepticus (pRSE) accounted for about 67 hours of the total recording and had BSP>0.1 for 27.8 hours or 41.5% of this time.
A. Significant Differences in Burst Suppression Probability Obtained from Data in Different Channels
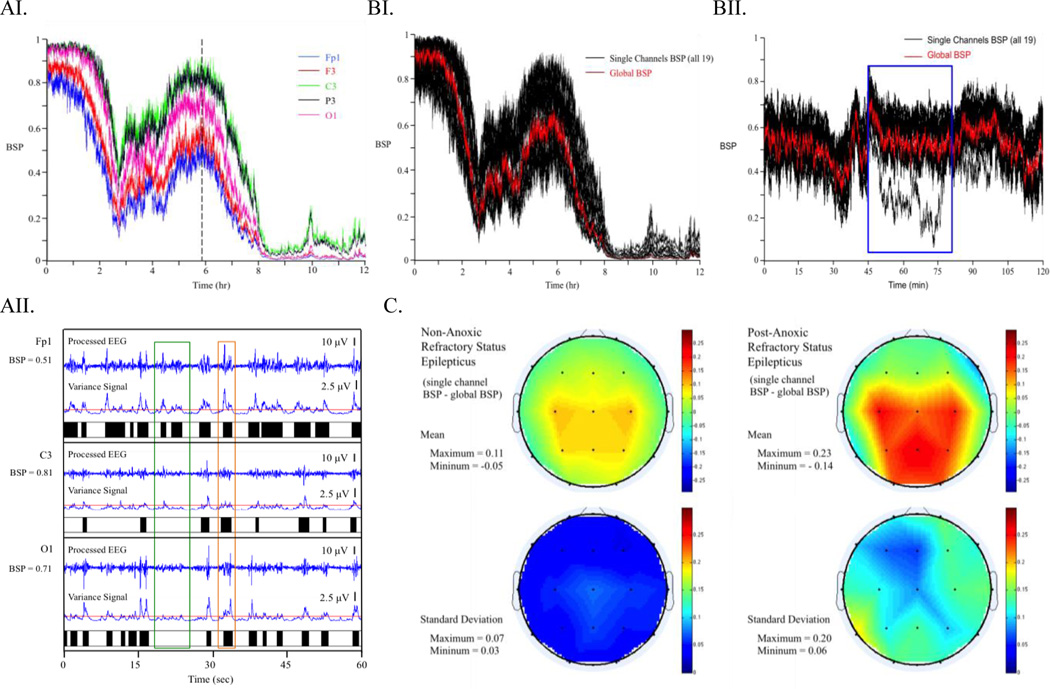
To study spatial variation in single channel BSP values we first studied the evolution of BSP for individual patients over time. Fig. 2AI shows a set of 5 representative 12-hour single channel BSPs estimated from an EEG recording of one pRSE patient. As in most other patients, it is evident from visual inspection that single channel BSP varied substantially between channels and the differences were consistent over time. Taking 1 min segments and comparing the variance signal obtained from processed EEG in the burst suppression detection algorithm (see example in Fig. 2AII) shows that while bursts can be seen globally, their magnitude can vary substantially and systematically. Some bursts are detected similarly across all channels while others are only registered by the automated detection algorithm in certain channels. This explains the source of variation in single channel BSP estimation among different channels.
Figure 2.
AI) Illustration of Variation in Single Channel BSP. Data from Fp1, F3 C3, P3 and O1 (left side medial anterior to posterior) channels of a patient with post-anoxic refractory status epilepticus. AII) Demonstration of Suppression Pattern Detection. This is a 1 min segment taken from the location of the vertical dashed line in Fig. 2AI. Note that the green box highlights a burst that is only observed in frontal lead Fp1 but not C3 or O1 while the orange box encases a burst that is similarly detected across all three channels. BI) Demonstration of Global BSP overlaid on all Single Channel BSP. Data from post anoxic RSE patient described in Fig 2AI and 2AII. BII) Demonstration of Global BSP Overlaid on All Single Channel BSP. Data from a non-anoxic RSE patient. Blue block indicates a location where one of the electrodes was an outlier. Global BSP was not affected by this outlier. C) Summary of Mean and Standard Deviation of Single Channel BSP difference from Global BSP Averaged Across pRSE and npRSE Groups. Note that pRSE patients have higher mean differenace and standard deviation in comparison to the npRSE group
B. Global Representation and Associated Burst Suppression Probability (BSP)
A global representation of the binary signals is obtained from multi-channel EEG recording by the voting method described earlier. The same binary filter algorithm applied to individual channels can then be used to estimate BSP from the global BSP. Fig. 2BI shows the global BSP overlaid on single channel BSPs from all 19 channels in the same pRSE patient. It can be seen that the global BSP tracks the overall trend of single channel BSPs. In a similar plot for a different patient (Fig. 2BII), we highlight that the global BSP remains relatively unaffected by an outlier BSP in one electrode.
This global representation also allows us to further investigate the extent of the variation in single channel BSP estimation as described in part A. We compared the single channel BSP estimates in each of the 19 channels with the global BSP for time points where the global BSP ≥ 0.1 and plotted the mean difference and standard deviation topographically (see Fig. 2C). pRSE and non-anoxic RSE (npRSE) patients are handled separately. In both groups, the frontal and temporal leads tend to report lower single channel BSP while the occipital and central leads tend to report higher single channel BSP. The expected deviation of single channel BSP from global BSP in pRSE patients is (0.13 ± 0.11), while that in npRSE patients is (0.06 ± 0.04). These findings suggest that (a) burst amplitudes tend to be higher in frontotemporal regions, leading to increased probability of burst detection and lower BSP values when using identical threshold values at all scalp locations, and (b) the degree of spatial heterogeneity in burst amplitude tends to be greater in patients with pRSE, at least in this cohort.
IV. Discussion
Developing quantitative, real-time algorithms to monitor the pattern of EEG burst suppression is a medical innovation necessitated by the need to safely and effectively maintain medically induced coma at a desired depth for extended periods. Burst suppression is quantified in real time using the burst suppression probability (BSP), which has been shown to have parametric sensitivity to the depth of coma. Although burst suppression is generally considered to be a spatially homogeneous phenomenon in scalp EEG, herein we observed that when a validated segmentation algorithm is applied significant differences between the burst suppression probabilities can result in different channels. A close analysis of the burst suppression detection procedure reveals that there are different types of bursts in the burst suppression state. Some bursts, although they visually seem to occur in all channels, have characteristically smaller amplitudes in certain channels such that the segmentation threshold is less often crossed or often crossed only briefly. This leads to systematic spatial differences in BSP estimated from different channels.
These spatial differences likely reflect the well-known functional differences that exist between regions of cortex, which are well described outside of burst suppression. These differences can manifest, e.g. in the relatively high amplitude of alpha (8–10 Hz) activity in posterior head regions during resting awake state, and the centrotemporal location of vertex waves in sleep. That such spatial differentiation persists in burst suppression is in keeping with recent theoretical work which views bursting activity as a continuation of processes active in the pre-burst-suppression state [1], [14].
The difference between single channel BSPs means that the location on the scalp where we collect data for monitoring matters. Using only forehead electrodes, such as in Bispectral Index monitoring, is likely to result in administering more anesthetic than if the patient is monitored with the global BSP summarized from a standard full scalp EEG, as the former would report a lower BSP than the latter.
Our observations on the use of a global representation of data obtained from individual channels by means of channel ‘voting’ indicates that this method may be more resilient to noise than single channel BSP. This method is therefore an improvement on previous methods for utilizing multichannel data. In those methods, signals from individual channels are simply integrated and would have resulted in data from the electrode with aberrant behavior being still included in the final result. Alternative enhancements to be explored in future work include using thresholds that vary as a function of head location, and the use of more sophisticated probabilistic detection methods as opposed to our current hard thresholding method of detecting suppressions.
Overall, we observed significant variation in the BSP recorded from different channels of a standard clinical EEG system. The variation follows a specific spatial pattern and is consistent over time. A global representation of information from individual channels that takes into account the burst suppression pattern recorded from multiple electrodes is likely to be useful for providing a more noise-resilient and accurate representation of the underlying brain state. Further work can be done to develop more robust ways of combining information from multiple electrodes for this purpose.
Acknowledgments
Funding: NIH-NINDS K23 NS090900, Rappaport Foundation, Andrew David Heitman Neuroendovascular Research Fund (MBW); DP2-OD006454 (to PLP), TR01-GM104948 (to ENB).
Contributor Information
Jingzhi An, Email: jzan@mit.edu, Harvard-MIT Division of Health Science and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139 USA, phone: 857-259-8208; fax: 303-555-5555.
Durga Jonnalagadda, Email: djonnalagadda@mgh.harvard.edu, Massachusetts General Hospital (MGH), Neurology Department, Clinical Neurophysiology Service, Harvard Medical School, Boston, MA 02114.
Valdery Moura, Junior, Email: vmoura@mgh.harvard.edu, Massachusetts General Hospital (MGH), Neurology Department, Clinical Neurophysiology Service, Harvard Medical School, Boston, MA 02114.
Patrick L. Purdon, Email: patrickp@nmr.mgh.harvard.edu, MGH Department of Anesthesia, Critical Care and Pain Medicine, Charlestown, MA 02129.
Emery N. Brown, Email: enb@neurostat.mit.edu, MGH Department of Anesthesia, Critical Care and Pain Medicine, and the Department of Brain and Cognitive Science, Massachusetts Institute of Technology, Cambridge, MA.
M. Brandon Westover, Email: mwestover@mgh.harvard.edu, Massachusetts General Hospital (MGH), Neurology Department, Clinical Neurophysiology Service, Harvard Medical School, Boston, MA 02114.
References
- 1.Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN. A neurophysiological–metabolic model for burst suppression. Proc. Natl. Acad. Sci. 2012 Feb.109(8):3095–3100. doi: 10.1073/pnas.1121461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, LaRoche SM, R JJ, Jr, Shutter L, Sperling MR, Treiman DM, Vespa PM N. C. S. S. E. G. W. Committee. Guidelines for the Evaluation and Management of Status Epilepticus. Neurocrit. Care. 2012 Apr.17(1):3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 3.Amzica F. Basic physiology of burst-suppression. Epilepsia. 2009 Dec.50(Suppl 12):38–39. doi: 10.1111/j.1528-1167.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 4.Chemali J, Ching S, Purdon PL, Solt K, Brown EN. Burst suppression probability algorithms: state-space methods for tracking EEG burst suppression. J. Neural Eng. 2013 Oct.10(5):056017. doi: 10.1088/1741-2560/10/5/056017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ching S, Liberman MY, Chemali JJ, Westover MB, Kenny J, Solt K, Purdon PL, Brown EN. Real-time Closed-loop Control in a Rodent Model of Medically-induced Coma Using Burst Suppression. Anesthesiology. 2013 Oct.119(4) doi: 10.1097/ALN.0b013e31829d4ab4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberman MY, Ching S, Chemali J, Brown EN. A closed-loop anesthetic delivery system for real-time control of burst suppression. J. Neural Eng. 2013 Aug.10(4):046004. doi: 10.1088/1741-2560/10/4/046004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanechi MM, Chemali JJ, Liberman M, Solt K, Brown EN. A Brain-Machine Interface for Control of Medically-Induced Coma. PLoS Comput Biol. 2013 Oct.9(10):e1003284. doi: 10.1371/journal.pcbi.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandon Westover M, Shafi MM, Ching S, Chemali JJ, Purdon PL, Cash SS, Brown EN. Real-time segmentation of burst suppression patterns in critical care EEG monitoring. J. Neurosci. Methods. 2013 Sep.219(1):131–141. doi: 10.1016/j.jneumeth.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Z, Wang Y, Ren Y, Li D, Voss L, Sleigh J, Li X. Detection of Burst Suppression Patterns in EEG Using Recurrence Rate. Sci. World J. 2014 Apr.2014:e295070. doi: 10.1155/2014/295070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Särkelä M, Mustola S, Seppänen T, Koskinen M, Lepola P, Suominen K, Juvonen T, Tolvanen-Laakso H, Jäntti V. Automatic Analysis and Monitoring of Burst Suppression in Anesthesia. J. Clin. Monit. Comput. 2002 Feb.17(2):125–134. doi: 10.1023/a:1016393904439. [DOI] [PubMed] [Google Scholar]
- 11.Löfhede J, Löfgren N, Thordstein M, Flisberg A, Kjellmer I, Lindecrantz K. Classification of burst and suppression in the neonatal electroencephalogram. J. Neural Eng. 2008 Dec.5(4):402. doi: 10.1088/1741-2560/5/4/005. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Agarwal R. Automatic detection of burst suppression. Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf. 2007;2007:553–556. doi: 10.1109/IEMBS.2007.4352350. [DOI] [PubMed] [Google Scholar]
- 13.Brenner RP. The electroencephalogram in altered states of consciousness. Neurol. Clin. 1985 Aug.3(3):615–631. [PubMed] [Google Scholar]
- 14.Lewis LD, Ching S, Weiner VS, Peterfreund RA, Eskandar EN, Cash SS, Brown EN, Purdon PL. Local cortical dynamics of burst suppression in the anaesthetized brain. Brain. 2013 Sep.136(9):2727–2737. doi: 10.1093/brain/awt174. [DOI] [PMC free article] [PubMed] [Google Scholar]