Abstract
Noninvasive brain stimulation techniques have been widely used for studying the physiology of the CNS, identifying the functional role of specific brain structures and, more recently, exploring large-scale network dynamics. Here we review key findings that contribute to our understanding of the mechanisms underlying the physiological and behavioral effects of these techniques. We highlight recent innovations using noninvasive stimulation to investigate global brain network dynamics and organization. New combinations of these techniques, in conjunction with neuroimaging, will further advance the utility of their application.
In the last two decades, modern noninvasive brain stimulation (NIBS) techniques have made remarkable contributions to neuroscience. The two most commonly used forms of NIBS are transcranial magnetic stimulation (TMS; Fig. 1a) and transcranial direct current stimulation (tDCS; Fig. 1b). Both TMS and tDCS are safe for use in human subjects and have been widely used to test hypotheses about the physiology of the CNS. They identify causal links between specific brain structures supporting cognitive, affective, sensory and motor functions. They also offer insight into local and global brain network organization, dynamics and experience-dependent plasticity.
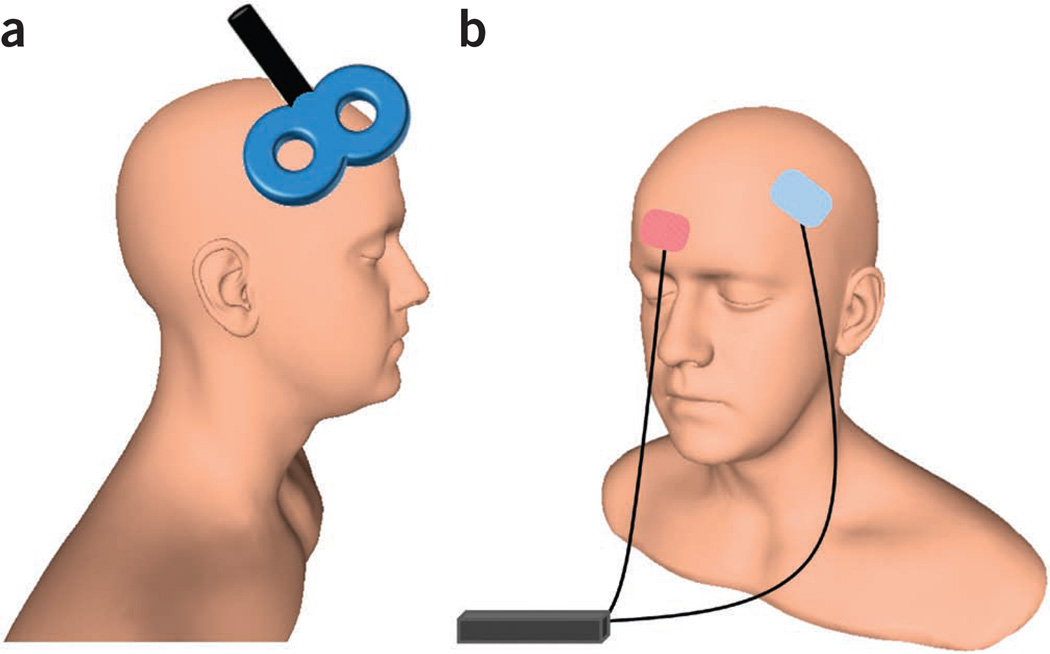
Figure 1.
Typical NIBS setups. (a) A standard figure-eight TMS coil placed on the scalp; here, over dorsolateral prefrontal cortex. (b) Bipolar tDCS electrode configuration, with one electrode over left dorsolateral prefrontal cortex and a reference electrode over the contralateral supraorbital region. Human head model from http://www.ir-ltd.net/. Used by Creative Commons license.
Abundant evidence supports the use of NIBS techniques as tools for enhancing motor skills and cognitive function in healthy subjects and as therapeutic agents for patients with neurological and psychiatric disorders1,2. Despite the unquestionable contribution of NIBS to cognitive, systems and translational neuroscience, the specific underlying mechanisms of stimulation-induced behavioral and physiological effects remain largely unknown. Still, evidence accumulated from animal models, sophisticated experimental designs involving neuropharmacological manipulations in humans, and the combination of NIBS with neuroimaging techniques has elucidated some of the possible neurophysiological underpinnings of NIBS effects. Here we review established and emerging NIBS techniques and propose underlying mechanisms. We also highlight more recent findings related to the effect of NIBS on brain network dynamics across spatial scales.
Brief historical overview
A series of experiments conducted by Faraday in 1831 led to the discovery of electromagnetic induction, in which an alternating magnetic field induces an electric current. More than 150 years later, it was shown that when a coil connected to a magnetic stimulator is placed on the human scalp over the motor cortex, current flow and neural activation in the targeted cortex are induced and movements of the contralateral upper or lower limb are easily elicited3. Since this seminal study, TMS has rapidly developed into a widely used technique for noninvasive exploration of human brain physiology, with emerging clinical implications. However, the biophysical mechanisms influenced by TMS are still not completely understood. The prevailing hypothesis is that axons are the most effective conductors in the CNS because they have the highest density of ion channels. Therefore, they are preferentially affected by the TMS pulse, which may activate both inhibitory and excitatory neurons4. TMS may suppress neural signal or generate random neuronal noise; however, its effects have been suggested to be activity dependent, suppressing the most active neurons and changing the balance between excitation and inhibition5,6.
The growing interest in noninvasive brain stimulation generated by TMS led to the revitalization of tDCS, a technique applied to animal models in the 1960s7. In 2000 it was demonstrated that tDCS induces cortical excitability changes in the human motor cortex8. This work used a battery-driven stimulator delivering weak (≤1 mA) currents between a pair of saline-soaked surface sponge electrodes, with one placed on the scalp over the motor cortex and the other over a reference location. Anodal stimulation produced excitation; cathodal stimulation, inhibition. Subsequent studies suggested that the immediate effects of tDCS on corticospinal excitability primarily depend on subthreshold resting membrane potential changes, whereas aftereffects of tDCS are due to shifts in intracortical inhibition and facilitation, and interactions with facilitatory corticospinal waves9. tDCS effects have been shown in a wide range of processes, spanning motor and sensory to cognitive functions7.
Stimulation protocols
TMS is commonly applied in single, paired or repetitive trains (Fig. 2a). Initial applications of TMS focused on the motor system and enabled mapping of functional representations in primary motor cortex (M1), with single-pulse TMS to M1 producing muscle contractions measured as motor-evoked potentials (MEPs)10. Single-pulse TMS has also been extended to the visual system: suprathreshold stimulation of occipital cortex (mainly primary visual cortex, V1) induces phosphenes (bright spots of light in the visual field) and transient scotomas, and TMS of area V5 enables the study of motion perception10. Furthermore, it has become standard practice to use these responses to inform the selection of appropriate stimulation parameters for other brain areas, from which there is no noninvasively measurable physiological response11.
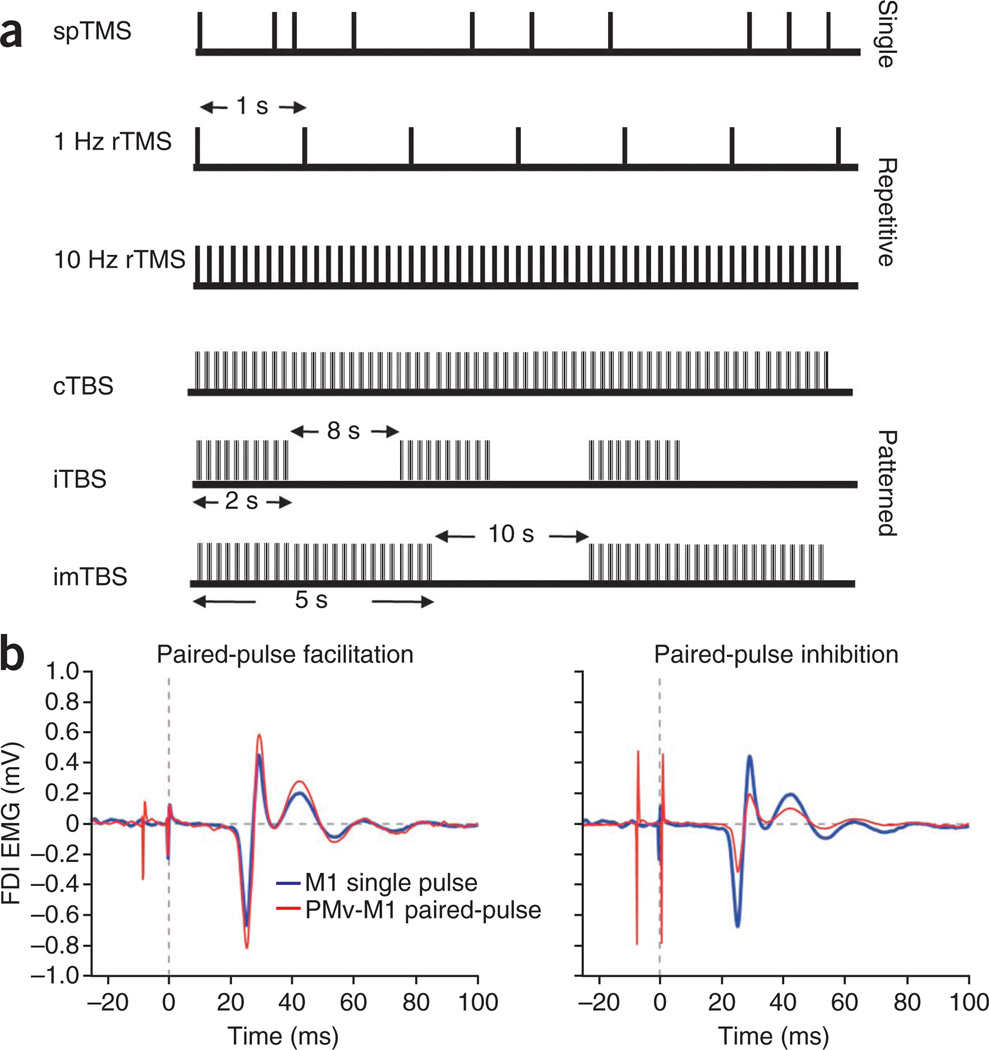
Figure 2.
TMS protocols. (a) TMS is commonly applied in single pulses (spTMS), multiple pulses or repetitively (rTMS, applied in low or high frequencies). An emerging form of rTMS is theta-burst stimulation (TBS), in which three 50-Hz pulses are applied at 5 Hz for 20–40 s (continuous TBS, cTBS) or each burst is applied for 2 s and repeated every 10 s for 190 s (intermittent TBS, iTBS). In a third variant, intermediate TBS (imTBS), 5-s burst trains are repeated every 15 s for a total of 110 s (ref. 31). (b) MEPs recorded from the first dorsal interosseous (FDI) muscle using surface electromyography (EMG) after spTMS to M1 and paired-pulse TMS to ventral premotor cortex (PMv) and M1.
Using the high temporal resolution of TMS, application of pulses during task execution can provide insights into the underlying neural substrates, and the time points during which such are engaged, in relation to the specific task performed12. For example, single-pulse TMS was used to show that a specific area in the parietal cortex mediates spatial orienting during distinct time periods after the onset of the behavioral event, suggesting that fast and slow visual pathways are necessary for orienting spatial attention13. Single-pulse TMS has also contributed to understanding mechanisms of motor learning14.
Paired-pulse TMS protocols enable further measurements of cortical physiology. Paired-pulse TMS can be used to study functional interactions within a single brain region or between two connected brain areas15. Single-region paired-pulse TMS is typically limited to M1 and involves the application of both a subthreshold conditioning stimulus and suprathreshold test stimulus to the same region. Variation of the precise latency between the conditioning and test stimuli can result in intracortical inhibitory (if the conditioning stimulus precedes the test stimulus by <5 ms) or intracortical facilitory (if the conditioning stimulus precedes the test stimulus by latencies between 6 and 25 ms, or if the conditioning stimulus succeeds the test stimulus at 1.5-ms intervals between approximately 1 and 4.5 ms) effects on corticospinal output10,16. Paired-pulse TMS can also be used to investigate interactions between two spatially distinct brain regions (Fig. 2b). In this model, a conditioning stimulus applied over one cortical area is followed by a test stimulus applied over a second, anatomically connected area. In the motor system, inhibitory or facilitatory effects on MEP sizes can be obtained when the test stimulus applied over M1 is preceded by a conditioning stimulus over contralateral M1, cerebellum, premotor and parietal regions17–19. In the visual system, the effects of paired-pulse TMS on phosphene threshold have been used to evaluate functional connectivity among V1, V5 and the frontal eye field20,21.
Repetitive TMS (rTMS) refers to a family of widely used NIBS techniques. rTMS can be applied using protocols in which stimulation and task performance are dissociated in time. In the most common approach, rTMS is applied over a site of interest for several minutes. The induced effects outlast the period of stimulation, giving insight into the role of the specific stimulated brain regions in plasticity and behavior. For example in the motor system, low-frequency (1 Hz) rTMS inhibits cortical excitability, creating a transient ‘virtual lesion’22. This resembles a reversible pharmacological lesion classically used in animal models to study the function of the targeted brain area in motor learning by applying inhibitory rTMS at different time points and to different brain regions along the learning process23–25. Alternatively, high-frequency (5–20 Hz) rTMS produces an increase in cortical excitability26, which can facilitate motor sequence learning27, though the effects may vary28. rTMS has been extended to probe cognitive processes as well, including spatial attention, working memory, episodic memory and decision making11,29,30.
Patterned stimulation protocols represent another established form of rTMS. Theta-burst stimulation (TBS), which involves the application of a burst of three 50-Hz pulses in trains repeated at 200-ms intervals, is the primary protocol in this class. Three TBS variants have been extensively explored. Continuous TBS (cTBS) involves the application of burst trains for 20–40 s and has an inhibitory effect on corticospinal excitability. Conversely, for intermittent TBS (iTBS), burst trains with a duration of 2 s are applied over a total of 190 s, with the trains repeating every 10 s. Intermediate TBS, a third variant including 5-s burst trains repeated every 15 s for a total of 110 s, is typically used as a negative control for cTBS and iTBS as it shows no effects on corticospinal excitibility31. These patterned stimulation protocols induce longer-lasting effects than conventional rTMS paradigms.
tDCS is most commonly applied at 1–2 mA for 5–20 min using 5-cm2 saline-soaked sponge electrodes and has been shown to have effects across various functions7. For example, anodal tDCS facilitates visual perception32, spatial tactile acuity33, visuospatial attention34 and also higher order cognitive functions35. Notably, beyond its short-term effects, application of tDCS in conjunction with task execution may result in prolonged improvements in performance. For example, application of anodal tDCS over M1 during multiple sessions enhanced motor skill learning36, and bilateral tDCS over posterior parietal cortex has been shown to enhance numerical processing37. Thus, overall, the underlying logic has been to induce by NIBS neural patterns that facilitate learning by coupling this activity with motor practice or perceptual stimulation, or modulate state-dependent activity (Box 1). This coupling might be useful for recalibrating or ameliorating neural functioning in clinical conditions.
Box 1. State dependency in brain stimulation.
The neural impact of an external stimulus is determined not only by the properties of that stimulus but also by the underlying state of the activated brain region. For example, the probability of phosphene perception induced by near-threshold TMS of the occipital cortex depends on the phase of ongoing alpha oscillations98. In accordance with the view of state-dependent stimulation effects, a new method called TMS adaptation (TMSA) has been introduced to increase the functional resolution of TMS. TMSA is based on the hypothesis that TMS acts differentially on neurons according to their initial neural activation state. An adapting stimulus, presented for a long time (usually 40–60 s), is used to induce habituation in a subset of cells that encode particular stimulus attributes, therefore making them a selective target for TMS11,99,100. TMSA predicts that TMS improves processing of attributes that are adapted, whereas it decreases performance for non-adapted attributes. However, the underlying mechanism of TMS and TMSA are still debated. To investigate these mechanisms, a recent study manipulated the brain state using contrast adaptation, a decrease in visual contrast sensitivity produced by repeated exposure to high-contrast stimuli6. TMS impaired perception when the visual cortex was not adapted but facilitated perception after adaptation. It has been proposed that TMS affects excitatory and inhibitory neural populations differentially, and that TMS has an activity-dependent suppressive effect on the inhibitory populations. Thus, overall, state dependency may be important in understanding the biological effects and mechanisms through which NIBS modulates neural activity.
Basic neurophysiological mechanisms
Although these two techniques are capable of producing similar physiological and behavioral effects, TMS and tDCS are believed to operate by different mechanisms. In searching for a feasible mechanistic framework underlying TMS, studies have mainly explored similarities with activity-dependent synaptic plasticity. Repetitive electrical stimulation of adult hippocampus in in vitro animal models induces NMDA receptor–dependent long-term potentiation (LTP) or depression (LTD)38,39. High frequency stimulation results in persistent increases in synaptic strength, whereas long trains of low frequency stimulation result in a lasting decrease in synaptic efficacy. In humans, similar in vivo LTP-like and LTD-like plasticity effects in the neocortex are largely reproduced by rTMS, with sustained changes in MEP amplitudes serving as a noninvasive probe for cortical excitability. Solid evidence linking human rTMS with LTP-like and LTD-like plasticity comes from studies using TBS protocols31,40, which are based on LTP and LTD stimulation protocols used in animal models. Another stimulation protocol, widely used for demonstrating LTP-like and LTD-like plasticity, is paired associative stimulation (PAS)41, in which low-frequency median nerve stimulation is coupled with TMS over the contralateral motor cortex. PAS protocols are of particular relevance because they demonstrate some of the characteristics of spike timing–dependent plasticity42, wherein the order and precise temporal interval between presynaptic and postsynaptic spikes determine the sign and magnitude of LTP-like or LTD-like synaptic changes43. More recently, PAS-like protocols have been extended to corticocortical pathways using repetitive paired-pulse TMS applied to directly connected cortical regions, producing similar effects44,45.
Pharmacological interventions provide further information about the possible mechanisms underlying TMS. The NMDA receptor antagonist memantine blocks the inhibitory effect of cTBS and the facilitory effect of iTBS46. Similarly, dextromethorphan, another NMDA receptor antagonist, blocks PAS-induced facilitation of MEP amplitudes47. It thus appears that, overall, the LTP-like and LTD-like effects of rTMS rely on NMDA receptor–mediated glutamatergic function.
The mechanisms by which tDCS exerts its effects are yet to be fully determined48. Unlike TMS, which is believed to induce action potentials in and around the stimulated neuronal tissue, tDCS is considered to have a modulatory effect, biasing cortical excitability8. More specifically, animal studies have established that anodal stimulation seems to increase neuronal excitability and spontaneous firing rate by depolarizing resting membrane potentials, whereas cathodal stimulation hyperpolarizes membrane potentials, leading to decreased neuronal firing rate and excitability. These effects are time and intensity dependent49. Similar polarity-specific shifts in cortical excitability have been observed in humans8, despite the added complexity associated with transcranial, rather than direct, application of stimulation.
Here too, pharmacological interventions have facilitated our understanding of the possible mechanisms of tDCS effects in humans, specifically helping to differentiate between short-lasting effects and longer-lasting aftereffects. In particular, ion-channel blockers seem to have differential polarity-specific effects on cortical excitability. Administration of the voltage-dependent sodium channel blocker carbamazepine50,51 or calcium channel blocker flunarizine51 eliminates only anodal LTP-like plasticity, both during and after stimulation51. Alternately, administration of the NMDA receptor antagonist dextromethorphan hinders the post-stimulation effects of tDCS in a non-polarity-specific manner51.
These findings were further elaborated in a recent ex vivo animal study in mice, in which anodal DCS applied to M1 slices was coupled with low-frequency synaptic stimulation inducing long-term synaptic plasticity52. Notably, these effects required activity-dependent brain-derived neurotrophic factor (BDNF) secretion52, a finding that is in agreement with previous demonstrations of the role of BDNF in NIBS-induced plasticity53,54 and its modulatory role in NMDA receptor-dependent LTP and LTD55,56. Other studies in humans have implicated the GABAergic system in tDCS-induced plasticity57,58. In particular, polarity-specific changes in GABA concentration, occurring with tDCS, were documented using magnetic resonance spectroscopy58. Taken together, these results indicate that the lasting aftereffects of tDCS may indeed reflect LTP-like and LTD-like plasticity, possibly through membrane polarization50,51. The excitatory effects of anodal tDCS seem to primarily involve NMDA receptor–dependent LTP but may also be mediated by a reduction in GABAergic inhibition58. Conversely, the inhibitory effects of cathodal tDCS seem to mainly reflect reduction in excitatory glutamatergic neurotransmission.
Recent studies have extended this mechanistic framework to establish the effect of certain neuromodulators on NIBS-induced plasticity. Although effects have been documented for the cholinergic, serotonergic and adrenergic systems, most investigations have focused on the dopaminergic system59. For example, administration of the D2 receptor antagonist sulpiride blocks the excitatory and inhibitory effects of iTBS and cTBS, respectively60. Similarly, low and high dosage l-DOPA administration abolishes tDCS-induced LTP-like and LTD-like plasticity61. Overall, these findings demonstrate that dopaminergic neurotransmission is required for NIBS-induced LTP-like and LTD-like plasticity, findings that are congruent with animal models59.
Inter-individual differences: challenges and possibilities
Given the complex set of biophysical interactions that NIBS rely on, it is perhaps not surprising that stimulation-induced behavioral, physiological and therapeutic effects are not uniform and tend to substantially vary among individuals. Indeed, several factors have been shown to modulate the magnitude of NIBS effects, including variation in brain and skull morphology, local brain oscillations, age, physical fitness and sex, to name a few62.
The presence of inter-individual difference in stimulation-induced effects poses a real challenge for studies and future therapeutic intervention using NIBS. Yet with careful methodological adjustments, the presence of such variation, rather than being a source of possible confounding effects, can be exploited as a source of information for gaining a greater mechanistic understanding of NIBS. For example, polymorphisms in genes related to dopamine63 and BDNF54 explain some of the variation in NIBS-induced plasticity and have thus pointed to mediation by these mechanisms. Furthermore, models of cortical surface folding patterns and white matter fiber architecture derived from structural neuroimaging data have recently been used to inform quantitative physical models of induced electrical fields generated by NIBS in individual subjects64. Thus, in the future, initial profiling of individuals that takes into account the presence of such gene polymorphisms or morphological structure could help inform individualized NIBS-based therapeutic interventions and improve possible outcomes.
Modulation of cortical network dynamics
The human brain is a complex neural network hierarchically organized on multiple, overlapping spatial scales65. One crucial principle of this organization is the strict competitive balance between segregated and integrated information exchange and processing, which is achieved through transient or long-lasting synchronization of oscillatory activity66. Experimental evidence obtained in both animals and humans suggests that NIBS techniques are capable of modulating such cortical oscillatory activity at each spatial scale67. Thus, they serve as powerful and complementary tools for investigating causal interactions in brain networks68.
At the small end of the spatial scale spectrum lie cortical microcircuits, which consist of layer V pyramidal neurons connected to glutamatergic corticocortical and corticofugal projection neurons and to heterogeneous GABAergic interneurons distributed in columnar fashion across different cortical laminae69. Several structural similarities exist between cortical microcircuits and central pattern generators classically identified with the spinal cord and brainstem, which support intrinsic and spontaneous oscillatory dynamics that can be modified by external inputs70. Paired-pulse TMS protocols that apply both stimuli to M1 through the same coil interrogate these intra- and inter-microcircuit dynamics. More recently, some researchers have begun using triple-pulse TMS protocols (which apply a combination of two standard conditioning stimuli) to gain insight into how different local network oscillations interact with one another71.
Paired- and triple-pulse techniques have also been used to interrogate networks on larger spatial scales, through the multi-regional protocols described previously. A single supra-threshold TMS pulse generates very high frequency, short-lasting and spatially specific oscillations that are phase-locked to the stimulus4. This combination of properties makes TMS useful for probing functional connectivity between connected brain regions, as distinct oscillations generated by a conditioning stimulus applied to a cortical region and a test stimulus applied to M1 can interact sufficiently to modulate motor output only in a small temporal window. Furthermore, the degree to which the phases of these oscillations are coupled determines the degree of modulation67. Initial applications along these lines studied interactions between right and left M1 (ref. 15). However, a pioneering application of paired-pulse stimulation72, as well as concurrent TMS–functional magnetic resonance imaging studies in which TMS applied to M1 or dorsal premotor cortex produces robust blood oxygen level–dependent modulation in remote, but connected, regions, laid the groundwork for expansion to other regions connected directly or indirectly to M1 (ref. 73).
Thus far, several interactions between M1 and other areas have been experimentally probed using this technique. These regions include ventral17,74 and dorsal premotor cortex75, pre-supplementary motor area76, dorsolateral prefrontal cortex77, posterior parietal cortex78 and the cerebellum79. Interactions between frontal eye fields and extrastriate visual cortex have also been explored using this technique21. Additionally, triple-pulse studies have made it possible to explore the effects of inter-regional inputs on local processing within M1 circuits71.
Across all of these studies, several consistent and robust findings have emerged17,74,76. First, inter-regional paired-pulse stimuli only show significant modulation of MEP amplitude at highly specific interstimulus intervals. For two regions that are directly connected anatomically, these effects are most common when the stimuli are separated in time by 6 or 8 ms. Although the interstimulus latencies producing significant modulation remain consistent whether measured at rest or in the context of a behavioral task, the sign of the modulation typically differs. In most cases, inter-regional paired-pulse stimulation results in inhibitory modulation of motor output (reduced MEP amplitude) when probed at rest74 but switches to facilitatory modulation when applied during a behavioral task75. Finally, the observed modulation during behavioral tasks is highly context specific17,74–76. Interpretation of the mechanisms mediating inhibitory and facilitatory modulation remains difficult, but it is clear that more expansive network interactions are crucial17.
The use of NIBS techniques in which stimulation and task performance are dissociated in time has yielded substantial information regarding functional specificity of individual network nodes during specific tasks through a ‘perturb and measure’ approach80–82. This approach has been used, for example, by combining TMS and subsequent neuroimaging measurements82 or by using low-frequency rTMS to modulate MEPs during specific tasks80,81. tDCS was also used in this manner to investigate changes in global inter-regional phase coupling at multiple frequency bands83. As expected, owing to the nonspecific frequency nature of tDCS, anodal tDCS applied to M1 resulted in increased synchronization within task-related networks across multiple frequency bands. When the effects of stimulation were assessed at rest, decreased synchronization was observed in the default-mode network83.
Recently, two NIBS techniques have been developed to gain a better understanding of the function of frequency-specific local and global cortical oscillatory dynamics in the production of behavior. Rhythmic TMS67 entrains specific brain oscillations by applying rTMS at the same frequency, resulting in a progressive increase in power in that band (Fig. 3a). Thus far, this technique has been used to successfully perturb theta, alpha, beta and gamma oscillatory activity67,84,85. Even more notably, it has for the first time allowed specific features of oscillatory dynamics to be causally linked to distinct perceptual processes in human subjects67,85.
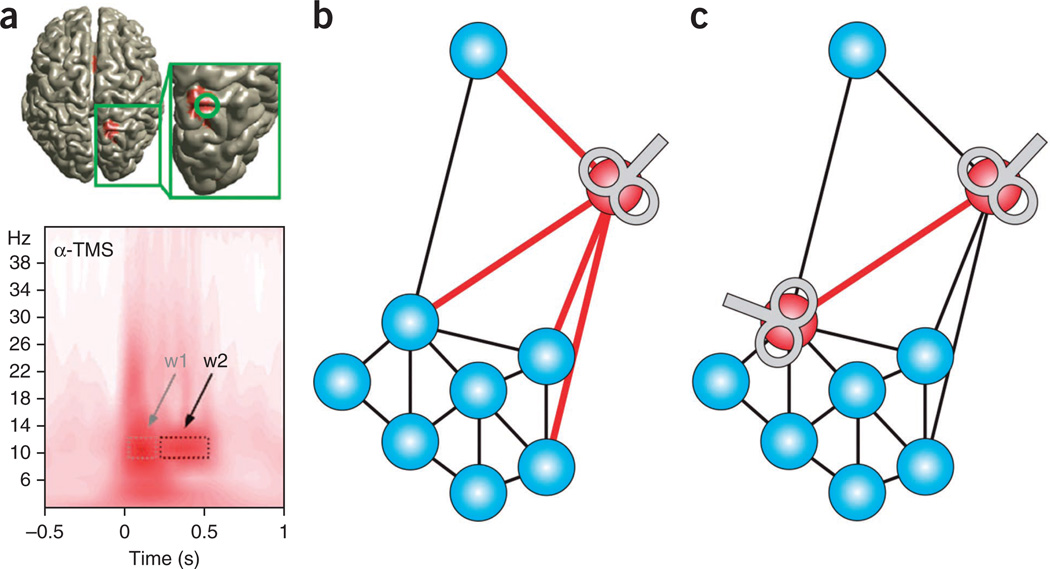
Figure 3.
Probing cortical network dynamics with NIBS. (a) Alpha-frequency rhythmic TMS applied to presumed generators of endogenous alpha oscillations in the posterior parietal cortex (inset, top; shown in red) entrains local endogenous oscillations that are specific to that frequency. This results in progressive increase in alpha power in early (w1) and late (w2) time windows (bottom). Modified from ref. 67 with permission. (b,c) rTMS protocols for investigating the role of nodes and connections in brain network dynamics. (b) A conventional rTMS protocol application that targets a specific brain region, or network node. In combination with functional neuroimaging, the effects of this stimulation on overall network dynamics can be assessed. (c) A recently developed repetitive paired-pulse TMS that seems to be capable of targeting a specific functional connectivity pathway (red). This will enable investigation of the roles of connections in network dynamics44.
In a separate experiment, transcranial alternating current stimulation, a second NIBS technique capable of influencing network dynamics in a frequency-specific manner, was used to investigate the effect of theta-band synchronization/desynchronization of parietofrontal regions on performance in a working memory task86. Synchronization resulted in performance improvement, whereas anti-phase desynchronization resulted in performance decreases relative to sham stimulation. In a similar vein, borrowing from methodologies developed in PAS protocols that pair cortical TMS with electrical stimulation of peripheral nerves, repetitive cortico-cortical paired-pulse TMS protocols have shown the ability to facilitate or inhibit specific pathways between two connected brain regions44,45 (Fig. 3b,c). This technique has yet to be implemented in combination with neuroimaging, however, so it is unknown how global network effects of repetitive cortico-cortical paired-pulse TMS on global network interactions differ from rTMS techniques targeting functional brain regions. Thus, this presents a new method that can be used to investigate the influences of specific functional connectivity pathways on network dynamics underlying a wide variety of behaviors.
Conclusions and future directions
Technological and methodological advances in NIBS bring about exciting new possibilities, as well as tackling some of the pitfalls associated with these techniques (Box 2 and Fig. 4). New TMS coils are being developed for stimulation of deep neural pathways87. Other technological advances have been introduced to generate more realistic sham stimulation conditions88. Another emerging trend is the combination of NIBS with other techniques; for instance, with positron-emission tomography and magnetic resonance spectroscopy to study the molecular mechanisms of action58 and with other imaging modalities (diffusion and functional MRI, electroencephalography or magnetoencephalography) to document large-scale stimulation-induced reorganization of structural and functional networks at rest or during task-related activity. Advances have also been made in applying new stimulation protocols able to increase the functional resolution of NIBS (Box 2).
Box 2. Technological challenges.
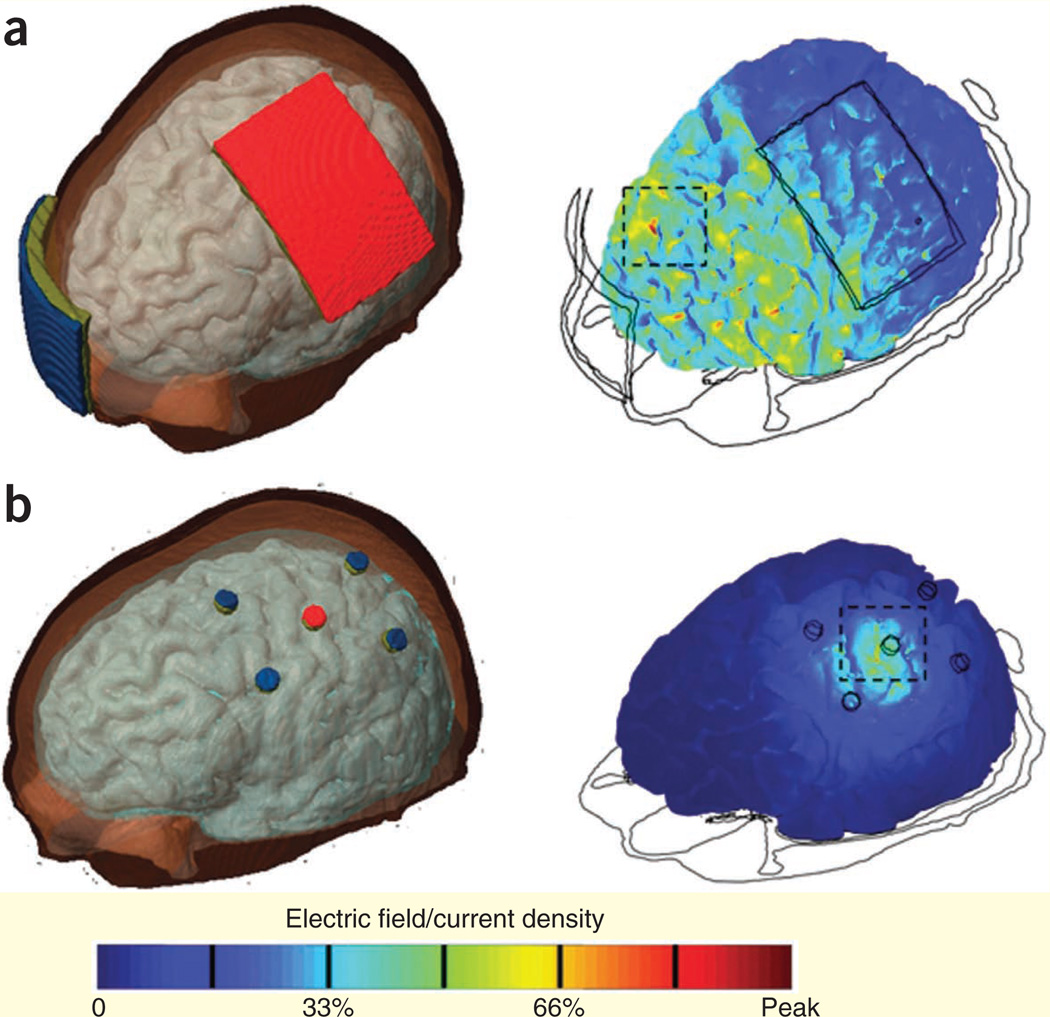
In light of the emerging interest in using paired-pulse TMS to evaluate inter-regional functional interactions, including cortical regions in the same hemisphere, the size of the coils has become a limitation. Mini-coils designed specifically for this application have been developed—for example, to probe connectivity between dorsal premotor cortex and M1 in the same hemisphere92. However, more generic developments are required to address this sophisticated challenge. For example, as the size of the coil is decreased, the current necessary for effective stimulation increases93. In addition, smaller coils heat more rapidly, limiting the duration and frequency of stimulation. For standard figure-eight coils, the problem of coil heating during repetitive stimulation might be addressed with active cooling system designs. A key technological challenge for TMS is improving stimulation focality and penetration depth, factors that typically show a tradeoff with one another94. The standard figure-eight coil is believed to stimulate a surface area ranging from 1 to 2 cm2, depending on coil type and tissue distribution95, but further efforts are needed to improve this focality. The electric field induced by TMS rapidly decays with distance, and thus the maximal effect of stimulation is limited to surface cortical, cerebellar and spinal cord structures. Stimulation of deeper structures, such as the cingulate gyrus, which may be of particular clinical promise, may possibly be achieved through the use of novel coil designs87. Improving the focality of stimulation is also a challenge for tDCS. One strategy is to reduce the size of the stimulation electrode while concurrently increasing the size of the reference electrode96. Another emerging class of innovations has been the use of novel electrode configurations97. A high-definition 4 × 1 ring configuration has recently been explored in computational models64,97, with this design resulting in increased spatial specificity and peak induced electric field magnitudes directly beneath the stimulating electrode (Fig. 4a,b). Addressing the challenge of tDCS focality will enable further advances in the use of tDCS to gain insight into intrinsic and larger scale functional architecture and network dynamics83,86.
Figure 4.
Stimulation focality of tDCS. (a) Cortical electric fields induced by a conventional tDCS electrode configuration. (b) Cortical electric fields induced by a 4 × 1 ring electrode configuration. Modified from ref. 97 with permission.
Other advances have been made in electrical stimulation of the brain, where emerging techniques are used presumably to induce or interfere with oscillations of cortical networks. These state-of-the-art techniques might be able to entrain task-related oscillatory activity, another important physiological determinant of cognitive processes89. Of these techniques, transcranial alternating current stimulation, as mentioned above, consists of an alternating current delivered in a frequency-specific fashion90. Transcranial random noise stimulation, by contrast, consists of an alternating current delivered to the cortex at random frequencies. Although the noise signal can contain all of the frequencies from 0.1 to 640 Hz, this spectrum can be also divided into the low frequency range (0.1–100 Hz) and high frequency range (100–640 Hz)91.
We have reviewed key findings that contribute to our understanding of the mechanisms underlying the physiological and behavioral effects of noninvasive brain stimulation techniques. Advances in the field reviewed here, as well as general advances in neuroscience, will lend further insight into these issues. Furthermore, some of these advances represent a paradigmatic shift in systems neuroscience, with a clear focus now on using NIBS to investigate and modulate complex network interactions, which may allow unresolved historical problems to be revisited with a fresh perspective.
Acknowledgments
We thank S.-L. Liew for suggestions. This work was supported by the Intramural Research Program of the US National Institute of Neurological Disorders and Stroke (NINDS; US National Institutes of Health) and by funding from US Department of Defense in the Center for Neuroscience and Regenerative Medicine to M.S. and E.R.B. N.C. was supported by an NINDS Ruth L. Kirschstein National Research Service Award.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Hummel F, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 2.Miniussi C, et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008;1:326–336. doi: 10.1016/j.brs.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;325:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 4.Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J. Neuroeng. Rehabil. 2009;6:7. doi: 10.1186/1743-0003-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasley BN, Allen EA, Freeman RD. State-dependent variability of neuronal responses to transcranial magnetic stimulation of the visual cortex. Neuron. 2009;62:291–303. doi: 10.1016/j.neuron.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perini F, Cattaneo L, Carrasco M, Schwarzbach JV. Occipital transcranial magnetic stimulation has an activity-dependent suppressive effect. J. Neurosci. 2012;32:12361–12365. doi: 10.1523/JNEUROSCI.5864-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utz KS, Dimova V, Oppenlander K, Kerkhoff G. Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology–a review of current data and future implications. Neuropsychologia. 2010;48:2789–2810. doi: 10.1016/j.neuropsychologia.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. (Lond.) 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nitsche MA, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J. Physiol. (Lond.) 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Sandrini M, Umilta C, Rusconi E. The use of transcranial magnetic stimulation in cognitive neuroscience: a new synthesis of methodological issues. Neurosci. Biobehav. Rev. 2011;35:516–536. doi: 10.1016/j.neubiorev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LG, et al. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- 13.Chambers CD, Payne JM, Stokes MG, Mattingley JB. Fast and slow parietal pathways mediate spatial attention. Nat. Neurosci. 2004;7:217–218. doi: 10.1038/nn1203. [DOI] [PubMed] [Google Scholar]
- 14.Bütefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J. Neurophysiol. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- 15.Reis J, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J. Physiol. (Lond.) 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J. Physiol. (Lond.) 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buch ER, Mars RB, Boorman ED, Rushworth MF. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J. Neurosci. 2010;30:1395–1401. doi: 10.1523/JNEUROSCI.4882-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch G, et al. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J. Neurosci. 2007;27:6815–6822. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann. Neurol. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- 20.Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- 21.Silvanto J, Lavie N, Walsh V. Stimulation of the human frontal eye fields modulates sensitivity of extrastriate visual cortex. J. Neurophysiol. 2006;96:941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- 22.Chen R, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 23.Censor N, Dimyan MA, Cohen LG. Modification of existing human motor memories is enabled by primary cortical processing during memory reactivation. Curr. Biol. 2010;20:1545–1549. doi: 10.1016/j.cub.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muellbacher W, et al. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- 25.Censor N, Cohen LG. Using repetitive transcranial magnetic stimulation to study the underlying neural mechanisms of human motor learning and memory. J. Physiol. (Lond.) 2011;589:21–28. doi: 10.1113/jphysiol.2010.198077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- 27.Kim YH, Park JW, Ko MH, Jang SH, Lee PK. Facilitative effect of high frequency subthreshold repetitive transcranial magnetic stimulation on complex sequential motor learning in humans. Neurosci. Lett. 2004;367:181–185. doi: 10.1016/j.neulet.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 28.Agostino R, et al. Effects of 5 Hz subthreshold magnetic stimulation of primary motor cortex on fast finger movements in normal subjects. Exp. Brain Res. 2007;180:105–111. doi: 10.1007/s00221-006-0838-3. [DOI] [PubMed] [Google Scholar]
- 29.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314:829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- 30.Rossi S, et al. Prefrontal cortex in long-term memory: an “interference” approach using magnetic stimulation. Nat. Neurosci. 2001;4:948–952. doi: 10.1038/nn0901-948. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Antal A, et al. Direct current stimulation over V5 enhances visuomotor coordination by improving motion perception in humans. J. Cogn. Neurosci. 2004;16:521–527. doi: 10.1162/089892904323057263. [DOI] [PubMed] [Google Scholar]
- 33.Ragert P, Vandermeeren Y, Camus M, Cohen LG. Improvement of spatial tactile acuity by transcranial direct current stimulation. Clin. Neurophysiol. 2008;119:805–811. doi: 10.1016/j.clinph.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparing R, et al. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain. 2009;132:3011–3020. doi: 10.1093/brain/awp154. [DOI] [PubMed] [Google Scholar]
- 35.Fregni F, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 36.Reis J, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. USA. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen Kadosh R, Soskic S, Iuculano T, Kanai R, Walsh V. Modulating neuronal activity produces specific and long-lasting changes in numerical competence. Curr. Biol. 2010;20:2016–2020. doi: 10.1016/j.cub.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. (Lond.) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J. Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Lazzaro V, et al. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J. Physiol. (Lond.) 2008;586:3871–3879. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 42.Wolters A, et al. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J. Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- 43.Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buch ER, Johnen VM, Nelissen N, O’Shea J, Rushworth MF. Noninvasive associative plasticity induction in a corticocortical pathway of the human brain. J. Neurosci. 2011;31:17669–17679. doi: 10.1523/JNEUROSCI.1513-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rizzo V, et al. Paired associative stimulation of left and right human motor cortex shapes interhemispheric motor inhibition based on a Hebbian mechanism. Cereb. Cortex. 2009;19:907–915. doi: 10.1093/cercor/bhn144. [DOI] [PubMed] [Google Scholar]
- 46.Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin. Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 47.Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J. Physiol. (Lond.) 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 49.Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J. Physiol. (Lond.) 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 51.Nitsche MA, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. (Lond.) 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fritsch B, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antal A, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010;3:230–237. doi: 10.1016/j.brs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Cheeran B, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J. Physiol. (Lond.) 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 56.Woo NH, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 57.Nitsche MA, et al. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur. J. Neurosci. 2004;19:2720–2726. doi: 10.1111/j.0953-816X.2004.03398.x. [DOI] [PubMed] [Google Scholar]
- 58.Stagg CJ, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nitsche MA, Müller-Dahlhaus F, Paulus W, Ziemann U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: building models for the clinical use of CNS active drugs. J. Physiol. (Lond.) 2012;590:4641–4662. doi: 10.1113/jphysiol.2012.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monte-Silva K, et al. D2 receptor block abolishes theta burst stimulation-induced neuroplasticity in the human motor cortex. Neuropsychopharmacology. 2011;36:2097–2102. doi: 10.1038/npp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monte-Silva K, Liebetanz D, Grundey J, Paulus W, Nitsche MA. Dosage-dependent non-linear effect of l-dopa on human motor cortex plasticity. J. Physiol. (Lond.) 2010;588:3415–3424. doi: 10.1113/jphysiol.2010.190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. (Lond.) 2010;588:2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plewnia C, et al. Effects of transcranial direct current stimulation (tDCS) on executive functions: Influence of COMT Val/Met polymorphism. Cortex. 2012 Nov 15; doi: 10.1016/j.cortex.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Bikson M, Rahman A, Datta A. Computational models of transcranial direct current stimulation. Clin. EEG Neurosci. 2012;43:176–183. doi: 10.1177/1550059412445138. [DOI] [PubMed] [Google Scholar]
- 65.Bullmore E, Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 66.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat. Rev. Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thut G, et al. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr. Biol. 2011;21:1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shafi MM, Westover MB, Fox MD, Pascual-Leone A. Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. Eur. J. Neurosci. 2012;35:805–825. doi: 10.1111/j.1460-9568.2012.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bastos AM, et al. Canonical microcircuits for predictive coding. Neuron. 2012;76:695–711. doi: 10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuste R. Origin and classification of neocortical interneurons. Neuron. 2005;48:524–527. doi: 10.1016/j.neuron.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Ni Z, Muller-Dahlhaus F, Chen R, Ziemann U. Triple-pulse TMS to study interactions between neural circuits in human cortex. Brain Stimul. 2011;4:281–293. doi: 10.1016/j.brs.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- 73.Bestmann S, et al. Mapping causal interregional influences with concurrent TMS-fMRI. Exp. Brain Res. 2008;191:383–402. doi: 10.1007/s00221-008-1601-8. [DOI] [PubMed] [Google Scholar]
- 74.Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J. Physiol. (Lond.) 2008;586:2735–2742. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MF. Functional specificity of human premotor-motor cortical interactions during action selection. Eur. J. Neurosci. 2007;26:2085–2095. doi: 10.1111/j.1460-9568.2007.05795.x. [DOI] [PubMed] [Google Scholar]
- 76.Mars RB, et al. Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict. J. Neurosci. 2009;29:6926–6931. doi: 10.1523/JNEUROSCI.1396-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hasan A, et al. Muscle and timing-specific functional connectivity between the dorsolateral prefrontal cortex and the primary motor cortex. J. Cogn. Neurosci. 2013;25:558–570. doi: 10.1162/jocn_a_00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koch G, et al. TMS activation of interhemispheric pathways between the posterior parietal cortex and the contralateral motor cortex. J. Physiol. (Lond.) 2009;587:4281–4292. doi: 10.1113/jphysiol.2009.174086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daskalakis ZJ, et al. Exploring the connectivity between the cerebellum and motor cortex in humans. J. Physiol. (Lond.) 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Avenanti A, Annella L, Candidi M, Urgesi C, Aglioti SM. Compensatory plasticity in the action observation network: virtual lesions of STS enhance anticipatory simulation of seen actions. Cereb. Cortex. 2013;23:570–580. doi: 10.1093/cercor/bhs040. [DOI] [PubMed] [Google Scholar]
- 81.Avenanti A, Bolognini N, Maravita A, Aglioti SM. Somatic and motor components of action simulation. Curr. Biol. 2007;17:2129–2135. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 82.Paus T. Inferring causality in brain images: a perturbation approach. Phil. Trans. R. Soc. Lond. B. 2005;360:1109–1114. doi: 10.1098/rstb.2005.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Polanía R, Nitsche MA, Paulus W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum. Brain Mapp. 2011;32:1236–1249. doi: 10.1002/hbm.21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chanes L, Quentin R, Tallon-Baudry C, Valero-Cabre A. Causal frequency-specific contributions of frontal spatiotemporal patterns induced by non-invasive neurostimulation to human visual performance. J. Neurosci. 2013;33:5000–5005. doi: 10.1523/JNEUROSCI.4401-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Romei V, Driver J, Schyns PG, Thut G. Rhythmic TMS over parietal cortex links distinct brain frequencies to global versus local visual processing. Curr. Biol. 2011;21:334–337. doi: 10.1016/j.cub.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Polanía R, Nitsche MA, Korman C, Batsikadze G, Paulus W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr. Biol. 2012;22:1314–1318. doi: 10.1016/j.cub.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 87.Roth Y, Amir A, Levkovitz Y, Zangen A. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J. Clin. Neurophysiol. 2007;24:31–38. doi: 10.1097/WNP.0b013e31802fa393. [DOI] [PubMed] [Google Scholar]
- 88.Rossi S, et al. A real electro-magnetic placebo (REMP) device for sham transcranial magnetic stimulation (TMS) Clin. Neurophysiol. 2007;118:709–716. doi: 10.1016/j.clinph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 89.Kuo MF, Nitsche MA. Effects of transcranial electrical stimulation on cognition. Clin. EEG Neurosci. 2012;43:192–199. doi: 10.1177/1550059412444975. [DOI] [PubMed] [Google Scholar]
- 90.Moliadze V, Antal A, Paulus W. Boosting brain excitability by transcranial high frequency stimulation in the ripple range. J. Physiol. (Lond.) 2010;588:4891–4904. doi: 10.1113/jphysiol.2010.196998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fertonani A, Pirulli C, Miniussi C. Random noise stimulation improves neuroplasticity in perceptual learning. J. Neurosci. 2011;31:15416–15423. doi: 10.1523/JNEUROSCI.2002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Groppa S, et al. The human dorsal premotor cortex facilitates the excitability of ipsilateral primary motor cortex via a short latency cortico-cortical route. Hum. Brain Mapp. 2012;33:419–430. doi: 10.1002/hbm.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cohen D, Cuffin BN. Developing a more focal magnetic stimulator. Part I: some basic principles. J. Clin. Neurophysiol. 1991;8:102–111. doi: 10.1097/00004691-199101000-00013. [DOI] [PubMed] [Google Scholar]
- 94.Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013;6:1–13. doi: 10.1016/j.brs.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wagner T, Rushmore J, Eden U, Valero-Cabre A. Biophysical foundations underlying TMS: setting the stage for an effective use of neurostimulation in the cognitive neurosciences. Cortex. 2009;45:1025–1034. doi: 10.1016/j.cortex.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nitsche MA, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J. Neurophysiol. 2007;97:3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- 97.Datta A, et al. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–207. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dugué L, Marque P, VanRullen R. The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. J. Neurosci. 2011;31:11889–11893. doi: 10.1523/JNEUROSCI.1161-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cattaneo L, Sandrini M, Schwarzbach J. State-dependent TMS reveals a hierarchical representation of observed acts in the temporal, parietal, and premotor cortices. Cereb. Cortex. 2010;20:2252–2258. doi: 10.1093/cercor/bhp291. [DOI] [PubMed] [Google Scholar]
- 100.Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]