Abstract
Schizophrenia risk has often been conceptualized using a model which requires two hits in order to generate the clinical phenotype—the first as an early priming in a genetically predisposed individual and the second a likely environmental insult. The aim of this paper was to review the literature and reformulate this binary risk-vulnerability model. We sourced the data for this narrative review from the electronic database PUBMED. Our search terms were not limited by language or date of publication. The development of schizophrenia may be driven by genetic vulnerability interacting with multiple vulnerability factors including lowered prenatal vitamin D exposure, viral infections, smoking intelligence quotient, social cognition cannabis use, social defeat, nutrition and childhood trauma. It is likely that these genetic risks, environmental risks and vulnerability factors are cumulative and interactive with each other and with critical periods of neurodevelopmental vulnerability. The development of schizophrenia is likely to be more complex and nuanced than the binary two hit model originally proposed nearly thirty years ago. Risk appears influenced by a more complex process involving genetic risk interfacing with multiple potentially interacting hits and vulnerability factors occurring at key periods of neurodevelopmental activity, which culminate in the expression of disease state. These risks are common across a number of neuropsychiatric and medical disorders, which might inform common preventive and intervention strategies across non-communicable disorders.
1. Introduction
Schizophrenia continues to impose a significant burden on global society. Data from the 2010 Global Burden of Disease Study suggests that mental and behavioral disorders account for a global disability adjusted life year (DALY) burden of 7.4% of the total, of which schizophrenia forms 0.6% of this (Murray et al., 2012). The modern theory of the development of schizophrenia has been built upon the neurodevelopmental theory, first elaborated upon by seminal papers by Weinberger and Murray and Robin almost thirty years ago. The initial neurodevelopmental theory speculated that a prenatal event could disrupt the normal maturation process of the brain which ultimately culminated in the clinical syndrome of schizophrenia later on during life, suggesting a “single hit” theory of schizophrenia (Weinberger, 1987; Murray and Lewis, 1987). As more research into the neurodevelopmental model occurred, it became apparent that such a static model was unable to explain features such as the longitudinal changes in brain volume that occur in schizophrenia (McGrath et al., 2003; Velakoulis et al., 2000). Research then began to focus on delineating the subsequent ‘second hits’ that were felt to be necessary to explain this gap in the neurodevelopmental model. However, as yet more work has gone into determining the nature of these second hits, it is becoming increasingly apparent that a two hit model is also inadequate to fully explain the considerable neuroanatomical heterogeneity present in schizophrenia (Gogtay et al., 2011; Pantelis et al., 2005). The notion that the timing of the hits during neurodevelopment causes differing outcomes, where early hits cause more widespread abnormalities as opposed to later hits causing more specific changes, has been previously explored as an extension of the original model (Pantelis et al., 2003). We propose that the notion of binary first and second hits oversimplified the model of schizophrenia pathogenesis and, based on modern epidemiology and neurobiology, we propose that a multi-hit threshold model interacting with key neurodevelopmental milestones might better represent the complex genetic, social and environmental interactions that have been explored as contributing to the development of schizophrenia.
Early developmental factors contributing to the neuroprogression of schizophrenia have been discussed elsewhere (Davis et al., 2014). There have been many potential non-genetic “second hits” proposed within the literature that act at differing periods of neurodevelopment (Owen et al., 2016). It may be that instead of being “second hits” per se, which implies that each factor occurs in a pre-specified binary sequence analogous to the development of malignant cells in cancer, given the complex interaction these factors have within a genetically at risk individual with the environment, they instead act as vulnerability factors. Such vulnerability factors likely have a relatively weak individual effect, but acting in concert at specific critical points of neurodevelopment in a genetically susceptible individual they may have sufficient magnitude to impact the development of the clinical syndrome of schizophrenia.
Factors suggested in the literature to be potential vulnerability factors include nutrition, perinatal vitamin D exposure, infection with human endogenous retroviruses (HERVs), smoking, intelligence quotient and social cognition, cannabis use, childhood trauma and social defeat.
This paper will provide concise summaries on selected vulnerability factors, with an exploration of the neurobiology of such factors involved in the complex causal chains to conceptualize a modern multiple hit theory for the pathogenesis of schizophrenia. The manuscript also explores prevention strategies which may serve to ameliorate the effects of some of these potential vulnerability factors.
2. Methods
Data for this review was sourced from electronic databases PUBMED, and was not limited by language or date of publication. The manuscripts were selected for relevance to the topics reviewed.
3. Cannabis and the endocannabinoid system
The use of cannabis is a potential vulnerability factor which is theorized to most likely to occur during the adolescent period. Cannabis use may represent one of the later non-genetic insults predisposing an individual to schizophrenia. According to the 2010 Global Burden of Disease study, cannabis usage worldwide is estimated to occur in 0.2% of the population; this is a figure that has stayed relatively constant since 1990, although the actual number of users has increased due to population growth (Degenhardt et al., 2013). There has also been an increase in cannabis potency due to changes in cannabis strains (Swift et al., 2013). Recent longitudinal studies suggest that there is up to a 40% greater risk of psychosis in individuals who have ever used cannabis, a value which persists even after controlling for such variables as premorbid personality traits, cigarette smoking, occupational function and poor social integration. There is also evidence of a dose-effect relationship between cannabis use and schizophrenia risk (Monteleone et al., 2014; Manrique-Garcia et al., 2012). Cannabis usage in adolescence has additionally been proposed to induce first episode psychosis at a younger age, with some authors suggesting that cannabis usage can induce the onset of psychosis up to 2.7 years earlier than in those who develop psychosis without a history of cannabis usage (Donoghue et al., 2014).
While evidence supporting the association between cannabis usage and the development of psychosis in susceptible individuals is relatively robust, the ongoing effects of cannabis usage on the course and symptoms of patients suffering from schizophrenia is less clear. Much of the literature surrounding ongoing cannabis usage in schizophrenia suggests that such co-morbid substance use is associated with worse outcomes including longer periods of hospitalization, higher relapse rates and less medication adherence (Malchow et al., 2013). On the other hand, a number of studies suggest that compared to non-users with schizophrenia, cannabis use may be associated with better cognitive and social functioning in schizophrenia; these are factors associated with positive clinical outcomes in psychosis (Koola et al., 2012; Lev-Ran et al., 2012). It has been argued that cannabis using patients have no significant difference in psychopathology or quality of life measures compared to non-using patients, even after adjustment for confounders such as length of illness and baseline illness severity (van Dijk et al., 2012). This may be due to the fact that cannabis can decrease blood concentrations of psychiatric medications such as antipsychotics, thus preventing some side effects related to negative and cognitive symptoms. However, it may aggravate positive symptoms, which is suggested by a higher incidence of relapse and re-hospitalization (van Dijk et al., 2012).
Evidence regarding the possible effect of cannabis use on the structural integrity of differing brain regions is inconclusive. The hippocampus is often reported to be structurally abnormal in schizophrenia, and some groups have demonstrated that chronic cannabis use in otherwise healthy individuals is associated with a smaller hippocampal and amygdala volumes and subclinical positive symptoms (Solowij et al., 2013; Yucel et al., 2008), as well as white matter tract reductions on diffusion tensor imaging in the region of the hippocampus (Zalesky et al., 2012). A recent study in these subjects also demonstrated that cannabis specifically affected medial temporal lobe structures (Lorenzetti et al., 2015), suggesting that cannabis use impacts hippocampus and amygdala structures specifically in normal individuals.
Patients with first episode psychosis who are regular cannabis users have a greater overall volume reduction during 5-year longitudinal follow-up, as opposed to individuals with schizophrenia who are non-users, with the greatest loss of volume pertaining to grey matter (Cohen et al., 2012a). In contrast, other authors have found larger brain volumes within both the right and left hippocampus in adolescents suffering from schizophrenia and comorbid cannabis use (Kumra et al., 2012). Other studies found increased grey matter density within the left dorsolateral prefrontal cortex and have suggested this alteration may in part explain the observation between cannabis use and cognitive function in those with schizophrenia (Schnell et al., 2012). Some of these conflicting findings of the effects in psychosis may in part stem from the observation that structural brain changes in schizophrenia may in fact represent a core feature of the illness, with volume loss within the thalami, hippocampus and prefrontal regions being already present in individuals who are at increased risk of developing schizophrenia, and that cannabis use is associated with further volume reductions and the potential transition from ultra-high risk to disease state in genetically disposed individuals (Welch et al., 2013).
4. Childhood trauma
Childhood trauma, including both physical and psychological maltreatment, childhood sexual abuse, parental loss or divorce, parental substance abuse, and poverty (Green et al., 2014), appears to be a potential vulnerability factor for the development of schizophrenia in later life. Meta-analyses suggest that individuals with a history of childhood trauma have nearly three times the risk of developing psychosis, with an estimated population attributable risk of 33% (Sahin et al., 2013). Although the field suffers from some methodological problems, including a lack of a clear definition of what constitutes childhood trauma, in general the findings suggest that the more severe the childhood trauma, the more severe the subsequent symptomatology which accompanies the illness (Braehler et al., 2013). Also, most studies were retrospective and therefore cannot defectively disentangle cause and response relationships. Interestingly, children with a family history of schizophrenia or those who display antecedents of schizophrenia were more likely to be exposed to major negative life events and daily stressors compared to their peers (Cullen et al., 2014). Importantly, a recent meta-analysis suggested that no one particular type of trauma confers greater risk of psychosis compared to others (Green et al., 2014).
Childhood trauma also appears to be associated with worse positive symptoms in individuals suffering from schizophrenia compared to those who have no history of childhood trauma, and childhood trauma is additionally associated with non-remission of positive symptoms (Cohen et al., 2012b). This non-remission of positive symptoms has been suggested to be in part due to increased hypothalamic pituitary adrenal (HPA) axis activity and cortisol secretion in patients with a history of trauma, although such a hypothesis is at present inconclusive (Corcoran et al., 2012). A study of children with either a family history of schizophrenia or those who displayed antecedents of schizophrenia showed no difference in pituitary volume on neuroimaging of children aged between 11 and 14 years in comparison to a control group, which suggests that HPA axis hyperactivity may develop later within the pathogenesis of schizophrenia (Cullen et al., 2015).
Brain derived neurotrophic factor (BDNF) is another potential candidate explanation for the proposed link between childhood trauma and the development of schizophrenia. Whilst the evidence in relation to BDNF in individuals suffering from schizophrenia remains somewhat inconclusive, recent systematic reviews have suggested that serum BDNF levels are decreased in both drug-naïve and medicated patients with schizophrenia (Fernandes et al., 2014). Exposure to trauma also appears to decrease the expression of BDNF messenger ribonucleic acid (mRNA) within some areas of the brain including the hippocampus and some have suggested that the hippocampal volume deficits seen within schizophrenia may be in part due to this decreased expression of BDNF (Bennett and Lagopoulos, 2014). Interestingly, BDNF mRNA expression in response to trauma was increased in the basal lateral amygdala, which may in part account for the inconsistent alterations seen in this region in imaging studies of schizophrenia patients with a history of childhood trauma.
In accordance with several other lines of evidence, maltreated children appear to demonstrate smaller intra-cerebral volumes in comparison to healthy controls, leading some authors to speculate that the volume reduction often observed in early schizophrenia may in part be due to the contribution of childhood trauma (Green et al., 2014). Volume reductions within the left hippocampus, a commonly reported finding in both first episode and patients with chronic schizophrenia, has also been reported in adult survivors of childhood abuse without psychosis (Picken and Tarrier, 2011). Other groups have suggested that the finding between a history of abuse and reduced hippocampal volume may only observed for male patients (Samplin et al., 2013). There has also been some suggestion within observational retrospective studies that amygdala volumes may be altered in schizophrenia in those patients with a history of childhood trauma, however this finding has not been reliably replicated (Hoy et al., 2012). In contrast to these findings, others have found that high-risk individuals with larger hippocampal volumes were more likely to develop psychosis than individuals with smaller or normal hippocampal volumes, and other prospective studies report no difference between schizophrenia and appropriately age-matched controls (Brambilla et al., 2013; Phillips et al., 2002). The noted aberrations may be secondary to inflammatory changes in this region during acute illness episodes (Cropley et al., 2013; Cropley and Pantelis, 2014). At present it remains unclear as to whether childhood trauma drives some structural brain changes seen in schizophrenia or if the noted changes antedate the development of psychosis and are simply a result of the traumatic process itself. Despite these unanswered questions, childhood trauma does appear to confer a significant vulnerability occurring early in life that may predispose to the later development of schizophrenia in the presence of other potential insults
5. Social defeat
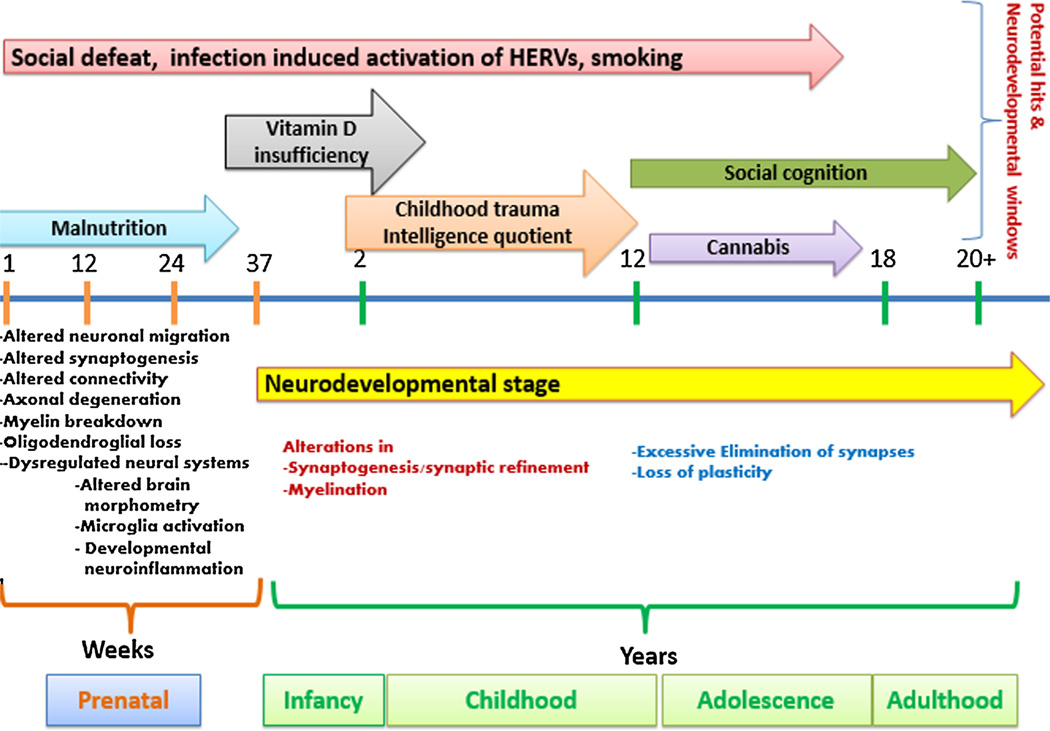
Social defeat, where social defeat is defined as losing a confrontation amongst any type of hostile dispute amongst humans, is a theory that is proposed by some to underlie part of the observed associations between vulnerability factors for schizophrenia development such as being part of a migrant population or having an urban upbringing. It needs to be noted that urbanicity is a however a broad proxy of many risk factors. Social defeat may act across multiple potential periods of neurodevelopment. The main crux of the argument for social defeat being a hit in schizophrenia is that social stressors, which are often perceived to be worse amongst marginalized and excluded social or ethnic groups, may potentiate an individual’s underlying genetic and other environmental risk for developing schizophrenia. Indeed people with mental health disorders often present a more vulnerable and socially excluded population who are more likely to be affected by discrimination, human rights violations or poverty, and exposed to other environmental risks (Susser and Patel, 2014). In addition, despite documentation of the effectiveness of a range of interventions from pharmacological to social, and the recent movement toward championing global mental health as a serious global disease burden, the majority of the world’s populace continues to have minimal to no access to such interventions (Patel and Saxena, 2014). In a similar way to childhood trauma, it appears that the more severe the social stressors an individual is exposed to the more likely they are to develop a psychotic disorder. Some authors have shown that the risk of schizophrenia is highest amongst immigrant groups who experience the least social integration, and subsequently are also likely to have the most poverty, alienation and social uprooting (Li et al., 2012). Similarly children from a different ethnic background were found to have a higher risk of subsequent psychosis if they were in schools with a higher percentage of native students (van Nierop et al., 2014). The social defeat theory may intertwine with that of childhood trauma, as some authors have proposed that early social adversities including trauma may induce negative schemas about the self, which later translates into an increased risk of psychosis (Stowkowy and Addington, 2012) (Fig. 1).
Fig. 1.
Neurodevelopmental windows across a multiple hit theory of schizophrenia. Schizophrenia may be conceptualized as a process which involves multiple vulnerability factors across numerous neurodevelopmental windows. Some hit such as under and over-nutrition are thought to act during early developmental periods, whereas others such as cannabis and social cognition act later with the neurodevelopmental period.
6. Nutrition
The hypothesis that malnutrition, specifically maternal malnutrition before and during pregnancy, is a potential vulnerability in the pathogenesis of schizophrenia mainly comes from three major studies of famine and the subsequent increases in cases of schizophrenia seen thereafter. Analysis of data obtained from two major famines, one being the Dutch Hunger Winter of 1944 and 1945, and the other occurring in China during 1959–1961 has demonstrated a close to 2-fold increase in the rate of schizophrenia in the offspring of mothers born during this period (McGrath et al., 2011; Xu et al., 2009). Several theories have been put forth as to why maternal malnutrition may increase the risk of schizophrenia in the offspring. One of the most widely hypothesized is that of a micronutrient deficiency, specifically the micronutrients vitamin D (see below), folate and/or iron may be involved. There is some evidence that increased third trimester plasma levels of homocysteine (a surrogate marker of decreased folate levels) and maternal anaemia are associated with a 2-fold and up to 4-fold increased risk of schizophrenia in the offspring even after controlling for variables such as ethnicity and education (McGrath et al., 2011). There remains a lack of longitudinal studies that look at the effects on long term neurocognitive outcome of supplementation of the aforementioned micronutrients, with studies reporting discordant results including improvement and no change (McGrath et al., 2011). Another potential theory is that micronutrient deficiency, and in this case specifically folate, may induce epigenetic changes in methylation patterns in maternally imprinted genes. Long term follow up of offspring born during the aforementioned famines have shown reduced methylation for the insulin-like growth factor 2 (IGF2) locus, a maternally imprinted gene with a crucial role during early development as well as ongoing cognitive processes throughout life (Kirkbride et al., 2012). Another potential cause comes from the finding of decreased neural aborization of serotonergic and dopaminergic neurons in the hippocampus and prefrontal cortex respectively of prenatally malnourished rats, an interesting observation given decreased neural aborization is postulated to occur during the pathogenesis of schizophrenia. Maternal diet before and during pregnancy may also be related to the risk for schizophrenia via its impact on metabolic conditions, such as overweight and gestational diabetes during pregnancy (Van Lieshout et al., 2011; Van Lieshout and Voruganti, 2008). At present it remains unclear whether it is protein-energy malnutrition itself which is the potential vulnerability factor for schizophrenia, its associated micronutrient deficiencies, or unhealthy dietary patterns that confer the vulnerability.
7. Vitamin D
Low levels of serum vitamin D, particularly during in utero development and infancy, have been suggested to underlie some of the other observed environmental vulnerability factors linked to the development of schizophrenia. These include seasonality of birth and difference in latitude and subsequent psychosis risk. Interestingly, low levels of vitamin D are proposed to underpin part of the increased risk for schizophrenia in migrant populations (as opposed to social defeat mentioned above, although it may be that the two are not mutually exclusive). This finding is more pronounced in darker skinned immigrants who subsequently emigrate to a higher latitude which risks lower vitamin D (Graham et al., 2014). It is somewhat problematic to apply this as a blanket statement however, as others have suggested that this observed association between low vitamin D in infancy and subsequent schizophrenia may only be true for those with lower levels of 25-hydroxyvitamin D and not the fully active form (1,25-dihydroxyvitamin D) (McGrath et al., 2010a). This led some to speculate as to whether variations within gene encoding proteins involved in vitamin D activation may be in part responsible for the observed effects of 25-hydroxvitamin D. Also interesting is the suggestion that this risk may be non-linear. Neonates with serum vitamin D levels in the lower quintile range have been suggested to have a 2-fold increased risk for schizophrenia (McGrath et al., 2010b). In support of this, long term observational data from a Finnish birth cohort study has suggested that mothers who used vitamin D supplementation of at least 2000 IU conferred an up to 77% risk reduction of subsequent schizophrenia for male infants as opposed to mothers who had no or supplementation of less than 2000 IU a day (McGrath et al., 2004). In contrast, another study suggested that the increased risk of developing schizophrenia among those with low vitamin D levels during infancy may bear little relation to maternal vitamin D levels prenatally (McGrath et al., 2010b). Vitamin D deficiency is likely to act as vulnerability factor for the development of schizophrenia and may underlie some of the observed environmental differences in risks for this disorder.
8. Infection induced activation of HERVs
Some environmental hits through activation of HERVs may act as vulnerability factors for the development of schizophrenia, although this data are premature and require further development (Arias et al., 2012; Krause et al., 2010; Yolken and Torrey, 1995). HERVs that exhibit similar characteristics as exogenous retroviruses constitute 8% of the human genome (Blikstad et al., 2008). Although HERV protein serves important functions in normal human physiology, recent understanding suggests that there is pathogenic potential of HERVs and the pathogenicity of these elements is mediated by the interactions between hereditary and environmental factors (Christensen, 2010; Young et al., 2013). The endogenous retroelements are responsive to external stimuli including environmental factors such as infectious agents and ultraviolet (UV) radiation (Hohenadl et al., 1999; Sutkowski et al., 2001). Genetic and/or epigenetic modulation of HERV elements at a particularly vulnerable development stage may lead to variable patterns of neurodevelopment and upon exposure to subsequent environmental triggers, such patterns may translate to a schizophrenia phenotype. Microbes that lead to immune cell activation through transcription of immune-related host genes may cause global modulation of endogenous retroelement transcription (Young et al., 2014). Certain copies of the HERV elements are activated by some infectious agents. For example, T. gondii and the influenza virus may activate HERV-W elements within human cell lines (Frank et al., 2006; Li et al., 2014; Nellaker et al., 2006). Several studies including a meta-analysis have indicated an increased prevalence of antibodies against T. gondii in schizophrenia (Torrey et al., 2007, 2012). The envelope protein released due to pathogenic activation of HERVs has been demonstrated to have the ability to activate innate immunity and induce the production of pro-inflammatory cytokines. This appears to be mediated through the cluster of differentiation 14 (CD14)/toll like receptor 4 (TLR4) pathway (Rolland et al., 2006). In addition, a study has also revealed significant correlation between group specific antigen (GAG) or envelope (ENV) antigenemia and C-reactive protein levels in schizophrenia (Perron et al., 2008). Elevated levels of HERV transcripts have been detected within red blood cells, plasma, cerebrospinal fluid and neural tissue of patients with first episode psychosis (Huang et al., 2006; Karlsson et al., 2001, 2004; Yao et al., 2008). Further, antibodies to retroviral proteins have been shown to be elevated in individuals with recent onset-psychosis (Dickerson et al., 2012). Recent studies in multiple sclerosis have demonstrated that simultaneous presence of endogenous retrovirus and herpes virus antigens have a profound impact on cell mediated immunity and may also translate into increased production of proinflammatory cytokines (Brudek et al., 2004,2008).The pathogenic glycoproteins of HERVs have been postulated to cause neuroinflammation as well as neurodegeneration (Antony et al., 2007; Perron and Lang, 2010), with one study finding that antibodies to HSV1 were associated with grey matter abnormalities (Whitford et al., 2012). Based on these observations, it is possible that environmental activation of HERVs can influence the onset and progression of schizophrenia by becoming a driver of the inflammatory process, although at present such data remains in relative infancy compared to other risk factors for the development of schizophrenia
9. Smoking
Smoking is known to influence the risk for other disorders, particularly depression (Pasco et al., 2008). A recent meta-analysis has suggested that smoking is both a vulnerability factor for schizophrenia and is associated with an earlier age of onset, with prospective studies showing a more than doubling of the risk for de-novo psychosis among daily smokers (Gage and Munafo, 2015). Smoking is also associated with cortical thinning, which is partially reversible with cessation (Karama et al., 2015). Theoretically, smoking along with other addictions may alter the regulatory set point of the dopaminergic and cholinergic neurotransmitter system, which are implicated in schizophrenia pathogenesis. Indeed, gene wide association studies (GWAS) have suggested that common variants in the cholinergic/nicotinic systems may be associated with the development of schizophrenia (McGrath, 2015; Young and Geyer, 2013). Data from animal models suggest that prenatal exposure to smoking increases risk for a psychotic phenotype in adulthood (Zugno et al., 2013). There is also some epidemiological data suggesting maternal smoking, but not paternal smoking, increases risk among offspring At illness onset, smoking is more prevalent among those with schizophrenia than among non-psychotic individuals (Samele et al., 2007), although the direction of this relationship is uncertain. There is some suggestion that tobacco use may be associated with (possibly subsyndromal) psychosis, given data from two large cross-sectional population surveys suggesting that both age of first tobacco use and daily use of tobacco are associated with psychotic like experiences (Rossler et al., 2015; Saha et al., 2011).
10. Intelligence quotient
A lower premorbid levels of intelligence quotient (IQ) is an often quoted potential vulnerability factor, although little is known about the mechanism for the association between the two or whether lower premorbid levels of IQ predict worse outcomes of illness. It is likely to be a mediating factor earlier in life. In a similar fashion to vitamin D, the risk of lower IQ and the risk of developing schizophrenia appears to be non-linear, with children aged 7 whose IQ is more than 2 standard deviations below the mean showing an eight times greater risk of developing schizophrenia than those with average IQ Also important to note is the lack of observed risk reduction for children who had an above average IQ further strengthening the suggestion that the link between IQ and schizophrenia is non-linear (Schulz et al., 2014). Despite this proposed non-linear relationship, it is unknown whether a lower premorbid IQ predicts a worse outcome, with inconsistent findings - particularly in longitudinal studies of first-episode psychosis (Leeson et al., 2009).The recent Dunedin cohort however suggested that there is substantial neuropsychological decline from the premorbid to the post onset period in schizophrenia, however this decline varied across various mental functions (Meier et al., 2014). However some authors have found no evidence of deterioration in IQ after a 10 year follow-up period. In contrast to the above findings, one study conducted in a Finnish population suggested that better performance at school may be linked to the subsequent development of schizophrenia, suggesting that a high IQ may also be a risk factor. There has also been a suggestion that people with schizophrenia with a higher IQ may represent a subtype of the disease spectrum who may suffer less negative symptoms (Cernis et al., 2015). Another systematic review has suggested that premorbid deficits in cognitive functioning including intelligence quotient (IQ), (see below) and behavioral, emotional and social problems within middle childhood and early adolescence identifies a cohort of children at risk for schizophrenia disorders in later adult life (Laurens et al., 2015). Overall the neurobiological link between these two factors remains to be fully elucidated.
11. Social cognition
Social cognition is a related but independent construct to IQ involving the perception, interpretation and processing of social information, which has received attention as a potential vulnerability factor contributing to the manifestation of schizophrenia. Social cognition refers to how people think about themselves and others in the social world (Penn et al., 2008). Preliminary findings point to relative stability of social cognition from the early through to the later stages of psychosis (Green et al., 2012). Social cognition provides phenotypic clues regarding underlying pathophysiology and is strongly related to functioning and disability in psychiatric disorders (Fett et al., 2011). It includes the domains of emotion recognition, theory of mind, and attributional style. In particular, the domain of emotion recognition has been extensively studied in people with schizophrenia, where Individuals with schizophrenia display deficits compared with nonclinical control participants (Couture et al., 2006; Edwards et al., 2002; Kohler et al., 2010). The greatest deficits are evident in the perception of negative emotions (compared with positive emotions). The deficit in emotion recognition is relatively stable over time and can be observed independent of the acute phase of the disorder. Notably, impairments in emotion recognition are present early in the course of psychotic illness (Amminger et al., 2012; Pinkham et al., 2007).
Impaired emotion recognition has been related to social dysfunction in people with schizophrenia (Hooker and Park, 2002), as well as in putatively prodromal individuals (Amminger et al., 2013). Impaired emotion recognition may therefore contribute to social impairment in people with schizophrenia, and may precede the onset of illness (Cannon et al., 2008; Nelson et al., 2013). However, whether it is a moderator, meditator or marker of risk remains uncertain. In social situations, inaccurate decoding of emotional expression may be a source of stress and a barrier to social interactions and communication (Bediou et al., 2007). Stress could exacerbate symptoms in people with schizophrenia, and possibly also play a role in the onset of frank psychosis in high-risk individuals (Phillips et al., 2006). Inaccurate decoding of emotions may also serve as a building block in delusion formation (Blackwood et al., 2001). This view is supported by a study that investigated emotion recognition as predictor of transition to psychosis in ultra-high risk individuals (Allott et al., 2014), showing that poorer identification of “neutral” emotion predicted transition. According to these findings, the positive symptoms of psychosis may arise because people read meaning where there is none.
The nature of the dysfunction underlying emotion recognition deficits in schizophrenia and other psychoses is only partially understood. Biological theories of psychosis note the tendency to misinterpret benign or ambiguous social cues. In psychosis, increased dopamine is observed in the mesolimbic pathway, with dopamine being a key neurochemical determinant of the significance of environmental cues to human motivations (Kapur, 2003). Abnormal increases in this neurotransmitter are proposed to lead to “aberrant salience” of environmental cues (Kapur, 2003). Thus, increased dopamine synthesis in prodromal individuals may influence the perceived salience of environmental cues, and in this instance, a tendency to interpret neutral faces as emotionally meaningful. Concordantly, recent findings show that elevated dopamine synthesis in prodromal individuals is correlated with the severity of psychotic (but not anxiety or depressive) symptoms (Howes et al., 2009) and also predicts later psychotic disorder transition (Howes et al., 2011).
12. Prevention strategies
Given the substantial economic, societal and global health impact that schizophrenia has, there have been recent attempts looking at preventing the onset of disease. Intervention during the prodromal stage or disease onset and during the critical period of the early years of illness has the potential to reduce the severity and impact of the subsequent disease state. This is particularly important if schizophrenia is conceptualized to involve the cumulative effect of a number of potential hits and vulnerability factors, as it provides multiple potential early therapeutic intervention targets. There is also a growing understanding that the appearance of the ultra-high risk stage seen during emerging adulthood often builds on latent vulnerability from earlier in life (Seidman and Nordentoft, 2015). It needs to be emphasized that risks for schizophrenia overlap with those for other psychiatric and non-communicable disorders, and feasible preventive approaches need to be integrated as part of a whole of health approach. Such interventions to potentially reduce the risk of non-communicable disorders including schizophrenia in vulnerable persons at risk may range from the care of pregnant patients with psychoses who may benefit from prenatal care and social support and family centered programs, to preemptive cognitive remediation and early intervention for children at risk. (Liu et al., 2015). Additionally, having a systemic approach which allows for multiple referral sources being established through networking with general practitioners, community mental health workers and school officials allows for the earlier recognition and referral of persons within the early phase of a psychotic disorder (Ho et al., 2005).
There is now a substantial evidence base regarding indicated prevention, with 11 randomised controlled trials to date in the ultra-high risk (UHR) prodrome. A recent meta-analysis concluded that preventative interventions aimed toward people at an ultrahigh risk of first episode psychosis are effective in reducing the risk of transition to psychosis by 50% in the first 12 months (van der Gaag et al., 2013). In addition, a previous randomised controlled trial has suggested that in people with an ultra-high risk of psychosis, early intervention with cognitive behavioral therapy may be safer and at least as efficacious as low dose anti-psychotic medications in this setting (McGorry et al., 2013). The use of omega 3 poly-unsaturated fatty acids for twelve weeks has been suggested to prevent the transition to a full-threshold psychotic disorder in younger adults in an at-risk mental state, a finding which is now the subject of a major replication study (Markulev et al., 2015). Data from the London Child Health and Development Study, which looked at 9–11 year old children identified a subset of children who are suggested to be at a greater risk of psychosis who report higher rates of psychotic like experiences, greater exposure and responsivity to stressors, and impairments in general intelligence may allow intervention at a younger age than has previously been employed with early adolescents (Laurens and Cullen, 2015).
There has also been investigation into the possible effects of the prevention of cannabis use in regards to subsequent conversion into schizophrenia. It has been estimated the number needed to prevent, that being the number of heavy cannabis users which would need to stop in order to prevent one episode of psychosis, is around 2800 in males aged 20–24 and 4700 in males aged 35–39, with female equivalent numbers being double both of these estimates (Brown and McGrath, 2011). However heavier use in the context of subthreshold psychotic symptoms would be a more cost effective target for intervention. It’s worth noting that current programs not aimed at psychiatric endpoints may indeed have efficacy in this domain; existing public health smoking campaigns and the Australian Royal Commission into Institutional Responses to Child Sexual Abuse being exemplars (Berk et al., 2014). A 2010 Cochrane review concluded that regular exercise programs are feasible in patients suffering from schizophrenia and have shown promising effects on both physical health, symptoms and cognition, as long as they have adequate intensity and duration (Gorczynski and Faulkner, 2010). Recently, some novel interventions have been developed that show promise in promoting physical activity and engagement in young, first episode patients with schizophrenia through the use of internet enabled mobile devices and interactive exercise applications (Killackey et al., 2011). Given the potential impact early life stressors and/or maternal under or over-nutrition may have on the subsequent pathogenesis of schizophrenia, it has been postulated that the delivery of health education, smoking cessation, substance use, nutritional and lifestyle interventions for women during the antenatal period may have long term benefits on diverse neurocognitive outcomes (Jacka and Berk, 2014; O’Neil et al., 2014). Others have shown that in order for a clinically meaningful risk-prediction for conversion to psychosis at first presentation to occur several investigative modalities including clinical interviewing, neurocognitive testing, structural imaging and electrophysiological testing would have to be performed In order to give a meaningful risk weighted profile (Clark et al., 2015). Specific structural abnormalities have been proposed to predict transition to psychosis. These include greater thinning of the grey matter, which is most pronounced within the pre-frontal cortex, as well as greater disrupted thalamo-cortical functional connectivity at baseline (Cannon, 2015), which may provide potential prognostic clues as to the risk of future progression within high risk populations. In order for any of the above early therapeutic interventions to be effective however, preventative paradigms need to be integrated within mental health in partnership with other medical disciplines and health promotion agencies (O’Neil et al., 2015). A greater understanding of the potential hits and vulnerability factors which work together to drive the pathophysiology of schizophrenia will be required in order to make such preventative programs feasible.
13. Conclusion
We have presented a summary of selected risk factors which demonstrate the considerable heterogeneity and complexity that the neuropathogenesis of schizophrenia displays. It is likely that multiple hits, with some conferring a greater risk than others, operate within a genetically primed individual across key periods of neurodevelopment to culminate in the clinical syndrome of schizophrenia. It may be that many vulnerability factors which contribute to the overall disease process are yet to be fully elucidated, just as many of these hits may have a small effect size which will make their detection and understanding of their overall contribution to the disease state a difficult task. It is likely, using the Framingham risk model for cardiovascular disease as an analogy, that these risks are cumulative and interactive, both with each other, and with critical periods of neurodevelopmental vulnerability. The genetic and molecular mechanisms underpinning such an association are only just beginning to be discovered, and we are likely to see further advances in understanding the complex interactions between these environmental factors and genetic influences in the pathogenesis of schizophrenia.
Acknowledgments
MB has received grant support from NIH, Simons Autism Foundation, Cancer Council of Victoria, CRC for Mental Health, Stanley Medical Research Foundation, MBF, NHMRC, Beyond Blue, Gee-long Medical Research Foundation, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Organon, Novartis, Mayne Pharma and Servier. MB has been a speaker for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen Cilag, Lundbeck, Merck, Pfizer, Sanofi Synthelabo, Servier, Solvay and Wyeth, and served as a consultant to Allergan, Astra Zeneca, Eli Lilly, GlaxoSmithKline, Janssen Cilag, Lundbeck, Merck, Pfizer and Servier. FNJ has received Grant/Research support from the Brain and Behaviour Research Institute, the National Health and Medical Research Council (NHMRC), Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, Eli Lilly, the Meat and Livestock Board and The University of Melbourne and has received speakers honoraria from Sanofi-Synthelabo, Janssen Cilag, Servier, Pfizer, Health Ed., Network Nutrition, Angelini Farmaceutica, and Eli Lilly. SD has received Grant/Research Support from the Stanley Medical Research Institute, NHMRC, Beyond Blue, ARHRF, Simons Foundation, Geelong Medical Research Foundation, Fondation FondaMental, Eli Lilly, GlaxoSmithKline, Organon, Mayne Pharma and Servier, speaker’s fees from Eli Lilly, advisory board fees from Eli Lilly and Novartis and conference travel support from Servier. SM has received grant support from NIMH (MH099431). OMD is a research fellow and has received grant support from the Brain and Behavior Foundation, Marion and EH Flack Trust, Simons Autism Foundation, Australian Rotary Health, Stanley Medical Research Institute, Deakin University, Brazillian Society Mobility Program Lilly, NHMRC and an ASBD/Servier Grant. She has also received kind support from BioMedica Nutracuticals, NutritionCare and Bioceuticals. JMcG was funded by the John Cade Fellowship from the National Health and Medical Research Council (APP1056929). CP was funded by a NHMRC Senior Principal Research Fellowship (628386), and a Brain and Behavior Research Foundation (NARSAD) Distinguished Investigator Award (US; Grant ID: 18722). CP has participated on Advisory Boards for Janssen-Cilag, Astra-Zeneca, Lundbeck, and Servier. He has received honoraria for talks presented at educational meetings organised by Astra-Zeneca, Janssen-Cilag, Eli-Lilly, Pfizer, Lundbeck and Shire. PMcG has received Investigator Initiated Research Grant support from Janssen Cilag, Astra Zeneca, Eli Lilly, Pfizer, and BMS, and speakers honoraria from Janssen CIlag, Eli Lilly, Pfizer, Lundbeck and Astra Zeneca. He is supported by a NHMRC Senior Principal Research Fellowship.
Abbreviations
- BDNF
Brain derived neurotrophic factor
- CD14
cluster of differentiation 14
- ENV
envelope
- GWAS
genome wide association studies
- GAG
group specific antigen
- HERVs
human endogenous retroviruses
- HPA
hypothalamic pituitary adrenal
- IGF2
insulin-like growth factor 2
- IQ
intelligence quotient
- mRNA
messenger ribonucleic acid
- TL4
toll like receptor 4
- UHR
ultra-high risk
- UV
ultraviolet
Footnotes
Declaration of interest
JD, HE, FJ, MD, MM and PA declare no conflict of interest.
References
- Allott KA, Schafer MR, Thompson A, Nelson B, Bendall S, Bartholomeusz CF, et al. Emotion recognition as a predictor of transition to a psychotic disorder in ultra-high risk participants. Schizophr. Res. 2014;153(1–3):25–31. doi: 10.1016/j.schres.2014.01.037. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Schlogelhofer M, Mossaheb N, et al. Emotion recognition in individuals at clinical high-risk for schizophrenia. Schizophr. Bull. 2012;38(5):1030–1039. doi: 10.1093/schbul/sbr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amminger GP, Allott K, Schlogelhofer M, Thompson A, Bechdolf A, Nelson B, et al. Affect recognition and functioning in putatively prodromal individuals. Schizophr. Res. 2013;147(2–3):404–405. doi: 10.1016/j.schres.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Antony JM, Ellestad KK, Hammond R, Imaizumi K, Mallet F, Warren KG, et al. The human endogenous retrovirus envelope glycoprotein, syncytin-1, regulates neuroinflammation and its receptor expression in multiple sclerosis: a role for endoplasmic reticulum chaperones in astrocytes. J. Immunol. 2007;179(2):1210–1224. doi: 10.4049/jimmunol.179.2.1210. [DOI] [PubMed] [Google Scholar]
- Arias I, Sorlozano A, Villegas E, de Dios Luna J, McKenney K, Cervilla J, et al. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr. Res. 2012;136(1–3):128–136. doi: 10.1016/j.schres.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Bediou B, Asri F, Brunelin J, Krolak-Salmon P, D’Amato T, Saoud M, et al. Emotion recognition and genetic vulnerability to schizophrenia. Br. J. Psychiatry. 2007;191:126–130. doi: 10.1192/bjp.bp.106.028829. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog. Neurobiol. 2014;112:80–99. doi: 10.1016/j.pneurobio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Berk M, Moylan S, Jacka FN. A royal gift to prevention efforts. Aust. N. Z.J. Psychiatry. 2014;48(2):110–111. doi: 10.1177/0004867413493524. [DOI] [PubMed] [Google Scholar]
- Blackwood NJ, Howard RJ, Bentall RP, Murray RM. Cognitive neuropsychiatric models of persecutory delusions. Am. J. Psychiatry. 2001;158(4):527–539. doi: 10.1176/appi.ajp.158.4.527. [DOI] [PubMed] [Google Scholar]
- Blikstad V, Benachenhou F, Sperber GO, Blomberg J. Evolution of human endogenous retroviral sequences: a conceptual account. Cell. Mol. Life Sci. 2008;65(21):3348–3365. doi: 10.1007/s00018-008-8495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braehler C, Valiquette L, Holowka D, Malla AK, Joober R, Ciampi A, et al. Childhood trauma and dissociation in first-episode psychosis, chronic schizophrenia and community controls. Psychiatry Res. 2013;210(1):36–42. doi: 10.1016/j.psychres.2013.05.033. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perlini C, Rajagopalan P, Saharan P, Rambaldelli G, Bellani M, et al. Schizophrenia severity, social functioning and hippocampal neuroanatomy: three-dimensional mapping study. Br. J. Psychiatry. 2013;202(1):50–55. doi: 10.1192/bjp.bp.111.105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, McGrath JJ. The prevention of schizophrenia. Schizophr. Bull. 2011;37(2):257–261. doi: 10.1093/schbul/sbq122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudek T, Christensen T, Hansen HJ, Bobecka J, Moller-Larsen A. Simultaneous presence of endogenous retrovirus and herpes virus antigens has profound effect on cell-mediated immune responses: implications for multiple sclerosis. AIDS Res. Hum. Retroviruses. 2004;20(4):415–423. doi: 10.1089/088922204323048168. [DOI] [PubMed] [Google Scholar]
- Brudek T, Christensen T, Hansen HJ, Petersen T, Moller-Larsen A. Synergistic immune responses induced by endogenous retrovirus and herpesvirus antigens result in increased production of inflammatory cytokines in multiple sclerosis patients. Scand. J. Immunol. 2008;67(3):295–303. doi: 10.1111/j.1365-3083.2007.02067.x. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch. Gen. Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD. Brain biomarkers of vulnerability and progression to psychosis. Schizophr. Bull. 2015 doi: 10.1093/schbul/sbv173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernis E, Vassos E, Brebion G, McKenna PJ, Murray RM, David AS, et al. Schizophrenia patients with high intelligence: a clinically distinct sub-type of schizophrenia? Eur. Psychiatry. 2015;30(5):628–632. doi: 10.1016/j.eurpsy.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Christensen T. HERVs in neuropathogenesis. J. Neuroimmune Pharmacol. 2010;5(3):326–335. doi: 10.1007/s11481-010-9214-y. [DOI] [PubMed] [Google Scholar]
- Clark SR, Schubert KO, Baune BT. Towards indicated prevention of psychosis: using probabilistic assessments of transition risk in psychosis prodrome. J. Neural Transm. 2015;122(1):155–169. doi: 10.1007/s00702-014-1325-9. [DOI] [PubMed] [Google Scholar]
- Cohen M, Rasser PE, Peck G, Carr VJ, Ward PB, Thompson PM, et al. Cerebellar grey-matter deficits, cannabis use and first-episode schizophrenia in adolescents and young adults. Int. J. Neuropsychopharmacol. 2012a;15(3):297–307. doi: 10.1017/S146114571100068X. [DOI] [PubMed] [Google Scholar]
- Cohen CI, Palekar N, Barker J, Ramirez PM. The relationship between trauma and clinical outcome variables among older adults with schizophrenia spectrum disorders. Am. J. Geriatric Psychiatry. 2012b;20(5):408–415. doi: 10.1097/JGP.0b013e318211817e. [DOI] [PubMed] [Google Scholar]
- Corcoran CM, Smith C, McLaughlin D, Auther A, Malaspina D, Cornblatt B. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr. Res. 2012;135(1–3):170–174. doi: 10.1016/j.schres.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr. Bull. 2006;32(Suppl. 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley VL, Pantelis C. Using longitudinal imaging to map the ‘relapse signature’ of schizophrenia and other psychoses. Epidemiol. Psychiatric Sci. 2014;23(3):219–225. doi: 10.1017/S2045796014000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley V, Wood SJ, Pantelis C. Brain structural, neurochemical and neuroinflammatory markers of psychosis onset and relapse: is there evidence for a psychosis relapse signature? Int. Clin. Psychopharmacol. 2013 [Google Scholar]
- Cullen AE, Fisher HL, Roberts RE, Pariante CM, Laurens KR. Daily stressors and negative life events in children at elevated risk of developing schizophrenia. Br. J. Psychiatry. 2014;204:354–360. doi: 10.1192/bjp.bp.113.127001. [DOI] [PubMed] [Google Scholar]
- Cullen AE, Day FL, Roberts RE, Pariante CM, Laurens KR. Pituitary gland volume and psychosocial stress among children at elevated risk for schizophrenia. Psychol. Med. 2015;45(15):3281–3292. doi: 10.1017/S0033291715001282. [DOI] [PubMed] [Google Scholar]
- Davis J, Moylan S, Harvey BH, Maes M, Berk M. Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust. N. Z. J. Psychiatry. 2014 doi: 10.1177/0004867414533012. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Ferrari AJ, Calabria B, Hall WD, Norman RE, McGrath J, et al. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One. 2013;8(10):e76635. doi: 10.1371/journal.pone.0076635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Lillehoj E, Stallings C, Wiley M, Origoni A, Vaughan C, et al. Antibodies to retroviruses in recent onset psychosis and multi-episode schizophrenia. Schizophr. Res. 2012;138(2–3):198–205. doi: 10.1016/j.schres.2012.03.037. [DOI] [PubMed] [Google Scholar]
- Donoghue K, Doody GA, Murray RM, Jones PB, Morgan C, Dazzan P, et al. Cannabis use, gender and age of onset of schizophrenia: data from the AESOP study. Psychiatry Res. 2014;215(3):528–532. doi: 10.1016/j.psychres.2013.12.038. [DOI] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin. Psychol. Rev. 2002;22(6):789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Steiner J, Berk M, Molendijk ML, Gonzalez-Pinto A, Turck CW, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol. Psychiatry. 2014 doi: 10.1038/mp.2014.117. [DOI] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Frank O, Jones-Brando L, Leib-Mosch C, Yolken R, Seifarth W. Altered transcriptional activity of human endogenous retroviruses in neuroepithelial cells after infection with Toxoplasma gondii. J. Infect. Dis. 2006;194(10):1447–1449. doi: 10.1086/508496. [DOI] [PubMed] [Google Scholar]
- Gage SH, Munafo MR. Smoking as a causal risk factor for schizophrenia. Lancet Psychiatry. 2015 doi: 10.1016/S2215-0366(15)00333-8. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr. Bull. 2011;37(3):504–513. doi: 10.1093/schbul/sbr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski P, Faulkner G. Exercise therapy for schizophrenia. Schizophr. Bull. 2010;36(4):665–666. doi: 10.1093/schbul/sbq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KA, Keefe RS, Lieberman JA, Calikoglu AS, Lansing KM, Perkins DO. Relationship of low vitamin D status with positive, negative and cognitive symptom domains in people with first-episode schizophrenia. Early Intervention Psychiatry. 2014 doi: 10.1111/eip.12122. [DOI] [PubMed] [Google Scholar]
- Green MF, Bearden CE, Cannon TD, Fiske AP, Hellemann GS, Horan WP, et al. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophr. Bull. 2012;38(4):854–864. doi: 10.1093/schbul/sbq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MJ, Chia TY, Cairns MJ, Wu J, Tooney PA, Scott RJ, et al. Catechol-O-methyltransferase (COMT) genotype moderates the effects of childhood trauma on cognition and symptoms in schizophrenia. J. Psychiatr. Res. 2014;49:43–50. doi: 10.1016/j.jpsychires.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Fuller R, Arndt S, Cadoret RJ. Secondary prevention of schizophrenia: utility of standardized scholastic tests in early identification. Ann. Clin. Psychiatry. 2005;17(1):11–18. doi: 10.1080/10401230590905272. [DOI] [PubMed] [Google Scholar]
- Hohenadl C, Germaier H, Walchner M, Hagenhofer M, Herrmann M, Sturzl M, et al. Transcriptional activation of endogenous retroviral sequences in human epidermal keratinocytes by UVB irradiation. J. Invest. Dermatol. 1999;113(4):587–594. doi: 10.1046/j.1523-1747.1999.00728.x. [DOI] [PubMed] [Google Scholar]
- Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112(1):41–50. doi: 10.1016/s0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry. 2009;66(1):13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. The Am. J. Psychiatry. 2011;168(12):1311–1317. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy K, Barrett S, Shannon C, Campbell C, Watson D, Rushe T, et al. Childhood trauma and hippocampal and amygdalar volumes in first-episode psychosis. Schizophr. Bull. 2012;38(6):1162–1169. doi: 10.1093/schbul/sbr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WJ, Liu ZC, Wei W, Wang GH, Wu JG, Zhu F. Human endogenous retroviral pol RNA and protein detected and identified in the blood of individuals with schizophrenia. Schizophr. Res. 2006;83(2–3):193–199. doi: 10.1016/j.schres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Berk M. Prevention of schizophrenia-will a broader prevention agenda support this aim? Schizophr. Bull. 2014;40(2):237–239. doi: 10.1093/schbul/sbt202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Karama S, Ducharme S, Corley J, Chouinard-Decorte F, Starr JM, Wardlaw JM, et al. Cigarette smoking and thinning of the brain’s cortex. Mol. Psychiatry. 2015;20(6):778–785. doi: 10.1038/mp.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H, Bachmann S, Schroder J, McArthur J, Torrey EF, Yolken RH. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2001;98(8):4634–4639. doi: 10.1073/pnas.061021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H, Schroder J, Bachmann S, Bottmer C, Yolken RH. HERV-W-related RNA detected in plasma from individuals with recent-onset schizophrenia or schizoaffective disorder. Mol. Psychiatry. 2004;9(1):12–13. doi: 10.1038/sj.mp.4001439. [DOI] [PubMed] [Google Scholar]
- Killackey E, Anda AL, Gibbs M, Alvarez-Jimenez M, Thompson A, Sun P, et al. Using internet enabled mobile devices and social networking technologies to promote exercise as an intervention for young first episode psychosis patients. BMC Psychiatry. 2011;11:80. doi: 10.1186/1471-244X-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkbride JB, Susser E, Kundakovic M, Kresovich JK, Davey Smith G, Relton CL. Prenatal nutrition, epigenetics and schizophrenia risk: can we test causal effects? Epigenomics. 2012;4(3):303–315. doi: 10.2217/epi.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr. Bull. 2010;36(5):1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koola MM, McMahon RP, Wehring HJ, Liu F, Mackowick KM, Warren KR, et al. Alcohol and cannabis use and mortality in people with schizophrenia and related psychotic disorders. J. Psychiatr. Res. 2012;46(8):987–993. doi: 10.1016/j.jpsychires.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D, Matz J, Weidinger E, Wagner J, Wildenauer A, Obermeier M, et al. The association of infectious agents and schizophrenia. World J. Biol. Psychiatry. 2010;11(5):739–743. doi: 10.3109/15622971003653246. [DOI] [PubMed] [Google Scholar]
- Kumra S, Robinson P, Tambyraja R, Jensen D, Schimunek C, Houri A, et al. Parietal lobe volume deficits in adolescents with schizophrenia and adolescents with cannabis use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(2):171–180. doi: 10.1016/j.jaac.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Cullen AE. Toward earlier identification and preventative intervention in schizophrenia: evidence from the London Child Health and Development Study. Soc. Psychiatry Psychiatr. Epidemiol. 2015 doi: 10.1007/s00127-015-1151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens KR, Luo L, Matheson SL, Carr VJ, Raudino A, Harris F, et al. Common or distinct pathways to psychosis? A systematic review of evidence from prospective studies for developmental risk factors and antecedents of the schizophrenia spectrum disorders and affective psychoses. BMC Psychiatry. 2015;15:205. doi: 10.1186/s12888-015-0562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson VC, Barnes TR, Hutton SB, Ron MA, Joyce EM. IQ as a predictor of functional outcome in schizophrenia: a longitudinal, four-year study of first-episode psychosis. Schizophr. Res. 2009;107(1):55–60. doi: 10.1016/j.schres.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Ran S, Segev A, Braw Y, Levkovitz Y. Neurocognitive functions of heavy cannabis using schizophrenia patients. Eur. Psychiatry. 2012;27(5):365–368. doi: 10.1016/j.eurpsy.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Li D, Law S, Andermann L. Association between degrees of social defeat and themes of delusion in patients with schizophrenia from immigrant and ethnic minority backgrounds. Transcult. Psychiatry. 2012;49(5):735–749. doi: 10.1177/1363461512464625. [DOI] [PubMed] [Google Scholar]
- Li F, Nellaker C, Sabunciyan S, Yolken RH, Jones-Brando L, Johansson AS, et al. Transcriptional derepression of the ERVWE1 locus following influenza A virus infection. J. Virol. 2014;88(8):4328–4337. doi: 10.1128/JVI.03628-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Keshavan MS, Tronick E, Seidman LJ. Perinatal risks and childhood premorbid indicators of later psychosis: next steps for early psychosocial interventions. Schizophr. Bull. 2015;41(4):801–816. doi: 10.1093/schbul/sbv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Whittle S, Fornito A, Lubman DI, Pantelis C, et al. Gross morphological brain changes with chronic, heavy cannabis use. Br. J. Psychiatry. 2015;206(1):77–78. doi: 10.1192/bjp.bp.114.151407. [DOI] [PubMed] [Google Scholar]
- Malchow B, Hasan A, Schneider-Axmann T, Jatzko A, Gruber O, Schmitt A, et al. Effects of cannabis and familial loading on subcortical brain volumes in first-episode schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(Suppl. 2):S155–S168. doi: 10.1007/s00406-013-0451-y. [DOI] [PubMed] [Google Scholar]
- Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol. Med. 2012;42(6):1321–1328. doi: 10.1017/S0033291711002078. [DOI] [PubMed] [Google Scholar]
- Markulev C, McGorry PD, Nelson B, Yuen HP, Schaefer M, Yung AR, et al. NEURAPRO-E study protocol: a multicentre randomized controlled trial of omega-3 fatty acids and cognitive-behavioural case management for patients at ultra high risk of schizophrenia and other psychotic disorders. Early Intervention Psychiatry. 2015 doi: 10.1111/eip.12260. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Nelson B, Phillips LJ, Yuen HP, Francey SM, Thampi A, et al. Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: twelve-month outcome. J. Clin. Psychiatry. 2013;74(4):349–356. doi: 10.4088/JCP.12m07785. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Feron FP, Burne TH, Mackay-Sim A, Eyles DW. The neurodevelopmental hypothesis of schizophrenia: a review of recent developments. Ann. Med. 2003;35(2):86–93. doi: 10.1080/07853890310010005. [DOI] [PubMed] [Google Scholar]
- McGrath J, Saari K, Hakko H, Jokelainen J, Jones P, Jarvelin MR. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr. Res. 2004;67(2–3):237–245. doi: 10.1016/j.schres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Burne TH, Feron F, Mackay-Sim A, Eyles DW. Developmental vitamin D deficiency and risk of schizophrenia: a 10-year update. Schizophr. Bull. 2010a;36(6):1073–1078. doi: 10.1093/schbul/sbq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JJ, Eyles DW, Pedersen CB, Anderson C, Ko P, Burne TH, et al. Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch. Gen. Psychiatry. 2010b;67(9):889–894. doi: 10.1001/archgenpsychiatry.2010.110. [DOI] [PubMed] [Google Scholar]
- McGrath J, Brown A, St Clair D. Prevention and schizophrenia-the role of dietary factors. Schizophr. Bull. 2011;37(2):272–283. doi: 10.1093/schbul/sbq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JJ. A Rosetta stone for epidemiology: genomic risk profile scores contain clues related to modifiable risk factors. Epidemiol. Psychiatric Sci. 2015;24(1):1–5. doi: 10.1017/S2045796014000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Reichenberg A, Keefe RS, Fisher HL, Harrington H, et al. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am. J. Psychiatry. 2014;171(1):91–101. doi: 10.1176/appi.ajp.2013.12111438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Di Filippo C, Fabrazzo M, Milano W, Martiadis V, Corrivetti G, et al. Flattened cortisol awakening response in chronic patients with schizophrenia onset after cannabis exposure. Psychiatry Res. 2014;215(2):263–267. doi: 10.1016/j.psychres.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br. Med. J. 1987;295(6600):681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Nellaker C, Yao Y, Jones-Brando L, Mallet F, Yolken RH, Karlsson H. Transactivation of elements in the human endogenous retrovirus W family by viral infection. Retrovirology. 2006;3:44. doi: 10.1186/1742-4690-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Yuen HP, Wood SJ, Lin A, Spiliotacopoulos D, Bruxner A, et al. Long-term follow-up of a group at ultra high risk (prodromal) for psychosis: the PACE 400 study. JAMA psychiatry. 2013;70(8):793–802. doi: 10.1001/jamapsychiatry.2013.1270. [DOI] [PubMed] [Google Scholar]
- O’Neil A, Itsiopoulos C, Skouteris H, Opie RS, McPhie S, Hill B, et al. Preventing mental health problems in offspring by targeting dietary intake of pregnant women. BMC Med. 2014;12:208. doi: 10.1186/s12916-014-0208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil A, Jacka FN, Quirk SE, Cocker F, Taylor CB, Oldenburg B, et al. A shared framework for the common mental disorders and non-communicable disease: key considerations for disease prevention and control. BMC Psychiatry. 2015;15:15. doi: 10.1186/s12888-015-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016 doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, McGorry PD, Velakoulis D. Early and late neurodevelopmental disturbances in schizophrenia and their functional consequences. Aust. N. Z. J. Psychiatry. 2003;37(4):399–406. doi: 10.1046/j.1440-1614.2003.01193.x. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr. Bull. 2005;31(3):672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Williams LJ, Jacka FN, Ng F, Henry MJ, Nicholson GC, et al. Tobacco smoking as a risk factor for major depressive disorder: population-based study. Br. J. Psychiatry. 2008;193(4):322–326. doi: 10.1192/bjp.bp.107.046706. [DOI] [PubMed] [Google Scholar]
- Patel V, Saxena S. Transforming lives, enhancing communities-innovations in global mental health. N. Engl. J. Med. 2014;370(6):498–501. doi: 10.1056/NEJMp1315214. [DOI] [PubMed] [Google Scholar]
- Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr. Bull. 2008;34(3):408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron H, Lang A. The human endogenous retrovirus link between genes and environment in multiple sclerosis and in multifactorial diseases associating neuroinflammation. Clin. Rev. Allergy Immunol. 2010;39(1):51–61. doi: 10.1007/s12016-009-8170-x. [DOI] [PubMed] [Google Scholar]
- Perron H, Mekaoui L, Bernard C, Veas F, Stefas I, Leboyer M. Endogenous retrovirus type W GAG and envelope protein antigenemia in serum of schizophrenic patients. Biol. Psychiatry. 2008;64(12):1019–1023. doi: 10.1016/j.biopsych.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, Velakoulis D, Pantelis C, Wood S, Yuen HP, Yung AR, et al. Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophr. Res. 2002;58(2–3):145–158. doi: 10.1016/s0920-9964(01)00392-9. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, McGorry PD, Garner B, Thompson KN, Pantelis C, Wood SJ, et al. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust. N. Z.J. Psychiatry. 2006;40(9):725–741. doi: 10.1080/j.1440-1614.2006.01877.x. [DOI] [PubMed] [Google Scholar]
- Picken A, Tarrier N. Trauma and comorbid posttraumatic stress disorder in individuals with schizophrenia and substance abuse. Compr. Psychiatry. 2011;52(5):490–497. doi: 10.1016/j.comppsych.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Perkins DO, Graham KA, Siegel M. Emotion perception and social skill over the course of psychosis: a comparison of individuals at-risk for psychosis and individuals with early and chronic schizophrenia spectrum illness. Cognit. Neuropsychiatry. 2007;12(3):198–212. doi: 10.1080/13546800600985557. [DOI] [PubMed] [Google Scholar]
- Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J. Immunol. 2006;176(12):7636–7644. doi: 10.4049/jimmunol.176.12.7636. [DOI] [PubMed] [Google Scholar]
- Rossler W, Ajdacic-Gross V, Haker H, Rodgers S, Muller M, Hengartner MP. Subclinical psychosis syndromes in the general population: results from a large-scale epidemiological survey among residents of the canton of Zurich, Switzerland. Epidemiol. Psychiatric Sci. 2015;24(1):69–77. doi: 10.1017/S2045796013000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Scott JG, Varghese D, McGrath JJ. The association between general psychological distress and delusional-like experiences: a large population-based study. Schizophr. Res. 2011;127(1–3):246–251. doi: 10.1016/j.schres.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Sahin S, Yuksel C, Guler J, Karadayi G, Akturan E, Gode E, et al. The history of childhood trauma among individuals with ultra high risk for psychosis is as common as among patients with first-episode schizophrenia. Early Intervention Psychiatry. 2013;7(4):414–420. doi: 10.1111/eip.12022. [DOI] [PubMed] [Google Scholar]
- Samele C, Patel M, Boydell J, Leese M, Wessely S, Murray R. Physical illness and lifestyle risk factors in people with their first presentation of psychosis. Soc. Psychiatry Psychiatr. Epidemiol. 2007;42(2):117–124. doi: 10.1007/s00127-006-0135-2. [DOI] [PubMed] [Google Scholar]
- Samplin E, Ikuta T, Malhotra AK, Szeszko PR, Derosse P. Sex differences in resilience to childhood maltreatment: effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. J. Psychiatr. Res. 2013;47(9):1174–1179. doi: 10.1016/j.jpsychires.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell T, Kleiman A, Gouzoulis-Mayfrank E, Daumann J, Becker B. Increased gray matter density in patients with schizophrenia and cannabis use: a voxel-based morphometric study using DARTEL. Schizophr. Res. 2012;138(2–3):183–187. doi: 10.1016/j.schres.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Schulz J, Sundin J, Leask S, Done DJ. Risk of adult schizophrenia and its relationship to childhood IQin the 1958 British birth cohort. Schizophr. Bull. 2014;40(1):143–151. doi: 10.1093/schbul/sbs157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Nordentoft M. New targets for prevention of schizophrenia: is it time for interventions in the premorbid phase? Schizophr. Bull. 2015;41(4):795–800. doi: 10.1093/schbul/sbv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Walterfang M, Lubman DI, Whittle S, Lorenzetti V, Styner M, et al. Alteration to hippocampal shape in cannabis users with and without schizophrenia. Schizophr. Res. 2013;143(1):179–184. doi: 10.1016/j.schres.2012.10.040. [DOI] [PubMed] [Google Scholar]
- Stowkowy J, Addington J. Maladaptive schemas as a mediator between social defeat and positive symptoms in young people at clinical high risk for psychosis. Early Intervention Psychiatry. 2012;6(1):87–90. doi: 10.1111/j.1751-7893.2011.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E, Patel V. Psychiatric epidemiology and global mental health: joining forces. Int. J. Epidemiol. 2014;43(2):287–293. doi: 10.1093/ije/dyu053. [DOI] [PubMed] [Google Scholar]
- Sutkowski N, Conrad B, Thorley-Lawson DA, Huber BT. Epstein-Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity. 2001;15(4):579–589. doi: 10.1016/s1074-7613(01)00210-2. [DOI] [PubMed] [Google Scholar]
- Swift W, Wong A, Li KM, Arnold JC, McGregor IS. Analysis of cannabis seizures in NSW, Australia cannabis potency and cannabinoid profile. PLoS One. 2013;8(7):e70052. doi: 10.1371/journal.pone.0070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr. Bull. 2007;33(3):729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr. Bull. 2012;38(3):642–647. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lieshout RJ, Voruganti LP. Diabetes mellitus during pregnancy and increased risk of schizophrenia in offspring: a review of the evidence and putative mechanisms. J. Psychiatry Neurosci. Jpn. 2008;33(5):395–404. [PMC free article] [PubMed] [Google Scholar]
- Van Lieshout RJ, Taylor VH, Boyle MH. Prepregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obesity Rev. 2011;12(5):e548–e549. doi: 10.1111/j.1467-789X.2010.00850.x. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, McGorry PD, Pantelis C. Evidence for progression of brain structural abnormalities in schizophrenia: beyond the neurodevelopmental model. Aust. N. Z. J. Psychiatry. 2000;34:S113–S126. doi: 10.1080/000486700231. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry. 1987;44(7):660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Welch KA, Moorhead TW, McIntosh AM, Owens DG, Johnstone EC, Lawrie SM. Tensor-based morphometry of cannabis use on brain structure in individuals at elevated genetic risk of schizophrenia. Psychol. Med. 2013;43(10):2087–2096. doi: 10.1017/S0033291712002668. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Wood SJ, Yung A, Cocchi L, Berger G, Shenton ME, et al. Structural abnormalities in the cuneus associated with Herpes Simplex Virus (type 1) infection in people at ultra high risk of developing psychosis. Schizophr. Res. 2012;135(1–3):175–180. doi: 10.1016/j.schres.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MQ, Sun WS, Liu BX, Feng GY, Yu L, Yang L, et al. Prenatal malnutrition and adult schizophrenia: further evidence from the 1959–1961 Chinese famine. Schizophr. Bull. 2009;35(3):568–576. doi: 10.1093/schbul/sbn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Schroder J, Nellaker C, Bottmer C, Bachmann S, Yolken RH, et al. Elevated levels of human endogenous retrovirus-W transcripts in blood cells from patients with first episode schizophrenia. Genes Brain Behav. 2008;7(1):103–112. doi: 10.1111/j.1601-183X.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Viruses, schizophrenia, and bipolar disorder. Clin. Microbiol. Rev. 1995;8(1):131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem. Pharmacol. 2013;86(8):1122–1132. doi: 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GR, Stoye JP, Kassiotis G. Are human endogenous retroviruses pathogenic? An approach to testing the hypothesis. BioEssays. 2013;35(9):794–803. doi: 10.1002/bies.201300049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GR, Mavrommatis B, Kassiotis G. Microarray analysis reveals global modulation of endogenous retroelement transcription by microbes. Retrovirology. 2014;11:59. doi: 10.1186/1742-4690-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Arch. Gen. Psychiatry. 2008;65(6):694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Solowij N, Yucel M, Lubman DI, Takagi M, Harding IH, et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135(Pt. 7):2245–2255. doi: 10.1093/brain/aws136. [DOI] [PubMed] [Google Scholar]
- Zugno AI, Fraga DB, De Luca RD, Ghedim FV, Deroza PF, Cipriano AL, et al. Chronic exposure to cigarette smoke during gestation results in altered cholinesterase enzyme activity and behavioral deficits in adult rat offspring: potential relevance to schizophrenia. J. Psychiatr. Res. 2013;47(6):740–746. doi: 10.1016/j.jpsychires.2013.02.001. [DOI] [PubMed] [Google Scholar]
- van Dijk D, Koeter MW, Hijman R, Kahn RS, van den Brink W. Effect of cannabis use on the course of schizophrenia in male patients: a prospective cohort study. Schizophr. Res. 2012;137(1–3):50–57. doi: 10.1016/j.schres.2012.01.016. [DOI] [PubMed] [Google Scholar]
- van Nierop M, van Os J, Gunther N, van Zelst C, de Graaf R, Ten Have M, et al. Does social defeat mediate the association between childhood trauma and psychosis? Evidence from the NEMESIS-2 Study. Acta Psychiatr. Scand. 2014;129(6):467–476. doi: 10.1111/acps.12212. [DOI] [PubMed] [Google Scholar]
- van der Gaag M, Smit F, Bechdolf A, French P, Linszen DH, Yung AR, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr. Res. 2013;149(1–3):56–62. doi: 10.1016/j.schres.2013.07.004. [DOI] [PubMed] [Google Scholar]