Abstract
OBJECTIVE
To evaluate the impact of comorbid depressive symptoms and/or stress on adverse cardiovascular (CV) outcomes in individuals with diabetes compared with those without diabetes.
RESEARCH DESIGN AND METHODS
Investigators examined the relationship between baseline depressive symptoms and/or stress in adults with and without diabetes and physician-adjudicated incident CV outcomes including stroke, myocardial infarction/acute coronary heart disease, and CV death over a median follow-up of 5.95 years in the national REGARDS cohort study.
RESULTS
Subjects included 22,003 adults (4,090 with diabetes) (mean age 64 years, 58% female, 42% black, and 56% living in the southeastern “Stroke Belt”). Elevated stress and/or depressive symptoms were more common in subjects with diabetes (36.8% vs. 29.5%; P < 0.001). In fully adjusted models, reporting either elevated stress or depressive symptoms was associated with a significantly increased incidence of stroke (HR 1.57 [95% CI 1.05, 2.33] vs. 1.01 [0.79, 1.30]) and CV death (1.53 [1.08, 2.17] vs. 1.12 [0.90, 1.38]) in subjects with diabetes but not in those without diabetes. The combination of both elevated stress and depressive symptoms in subjects with diabetes was associated with a higher incidence of CV death (2.15 [1.33, 3.47]) than either behavioral comorbidity alone (1.53 [1.08, 2.17]) and higher than in those with both elevated stress and depressive symptoms but without diabetes (1.27 [0.86, 1.88]).
CONCLUSIONS
Comorbid stress and/or depressive symptoms are common in individuals with diabetes and together are associated with progressively increased risks for adverse CV outcomes.
Introduction
The prevalence of diabetes in the U.S. has increased (1), and cardiovascular (CV) complications remain the major cause of morbidity and mortality in persons with type 2 diabetes, contributing substantially to increased health care costs (2). As individuals attempt to manage their diabetes, there is increasing recognition that comorbid behavioral problems, including both depressive symptoms and stress, are associated with poor glycemic control, poor lifestyle behaviors, and increased health services utilization (3–10). We (11) and others (3,4) have shown that these behavioral challenges are associated with inadequate medication adherence, which may be associated with adverse outcomes. Similarly, poor meta-bolic control may worsen depressive symptoms (5), and the relationship appears to be bidirectional (8). The prevalence of these comorbidities and their potential to worsen disease management have led some to develop interventions designed to address comorbid depressive symptoms or stress in persons with diabetes (12–14).
While many studies have documented the cross-sectional presence of these comorbidities and the effect on glycemic control in subjects with diabetes, only a limited number of studies have examined the potential impact on CV outcomes. Most of these studies have been limited to patients with depression recruited from established health care settings, and the true population-level impact is unknown. Likewise, there has not been adequate comparison of individuals with and without diabetes who have concurrent depressive symptoms and/or elevated levels of perceived stress to examine the unique consequences of behavioral and medical comorbidity. The objective of the present report was to examine the association between the presence of depressive symptoms and/or increased levels of perceived stress, determined at baseline, and risk of incident CV events over 5 years of follow-up in a major national cohort study that used population-based sampling methods, collecting data in the home both in subjects with and without diabetes.
Research Design and Methods
Study Design, Cohort Description, and Medication Information
The description of the population for this analysis has previously been reported (15). In short, the REasons for Geographic And Racial Differences in Stroke (REGARDS) study is a population-based, prospective, longitudinal cohort study of 30,239 subjects aged >45 years, 45% of whom are male, 55% female, 41% black, 59% white, 55% from the “Stroke Belt” region, and 45% from the rest of the continental U.S. It was designed to examine factors associated with the geographic and racial differences in stroke incidence as well as excess stroke mortality in the southeastern U.S. (i.e., Stroke Belt) relative to the rest of the nation and among blacks relative to whites. The REGARDS study included relevant measures for perceived stress and depressive symptoms and provides a unique opportunity to examine the impact of these behavioral comorbidities in individuals with and without diabetes and the incidence of adverse CV outcomes in a national sample of black and white adults. The REGARDS cohort was recruited between January 2003 and October 2007 and is evaluated by computer-assisted telephone interview every 6 months. The initial recruitment call was used to obtain verbal consent and collect CV history, risk factors, and demographic characteristics, including age, sex, and race (each subject self-reported race, and by design, the study compared only non-Hispanic black and white subjects, excluding other racial/ethnic groups).
The study also included an in-home visit by a trained health professional for data collection. Participants were asked to provide all prescription and nonprescription medications they had taken in the past 2 weeks, and medication names were recorded during the in-home visit and subsequently coded into drug classes. Written informed consent was obtained during the in-person evaluation. The institutional review boards of participating institutions approved the study methods. The present analysis included all members of the cohort who had complete follow-up data.
Physical Examination and Laboratory Measures
A brief physical exam including blood pressure (assessed as the average of two measurements obtained with an aneroid sphygmomanometer after the subject was in the seated position for at least 3 min with both feet on the floor) and blood and urine samples, and an electrocardiogram (ECG) was conducted during an in-home visit by a trained health professional 3–4 weeks after the telephone interview. Height and weight was measured via standard procedures, and BMI was computed as the body weight in kilograms divided by the square of height in meters (2). All ECGs were read by a trained cardiologist in a centralized ECG reading laboratory at the Wake Forest University/Baptist Medical Center in Winston-Salem, NC, using a predefined interpretation protocol (16). Atrial fibrillation was determined to be present if there was a self-reported history (asked as “Has a doctor or other health professional ever told you that you had atrial fibrillation?”) or if characteristic findings were present on the ECG as defined in the protocol. Left ventricular hypertrophy was defined to be present or absent based on ECG findings using the Sokolow-Lyon limb lead criteria (17) as specified in the protocol. hs-CRP was determined by particle-enhanced immunonephelometry using the BNII nephelometer (N High Sensitivity CRP; Dade Behring, Deerfield, IL), while total and HDL cholesterol and glucose were measured by colorimetric reflectance spectrophotometry using the Ortho Clinical Vitros 950/IRC Chemistry System (Johnson & Johnson Clinical Diagnostics, New Brunswick, NJ) in accordance with the National Cholesterol Education Program guidelines (18).
Diabetes, Depressive Symptoms, and Stress Measures
Diabetes was defined as self-reported diabetes obtained during the telephone interview or a fasting glucose ≥126 mg/dL, a nonfasting glucose ≥200 mg/dL, or the presence of oral hypoglycemic medication or insulin—each obtained during the home visit. The primary exposure of interest was the presence, at baseline, of depressive symptoms and/or elevated levels of perceived stress among individuals with diabetes at baseline compared with those without diabetes. The presence of depressive symptoms was assessed using the previously validated four-item Center for Epidemiologic Studies Depression (CESD) questionnaire (19). The CESD-4 is derived from the original 20-item CESD (20) and has been found to be highly correlated at 0.87 (19). These questions included responses that ranged from 0 to 12 based on the number of days the subject reported experiencing those feelings in the prior week, with higher scores indicating more depressive symptoms. Subjects with a CESD score ≥4 were determined to have an elevated level of depressive symptoms. Perceived level of stress was measured using a four-item version of the Cohen Perceived Stress Scale (21), a validated and previously used (22) instrument for measuring the perception of personal stress. It measures the extent to which respondents perceive their lives as unpredictable, uncontrollable, and/or overloaded. Subjects with Cohen Perceived Stress Scale scores >4 were determined to have high levels of psychological stress, corresponding to the highest quartile of scores.
CV Outcome Measures and Death
The primary outcomes of interest were physician-adjudicated CV events including stroke, myocardial infarction (MI)/acute coronary heart disease (CHD), and CV death. Living participants or their proxies were contacted every 6 months via telephone to assess new-onset stroke, CHD events, and CV mortality. A trained interviewer administered a standardized questionnaire that specifically asks whether, since the last follow-up, they had been hospitalized for stroke or heart disease. For each positive response, the date and time of each event were recorded. Medical records were retrieved for all potential stroke- and CHD-related hospitalizations and deaths.
For suspected stroke, each event was adjudicated via medical record review by a neurologist-led medical review team. Stroke events were defined following the World Health Organization definition (23) but also included events with symptoms lasting <24 h with neuroimaging consistent with acute ischemia or hemorrhage and cases where adjudicators agreed that the event was likely a stroke or death related to stroke but information was incomplete for World Health Organization or clinical classification.
Acute CHD events included were fatal or nonfatal MI. After a report of a hospitalization or death that potentially could be related to CVD, medical records were retrieved, and the event was adjudicated by a physician-led medical review team, following published guidelines (24,25). Specifically, medical records were examined for the presence of signs or symptoms suggestive of ischemia; a rising and/or falling pattern in cardiac troponin or creatine phosphokinase-MB over 6 or more hours with a peak value greater than or equal to twice the upper limit of normal (diagnostic cardiac enzymes); and ECG changes consistent with ischemia or MI, guided by the Minnesota code and classified as evolving diagnostic, positive, nonspecific, or not consistent with ischemia (26,27).
Participant deaths were detected by report of next of kin, online sources (e.g., the Social Security Death Index), and the National Death Index. For information surrounding the circumstances of participant death, proxies or next of kin were interviewed. Additionally, following published guidelines, medical records in the last year of life, death certificates, and autopsy reports were collected and reviewed by the physician-led adjudicators to determine whether the death was a CVD death (24,25).
Measurement of Potential Confounders
Data on traditional CV risk factors and socioeconomic factors were also collected in order to adjust for potential confounding in our analyses of the relationship between the presence of comorbid behavioral problems, diabetes, and adverse CV outcomes. Additional data collected included the following: annual household income (<$20,000/year, $20,000–$35,000/year, $35,000–$75,000/year, and >$75,000/year), education level (less than high school education, high school graduate, some college, and college graduate or higher), and health insurance (yes or no). Also collected was a health history including a history of stroke or heart disease (self-reported MI, coronary artery bypass grafting, bypass, angioplasty, stenting, or evidence of myocardial infarction on the study ECG). Smoking behavior was collected and categorized as nonsmoker, past smoker, or current smoker.
Analysis
The current study examined the relationship between comorbid depressive symptoms and/or elevated levels of perceived stress, both measured at baseline, in subjects with and without diabetes and the adjudicated incidence of stroke, acute CHD, and CVD death for events occurring through 30 December 2010. Follow-up time for each participant was calculated from the date of the in-home visit to date of first stroke, acute CHD, death, or last telephone follow-up. The analysis cohort for this report was 22,003 after exclusion of participants with a history of stroke or heart disease at baseline (N = 7,164), missing diabetes status at baseline (N = 845), or missing follow-up data (N = 171). The initial analysis compared the demographic, socioeconomic, and CV risk characteristics of subjects with and without diabetes in three groups: 1) those reporting no comorbid depressive symptoms and without elevated levels of perceived stress, 2) those reporting either depressive symptoms or elevated levels of perceived stress, and 3) those reporting both depressive symptoms and elevated levels of perceived stress. Age-adjusted incidence rates were then compared for each type of CV event in individuals with and without diabetes in the same three comparison groups. The longitudinal relationship between baseline behavioral morbidity (i.e., the three comparison groups), in both those with and without diabetes, and subsequent adjudicated CV events during follow-up was then examined in a series of Cox proportional hazards models. These models examined the relationships first in an unadjusted (crude) model and then in additional models that incrementally added the following groups of variables: 1) demographic characteristics (race, age, sex, and region [Stroke Belt vs. non–Stroke Belt]), 2) socioeconomic factors (income, education, and health insurance), and, finally, 3) CV risk factors (current smoking, history of heart disease, left ventricular hypertrophy, atrial fibrillation, BMI, systolic blood pressure, total cholesterol, hs-CRP, and statin use). All analyses were performed using SAS (SAS, Cary, NC).
Results
The current study included a total of 22,003 subjects, of whom 42% were black, 58% female, and 56% living in the southeastern U.S. Stroke Belt. Among all subjects at baseline, 18.6% subjects (n = 4,090) had diabetes, 10% (n = 2,202) reported increased depressive symptoms, and 28% (n = 6,132) reported elevated levels of stress. Subjects with diabetes were more likely to report either elevated depressive symptoms or stress or both than were subjects without diabetes (36.8% vs. 29.5%; P < 0.001). Detailed demographic characteristics for the study group are given in Table 1, separated into those with and without diabetes and stratified by the presence of behavioral comorbidities. Among subjects with diabetes, those reporting either increased depressive symptoms or stress or the combination were more likely to be women, black, live in the Stroke Belt, and have limited income. Subjects reporting either elevated depressive symptoms or stress or both had a higher prevalence of elevated baseline hs-CRP values. Across each category of behavioral comorbidity (i.e., none, either elevated depressive symptoms or stress, both elevated), subjects with diabetes had a higher prevalence of elevated baseline hs-CRP values, higher mean systolic BP, and greater prevalence of statin use than those without diabetes.
Table 1.
Baseline characteristics of REGARDS participants with or without diabetes, by depressive symptom/stress category
| Diabetes (n = 4,090) |
No diabetes (n = 17,913) |
P* | |||||
|---|---|---|---|---|---|---|---|
| No depressive symptoms, no/low stress | Depressive symptoms or moderate/severe stress | Depressive symptoms and moderate/severe stress | No depressive symptoms, no/low stress | Depressive symptoms or moderate/severe stress | Depressive symptoms and moderate/severe stress | ||
| N | 2,583 | 1,091 | 416 | 12,620 | 4,175 | 1,118 | |
| Age (years), mean (SD) | 65.2 (8.5) | 64.8 (9.3) | 62.1 (8.6) | 64.1 (9.2) | 63.1 (9.7) | 61.0 (9.8) | <0.001 |
| Female, n (%) | 1,335 (51.7) | 703 (64.4) | 324 (77.9) | 6,828 (54.1) | 2,798 (67.0) | 827 (74.0) | <0.001 |
| Black, n (%) | 1,510 (58.5) | 712 (65.3) | 279 (67.1) | 4,341 (34.4) | 1,760 (42.2) | 557 (49.8) | <0.001 |
| Stroke Belt residence, n (%) | 1,477 (57.2) | 646 (59.2) | 280 (67.3) | 6,773 (53.7) | 2,376 (56.9) | 675 (60.4) | <0.001 |
| High school or more education, n (%) | 2,202 (85.3) | 837 (76.9) | 295 (71.2) | 11,682 (92.6) | 3,696 (88.6) | 881 (78.8) | <0.001 |
| Annual household income ≥$35,000, n (%) | 1,088 (47.7) | 313 (33.0) | 80 (22.1) | 6,937 (62.2) | 1,847 (50.8) | 292 (29.9) | <0.001 |
| Health insurance, n (%) | 2,412 (93.5) | 994 (91.1) | 356 (85.8) | 11,895 (94.4) | 3,804 (91.2) | 933 (83.5) | 0.04 |
| Current smoker, n (%) | 328 (12.7) | 141 (13.0) | 91 (22.0) | 1,551 (12.3) | 654 (15.7) | 315 (28.3) | 0.54 |
| BMI <25 kg/m2, n (%) | 284 (11.1) | 111 (10.3) | 30 (7.3) | 3,583 (28.5) | 1,191 (28.6) | 285 (25.6) | <0.001 |
| BMI 25–29.9 kg/m2, n (%) | 788 (30.9) | 301 (28.0) | 109 (26.6) | 5,026 (39.9) | 1,448 (34.8) | 364 (32.7) | |
| BMI 30–40 kg/m2, n (%) | 1,186 (46.5) | 497 (46.2) | 195 (47.6) | 3,465 (27.5) | 1,285 (30.9) | 382 (34.3) | |
| BMI >40 kg/m2, n (%) | 292 (11.5) | 167 (15.5) | 76 (18.5) | 510 (4.1) | 236 (5.7) | 84 (7.5) | |
| hs-CRP <1 mg/dL, n (%) | 507 (21.1) | 200 (19.6) | 56 (14.6) | 3,678 (29.8) | 1,061 (26.1) | 222 (20.3) | <0.001 |
| hs-CRP 1–3 mg/dL, n (%) | 765 (31.9) | 271 (26.6) | 86 (22.3) | 4,244 (34.4) | 1,374 (33.8) | 351 (32.1) | |
| hs-CRP ≥3 mg/dL, n (%) | 1,130 (47.0) | 548 (53.8) | 243 (63.1) | 4,420 (35.8) | 1,634 (40.2) | 521 (47.6) | |
| Left ventricular hypertrophy, n (%) | 321 (12.4) | 154 (14.1) | 56 (13.5) | 975 (7.7) | 349 (8.4) | 107 (9.6) | <0.001 |
| Statin use, n (%) | 1,038 (40.2) | 461 (42.3) | 155 (37.3) | 2,755 (21.8) | 843 (20.2) | 232 (20.8) | <0.001 |
| Systolic blood pressure (mmHg), mean (SD) | 131.1 (16.4) | 131.9 (17.2) | 131.2 (18.6) | 126.0 (15.9) | 125.4 (16.5) | 126.2 (17.2) | <0.001 |
| Total cholesterol (mg/dL), mean (SD) | 182.7 (39.7) | 185.3 (42.7) | 190.3 (44.2) | 197.5 (37.7) | 199.0 (38.4) | 201.3 (40.4) | <0.001 |
*P value for comparison of subjects with diabetes vs. subjects with no diabetes.
Table 2 provides age-adjusted incidence rates per 1,000 person-years of follow-up for each of the three independent CV outcomes, broken out by the presence of behavioral comorbidities in those with and without diabetes. With respect to individual CV outcomes, the pattern of age-adjusted incidence rates was approximately twofold higher in those with diabetes than in those without diabetes across the range of comorbid behavioral illness. Among individuals with diabetes and one or both behavioral comorbidities, age-adjusted incidence rates were highest for acute CHD, followed by CV death, and stroke. Among those without diabetes but with one or more behavioral comorbidities, incidence rates were again highest for acute CHD but were followed by stroke and CV death.
Table 2.
Event numbers, age-adjusted incidence rates per 1,000 person-years, and crude and adjusted HRs for stroke, acute CHD, and CV disease death for individuals with stress or depressive symptoms and with stress and depressive symptoms compared with those with neither, for individuals with and without diabetes
| Diabetes |
No diabetes |
|||||
|---|---|---|---|---|---|---|
| No depressive symptoms, no/low stress (n = 2,583) | Depressive symptoms or moderate/severe stress(n = 1,091) | Depressive symptoms and moderate/severe stress (n = 416) | No depressive symptoms, no/low stress (n = 12,620) | Depressive symptoms or moderate/severe stress (n = 4,175) | Depressive symptoms and moderate/severe stress (n = 1,118) | |
| Stroke | ||||||
| Events (N) | 68 | 42 | 14 | 257 | 81 | 24 |
| Age-adjusted incidence rt./1,000 person-years | 3.9 | 4.4 | 5.5 | 2.7 | 3.1 | 3.9 |
| Crude HR (95% CI) | Ref. | 1.55 (1.05, 2.29) | 1.40 (0.79, 2.49) | Ref. | 1.02 (0.80, 1.31) | 1.15 (0.76, 1.74) |
| Model 1 HR (95% CI) | Ref. | 1.61 (1.09, 2.39) | 1.75 (0.97, 3.17) | Ref. | 1.09 (0.85, 1.40) | 1.40 (0.92, 2.14) |
| Model 2 HR (95% CI) | Ref. | 1.56 (1.05, 2.31) | 1.60 (0.88, 2.92) | Ref. | 1.03 (0.80, 1.32) | 1.25 (0.82, 1.90) |
| Full model HR (95% CI) | Ref. | 1.57 (1.05, 2.33) | 1.57 (0.86, 2.87) | Ref. | 1.01 (0.79, 1.30) | 1.14 (0.75, 1.75) |
| Acute CHD | ||||||
| Events (N) | 117 | 62 | 24 | 329 | 98 | 28 |
| Age-adjusted incidence rt./1,000 person-years | 7.4 | 8.0 | 9.8 | 3.9 | 4.2 | 5.1 |
| Crude HR (95% CI) | Ref. | 1.29 (0.96, 1.73) | 1.39 (0.92, 2.10) | Ref. | 1.06 (0.84, 1.34) | 1.27 (0.79, 2.02) |
| Model 1 HR (95% CI) | Ref. | 1.35 (1.00, 1.82) | 1.70 (1.12, 2.58) | Ref. | 1.18 (0.95, 1.45) | 1.56 (1.06, 2.30) |
| Model 2 HR (95% CI) | Ref. | 1.30 (0.96, 1.75) | 1.57 (1.02, 2.40) | Ref. | 1.13 (0.91, 1.39) | 1.37 (0.94, 2.01) |
| Full model HR (95% CI) | Ref. | 1.29 (0.96, 1.74) | 1.48 (0.96, 2.27) | Ref. | 1.12 (0.90, 1.38) | 1.27 (0.86, 1.88) |
| CV death | ||||||
| Events (N) | 81 | 51 | 23 | 237 | 88 | 23 |
| Age-adjusted incidence rt./1,000 person-years | 3.8 | 5.0 | 6.7 | 2.1 | 2.7 | 3.6 |
| Crude HR (95% CI) | Ref. | 1.58 (1.12, 2.24) | 1.93 (1.22, 3.06) | Ref. | 1.24 (0.96, 1.62) | 1.28 (0.83, 1.97) |
| Model 1 HR (95% CI) | Ref. | 1.60 (1.13, 2.26) | 2.35 (1.47, 3.76) | Ref. | 1.30 (1.00, 1.70) | 1.40 (0.90, 2.17) |
| Model 2 HR (95% CI) | Ref. | 1.52 (1.07, 2.16) | 2.13 (1.33, 3.43) | Ref. | 1.20 (0.92, 1.56) | 1.13 (0.73, 1.74) |
| Full model HR (95% CI) | Ref. | 1.53 (1.08, 2.17) | 2.15 (1.33, 3.47) | Ref. | 1.12 (0.90, 1.38) | 1.27 (0.86, 1.88) |
Crude model adjusted for depressive symptom and stress category. Model 1 adjusted for crude model plus demographic factors (race, sex, age, region). Model 2 adjusted for crude model plus demographic factors plus social and economic factors (income, education, health insurance). Full model adjusted for crude model plus demographic factors, social and economic factors, and risk factors (BMI, total cholesterol, hs-CRP, atrial fibrillation, left ventricular hypertrophy, systolic blood pressure, statin use, current cigarette smoking). Boldface type indicates statistical significance. Ref., reference; rt., rate.
Table 2 also provides the results of a series of crude and progressively adjusted hazard ratio (HR) models for each of the three independent CV outcomes, demonstrating the progressive impact of comorbidity on acute CHD and CV death among subjects with diabetes, relative to those without diabetes, even in fully adjusted models that accounted for demographic, socioeconomic, and CV risk factors. Unlike the pattern for acute CHD and CV death, the magnitude of the HRs for stroke was very similar in subjects with one versus both behavioral comorbidities and diabetes.
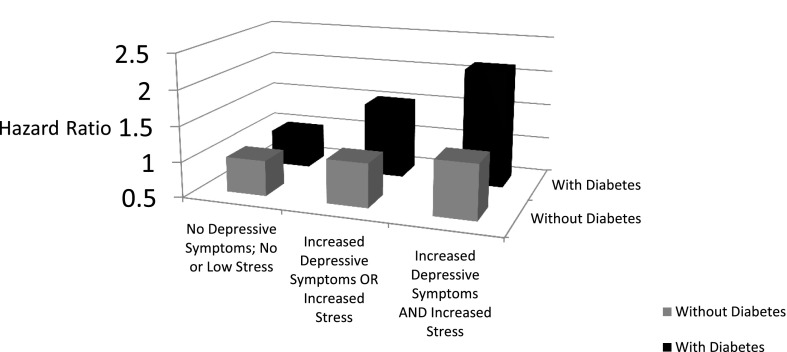
Figure 1 illustrates this significant and progressive pattern of worsening fully adjusted HRs for CV death among those with behavioral comorbidities, relative to those with no behavioral comorbidities, in subjects with diabetes and in comparison with those who do not have diabetes. Among individuals with diabetes, the presence of a single behavioral comorbidity—either increased depressive symptoms or stress—increased the risk of CV death by 53% relative to individuals with diabetes but without either behavioral comorbidity. Among individuals with diabetes, the presence of both behavioral comorbidities—increased depressive symptoms and stress—increased the risk of CV death by 115% relative to individuals with neither behavioral comorbidity, even after adjustment for a wide range of demographic and CV risk factors. While there was a pattern of increasing risk of CV death among those with one or both behavioral comorbidities (vs. neither) in subjects without diabetes, the differences were substantially smaller (12% and 27% increases, respectively) and were not statistically significant.
Figure 1.
Consequences of comorbid diabetes and elevated depressive symptoms and/or stress on CV death during follow-up among participants in the REGARDS study.
Conclusions
This article joins a growing body of literature that demonstrates a compelling pattern of augmented risk for CV events or CV death associated with comorbid behavioral illness among individuals with diabetes that far exceeds that observed in individuals with these same illnesses but without diabetes. While several studies have shown that formally diagnosed depression and/or depressive symptoms in subjects who are patients in an existing health care system are associated with incident CHD, this is among the first studies to compare the impact of these behavioral comorbidities on CV outcomes and mortality in a population-based sample of subjects with versus without diabetes in the same study. It is the first study to compare the effects of both depressive symptoms and stress together and separately in subjects with versus without diabetes, obtained by population-based sampling rather than recruitment in established health care settings. Further, the REGARDS study has a large sample size; includes oversampling of African Americans in the Stroke Belt region, allowing important racial comparisons; and also includes rich data obtained in the subject’s home environment. The study is novel in that, as a community-sampled study, it provides more precise estimates of the population burden and racial disparities associated with these comorbidities and their CV consequences than other studies in which patients were recruited in health care settings. The study provides a much larger sample of African Americans and illustrates the greater prevalence of comorbidities among African American women in particular, suggesting the need for more careful screening. Further, CV outcomes and CV death were carefully adjudicated events in the current study; by contrast, no adjudication process occurred in many other studies.
Our findings suggest that the adverse consequences observed in individuals with behavioral comorbidities and diabetes are best understood in a larger context or spectrum of linkages between physical and behavioral illness and subsequent outcomes. Elevated levels of stress and depressive symptoms, alone and together, were associated with a pattern of increased HRs for acute coronary disease and CV death in adjusted models both in subjects with and in subjects without diabetes, achieving statistical significance in those with diabetes. Together this pattern suggests the potential for progressive impact of single and multiple behavioral comorbidities on adverse CV outcomes and mortality that is of greatest concern in subjects with comorbid diabetes.
There was a significant pattern of increasing HRs for acute CHD and CV death in those with diabetes and with one or both behavioral comorbidities (none, stress or depressive symptoms alone, or both together). While diabetes has long been known to increase CV outcomes, our findings suggest that, relative to those individuals with diabetes who have no behavioral comorbidity, those with either depressive symptoms or elevated levels of stress as well as those with both together have progressively increased risks for acute CHD events and CV mortality during 5 years of follow-up, even when other risk factors are controlled for. These findings have important implications for the management of individuals with diabetes. In particular, behavioral comorbidities such as stress or depressive symptoms are not usually screened for in busy primary care practices, and many patients are reluctant to share these symptoms with busy primary care providers. Further, many individuals are reluctant to seek care from mental health providers. Among the demographic group in this study shown to have the highest prevalence of comorbid behavioral disease and diabetes (i.e., black females with limited income in the southeastern U.S.), there may also be distrust of the traditional health care delivery system. As a result, these comorbidities may go unrecognized and unmanaged. This study therefore provides strong evidence for adverse consequences associated with these comorbidities and suggests the need for more careful research to identify optimal screening and treatment strategies that work in busy primary care practices or other community settings.
Interestingly, while the presence of any behavioral comorbidity resulted in important increases in the HR for stroke, the increased HRs for one versus both behavioral comorbidities in subjects with diabetes were the same in fully adjusted models. This may suggest that a different mechanism is operative in the increased relationship of behavioral comorbidities and stroke.
These findings build on and expand an established literature that links the cross-sectional and longitudinal presence of stress, depressive symptoms, and/or established depression with CV outcomes and/or mortality including in individuals with comorbid diabetes (10,28–32). Because our study measured these symptoms at baseline and then followed patients longitudinally, it suggests the potential for the chronic influence of these behavioral comorbidities in subjects with diabetes to across time lead to adverse CV outcomes. Indeed, the chronic nature of depressive symptoms and stress in individuals with type 2 diabetes has been described by Fisher et al. (10,33), who called for a more careful understanding of the conjoint physical and emotional burden in these patients. Further, these authors join our findings in calling for integrating the screening and management of the emotional side of diabetes into regular diabetes care.
In addition, Hamer et al. (34) demonstrated an association between stress and CV events with HR of ∼1.5 and with the suggestion that behavioral processes explained the largest proportion of the variance in the hazards model. This raises an important question regarding the mechanism(s) through which behavioral comorbidities result in increased CV events and/or CV death. An early review by Plante (35) suggested that stress may lead to depressive symptoms that are subsequently associated with accelerated atherosclerosis, endothelial dysfunction, inflammatory reactions, and interstitial disturbances that may predispose the subject to premature CV disease or death. In the current study, hs-CRP levels were elevated in subjects with diabetes relative to those without diabetes, but among subjects with diabetes, there was a clearly progressive pattern of higher hs-CRP levels among those with either versus both behavioral comorbidities. These elevated hs-CRP levels may suggest a pattern of worsening atherosclerotic disease in subjects with diabetes with one or both behavioral comorbidities relative to those with no comorbidity. A recent article by Pizzi et al. (36) supports this and shows that depressive symptoms are associated with progressive longitudinal increases in carotid intima-media thickness relative to those who did not report these symptoms. This progressive pattern of atherosclerosis associated with these symptoms or potentially other behavioral problems might be expected to be more common in subjects with diabetes and therefore contribute to adverse CV outcomes.
Behavioral comorbidities may also interfere with self-care behaviors. Among individuals with diabetes, a study by Bonnet et al. (37) showed that those with anxiety or depressive symptoms were less likely to engage in healthy behaviors including physical activity, proper eating behaviors, and avoidance of smoking than were those without anxiety or depressive symptoms. Similarly, a meta-analysis by Gonzalez et al. (3) that included studies of individuals with depressive symptoms showed that those with depressive symptoms or depression were more likely to be nonadherent with medications as well as with the larger treatment regimen. Data by Fisher et al. (38) suggest a stronger relationship between diabetes-related distress and glycemic control than between depressive symptoms and glycemic control, and recent data by this group (39) show that specific reductions in regimen-related distress can result in improvements in medication adherence, physical activity, and glycemic control, all of which may influence CV risk.
The scope of the implications from these important associations with CV outcomes should be considered in the context of the recent Second Diabetes Attitudes, Wishes and Needs (DAWN2) study, which shows that these behavioral comorbidities know no geographic or ethnic boundaries and are highly prevalent in subjects with diabetes in multiple countries around the world (6). This suggests the need for additional research to understand the mechanisms by which these behavioral comorbidities are associated with CV outcomes in subjects with diabetes as well as to understand innovative intervention strategies that will not only improve the behavioral comorbidities but will also decrease the risk for CV outcomes. Clearly, more research is needed to understand whether the effective treatment of depressive symptoms and stress can lower CVD events in individuals with diabetes. And, as noted above, more work needs to be done to establish appropriate screening strategies for behavioral comorbidities that can be effectively disseminated in busy primary care settings where most individuals with diabetes are managed.
The current study has several limitations. This study is a prospective cohort study and not a randomized clinical trial, and the incident outcomes should be understood as associations with baseline characteristics without clear evidence of causality. The study was carried out in the U.S. and, by design, included only non-Hispanic white and black subjects; extrapolation to other ethnic groups or to other nations should not be undertaken. The current study did not include a measure of diabetes duration, which may have influenced the risk of CV events. The study included data on a large number of CV risk factors that were used to help minimize the influence of confounders; however, there may have been other unmeasured risk factors that were associated with adverse CV outcomes.
The presence of elevated stress or depressive symptoms in community-dwelling subjects with diabetes was associated with an increased risk for acute CHD or CV death. Moreover, subjects with diabetes who reported both stress and depressive symptoms together had the greatest risk for acute CHD and CV death. The presence of either or both behavioral comorbidities in subjects with diabetes was also associated with increased HRs for stroke. Elevated stress and/or depressive symptoms in subjects without diabetes resulted in increased HRs in fully adjusted models that were much smaller in magnitude and not statistically significant. These findings demonstrate the persistent disparities and negative CV impact of these comorbidities at the population level and suggest the need for more careful integration of behavioral screening and management in primary care settings, where most patients with type 2 diabetes are managed.
Supplementary Material
Article Information
Acknowledgments. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Funding. This research project is supported by a cooperative agreement from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH), and the U.S. Department of Health and Human Services (U01-NS-041588). D.M.C. is also supported by NIH grant P30-DK-093002. S.E.J. is also supported by the following NIH grants: HL R01080477 and K24 HL111154. N.R. is also supported by NIH grant HL R01080477.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the NIH. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of data.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.M.C. and K.K. wrote and revised the manuscript. G.H., V.H., P.M., B.K., N.R., S.E.J., and M.M.S. reviewed the manuscript and provided suggestions for revision. Y.Y. performed data analysis, reviewed the manuscript, and provided suggestions for revision. D.M.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
Footnotes
A slide set summarizing this article is available online.
References
- 1.Geiss LS, Wang J, Cheng YJ, et al. . Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 2014;312:1218–1226 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes--2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez JS, Peyrot M, McCarl LA, et al. . Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care 2008;31:2398–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin EHB, Von Korff M, Ciechanowski P, et al. . Treatment adjustment and medication adherence for complex patients with diabetes, heart disease, and depression: a randomized controlled trial. Ann Fam Med 2012;10:6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications 2005;19:113–122 [DOI] [PubMed] [Google Scholar]
- 6.Nicolucci A, Kovacs Burns K, Holt RI, et al.; DAWN2 Study Group . Diabetes Attitudes, Wishes and Needs second study (DAWN2™): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med 2013;30:767–777 [DOI] [PubMed] [Google Scholar]
- 7.Katon WJ, Young BA, Russo J, et al. . Association of depression with increased risk of severe hypoglycemic episodes in patients with diabetes. Ann Fam Med 2013;11:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan A, Lucas M, Sun Q, et al. . Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med 2010;170:1884–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher L, Skaff MM, Mullan JT, et al. . Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care 2007;30:542–548 [DOI] [PubMed] [Google Scholar]
- 10.Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabet Med 2008;25:1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings DM, Lutes L, Littlewood K, et al. . Regimen-related distress, medication adherence, and glycemic control in rural African American women with type 2 diabetes mellitus. Ann Pharmacother 2014;48:970–977 [DOI] [PubMed] [Google Scholar]
- 12.Fisher L, Hessler D, Glasgow RE, et al. . REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care 2013;36:2551–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogner HR, Morales KH, de Vries HF, Cappola AR. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Ann Fam Med 2012;10:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katon WJ, Lin EH, Von Korff M, et al. . Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard VJ, Cushman M, Pulley L, et al. . The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–143 [DOI] [PubMed] [Google Scholar]
- 16.ECG Reading Laboratory, Wake Forest University/Baptist Medical Center. Available from https://epicare.phs.wakehealth.edu/public/Epicare_Home.cfm#. Accessed 14 December 2012
- 17.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;37:161–186 [DOI] [PubMed] [Google Scholar]
- 18.National Cholesterol Education Program Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel: Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), Final Report. Bethesda, MD, National Cholesterol Education Program, National Heart, Lung, and Blood Institute, National Institutes of Health, 2002 (NIH publ. no. 02–5215)
- 19.Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas 1993;53:1117–1125 [Google Scholar]
- 20.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;3:385–401 [Google Scholar]
- 21.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–396 [PubMed] [Google Scholar]
- 22.Redmond N, Richman J, Gamboa CM, et al. . Perceived stress is associated with incident coronary heart disease and all-cause mortality in low- but not high-income participants in the Reasons for Geographic And Racial Differences in Stroke study. J Am Heart Assoc 2013;2:e000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 1989;20:1407–1431 [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, White HD, et al.; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction . Universal definition of myocardial infarction. Circulation 2007;116:2634–2653 [DOI] [PubMed] [Google Scholar]
- 25.Luepker RV, Apple FS, Christenson RH, et al.; AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute . Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003;108:2543–2549 [DOI] [PubMed] [Google Scholar]
- 26.Prineas R, Crow R, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Boston, MA, Wright-OSG, 1982 [Google Scholar]
- 27.Prineas R, Crow R, Zhang ZM. Minnesota Code Manual of Electrocardiographic Findings. 2nd ed. London, U.K., Springer-Verlag, 2010 [Google Scholar]
- 28.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006;27:2763–2774 [DOI] [PubMed] [Google Scholar]
- 29.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 2007;22:613–626 [DOI] [PubMed] [Google Scholar]
- 30.Whang W, Kubzansky LD, Kawachi I, et al. . Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. J Am Coll Cardiol 2009;53:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman SM, Katon W, Lin E, Von Korff M. Depression and death in diabetes; 10-year follow-up of all-cause and cause-specific mortality in a diabetic cohort. Psychosomatics 2013;54:428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park M, Katon WJ, Wolf FM. Depression and risk of mortality in individuals with diabetes: a meta-analysis and systematic review. Gen Hosp Psychiatry 2013;35:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med 2014;31:764–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamer M, Molloy GJ, Stamatakis E. Psychological distress as a risk factor for cardiovascular events: pathophysiological and behavioral mechanisms. J Am Coll Cardiol 2008;52:2156–2162 [DOI] [PubMed] [Google Scholar]
- 35.Plante GE. Depression and cardiovascular disease: a reciprocal relationship. Metabolism 2005;54(Suppl. 1):45–48 [DOI] [PubMed] [Google Scholar]
- 36.Pizzi C, Costa GM, Santarella L, et al. . Depression symptoms and the progression of carotid intima-media thickness: a 5-year follow-up study. Atherosclerosis 2014;233:530–536 [DOI] [PubMed] [Google Scholar]
- 37.Bonnet F, Irving K, Terra JL, Nony P, Berthezène F, Moulin P. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis 2005;178:339–344 [DOI] [PubMed] [Google Scholar]
- 38.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care 2010;33:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hessler D, Fisher L, Glasgow RE, et al. . Reductions in regimen distress are associated with improved management and glycemic control over time. Diabetes Care 2014;37:617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.