Acetaminophen (paracetamol) interferes with continuous glucose monitor (CGM) sensing, resulting in falsely elevated CGM glucose values in both sensors currently approved by the U.S. Food and Drug Administration (FDA). In amperometric glucose biosensors, particularly those measuring hydrogen peroxide, acetaminophen’s phenolic moiety is oxidized at the sensing electrode, producing an electrochemical signal not related to glucose (1). Limited published data exist documenting the magnitude of the effect of acetaminophen on CGM glucose (2), especially in the outpatient setting with contemporary sensor technology. Currently, the FDA recommends that insulin dosing decisions are based on blood glucose (BG) meter values, not CGM glucose values. Given the common use of acetaminophen, its interference with CGM sensing has significant clinical implications for patients who use CGM. To better understand this effect, we performed an acetaminophen challenge as part of an outpatient study designed to investigate the potential challenges to closed-loop systems, which use CGM sensor glucose values to automate insulin delivery. We hypothesized that acetaminophen would cause elevation of CGM glucose values in relationship to BG meter values.
Institutional review board approval at both sites and informed consent or assent plus guardian consent for subjects aged <18 years were obtained for all participants. Data from 40 subjects (43% male, age 28.5 ± 8.4 years, HbA1c 7.3 ± 0.8%) were included in this analysis. At breakfast, participants using a Dexcom G4 CGM system ingested 1,000 mg of acetaminophen and obtained BG meter (Bayer CONTOUR NEXT) readings at baseline, 0.5, 1, 2, 4, 6, and 8 h after acetaminophen ingestion. If the CGM was calibrated during the 8 h following acetaminophen ingestion, then the postcalibration data were excluded. The BG meter value closest to but no more than 15 min from the target time was selected (with the exception of the 0 min time point, which was allowed to be up to 30 min prior to acetaminophen ingestion). Next, the CGM glucose values were adjusted for baseline bias by calculating the difference between the 0 min CGM and BG meter values and then correcting the subsequent CGM values for the baseline bias.
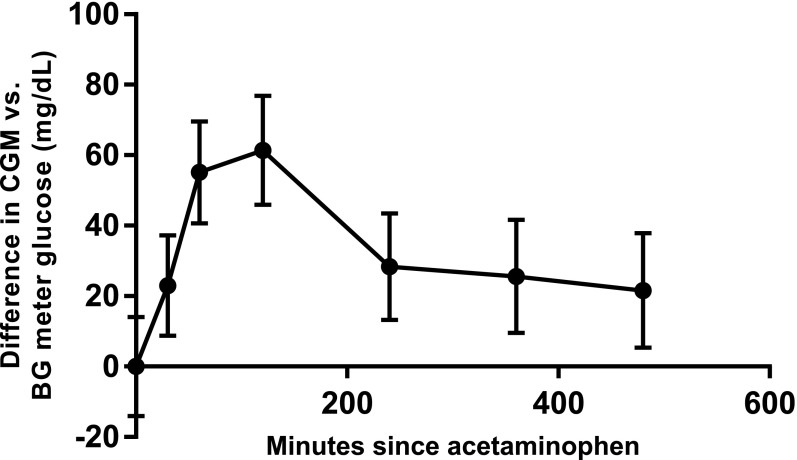
Least squares means and 95% CIs from a mixed model of the difference at each time point are shown in Fig. 1. Significant differences existed for 8 h after acetaminophen ingestion (P ≤ 0.01 for all), consistent with expected acetaminophen pharmacokinetics. The greatest mean difference was 61 mg/dL with an upper 95% CI limit of 77 mg/dL at 120 min (BG meter 171 ± 37 mg/dL). Notable individual variation was seen with 50% of relative differences within 20% and an additional 26% within 40% over 8 h. Three subjects had a BG meter value of <70 mg/dL with higher CGM glucose readings (63 vs. 138 mg/dL, 46 vs. 175 mg/dL, and 51 vs. 184 mg/dL, respectively), whereas 10 subjects had a CGM glucose >180 mg/dL and a BG meter value more than 100 mg/dL lower. Age, BMI, HbA1c, and number of boluses for food ingestion did not affect the observed difference, but a greater effect was seen in women (P = 0.04).
Figure 1.
Least squares means and 95% CIs for difference between CGM glucose and BG meter glucose by time point.
Acetaminophen falsely elevates CGM glucose values compared with BG meter values. These data have implications for use of CGM glucose as a replacement for meter glucose for diabetes decision making and for closed-loop systems using CGM glucose values for automated insulin delivery.
Article Information
Funding. The authors would like to acknowledge JDRF (grant 17-2013-471) and the Clinical and Translational Science Award program, funded by the National Center for Advancing Translational Sciences (grant UL1 TR000093) at the National Institutes of Health.
Duality of Interest. D.M.M. is on the advisory board for Insulet. R.P.W. is a consultant for Medtronic and receives research support from Novo Nordisk. B.B. is on medical advisory boards for Sanofi; Novo Nordisk; Becton, Dickinson and Company; Unomedical; and Medtronic. He has received research grant and/or material support from Medtronic, Dexcom, LifeScan, Insulet, Bayer, Unomedical, and Tandem. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.M.M. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. D.D., L.P., T.L., L.M., P.C., E.W., R.P.W., and B.B. researched data, contributed to discussion, and reviewed and edited the manuscript. D.M.M. and B.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Zhang Y, Hu Y, Wilson GS, Moatti-Sirat D, Poitout V, Reach G. Elimination of the acetaminophen interference in an implantable glucose sensor. Anal Chem 1994;66:1183–1188 [DOI] [PubMed] [Google Scholar]
- 2.Moatti-Sirat D, Poitout V, Thomé V, et al. Reduction of acetaminophen interference in glucose sensors by a composite Nafion membrane: demonstration in rats and man. Diabetologia 1994;37:610–616 [DOI] [PubMed] [Google Scholar]