The notion that central nervous system (CNS) insulin action plays an important role in mediating the inhibition of endogenous glucose production (EGP) is becoming increasingly accepted (1–5). In the rodent, insulin’s effect in the brain involves transport of insulin across the blood–brain barrier, activation of insulin signaling, opening of neuronal ATP-sensitive potassium (KATP) channels, signaling via vagal hepatic efferents, phosphorylation of liver STAT3, and suppression of gluconeogenic gene expression, with subsequent reduction of EGP due to inhibition of gluconeogenesis but not glycogenolysis (6–10). The effect was relatively slow in onset (requiring several hours to appear) and was evident under nonphysiological circumstances because infusion of insulin into a peripheral vein results in absolute or relative hepatic insulin deficiency (Fig. 1) (11,12). In addition, glucagon was not replaced, raising the possibility that insulin’s brain–liver effect is only manifest when the liver is deprived of other normal regulatory inputs. Despite such limitations, these studies have led some to conclude that brain insulin action is “required,” “necessary,” or even “essential” for the suppression of EGP by insulin (2,5,7–10).
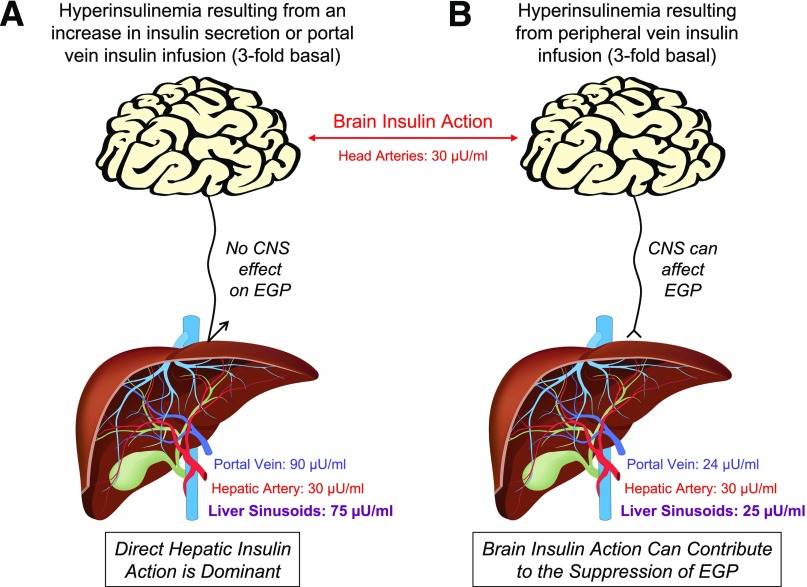
Figure 1.
In the basal state, arterial and hepatic portal vein insulin concentrations are approximately 10 and 30 µU/mL, respectively, such that the concentration of insulin in blood entering the hepatic sinusoids is ∼25 µU/mL. A threefold increase in basal insulin secretion or portal vein insulin infusion (A) increases both arterial (brain) and liver insulin concentrations by threefold. When insulin is acutely elevated in this way, insulin’s direct hepatic effect drives the rapid suppression of EGP, and the CNS effects of insulin on the liver are masked. In response to a threefold rise in insulin brought about by infusion into a peripheral vein (B), arterial (brain) insulin concentrations are also elevated threefold, but in this case hepatic sinusoidal insulin levels remain at the basal level (∼25 μU/mL) because endogenous insulin secretion falls (exogenous insulin infusion inhibits insulin secretion in the human, dog, and rodent [12,40,41]) and the gut destroys 20% of the insulin in the blood perfusing it. Clearly, when insulin is administered intranasally, by direct infusion into the brain, or via a peripheral vein, the normal physiological insulin gradient between the brain and liver is lost. Thus, although brain insulin action can impact hepatic glucose production (albeit slowly) in the deficiency of insulin signaling at the liver, it cannot do so under circumstances in which the direct insulin signal is normal.
As in the rodent, the canine brain–liver insulin axis has been shown to involve CNS insulin signaling and KATP channel activation, a neurally mediated increase in hepatic STAT3 phosphorylation, and changes in glucoregulatory gene expression in the liver (13,14). In one study, a selective increase in brain insulin, brought about by insulin infusion into the carotid and vertebral arteries at a rate that raised insulin in the head but maintained basal insulin levels at the liver, decreased the transcription of gluconeogenic genes but did not suppress EGP under euglycemic clamp conditions (14). Lack of correlation between gluconeogenic gene expression and glucose flux is not surprising given the poor control strength of enzymes such as PEPCK across species (15–17). After several hours, however, there was a modest increase in the ability of the liver to take up glucose. Notably, all of insulin’s central effects were blocked by third ventricle infusion of a phosphatidylinositol 3-kinase (PI3K) inhibitor or a KATP channel blocker (14), the latter of which would block insulin's effects through both the PI3K and mitogen-activated protein kinase (MAPK) pathways (18).
As excess EGP contributes to hyperglycemia in humans with diabetes, it is imperative that regulation of the process be fully understood. In that regard it is necessary to determine whether a brain–liver insulin axis controlling EGP exists in the human, and if so, to what extent it is relevant. These are significant issues because targeting the brain–liver insulin axis may be of therapeutic value, especially if hypothalamic insulin resistance contributes to metabolic dysfunction (5). Although studying brain insulin action in the human is technically challenging, intranasal insulin administration is known to increase cerebrospinal fluid insulin concentrations and to affect cognitive performance, food intake, and satiety (19). Thus, it is a tool with which to address the above questions. Two articles, published in the current issue of Diabetes (20,21), describe the use of intranasal insulin to investigate the impact of brain insulin action on human glucose metabolism.
In the study by Dash et al. (20), insulin was administered intranasally (40 IU) on the background of a pancreatic clamp using somatostatin (insulin and glucagon were infused into a peripheral vein to clamp their levels at basal arterial values, meaning that the liver was deficient in both). After 3 h, a modest suppression of EGP became evident (36% reduction at 240 min and 15% during the last hour) in the test group relative to a control group in which insulin was infused peripherally to account for the leakage of intranasally delivered insulin into the bloodstream. This observation indicates that a pharmacological dose of insulin given into the head can inhibit EGP in the human. Nevertheless, considering the slow onset of the effect (>3 h), Dash et al. (20) concluded that CNS insulin action cannot explain the rapid (minutes) suppression of EGP that is consistently seen during hyperinsulinemic clamps across species (11,12,22). Thus, even though these data support the existence of a brain–liver insulin axis in the human, they also clearly indicate that an acute increase in brain insulin action is not essential for the suppression of EGP by hyperinsulinemia.
Based on the observation that a large dose of intranasal insulin (160 IU) increased the glucose infusion rate required to maintain euglycemia during a hyperinsulinemic clamp, Heni et al. (23) recently concluded that brain insulin action rapidly (within 15 min) increases peripheral insulin sensitivity in the human. This finding disagrees with previous human, dog, and rodent studies, which have consistently shown that brain insulin action requires several hours to manifest effects on glucose metabolism (6–10,14,18,20). It should be noted that Dash et al. (20) measured significant peripheral spillover of insulin into the circulation after even a 10 IU intranasal dose of insulin. Unfortunately, this leakage was not accounted for in the study of Heni et al. (23), and this most likely explains the increase in glucose infusion that was observed, rather than a brain insulin effect.
In an earlier attempt to identify a brain–liver insulin effect in the human, Kishore et al. (24) administered a KATP channel activator (diazoxide) orally to see if mimicking insulin action in the CNS would have any effect on EGP (diazoxide was given 3 h prior to a 4-h peripheral insulin clamp in which arterial insulin was increased threefold, with basal insulin levels at the liver). EGP did not change for 5 h, but decreased by 30% 6–7 h after dosing. Thus, although the studies of Dash et al. (20) and Kishore et al. (24) support the concept that brain insulin action can regulate EGP in the human, albeit slowly, both studies were pharmacological in nature and were carried out with the liver in a relatively insulin-deficient state, leaving open the question of the physiological relevance of brain insulin action in control of EGP in the human.
Unfortunately, the difficulty of hepatic portal vein access makes it challenging to create a normal insulin gradient between the liver and brain during a clamp in the human or rodent. It should be noted that the normal 3:1 ratio of insulin that exists between the liver and brain is always eliminated when insulin is administered intranasally, by infusion directly into the brain, or via a peripheral vein. Studying the effect of neural input to the liver at a time when hepatic insulin and/or glucagon levels are inappropriately low, relative to those in the brain, complicates the interpretation of results regarding the physiological importance of the perturbation in question. A threefold rise in arterial insulin brought about by peripheral insulin infusion (6–10,24) is often considered hyperinsulinemic (Fig. 1), even though hepatic insulin levels are basal (11,12), and in such a case, it would be no surprise for the “hyperinsulinemic” effects of insulin to be entirely nonhepatic (25,26). Even a sixfold rise in the arterial (brain) insulin level produces only a twofold rise at the liver, again making interpretation of the results difficult. Thus, when determining the physiological relevance of brain insulin action on EGP, the appropriate hormonal gradient between the liver and brain must be preserved.
To our knowledge, only one study has examined the impact of acute brain insulin signaling on EGP during a normal physiological hyperinsulinemic clamp (i.e., when the same magnitude of rise in insulin occurred at all tissues) (13). In that dog study, we found that the noncentral effects of insulin fully explained the suppression of EGP, such that there was no contribution of CNS insulin action to the rapid decrease in EGP. Likewise, when the normal brain–liver insulin gradient was maintained under basal insulin conditions, selectively increasing or blocking brain insulin signaling (via third ventricle infusion of insulin or PI3K/KATP channel inhibitors, respectively) had no effect on EGP (14). On the other hand, in another study a selective increase in insulin at the liver (but not brain) suppressed EGP within 15 min (22). While it has been postulated that insulin’s central and direct hepatic effects are redundant and equally sufficient for the inhibition of EGP in the rodent (4), the relevance of CNS insulin action in the response to physiological hyperinsulinemia (normal distribution of insulin and glucagon between the liver and brain) has never been measured in the rodent. The available data in the dog suggest that acute activation of brain insulin signaling does not have a meaningful impact on hepatic glucose metabolism under such conditions.
While the results of Dash et al. (20) provide hope that pharmacological activation of the brain−liver insulin axis may be useful clinically in the treatment of excess EGP in diabetes, the results of Ott et al. (21) question the efficacy of such an approach. In unclamped experiments, where neither somatostatin nor glucose were infused, Ott et al. created a prolonged elevation of central insulin by dosing healthy men with 10 or 20 IU of insulin intranasally every 15 min over 6 h (a total of 210 or 420 IU). Fasting plasma glucose levels decreased slightly (∼5 mg/dL), but a similar drop occurred when intranasal insulin spillover was simulated by peripheral insulin infusion in a control group. Subtle alterations in endogenous insulin and glucagon secretion could have masked small effects on the liver, although if EGP was suppressed by CNS insulin action (glucose kinetics were not determined), it had no net impact on basal glucose levels. Other studies in humans given intranasal insulin have shown either no change in arterial insulin, C-peptide, or glucose concentrations (27–30), or at most a 5% decrease in plasma glucose (31,32), probably resulting from insulin spillover, although those studies were only carried out for 3 h. Even chronic treatment with intranasal insulin (4 × 40 IU/day for 8 weeks) did not affect plasma insulin or glucose concentrations in healthy normal-weight subjects (33). Finally, hepatic denervation had little to no effect on hepatic insulin action in liver transplant patients despite complete lack of neural input to the liver (34–36). Thus, while it seems likely that brain insulin action can signal the liver across species, as of yet there are no studies demonstrating that it has a meaningful impact on EGP when the liver is receiving other direct signals normally.
In summary, in vitro (37), ex vivo (38), and in vivo (13,22) data across species clearly demonstrate that acute CNS insulin action is not essential for the rapid suppression (within minutes) of EGP by hyperinsulinemia. Nevertheless, findings in the rodent, dog, and now human suggest that brain insulin action has the potential to slowly alter hepatic glucose metabolism, leaving open the question of whether targeting brain insulin action could be of therapeutic value. It would appear that when proportional increases in insulin occur simultaneously at the liver and brain, as occurs normally, the direct effect of insulin on the liver is dominant and determines the rapid and predominantly antiglycogenolytic effect of the hormone (13,39). It remains to be seen, however, if chronic modulation of brain insulin action can alter the gluconeogenic tone of the liver such that the hepatic response to various factors, including the direct effects of insulin, might be altered. Further studies are required to determine when brain insulin action has physiological, pathophysiological, or therapeutic relevance in the regulation of hepatic glucose production.
Article Information
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
References
- 1.Carey M, Kehlenbrink S, Hawkins M. Evidence for central regulation of glucose metabolism. J Biol Chem 2013;288:34981–34988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grayson BE, Seeley RJ, Sandoval DA. Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat Rev Neurosci 2013;14:24–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz MW, Seeley RJ, Tschöp MH, et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 2013;503:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas JM, Schwartz MW. Control of hepatic glucose metabolism by islet and brain. Diabetes Obes Metab 2014;16(Suppl. 1):33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogt MC, Brüning JC. CNS insulin signaling in the control of energy homeostasis and glucose metabolism—from embryo to old age. Trends Endocrinol Metab 2013;24:76–84 [DOI] [PubMed] [Google Scholar]
- 6.Inoue H, Ogawa W, Asakawa A, et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab 2006;3:267–275 [DOI] [PubMed] [Google Scholar]
- 7.Könner AC, Janoschek R, Plum L, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 2007;5:438–449 [DOI] [PubMed] [Google Scholar]
- 8.Lin HV, Plum L, Ono H, et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 2010;59:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002;8:1376–1382 [DOI] [PubMed] [Google Scholar]
- 10.Pocai A, Lam TK, Gutierrez-Juarez R, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature 2005;434:1026–1031 [DOI] [PubMed] [Google Scholar]
- 11.Edgerton DS, Moore MC, Winnick JJ, et al. Changes in glucose and fat metabolism in response to the administration of a hepato-preferential insulin analog. Diabetes 2014;63:3946–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farmer TD, Jenkins EC, O'Brien TP, et al. A comparison of the physiological relevance of systemic versus portal Insulin delivery to evaluate whole body glucose flux during an insulin clamp. Am J Physiol Endocrinol Metab. 16 December 2014 [Epub ahead of print]. DOI: 10.1152/ajpendo.00406.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramnanan CJ, Kraft G, Smith MS, et al. Interaction between the central and peripheral effects of insulin in controlling hepatic glucose metabolism in the conscious dog. Diabetes 2013;62:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramnanan CJ, Saraswathi V, Smith MS, et al. Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. J Clin Invest 2011;121:3713–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess SC, He T, Yan Z, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab 2007;5:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramnanan CJ, Edgerton DS, Rivera N, et al. Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes 2010;59:1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuel VT, Beddow SA, Iwasaki T, et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc Natl Acad Sci U S A 2009;106:12121–12126 [DOI] [PMC free article] [PubMed]
- 18.Filippi BM, Yang CS, Tang C, Lam TK. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab 2012;16:500–510 [DOI] [PubMed] [Google Scholar]
- 19.Chapman CD, Frey WH 2nd, Craft S, et al. Intranasal treatment of central nervous system dysfunction in humans. Pharm Res 2013;30:2475–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes 2015;64:766–774 [DOI] [PubMed]
- 21.Ott V, Lehnert H, Staub J, Wönne K, Born J, Hallschmid M. Central nervous insulin administration does not potentiate the acute glucoregulatory impact of concurrent mild hyperinsulinemia. Diabetes 2015;64:760–765 [DOI] [PubMed]
- 22.Sindelar DK, Balcom JH, Chu CA, Neal DW, Cherrington AD. A comparison of the effects of selective increases in peripheral or portal insulin on hepatic glucose production in the conscious dog. Diabetes 1996;45:1594–1604 [DOI] [PubMed] [Google Scholar]
- 23.Heni M, Wagner R, Kullmann S, et al. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes 2014;63:4083–4088 [DOI] [PubMed] [Google Scholar]
- 24.Kishore P, Boucai L, Zhang K, et al. Activation of K(ATP) channels suppresses glucose production in humans. J Clin Invest 2011;121:4916–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto H, Obici S, Accili D, Rossetti L. Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action. J Clin Invest 2005;115:1314–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buettner C, Patel R, Muse ED, et al. Severe impairment in liver insulin signaling fails to alter hepatic insulin action in conscious mice. J Clin Invest 2005;115:1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohringer A, Schwabe L, Richter S, Schachinger H. Intranasal insulin attenuates the hypothalamic-pituitary-adrenal axis response to psychosocial stress. Psychoneuroendocrinology 2008;33:1394–1400 [DOI] [PubMed] [Google Scholar]
- 28.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002;5:514–516 [DOI] [PubMed] [Google Scholar]
- 29.Jauch-Chara K, Friedrich A, Rezmer M, et al. Intranasal insulin suppresses food intake via enhancement of brain energy levels in humans. Diabetes 2012;61:2261–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krug R, Benedict C, Born J, Hallschmid M. Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory. J Clin Endocrinol Metab 2010;95:E468–E472 [DOI] [PubMed] [Google Scholar]
- 31.Benedict C, Brede S, Schiöth HB, et al. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes 2011;60:114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heni M, Kullmann S, Ketterer C, et al. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia 2012;55:1773–1782 [DOI] [PubMed] [Google Scholar]
- 33.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes 2004;53:3024–3029 [DOI] [PubMed] [Google Scholar]
- 34.Luzi L, Perseghin G, Regalia E, et al. Metabolic effects of liver transplantation in cirrhotic patients. J Clin Invest 1997;99:692–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perseghin G, Regalia E, Battezzati A, et al. Regulation of glucose homeostasis in humans with denervated livers. J Clin Invest 1997;100:931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneiter P, Gillet M, Chioléro R, Wauters JP, Berger M, Tappy L. Postprandial hepatic glycogen synthesis in liver transplant recipients. Transplantation 2000;69:978–981 [DOI] [PubMed] [Google Scholar]
- 37.Pilkis SJ, el-Maghrabi MR, Claus TH. Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Biochem 1988;57:755–783 [DOI] [PubMed] [Google Scholar]
- 38.Hausler N, Browning J, Merritt M, et al. Effects of insulin and cytosolic redox state on glucose production pathways in the isolated perfused mouse liver measured by integrated 2H and 13C NMR. Biochem J 2006;394:465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edgerton DS, Lautz M, Scott M, et al. Insulin’s direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest 2006;116:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cherrington AD. Control of Glucose Production in vivo by insulin and glucagon. In Handbook of Physiology: A Critical, Comprehensive Presentation of Physiological Knowledge and Concepts: Section 7: The Endocrine System, Volume 2: The Endocrine Pancreas and Regulation of Metabolism. Jefferson LSC, Cherrington A.D., Eds. New York, Oxford University Press, 2001, p. 759–785 [Google Scholar]
- 41.Prager R, Wallace P, Olefsky JM. Direct and indirect effects of insulin to inhibit hepatic glucose output in obese subjects. Diabetes 1987;36:607–611 [DOI] [PubMed] [Google Scholar]