Abstract
The FTO gene harbors variation with the strongest effect on adiposity and obesity risk. Previous data support a role for FTO variation in influencing food intake. We conducted a combined analysis of 16,094 boys and girls aged 1–18 years from 14 studies to examine the following: 1) the association between the FTO rs9939609 variant (or a proxy) and total energy and macronutrient intake; and 2) the interaction between the FTO variant and dietary intake, and the effect on BMI. We found that the BMI-increasing allele (minor allele) of the FTO variant was associated with increased total energy intake (effect per allele = 14.3 kcal/day [95% CI 5.9, 22.7 kcal/day], P = 6.5 × 10−4), but not with protein, carbohydrate, or fat intake. We also found that protein intake modified the association between the FTO variant and BMI (interactive effect per allele = 0.08 SD [0.03, 0.12 SD], P for interaction = 7.2 × 10−4): the association between FTO genotype and BMI was much stronger in individuals with high protein intake (effect per allele = 0.10 SD [0.07, 0.13 SD], P = 8.2 × 10−10) than in those with low intake (effect per allele = 0.04 SD [0.01, 0.07 SD], P = 0.02). Our results suggest that the FTO variant that confers a predisposition to higher BMI is associated with higher total energy intake, and that lower dietary protein intake attenuates the association between FTO genotype and adiposity in children and adolescents.
Introduction
Common single nucleotide polymorphisms (SNPs) located in the first intron of the gene associated with fat mass and obesity (FTO) are the first adiposity/BMI-associated variants identified through genome-wide association studies (GWASs) (1–3), and to date this remains the locus with the largest influence on BMI in adults, as well as in children and adolescents (4). The mechanism by which FTO variants influence adiposity is unclear. Previous animal studies have suggested a role of Fto in regulating energy homeostasis, but it is unknown whether it influences energy intake (5,6) or energy expenditure (7,8). In addition, it is not clear which gene’s function is affected by the functional variants at this locus: FTO itself or another gene located downstream or upstream of FTO, such as IRX3 (9) and RPGRIP1L (10).
In many human studies (11–20), the BMI-increasing allele of FTO variants has been reported to be associated with increased food intake, total energy intake, fat or protein intake, suggesting that diet mediates the association with BMI. However, these associations have not been replicated in a number of other studies (21–35). In addition, there is an increasing interest in examining whether lifestyle factors influence the associations between FTO variants and adiposity. While there is evidence that physical activity reduces the effect of FTO on BMI, at least in adults (36), the few studies (12,20,26,32,34,35,37,38) that have investigated interaction with dietary factors in relation to BMI/obesity have generated conflicting results regarding potential interactions. Our recent large-scale meta-analysis (39) indicated that FTO variants were associated with protein intake in adults and that under-reporting of dietary intake in obese participants might be a major issue in the analysis. Studies in children are of particular interest in this regard, since this population is less biased by comorbidities, and their treatment and exposure to environmental contributors is shorter.
The relatively small sample size of individual studies, the modest genetic effect size, and the inevitable measurement errors might be major reasons for these inconsistent observations. Thus, studies with larger sample sizes are needed to clarify interrelations among FTO variants, dietary intake, and adiposity. Herein we report the results of a combined analysis of 16,094 children and adolescents from 14 studies to examine the following: 1) whether the FTO rs9939609 variant (or a proxy SNP) is associated with dietary intake of total energy and macronutrients (protein, carbohydrate, and fat); and 2) whether dietary intake influences the association between the FTO variant and BMI.
Research Design and Methods
Study Participants
The current analysis included cross-sectional data on 16,094 children and adolescents (15,352 whites, 478 African Americans, and 267 Asians) aged 1–18 years from 14 studies (Supplementary Table 1). The study design, recruitment of participants, and data collection of individual studies have been described in detail previously (14,23,24,40–50). In each study, informed consent was obtained from subjects’ parents or guardians and subjects (if appropriate). Each study was reviewed and approved by the local institutional review board.
Study-specific characteristics for each study are shown in Supplementary Table 2. The ranges of mean values across studies were as follows: age 1.1–16.4 years; BMI 16.2–24.7 kg/m2; total energy intake 1,017–2,423 kcal/day; total protein intake 12.9–16.8% (percentage of total energy intake); total carbohydrate 43.4–59.0%; and total fat intake 28.1–40.0%.
Assessment of BMI and Dietary Intake
BMI was calculated as body weight (kg)/height (m2). Body weight and height were measured in all studies except for one study which used self-reported data in a subsample (Supplementary Table 3). For two studies (43,48) with children younger than 2 years of age, length (height) was measured to the nearest millimeter with children in a supine position. Dietary intake (total energy, protein, carbohydrate, and fat) was assessed using validated food frequency questionnaires (four studies), multiple-day dietary/food records (three studies), multiple-day 24-h recalls (four studies), both dietary records and 24-h recalls (one study), diet history determined by consulting and information system (one study), or a brief-type self-administered diet history questionnaire (one study) (Supplementary Table 3). Macronutrient intake was expressed as the percentage of total energy intake.
Genotyping
FTO SNP rs9939609 or a proxy (linkage disequilibrium r2 = 1 in the corresponding ethnic group) was genotyped using direct genotyping methods or Illumina genome-wide genotyping arrays, or imputed using MACH (http://csg.sph.umich.edu/abecasis/MACH/) with a high imputation quality (r2 = 1) (Supplementary Table 4). The studies provided summary statistics based on data that met their quality control criteria for genotyping call rate, concordance in duplicate samples, and Hardy-Weinberg equilibrium P value.
Statistical Analysis
A standardized analytical plan, which is described below, was sent to study analysts from the 14 studies, and analyses were performed locally. BMI was transformed to age-standardized z score by sex in each study before analysis. A linear regression model under additive allelic effects was applied to examine associations of FTO variant with BMI, total energy intake, and intake of fat, protein, and carbohydrate (expressed as the percentage of total energy), adjusted for pubertal status (if available), physical activity (if available), and eigenvectors (data from GWASs only). We additionally adjusted for BMI when evaluating the association between FTO variant and dietary intake. In addition, the difference in BMI between the low– and high–dietary intake groups (dichotomized at medians in each study) was also examined. Interactions between FTO genotype and dietary intake and their effect on BMI were tested by including the respective interaction terms in the models (e.g., interaction term = rs9939609 SNP × total energy intake [dichotomized at the medians in each study]). We examined the association between FTO variant and BMI stratified by low– and high–dietary intake groups (dichotomized at medians in each study). All of the analyses were conducted in boys and girls separately, except for one study that combined the data from boys and girls, with sex as a covariate. Analyses were also conducted in each race, and in cases and controls separately if studies included multiple ancestries or had a case-control design.
Detailed summary statistics from each study were subsequently collected, and we pooled β-coefficients and SEs from individual studies using the Mantel-Haenszel fixed-effects method, as well as the DerSimonian and Laird random-effects method implemented in Stata, version 12 (StataCorp LP, College Station, TX). The significant P value was 0.005 after Bonferroni adjustment for 10 independent tests: FTO-BMI association (1 test); diet-BMI associations (3 tests; we considered total energy, protein, carbohydrate, and fat intake as 3 independent variables); FTO-diet associations (3 tests); and FTO-diet interactions (3 tests). Between-study heterogeneity was tested by the Cochran Q statistic and quantified by the values for the proportion of variance explained by interstudy differences (I2). Low heterogeneity was defined as an I2 value of 0–25%, moderate heterogeneity as an I2 of 25–75%, and high heterogeneity as an I2 of 75–100%. The P value for heterogeneity was derived from a χ2 test. We also performed stratified meta-analyses in subgroups according to ethnicity (whites, African Americans, or Asians), sex, age group (mean age <10 vs. ≥10 years), geographic region (North America, Europe, or Asia), study sample size (n < 500 vs. n ≥ 500), study design (population based vs. case-control), dietary intake assessment method (dietary records or 24-h recalls vs. food frequency questionnaire or others), and adjustment for physical activity (yes vs. no).
Results
FTO Variants and BMI
We found a significant association between the minor allele (A-allele) of the FTO SNP rs9939609 (or its proxies) and a higher BMI in all participants combined (effect per allele = 0.07 SD [95% CI 0.05, 0.09 SDs], P = 4.7 × 10−10) (Table 1). The association was significant in 15,352 whites (effect per allele = 0.08 SD [0.05, 0.10 SDs], P = 2.9 × 10−11), but not in 478 African Americans (effect per allele = −0.12 SD [−0.26, 0.02 SDs], P = 0.08) or 267 Asians (effect per allele = 0.11 SD [−0.12, 0.09 SDs], P = 0.87), separately.
Table 1.
Associations between FTO SNP rs9939609, BMI, and dietary intake in a fixed-effects meta-analysis of 16,097 children and adolescents
| Model 1* |
Model 2† |
|||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | I2 | β (95% CI) | P | I2 | |
| BMI z score | 0.07 (0.05, 0.09) | 4.7 × 10−10 | 40% | |||
| Total energy (kcal/day) | 14.6 (6.3, 23.1) | 6.5 × 10−4 | 0% | 14.7 (6.3, 23.1) | 6.5 × 10−4 | 6% |
| Protein (% of energy) | 0.0 (−0.1, 0.0) | 0.10 | 0% | 0.0 (−0.1, 0.0) | 0.09 | 0% |
| Carbohydrate (% of energy) | 0.0 (−0.1, 0.1) | 0.96 | 24% | 0.0 (−0.1, 0.1) | 0.92 | 15% |
| Fat (% of energy) | 0.1 (−0.1, 0.2) | 0.40 | 34% | 0.1 (−0.1, 0.2) | 0.35 | 29% |
Data are β-coefficients (95% CI) per minor allele of FTO rs9939609 or a proxy (r2 = 1) are given for each trait. Analyses from individual studies were conducted separately and then combined by meta-analysis of 16,097 children and adolescents (15,352 whites, 478 African Americans, and 267 Asians). I2 values are also given.
*Adjusted for age, pubertal status (if available), physical activity (if available), region (if available), and eigenvectors (GWAS data only).
†Further adjusted for BMI based on model 1.
FTO Variants and Dietary Intake
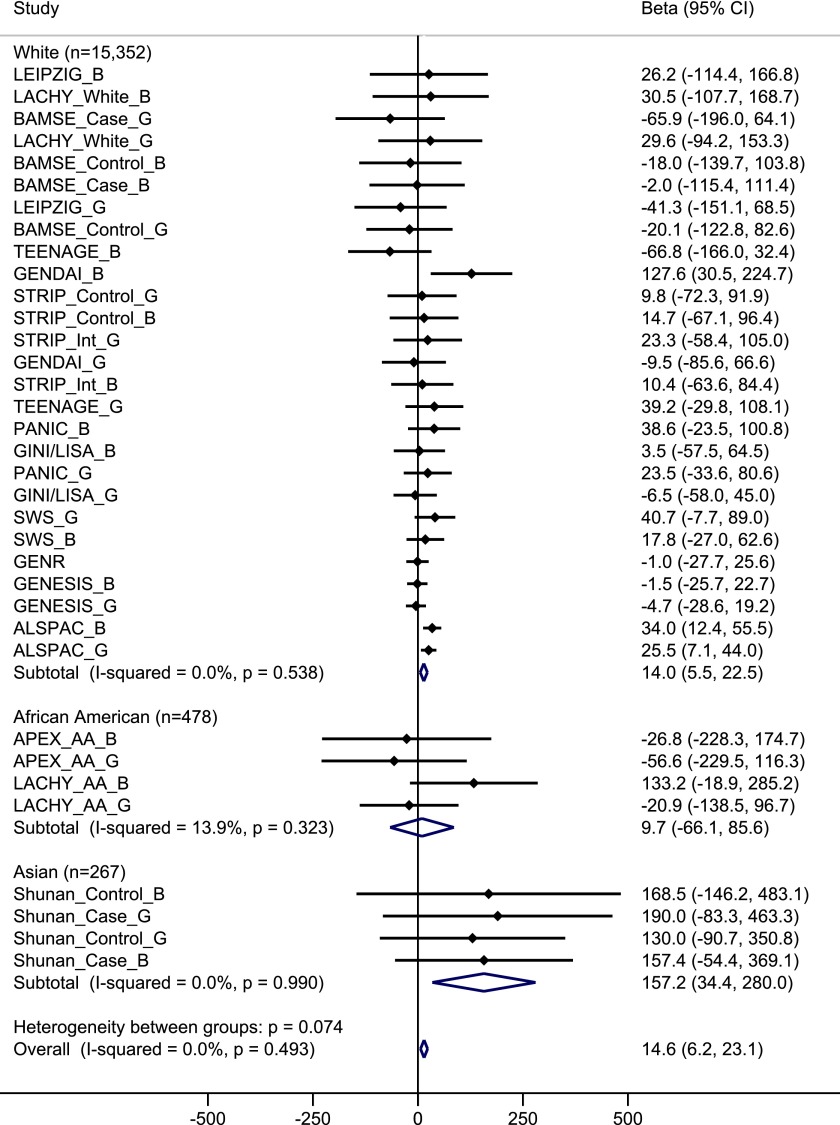
The minor allele of the FTO variant was significantly associated with higher total energy intake in all participants combined (effect per allele = 14.6 kcal/day [6.3, 23.1 kcal/day], P = 6.5 × 10−4), with no heterogeneity among studies (I2 = 0%) (Table 1). This association was unchanged after further adjustment for BMI (effect per allele = 14.7 kcal/day [6.3, 23.1 kcal/day], P = 6.5 × 10−4). The association between FTO variant and total energy intake was found in whites (P = 0.001) and Asians (P = 0.01), but not in African Americans (P = 0.80), although directions of associations were consistent across ethnicities (P for heterogeneity = 0.07) (Fig. 1). In stratified meta-analyses according to sex, age group, geographic region, study design, dietary intake assessment method, and adjustment for physical activity (Supplementary Fig. 1), the directions of the associations between FTO variant and total energy intake were consistent across subgroups. Of note, the association was stronger in studies with a mean age for participants of ≥10 years than in studies with a mean age of <10 years (effect per allele = 25.3 vs. 4.2 kcal/day, P for heterogeneity = 0.014). Since most studies had a mean age for participants of >7.5 years and three studies had a mean age between 1.0 and 3.5 years, we further examined the association between FTO variant and total energy intake according to the following three categories of age: studies with a mean age for participants between 1.0 and 3.5 years (effect per allele = 2.4 kcal/day); studies with a mean age for participants between 7.5 and 10 years (effect per allele = 10.6 kcal/day); and studies with a mean age for participants of ≥10 years old (effect per allele = 25.3 kcal/day).
Figure 1.
Forest plot of the association between FTO SNP rs9939609 and total energy intake in a fixed-effects meta-analysis of 16,097 children and adolescents. The studies are shown in boys (_B), girls (_G), or mixed case patients (_Case) and control subjects (_Control) for case-control studies and whites (_White) and African Americans (_AA) for studies with multiple ethnicities separately, sorted by sample size (smallest to largest). The β represents the difference in total energy intake per minor allele of SNP rs9939609 or a proxy (r2 = 1), adjusted for age, pubertal status (if available), physical activity (if available), region (if available), and eigenvectors (GWAS data only).
We did not find evidence for associations between FTO variant and intake of protein (P = 0.10), carbohydrate (P = 0.96), or fat (P = 0.40), and there was a low or moderate heterogeneity among studies (I2 = 0%, 24%, and 34%, respectively) (Table 1 and Supplementary Figs. 2, 3, and 4). Further adjustment for BMI did not notably change the results.
We also performed meta-analyses for FTO variant and dietary intake using the random-effects method, resulting in similar findings (Supplementary Table 5).
Dietary Intake and BMI
Higher total energy and protein intake were significantly associated with higher BMI (Supplementary Table 6). Difference in BMI between the high and low energy intake groups was 0.04 SD (95% CI 0.01, 0.02 SDs, P = 0.004), and difference in BMI between the high–protein intake and low–protein intake groups was 0.09 SD (0.07, 0.12 SDs, P = 5.0 × 10−10). There was no significant difference in BMI between the high–carbohydrate intake and low–carbohydrate intake groups (difference in BMI = −0.02 SD [−0.05, 0.01 SDs], P = 0.12), and a nominally significant difference in BMI between the high–fat intake and low–fat intake groups (difference in BMI = −0.03 SD [−0.06, −0.001 SDs], P = 0.04).
Interaction Between FTO Variants and Dietary Intake on BMI
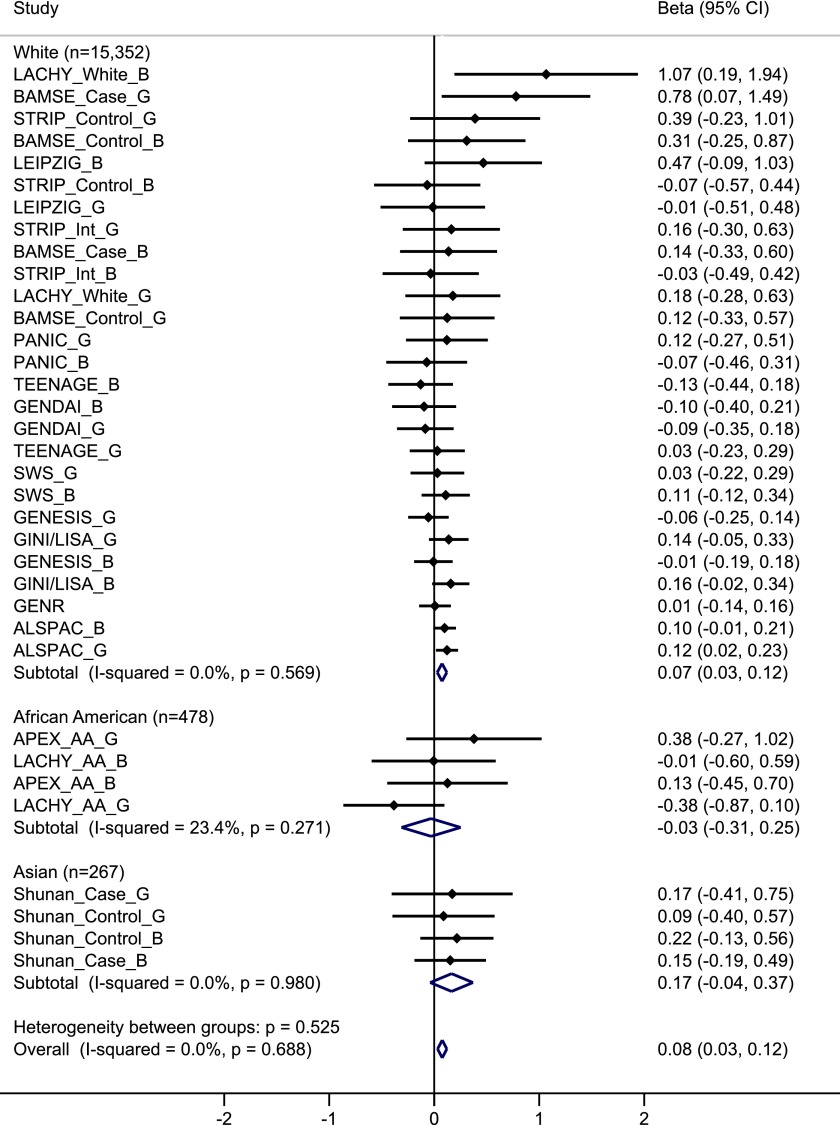
We observed a significant interaction between FTO variant and dietary protein intake on BMI in all participants combined (effect per allele for interaction = 0.08 SD [95% CI 0.03, 0.12 SDs], P for interaction = 7.2 × 10−4), showing that lower protein intake attenuated the association between the FTO variant and BMI, with no heterogeneity among studies (I2 = 0%) (Table 2). In stratified analysis by low–protein intake and high–protein intake groups (dichotomized at medians of protein intake in each study: ranging from 12.9% to 16.8% across studies). The association between FTO variant and BMI among participants in the low–protein intake group (effect per allele = 0.04 SD [95% CI 0.01, 0.07 SDs], P = 0.02) was significantly weaker than that in the high–protein intake group (effect per allele = 0.10 SD [0.07, 0.13 SDs], P = 8.2 × 10−10) (Table 2). Although the interaction was found in whites (P for interaction = 0.001) but not in African Americans (P = 0.84) or Asians (P = 0.11) separately, there was no significant heterogeneity among these ethnic groups (P for heterogeneity = 0.53) (Fig. 2). In stratified meta-analyses (Supplementary Fig. 5), we found similar interaction patterns between FTO variant and protein intake on BMI across subgroups divided by sex, age group, geographic region, study design, dietary intake assessment method, and adjustment for physical activity (all P for heterogeneity > 0.11).
Table 2.
Interaction between FTO SNP rs9939609 and dietary intake on BMI in a fixed-effects meta-analysis of 16,097 children and adolescents
| Association between FTO variant and BMI |
Interaction effect |
|||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | I2 | β (95% CI) | P | I2 | |
| Total energy (kcal/day) | −0.03 (−0.07, 0.02) | 0.20 | 0% | |||
| Low-intake group | 0.08 (0.05, 0.12) | 2.9 × 10−7 | 25% | |||
| High-intake group | 0.05 (0.02, 0.08) | 8.0 × 10−4 | 25% | |||
| Protein (% of total energy intake) | 0.08 (0.03, 0.12) | 7.2 × 10−4 | 0% | |||
| Low-intake group | 0.04 (0.01, 0.07) | 0.02 | 0% | |||
| High-intake group | 0.10 (0.07, 0.13) | 8.2 × 10−10 | 34% | |||
| Carbohydrate (% of total energy intake) | 0.00 (−0.04, 0.04) | 0.98 | 10% | |||
| Low-intake group | 0.08 (0.05, 0.11) | 1.6 × 10−6 | 20% | |||
| High-intake group | 0.07 (0.04, 0.10) | 9.9 × 10−6 | 26% | |||
| Fat (% of total energy intake) | 0.00 (−0.05, 0.05) | 0.89 | 0% | |||
| Low-intake group | 0.08 (0.05, 0.11) | 6.7 × 10−7 | 24% | |||
| High-intake group | 0.07 (0.03, 0.10) | 4.1 × 10−5 | 34% | |||
Data are β-coefficients (95% CI) per minor allele of FTO rs9939609 or a proxy (r2 = 1) for BMI (z score), adjusted for age, pubertal status (if available), physical activity (if available), region (if available), and eigenvectors (GWAS data only). Analyses from individual studies were conducted separately and then combined by meta-analysis of 16,097 children and adolescents (15,352 whites, 478 African Americans, and 267 Asians). I2 values are also given. High- and low-intake groups were defined by medians of each dietary intake in each study. Medians of total energy intake ranged from 1,160 to 2,422 kcal/day, medians of protein intake ranged from 12.9% to 16.8%, medians of carbohydrate intake ranged from 44.2% to 59.0%, and medians of fat intake ranged from 28.0% to 41.0% across studies.
Figure 2.
Forest plot of the interaction between FTO SNP rs9939609 and dietary protein intake on BMI in a fixed-effects meta-analysis of 16,097 children and adolescents. The studies are shown in boys (_B), girls (_G), or mixed case patients (_Case) and control subjects (_Control) for case-control studies and whites (_White) and African Americans (_AA) for studies with multiple ethnicities separately, sorted by sample size (smallest to largest). The β represents the difference in BMI per minor allele of SNP rs9939609 or a proxy (r2 = 1) comparing participants in the high–protein intake group to those in the low–protein intake group, adjusted for age, pubertal status (if available), physical activity (if available), region (if available), and eigenvectors (GWAS data only).
We did not find substantive evidence for interactions between FTO variant and total energy intake (P for interaction = 0.20), carbohydrate intake (P for interaction = 0.98), or fat intake (P for interaction = 0.89) on BMI (Table 2 and Supplementary Figs. 6, 7, and 8). The heterogeneity among studies was low (I2 = 0%, 15%, and 5%, respectively). In analyses stratified by levels of dietary intake, associations between FTO variant and BMI were similar in high- and low-intake groups (Table 2).
In addition, since there was little or no heterogeneity in interactions between FTO variant and dietary intake on BMI across studies, the results were similar when we performed meta-analyses using the random-effects method (Supplementary Table 7).
Discussion
We confirmed the association between an index SNP in the FTO gene, rs9939609 (or its proxy), and BMI in white children and adolescents and in all participants combined, but did not detect significant association in African American or Asian children and adolescents. This might be due to the relatively small sample size used by studies of African Americans or Asians included in the current analysis and/or to different linkage disequilibrium patterns across FTO intron 1 between different ethnic groups, particularly in populations of African ancestry (4,51). Other index SNPs within FTO locus might be needed in future studies of African American children and adolescents.
Although studies of FTO association with dietary intake in adults have been more numerous and often better powered with larger sample sizes than similar studies conducted in children and adolescents, the reported results have been inconsistent (16–20,25–34). Our and other studies even observed an inverse association between FTO variant and total energy intake in adults, which might be partly due to under-reporting of total energy intake among individuals with a higher BMI (19,20,39). In the current analysis, we demonstrated an association between the BMI-increasing allele of the FTO variant and higher total energy intake. However, we did not observe a significant association between FTO variants and percentages of energy derived from protein, which has been observed in adults (39), or other macronutrients.
An apparently stronger, and more consistently reported, effect of FTO on total energy intake in children and adolescents could have several explanations. The influence of social desirability bias and the under-reporting issues are smaller in children than in adults (52–54). It is possible that the effect of FTO variation on appetite may be stronger in children and adolescents than in adults. Consistent with this hypothesis and with the idea that FTO genetic effects might vary over the life course, previous studies (49,55–60) have reported an increasing effect of FTO variants on BMI from early childhood to adolescence, with a subsequently decreasing effect throughout adulthood. Our result is also consistent with this, as we observed a stronger association between FTO variant and total energy intake in studies of older children than in studies of younger children.
Several lines of evidence from animal and in vitro studies are consistent with the observed association between FTO variant and total energy intake in humans. It has been reported that overexpression of Fto in mice led to increased food intake (5), and Fto expression in hypothalamus was regulated by feeding, fasting, and energy restriction (61–67). Further studies showed that glucose and amino acid deprivation decreases Fto expression, suggesting a role of FTO in cellular nutrient sensing (68,69), possibly acting via hypothalamic mammalian target of rapamycin pathways known to regulate food intake (70). A recent study (71) suggested a link among FTO, ghrelin (a key mediator of ingestive behavior), and impaired brain food-cue responsivity in both animals and humans. Interestingly, a recent study (9) has challenged the established view of FTO as the major gene associated with BMI and risk of obesity, reporting that the region of FTO intron 1 harboring the BMI-associated variants are strongly associated with IRX3 gene (500 kbp downstream of FTO intron 1) expression in cerebellar brain samples. However, it has been pointed out that the cerebellum is not primarily involved in food intake or appetite regulation and FTO expression may function in a site-dependent manner (72). In addition, another study (10) suggested that RPGRIP1L, located >100 bp 5′ in the opposite transcriptional orientation of FTO, may be partly or exclusively responsible for the obesity susceptibility signal at the FTO locus.
One novel finding of our study is the interaction between the FTO variant and dietary protein intake on BMI. The effect size of FTO variant on BMI in children with a low–protein intake was much smaller than in children with a high–protein intake, suggesting that low–protein intake may attenuate the influence of FTO variation on BMI. A study of 354 Spanish children and adolescents reported a significant interaction between the FTO-rs9939609 variant and dietary saturated fat intake on BMI (38), and several adult studies also found interactions between the FTO variant and total fat or saturated fat intake on BMI and obesity risk (20,26,34), while no significant interaction between the FTO variant and dietary intake was observed in our meta-analysis of adult data (39). In addition, we previously found that dietary protein intake might modify the effects of FTO variants on changes in body composition, fat distribution, and appetite in a 2-year weight-loss trial (73,74). A recent mouse study (6) showed that loss of Fto gene altered protein utilization and body composition; and consistently, other studies (68,69) also suggest that FTO may influence body composition through cellular sensing of amino acids. Given the increasing evidence supporting the role of FTO in protein metabolism and body composition, future investigations on this topic might help to clarify the mechanisms underlying the observed interaction between the FTO variant and protein intake, and its effect on BMI.
Major strengths of our study include a large sample size of >16,000 children and adolescents from 14 studies, a wide range of studies with data from early childhood to late adolescence, and the standardized analytical plan across studies. There are some limitations in our study. Our analysis was conducted based on cross-sectional data. Measurement errors in dietary assessment are inevitable since self-reported data on dietary intake are all subject to bias. We only included dietary data on total energy and macronutrient intake, but no data on specific foods or more specific types of fatty acids or micronutrients, which may potentially interact with the FTO variant as suggested previously (26,34,38). We were unable to examine other adiposity proxies, but were limited to the consideration of BMI, which cannot distinguish body composition and does not give any indication about body fat distribution. To the best of our knowledge, this is to date the largest analysis of FTO variant and dietary intake in children and adolescents, though more data are needed to further confirm our results. In particular, most of the children and adolescents included in our analysis are individuals of European ancestry (95% of all samples), and it is unknown whether our results can be generalized to other ethnic groups.
In summary, we demonstrated an association between the BMI-increasing allele of FTO variant and total energy intake based on data from 16,094 children and adolescents. Our data also show that dietary protein intake may modify the influence of FTO variants on BMI, offering new insight into the interrelationships between FTO genetic variants, dietary intake, and obesity.
Article Information
Acknowledgments and Funding. There was no specific funding for this project. Funding sources for the individual authors and for the studies included in the analysis are listed as follows. The ALSPAC study was supported by Medical Research Council grant MC_UU_12013/1-9. The APEX project was supported by National Institutes of Health (NIH) grant HL64972. The authors thank Haidong Zhu, Bernard Gutin, Inger S. Stallmann-Jorgensen, and Yanbin Dong (Georgia Prevention Center, Georgia Regents University, Augusta, GA) for their contributions to conducting the study and data collection. The BAMSE study was funded by The Swedish Research Council, The Swedish Heart-Lung Foundation, Stockholm County Council (ALF), and the SFO Epidemiology Program at Karolinska Institutet. The GENDAI study was partially supported by a research grant from Coca-Cola Hellas. The GENR study is being conducted by the Erasmus Medical Center and Erasmus University Rotterdam in close collaboration with the Municipal Health Service Rotterdam area, Rotterdam, and the Stichting Trombosedienst en Artsenlaboratorium Rijnmond, Rotterdam. The authors thank the children and their parents, general practitioners, hospitals, midwives, and pharmacies in Rotterdam for their contribution. The generation and management of GWAS genotype data for the Generation R Study were performed at the Genetic Laboratory of the Department of Internal Medicine, Erasmus Medical Center, the Netherlands. The authors thank Karol Estrada, Dr. Tobias A. Knoch, Anis Abuseiris, Luc V. de Zeeuw, and Rob de Graaf for their help in creating GRIMP, BigGRID, MediGRID, and Services@MediGRID/D-Grid (funded by the German Bundesministerium fuer Forschung und Technology, grants 01 AK 803 A-H, 01 IG 07015 G) for access to their grid computing resources. The authors also thank Mila Jhamai, Manoushka Ganesh, Pascal Arp, Marijn Verkerk, Lizbeth Herrera, and Marjolein Peters for their help in the creation, management, and quality control of the GWAS database. Also, the authors thank Karol Estrada and Carolina Medina-Gomez for their support in the creation and analysis of imputed data. In addition, the authors thank Henriette Moll for her support in calculating the dietary intakes. The Generation R Study receives financial support from the Erasmus University Medical Center, Rotterdam, and The Netherlands Organisation for Health Research and Development (ZonMw). V.W.V.J. received an additional grant from ZonMw (ZonMw VIDI: 016.136.361). Additional support was provided by a grant from the Dutch Kidney Foundation (C08.2251). The GINI study team thanks the following: Helmholtz Zentrum München-German Research Center for Environmental Health, Institute of Epidemiology I, Munich (J. Heinrich, H.E. Wichmann, S. Sausenthaler, C.-M. Chen, E. Thiering, C.M.T. Tiesler, M. Schnappinger, and P. Rzehak); Department of Pediatrics, Marien-Hospital, Wesel (D. Berdel, A. von Berg, C. Beckmann, and I. Gross); Department of Pediatrics, Ludwig Maximilians University, Munich (S. Koletzko, D. Reinhardt, and S. Krauss-Etschmann); Department of Pediatrics, Technical University, Munich (C.P. Bauer, I. Brockow, A. Grübl, and U. Hoffmann); IUF-Leibniz Research Institute for Environmental Medicine, Düsseldorf (U. Krämer, E. Link, and C. Cramer); and the Centre of Allergy & Environment, Technical University, Munich (H. Behrendt). The LISA study team wishes to acknowledge the following: Helmholtz Zentrum München-German Research Center for Environmental Health, Institute of Epidemiology I, Neuherberg (J. Heinrich, H.E. Wichmann, S. Sausenthaler, C.-M. Chen, and C.M.T. Tiesler); University of Leipzig, Department of Pediatrics (M. Borte); Department of Environmental Medicine and Hygiene (O. Herbarth); Department of Pediatrics, Marien Hospital, Wesel (A. von Berg); Bad Honnef (B. Schaaf); UFZ-Centre for Environmental Research Leipzig-Halle, Department of Environmental Immunology (I. Lehmann); IUF-Leibniz Research Institute for Environmental Medicine, Düsseldorf (U. Krämer); and Department of Pediatrics, Technical University, Munich (C.P. Bauer and U. Hoffman). This work was supported financially in part by the “Kompetenznetz Adipositas” (“Competence Network Obesity”), which is funded by the German Federal Ministry of Education and Research (FKZ: 01GI0826) and by the Munich Center of Health Sciences (MCHEALTH). The LACHY project was supported by NIH grant HL64157. The LEIPZIG study was supported by German Research Council (DFG) CRC 1052 “Obesity Mechanisms” C05, the Integrated Research and Treatment Centre (IFB) Adiposity Diseases, the European Commission Seventh Framework Programme (FP7/2007-2013) project Beta-JUDO under grant agreement no. 279153, and the LIFE-Leipzig Research Center for Civilization Diseases, Universität Leipzig, subproject B1 LIFE Child, funded by the European Union, by the European Regional Development Fund (EFRE), and by the Free State of Saxony within the framework of the excellence initiative. The PANIC study was financially supported by grants from the Ministry of Social Affairs and Health of Finland, the Ministry of Education and Culture of Finland, the University of Eastern Finland, the Finnish Innovation Fund Sitra, the Social Insurance Institution of Finland, the Finnish Cultural Foundation, the Juho Vainio Foundation, the Foundation for Paediatric Research, the Paavo Nurmi Foundation, the Paulo Foundation, the Diabetes Research Foundation, the City of Kuopio, the Kuopio University Hospital (EVO-funding no. 5031343), and the Research Committee of the Kuopio University Hospital Catchment Area (the State Research Funding). The authors thank Professor Satoshi Sasaki at the University of Tokyo for dietary assessment and Naoko Okayama of Yamaguchi University for genotyping in the SHUNAN study. The SWS study was supported by the Medical Research Council, the British Heart Foundation, the Food Standards Agency, and Arthritis Research UK. The authors thank the members of the Southampton Women's Survey team, including Cyrus Cooper, Keith Godfrey, and Sian Robinson, and the team of dedicated research nurses and ancillary staff. The authors thank the participants in the Southampton Women's Survey, who gave us so much of their time. The STRIP study was financially supported by the Academy of Finland (grants 206374 and 251360), the Juho Vainio Foundation, the Finnish Foundation for Cardiovascular Research, the Finnish Cultural Foundation, the Ministry of Education and Culture of Finland, the Sigrid Juselius Foundation, the Yrjö Jahnsson Foundation, the C.G. Sundell Foundation, Special Governmental Grants for Health Sciences Research, Turku University Hospital, the Foundation for Pediatric Research, and the Turku University Foundation. The TEENAGE study has been cofinanced by the European Union (European Social Fund [ESF]) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)-Research Funding Program: Heracleitus II. Investing in Knowledge Society Through the European Social Fund. This work was funded by the Wellcome Trust (grant 098051). The authors thank all study participants and their families, as well as all volunteers for their contribution to this study. T.O.K. was supported by grant no. DFF-1333-00124 from the Danish Council for Independent Research. V.B. is supported by the Unity Through Knowledge Fund CONNECTIVITY PROGRAM (“Gaining Experience” grant 2A) and the National Foundation for Science, Higher Education and Technological Development of the Republic of Croatia (BRAIN GAIN-Postdoctoral fellowship).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Q.Q. designed the study, researched data, and wrote the manuscript. M.K.D., T.O.K., H.R.T., S.J.B., I.N., M.S., V.B., V.H., J.C.K.-d.J., A.K., T.A.L., G.L., J.M., M.O., O.R., R.R., R.A.S., M.E.S.B., K.S., J.W.H., H.I., V.W.V.J., J.L., V.L., E.M., Y.P., N.P., H.S., J.H., N.J.T., T.W., H.Y., E.Z., and G.V.D. researched data and edited and reviewed the manuscript. C.R.I., Y.M.-R., R.C.K., J.W.-R., R.J.F.L., and F.B.H. contributed to discussion and edited and reviewed the manuscript. L.Q. designed the study, contributed to discussion, and edited and reviewed the manuscript. Q.Q. and L.Q. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-1629/-/DC1.
References
- 1.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 2007;3:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 2007;39:724–726 [DOI] [PubMed] [Google Scholar]
- 4.Loos RJ, Yeo GS. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol 2014;10:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church C, Moir L, McMurray F, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet 2010;42:1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMurray F, Church CD, Larder R, et al. Adult onset global loss of the fto gene alters body composition and metabolism in the mouse. PLoS Genet 2013;9:e1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer J, Koch L, Emmerling C, et al. Inactivation of the Fto gene protects from obesity. Nature 2009;458:894–898 [DOI] [PubMed] [Google Scholar]
- 8.Church C, Lee S, Bagg EA, et al. A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet 2009;5:e1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smemo S, Tena JJ, Kim KH, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 2014;507:371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stratigopoulos G, Martin Carli JF, O’Day DR, et al. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab 2014;19:767–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med 2008;359:2558–2566 [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Kim IK, Kang JH, et al. Effects of common FTO gene variants associated with BMI on dietary intake and physical activity in Koreans. Clin Chim Acta 2010;411:1716–1722 [DOI] [PubMed] [Google Scholar]
- 13.Tanofsky-Kraff M, Han JC, Anandalingam K, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am J Clin Nutr 2009;90:1483–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timpson NJ, Emmett PM, Frayling TM, et al. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr 2008;88:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obes (Lond) 2009;33:42–45 [DOI] [PubMed] [Google Scholar]
- 16.Brunkwall L, Ericson U, Hellstrand S, Gullberg B, Orho-Melander M, Sonestedt E. Genetic variation in the fat mass and obesity-associated gene (FTO) in association with food preferences in healthy adults. Food Nutr Res 2013;57:20028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haupt A, Thamer C, Staiger H, et al. Variation in the FTO gene influences food intake but not energy expenditure. Exp Clin Endocrinol Diabetes 2009;117:194–197 [DOI] [PubMed] [Google Scholar]
- 18.McCaffery JM, Papandonatos GD, Peter I, et al.; Genetic Subgroup of Look AHEAD; Look AHEAD Research Group . Obesity susceptibility loci and dietary intake in the Look AHEAD Trial. Am J Clin Nutr 2012;95:1477–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SL, Cheng I, Pendergrass SA, et al. Association of the FTO obesity risk variant rs8050136 with percentage of energy intake from fat in multiple racial/ethnic populations: the PAGE study. Am J Epidemiol 2013;178:780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfält E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am J Clin Nutr 2009;90:1418–1425 [DOI] [PubMed] [Google Scholar]
- 21.da Silva CF, Zandoná MR, Vitolo MR, et al. Association between a frequent variant of the FTO gene and anthropometric phenotypes in Brazilian children. BMC Med Genet 2013;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakanen M, Raitakari OT, Lehtimäki T, et al. FTO genotype is associated with body mass index after the age of seven years but not with energy intake or leisure-time physical activity. J Clin Endocrinol Metab 2009;94:1281–1287 [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Zhu H, Lagou V, et al. FTO variant rs9939609 is associated with body mass index and waist circumference, but not with energy intake or physical activity in European- and African-American youth. BMC Med Genet 2010;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuda M, Hinoda Y, Okayama N, et al. Association between the FTO gene and overweight in Japanese children and adolescents. Pediatr Diabetes 2011;12:494–500 [DOI] [PubMed] [Google Scholar]
- 25.Bauer F, Elbers CC, Adan RA, et al. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr 2009;90:951–959 [DOI] [PubMed] [Google Scholar]
- 26.Corella D, Arnett DK, Tucker KL, et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J Nutr 2011;141:2219–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Do R, Bailey SD, Desbiens K, et al. Genetic variants of FTO influence adiposity, insulin sensitivity, leptin levels, and resting metabolic rate in the Quebec Family Study. Diabetes 2008;57:1147–1150 [DOI] [PubMed] [Google Scholar]
- 28.Hasselbalch AL, Angquist L, Christiansen L, Heitmann BL, Kyvik KO, Sørensen TI. A variant in the fat mass and obesity-associated gene (FTO) and variants near the melanocortin-4 receptor gene (MC4R) do not influence dietary intake. J Nutr 2010;140:831–834 [DOI] [PubMed] [Google Scholar]
- 29.Hubacek JA, Pikhart H, Peasey A, Kubinova R, Bobak M. FTO variant, energy intake, physical activity and basal metabolic rate in Caucasians. The HAPIEE study. Physiol Res 2011;60:175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonassaint CR, Szatkiewicz JP, Bulik CM, et al. Absence of association between specific common variants of the obesity-related FTO gene and psychological and behavioral eating disorder phenotypes. Am J Med Genet B Neuropsychiatr Genet 2011;156B:454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karasawa S, Daimon M, Sasaki S, et al. Association of the common fat mass and obesity associated (FTO) gene polymorphism with obesity in a Japanese population. Endocr J 2010;57:293–301 [DOI] [PubMed] [Google Scholar]
- 32.Lappalainen T, Lindström J, Paananen J, et al. Association of the fat mass and obesity-associated (FTO) gene variant (rs9939609) with dietary intake in the Finnish Diabetes Prevention Study. Br J Nutr 2012;108:1859–1865 [DOI] [PubMed] [Google Scholar]
- 33.Lear SA, Deng WQ, Paré G, Sulistyoningrum DC, Loos RJ, Devlin A. Associations of the FTO rs9939609 variant with discrete body fat depots and dietary intake in a multi-ethnic cohort. Genet Res 2011;93:419–426 [DOI] [PubMed] [Google Scholar]
- 34.Phillips CM, Kesse-Guyot E, McManus R, et al. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity-associated gene in adults. J Nutr 2012;142:824–831 [DOI] [PubMed] [Google Scholar]
- 35.Scott RA, Bailey ME, Moran CN, et al. FTO genotype and adiposity in children: physical activity levels influence the effect of the risk genotype in adolescent males. Eur J Hum Genet 2010;18:1339–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kilpeläinen TO, Qi L, Brage S, et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med 2011;8:e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corella D, Ortega-Azorín C, Sorlí JV, et al. Statistical and biological gene-lifestyle interactions of MC4R and FTO with diet and physical activity on obesity: new effects on alcohol consumption. PLoS ONE 2012;7:e52344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moleres A, Ochoa MC, Rendo-Urteaga T, et al.; GENOI . Dietary fatty acid distribution modifies obesity risk linked to the rs9939609 polymorphism of the fat mass and obesity-associated gene in a Spanish case-control study of children. Br J Nutr 2012;107:533–538 [DOI] [PubMed] [Google Scholar]
- 39.Qi Q, Kilpeläinen TO, Downer MK, et al. FTO genetic variants, dietary intake and body mass index: insights from 177,330 individuals. Hum Mol Genet 2014;23:6961–6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simell O, Niinikoski H, Rönnemaa T, et al. STRIP Study Group . Cohort profile: the STRIP Study (Special Turku Coronary Risk Factor Intervention Project), an Infancy-onset Dietary and Life-style Intervention Trial. Int J Epidemiol 2009;38:650–655 [DOI] [PubMed] [Google Scholar]
- 41.Papoutsakis C, Vidra NV, Hatzopoulou I, et al. The Gene-Diet Attica investigation on childhood obesity (GENDAI): overview of the study design. Clin Chem Lab Med 2007;45:309–315 [DOI] [PubMed] [Google Scholar]
- 42.Ntalla I, Giannakopoulou M, Vlachou P, et al. Body composition and eating behaviours in relation to dieting involvement in a sample of urban Greek adolescents from the TEENAGE (TEENs of Attica: Genes & Environment) study. Public Health Nutr 2014;17:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manios Y. Design and descriptive results of the “Growth, Exercise and Nutrition Epidemiological Study In preSchoolers”: the GENESIS study. BMC Public Health 2006;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Körner A, Berndt J, Stumvoll M, Kiess W, Kovacs P. TCF7L2 gene polymorphisms confer an increased risk for early impairment of glucose metabolism and increased height in obese children. J Clin Endocrinol Metab 2007;92:1956–1960 [DOI] [PubMed] [Google Scholar]
- 45.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C; SWS Study Group . Cohort profile: The Southampton Women’s Survey. Int J Epidemiol 2006;35:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eloranta AM, Lindi V, Schwab U, et al. Dietary factors and their associations with socioeconomic background in Finnish girls and boys 6-8 years of age: the PANIC Study. Eur J Clin Nutr 2011;65:1211–1218 [DOI] [PubMed] [Google Scholar]
- 47.Berg A, Kramer U, Link E, et al.; GINIplus Study Group . Impact of early feeding on childhood eczema: development after nutritional intervention compared with the natural course—the GINIplus study up to the age of 6 years. Clin Exp Allergy 2010;40:627–636 [DOI] [PubMed] [Google Scholar]
- 48.Jaddoe VW, van Duijn CM, Franco OH, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol 2012;27:739–756 [DOI] [PubMed] [Google Scholar]
- 49.Rzehak P, Scherag A, Grallert H, et al.; GINI and LISA Study Group . Associations between BMI and the FTO gene are age dependent: results from the GINI and LISA birth cohort studies up to age 6 years. Obes Facts 2010;3:173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melen E, Granell R, Kogevinas M, et al. Genome-wide association study of body mass index in 23 000 individuals with and without asthma. Clin Exp Allergy 2013;43:463–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monda KL, Chen GK, Taylor KC, et al.; NABEC Consortium; UKBEC Consortium; BioBank Japan Project; AGEN Consortium . A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nat Genet 2013;45:690–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ventura AK, Loken E, Mitchell DC, Smiciklas-Wright H, Birch LL. Understanding reporting bias in the dietary recall data of 11-year-old girls. Obesity (Silver Spring) 2006;14:1073–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansson L, Solvoll K, Bjørneboe GE, Drevon CA. Under- and overreporting of energy intake related to weight status and lifestyle in a nationwide sample. Am J Clin Nutr 1998;68:266–274 [DOI] [PubMed] [Google Scholar]
- 54.Hebert JR, Ma Y, Clemow L, et al. Gender differences in social desirability and social approval bias in dietary self-report. Am J Epidemiol 1997;146:1046–1055 [DOI] [PubMed] [Google Scholar]
- 55.Haworth CM, Carnell S, Meaburn EL, Davis OS, Plomin R, Wardle J. Increasing heritability of BMI and stronger associations with the FTO gene over childhood. Obesity (Silver Spring) 2008;16:2663–2668 [DOI] [PubMed] [Google Scholar]
- 56.Hardy R, Wills AK, Wong A, et al. Life course variations in the associations between FTO and MC4R gene variants and body size. Hum Mol Genet 2010;19:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cauchi S, Stutzmann F, Cavalcanti-Proença C, et al. Combined effects of MC4R and FTO common genetic variants on obesity in European general populations. J Mol Med (Berl) 2009;87:537–546 [DOI] [PubMed] [Google Scholar]
- 58.Jess T, Zimmermann E, Kring SII, et al. Impact on weight dynamics and general growth of the common FTO rs9939609: a longitudinal Danish cohort study. Int J Obes (Lond) 2008;32:1388–1394 [DOI] [PubMed] [Google Scholar]
- 59.Qi L, Kang K, Zhang C, et al. Fat mass-and obesity-associated (FTO) gene variant is associated with obesity: longitudinal analyses in two cohort studies and functional test. Diabetes 2008;57:3145–3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sovio U, Mook-Kanamori DO, Warrington NM, et al.; Early Growth Genetics Consortium . Association between common variation at the FTO locus and changes in body mass index from infancy to late childhood: the complex nature of genetic association through growth and development. PLoS Genet 2011;7:e1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerken T, Girard CA, Tung YC, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007;318:1469–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stratigopoulos G, Padilla SL, LeDuc CA, et al. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol 2008;294:R1185–R1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fredriksson R, Hägglund M, Olszewski PK, et al. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology 2008;149:2062–2071 [DOI] [PubMed] [Google Scholar]
- 64.Olszewski PK, Fredriksson R, Olszewska AM, et al. Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC Neurosci 2009;10:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang P, Yang FJ, Du H, et al. Involvement of leptin receptor long isoform (LepRb)-STAT3 signaling pathway in brain fat mass- and obesity-associated (FTO) downregulation during energy restriction. Mol Med 2011;17:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boender AJ, van Rozen AJ, Adan RA. Nutritional state affects the expression of the obesity-associated genes Etv5, Faim2, Fto, and Negr1. Obesity (Silver Spring) 2012;20:2420–2425 [DOI] [PubMed] [Google Scholar]
- 67.Tung YC, Ayuso E, Shan X, et al. Hypothalamic-specific manipulation of Fto, the ortholog of the human obesity gene FTO, affects food intake in rats. PLoS One 2010;5:e8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheung MK, Gulati P, O’Rahilly S, Yeo GS. FTO expression is regulated by availability of essential amino acids. Int J Obes (Lond) 2013;37:744–747 [DOI] [PubMed] [Google Scholar]
- 69.Gulati P, Cheung MK, Antrobus R, et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc Natl Acad Sci U S A 2013;110:2557–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cota D, Proulx K, Smith KA, et al. Hypothalamic mTOR signaling regulates food intake. Science 2006;312:927–930 [DOI] [PubMed] [Google Scholar]
- 71.Karra E, O’Daly OG, Choudhury AI, et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest 2013;123:3539–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cedernaes J, Benedict C. Human obesity: FTO, IRX3, or both? Mol Metab 2014;3:505–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang T, Qi Q, Li Y, et al. FTO genotype, dietary protein, and change in appetite: the Preventing Overweight Using Novel Dietary Strategies trial. Am J Clin Nutr 2014;99:1126–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang X, Qi Q, Zhang C, et al. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: the POUNDS LOST Trial. Diabetes 2012;61:3005–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]