Abstract
Cystic fibrosis (CF) is the result of mutations in the cystic fibrosis transmembrane conductance regulator (CFTR). CF-related diabetes affects 50% of adult CF patients. How CFTR deficiency predisposes to diabetes is unknown. Herein, we examined the impact of the most frequent cftr mutation in humans, deletion of phenylalanine at position 508 (ΔF508), on glucose homeostasis in mice. We compared ΔF508 mutant mice with wild-type (WT) littermates. Twelve-week-old male ΔF508 mutants had lower body weight, improved oral glucose tolerance, and a trend toward higher insulin tolerance. Glucose-induced insulin secretion was slightly diminished in ΔF508 mutant islets, due to reduced insulin content, but ΔF508 mutant islets were not more sensitive to proinflammatory cytokines than WT islets. Hyperglycemic clamps confirmed an increase in insulin sensitivity with normal β-cell function in 12- and 18-week-old ΔF508 mutants. In contrast, 24-week-old ΔF508 mutants exhibited insulin resistance and reduced β-cell function. β-Cell mass was unaffected at 11 weeks of age but was significantly lower in ΔF508 mutants versus controls at 24 weeks. This was not associated with gross pancreatic pathology. We conclude that the ΔF508 CFTR mutation does not lead to an intrinsic β-cell secretory defect but is associated with insulin resistance and a β-cell mass deficit in aging mutants.
Introduction
Cystic fibrosis (CF) is the most frequent autosomal recessive disorder in the Caucasian population. It results from loss-of-function mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). Major improvements in the treatment of CF in the last decades have led to a remarkable increase in life expectancy of the patients, from 14 years in the 1980s to >37 years today (1). This is associated with increased prevalence of complications and comorbidities, such as CF-related diabetes (CFRD), which affects 50% of adult CF patients (1). Clinically, CFRD shares features of both types of diabetes, and gene variants associated with type 1 (2) and type 2 (3) diabetes increase the risk of CFRD. CFRD is considered its own clinical entity (4) and is believed to result primarily from defective insulin secretion from the pancreatic β-cell (5–12) with a secondary, aggravating effect of insulin resistance (5,13–15) both in the liver (16,17) and in peripheral tissues (14,18). Thus, the β-cell plays a key role in the pathogenesis of CFRD, yet surprisingly little is known regarding the mechanisms underlying its functional defect in CF. CFTR is expressed in islets including β-cells (19), but its functional importance in this tissue is unclear. Postmortem examination of pancreata from CF patients has suggested that islet dysfunction might be secondary to fibrosis and fatty infiltration (20,21) or amyloid deposits (22); however, islets from CF patients who develop diabetes are not more damaged than those who remain normoglycemic (23), and CF children exhibit impaired insulin secretion independent of pancreatic exocrine deficiency (24). These studies are in agreement with several observations in preclinical models suggesting that a primary, modest impairment of β-cell function remains clinically silent initially and becomes more severe as systemic inflammation develops and the disease progresses (25). Accordingly, cftr-null mice are more susceptible to streptozotocin-induced diabetes (26), and cftr-null ferrets display an early β-cell secretory defect that is already present at birth and precedes overt pancreas pathology (27). Finally, recent studies suggest a role of CFTR in the regulation of insulin secretion (28,29). Together, these findings indicate that defective CFTR function might affect pancreatic β-cell function.
Although most preclinical studies on the mechanisms of CFRD used cftr-null models, the most frequent cftr mutation, which affects ∼70% of CF patients, is a deletion of phenylalanine at position 508 (ΔF508) resulting in misfolding and altered intracellular trafficking of the protein (30). This in turn results in endoplasmic reticulum (ER) stress (31) which, given the high susceptibility of pancreatic β-cells to ER stress, has been proposed as a possible cause of the insulin secretory defect (32). Thus, the impact of the ΔF508 mutation on the β-cell is likely different from that of complete deletion of the protein. Elucidating the impact of the ΔF508 mutation on β-cell function has important clinical implications. However, to our knowledge ΔF508 mutant mice have not been characterized with respect to glucose homeostasis. In this study, we tested the hypothesis that the ΔF508 mutation alters glucose homeostasis in an age-dependent manner. To this aim, we systematically examined insulin secretion and sensitivity in ΔF508 mutant mice. Specifically, we asked the following questions: 1) Is the ΔF508 mutation associated with impaired glucose tolerance and defective insulin secretion and/or insulin sensitivity in mice? 2) Do islets from ΔF508 mutant mice have impaired insulin secretion under normal or proinflammatory conditions? And 3) Do β-cell function and mass and insulin sensitivity decline with age in ΔF508 mutant mice?
Research Design and Methods
Animals and Diet
All procedures using animals were approved by the Institutional Committee for the Protection of Animals at Centre Hospitalier de l’Université de Montréal. Mice heterozygote (HET) for the ΔF508 mutation (cftrWT/ΔF508) backcrossed onto the FVB genetic background for >12 generations were kindly provided by Dr. B.J. Scholte (Erasmus University, Rotterdam, the Netherlands [33]) and bred to generate homozygous (ΔF508) mutants (cftrΔF508/ΔF508) and wild-type (WT) littermates (cftrWT/WT). ΔF508 mutants were genotyped as previously described (33). Male and female mice were housed under controlled temperature (21°C) on a 12-h light-dark cycle with unrestricted access to food and water. For prevention of intestinal obstruction, CF mice were fed a high-protein diet. In a pilot study, we compared male and female WT mice fed standard chow versus a high-protein diet (59% carbohydrates, 26% protein, and 15% fat on a caloric basis; Research Diets, Inc., New Brunswick, NJ) (Supplementary Fig. 1). There was no effect of the diet on growth curves (Supplementary Fig. 1A and B), fat mass (Supplementary Fig. 1C and D), or fed or fasted blood glucose levels (Supplementary Fig. 1E and F) in either male or female mice. There was a tendency for glucose intolerance in mice fed a diet high in protein (Supplementary Fig. 1G and H), although the difference only reached statistical significance at the 60-min time point in female mice. For the remainder of the study, both WT and ΔF508 mutant mice were fed the high-protein diet.
Body Weight, Body Fat, and Metabolic Parameters
Body weight of male and female mice was determined weekly from weaning to 14 weeks of age. The percentage of body fat in 11-week-old fed WT, HET, and ΔF508 mice was assessed using an EchoMRI-700 (Echo Medical Systems, Houston, TX). Respiratory Exchange Ratio (RER), heat production, food and water consumption, and locomotor activity were determined in 12-week-old WT and ΔF508 mice using a Comprehensive Laboratory Animal Monitoring System (CLAMS; Colombus Instruments, Columbus, OH). Body size, fasting blood glucose, plasma insulin and glucagon, triglycerides (TG), and total cholesterol were determined at 14 and 24 weeks of age.
Mouse Islet Isolation and Assessment of Insulin Secretion Ex Vivo
Mouse islets were isolated as previously described (34), recovered overnight at 11.1 mmol/L glucose, and then cultured for 24 h in various experimental conditions as described in results. Insulin secretion was assessed in 1-h static incubations. Triplicate batches of 10 islets each were washed twice in Krebs-Ringer buffer containing 0.1% BSA and 2.8 mmol/L glucose for 20 min at 37°C and then incubated for 1 h at 37°C in either 2.8 or 16.7 mmol/L glucose. Secreted insulin was measured in the supernatant by radioimmunoassay (Millipore Corporation, Billerica, MA), and intracellular insulin content was determined after acidified ethanol extraction.
Glucose and Insulin Tolerance Tests and Hyperglycemic Clamps
Oral glucose tolerance tests (OGTTs) were performed in overnight-fasted mice at 10 weeks of age by administration of 2 g/kg glucose by gavage. Insulin tolerance was assessed in 5-h-fasted animals after administration of 0.6 units/kg i.p. human insulin at 12 weeks of age. Insulin secretion in vivo was measured using hyperglycemic clamps. Mice underwent catheterization of the right jugular vein under general anesthesia. After a 5-day recovery, conscious mice were subjected to one-step hyperglycemic clamps. A 20% dextrose solution (McKesson Canada, Montreal, QC, Canada) was infused to clamp blood glucose at ∼22 mmol/L for 60–80 min (ACCU-CHEK; Roche, Indianapolis, IN). Plasma samples were collected from the tail for measurements of insulin and C-peptide using mouse ELISA kits (Alpco Diagnostics, Salem, NH). The insulin sensitivity index (M/I) was calculated as the glucose infusion rate (M) divided by the average insulinemia during the last 30 min of the clamp (I). The disposition index (DI), an index of β-cell function taking into account the prevailing level of insulin sensitivity developed in humans (35) and validated in rodents (36), was calculated by multiplying M/I by C-peptide levels during the last 30 min of the clamp.
Analysis of β-Cell Mass and Pancreas Histology
Pancreata were trimmed of fat, weighed, fixed in 10% buffered formalin (vol/vol), and embedded in paraffin. Paraffin sections (5 μm) including the head, neck, and tail of the pancreas were made, and every 10th section, for a total of 6, was mounted on glass slides for immunohistochemical and β-cell mass analyses after insulin immunostaining with guinea pig anti-insulin IgG (DAKO) and hematoxylin counterstaining as previously described (37). The β-cell surface and whole pancreas areas were determined using ImageJ software (National Institutes of Health), and β-cell mass was calculated by multiplying the relative β-cell surface area by the weight of pancreas. Pancreatic histology was analyzed by light microscopy after hematoxylin phloxine saffron (HPS) staining of paraffin sections.
Analytical Measurements
Blood glucose was assessed using a handheld glucometer, nonesterified fatty acid levels were assessed using the Wako NEFA C kit (Wako Chemical, Osaka, Japan), and glucagon was assessed by ELISA (Alpco Diagnostics), TG by the GPO Trinder kit (Sigma Aldrich, Saint Louis, MO), and total cholesterol with the Amplex red assay kit (Molecular Probes, Eugene, OR). Plasma levels of an array of inflammatory cytokines were determined using the Mouse Inflammatory Cytokines Multi-Analyte ELISArray kit (Qiagen, Hilden, Germany).
Statistical Analysis
Data are expressed as means ± SEM and were analyzed by Student t test or ANOVA followed by two-by-two comparisons with Bonferroni post hoc adjustments, as appropriate, using GraphPad Instat (GraphPad Software, San Diego, CA). P < 0.05 was considered significant.
Results
Energy Metabolism in ΔF508 Mutant Mice
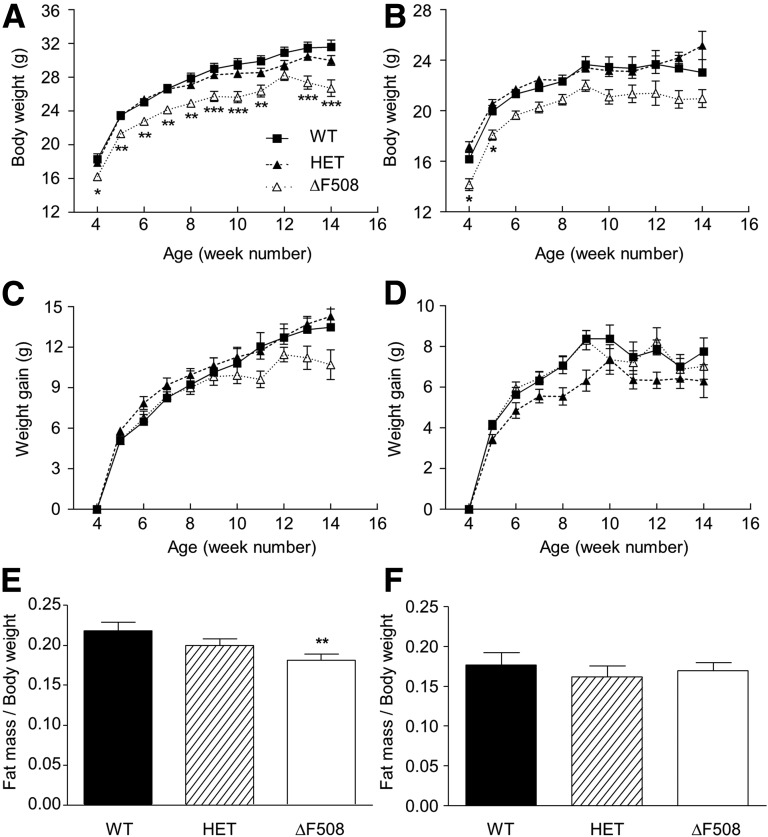
Body weight was lower in both male (Fig. 1A) and female (Fig. 1B) ΔF508 mice compared with WT and HET controls from the beginning of the study (4 weeks of age), and this difference was maintained until 14 weeks of age (P < 0.001). There was a nonsignificant trend in ΔF508 males (Fig. 1C) but not females (Fig. 1D) to gain less weight than controls. The percentage of fat mass was lower in male (Fig. 1E) but not female (Fig. 1F) ΔF508 mice. Body weight and fat mass in HETs were indistinguishable from the WT (Fig. 1). Body length was similar in all groups (data not shown). RER, heat production, food intake, and water consumption were similar between 12-week-old WT and ΔF508 male mice (Supplementary Fig. 2A–D). Male ΔF508 mice exhibited a significant reduction in locomotor activity (Supplementary Fig. 2E).
Figure 1.
Body weight and fat mass in ΔF508 mutant mice. A–D: Body weight (A and B) and weight gain (C and D) were measured from 4 to 14 weeks of age in male (A and C) and female (B and D) WT, HET, and ΔF508 mice. Data are expressed as mean ± SEM of 7–15 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT. E and F: Fat mass content relative to body weight was assessed by EchoMRI in 11-week-old WT, HET, and ΔF508 male (E) and female (F) mice. Data are mean ± SEM of 9–20 mice per group. **P < 0.01 vs. WT.
Insulin Secretion Under Normal and Proinflammatory Conditions in Islets From ΔF508 Mutant Mice
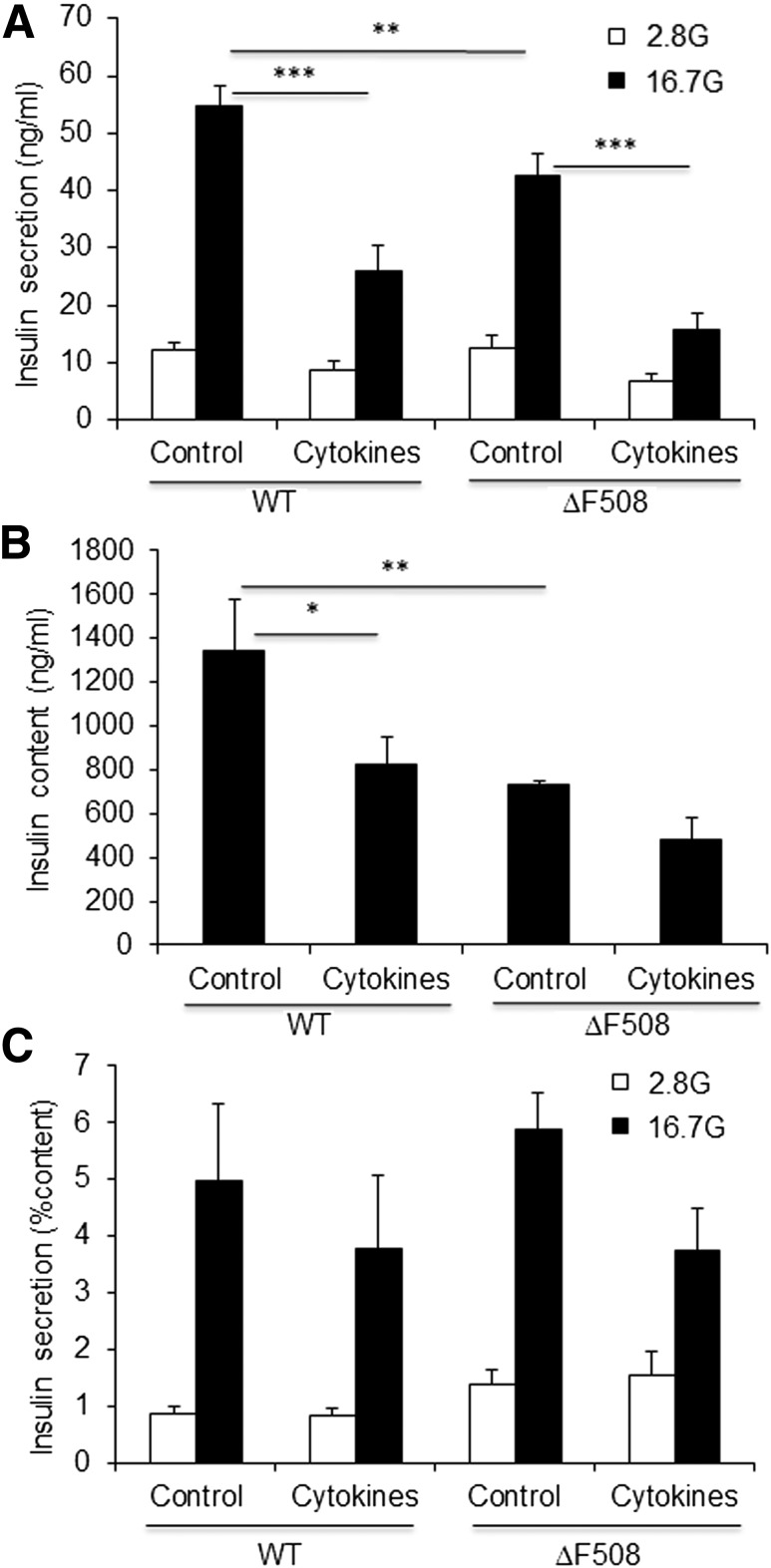
Recent studies suggest that CFTR in β-cells is implicated in glucose-induced electrical activities governing insulin secretion (28) and the potentiating effect of cAMP (29). Furthermore, insulin secretion is compromised in islets from CFTR-null ferrets (27). Insulin secretion in response to glucose was assessed ex vivo in isolated islets from 14-week-old WT and ΔF508 mice (Fig. 2). Basal insulin secretion was similar, but glucose-induced insulin secretion was slightly but significantly reduced in islets from ΔF508 mice (Fig. 2A). This was associated with a reduction in intracellular insulin content (Fig. 2B), such that when expressed as a percentage of insulin content, glucose-induced insulin secretion was not significantly different between WT and ΔF508 mutant islets (Fig. 2C). These data suggest that under normal conditions, the mild insulin secretory defect observed in ΔF508 mutant islets is due to a decrease in insulin content.
Figure 2.
Insulin secretion and content under normal and proinflammatory conditions in ΔF508 mutant islets. Insulin secretion (A), insulin content (B), and insulin secretion normalized by insulin content (C) as assessed in 1-h static incubations in isolated islets from 14-week-old ΔF508 mutant and WT mice in response to 2.8 or 16.7 mmol/L glucose (G) after a 24-h exposure to 16.7 mmol/L glucose in the presence or absence of 1 ng/mL interleukin-1β plus 5 ng/mL interferon-γ. Data are mean ± SEM of 5 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
The CFTR mutation in mice is associated with mild pancreatic insufficiency and inflammation (38,39). Further, the ΔF508 CFTR mutation leads to ER stress (31). Therefore, it is possible that the ΔF508 mutation renders β-cells more susceptible to inflammatory stress and induces a functional defect that is only revealed under proinflammatory conditions, normally not seen in mice bred in a highly controlled environment. For examination of this possibility, insulin secretion in response to glucose was assessed ex vivo in isolated islets from 14-week-old WT and ΔF508 mice after a 24-h exposure to proinflammatory cytokines (interleukin-1β plus interferon-γ [1 and 5 ng/mL, respectively]) (Fig. 2). As expected, exposure to proinflammatory cytokines led to a significant reduction in glucose-induced insulin secretion (Fig. 2A) and intracellular insulin content (Fig. 2B) in WT islets. The inhibitory effect of proinflammatory cytokines was also observed, but not more pronounced, in ΔF508 mutant islets (38 ± 6% reduction vs. 47 ± 7% reduction in WT islets; n = 5; nonsignificant). Altogether, these data suggest that the ΔF508 mutation in mice is not associated with an intrinsic β-cell secretory defect under normal or proinflammatory conditions.
Glucose Homeostasis in ΔF508 Mutant Mice
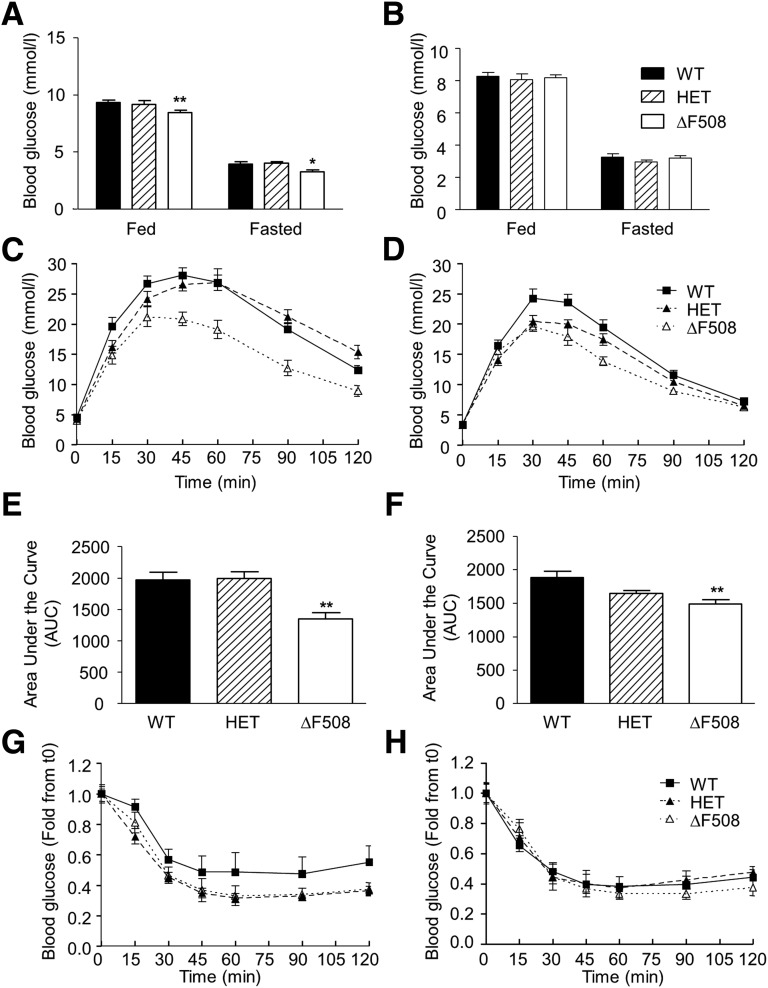
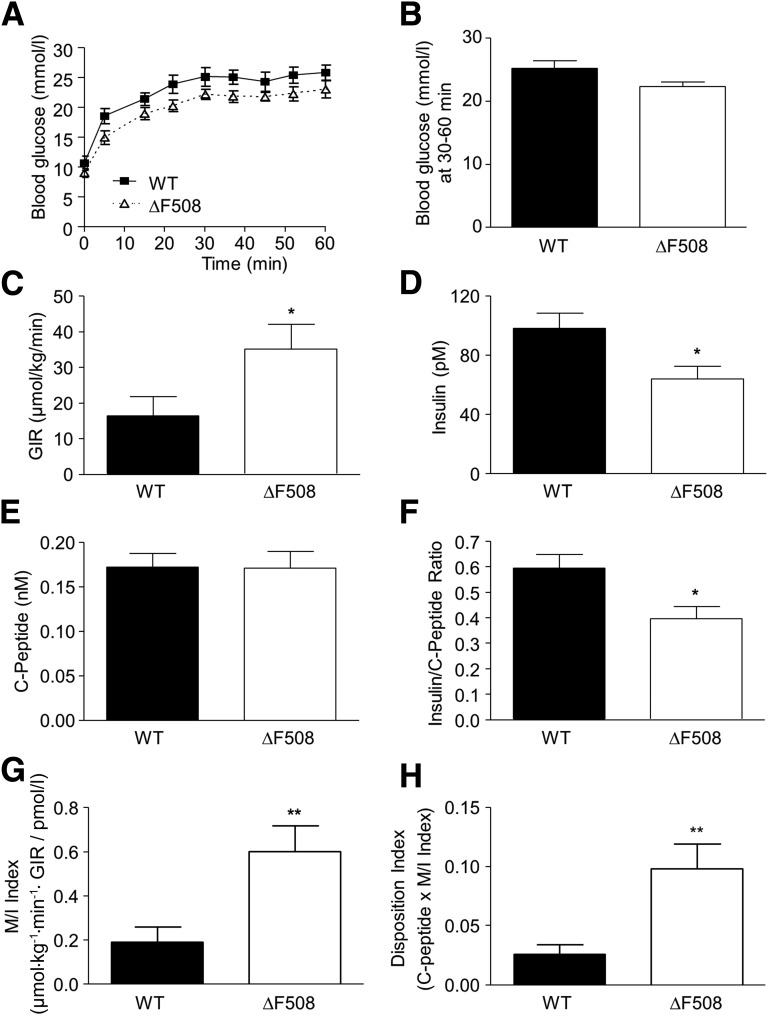
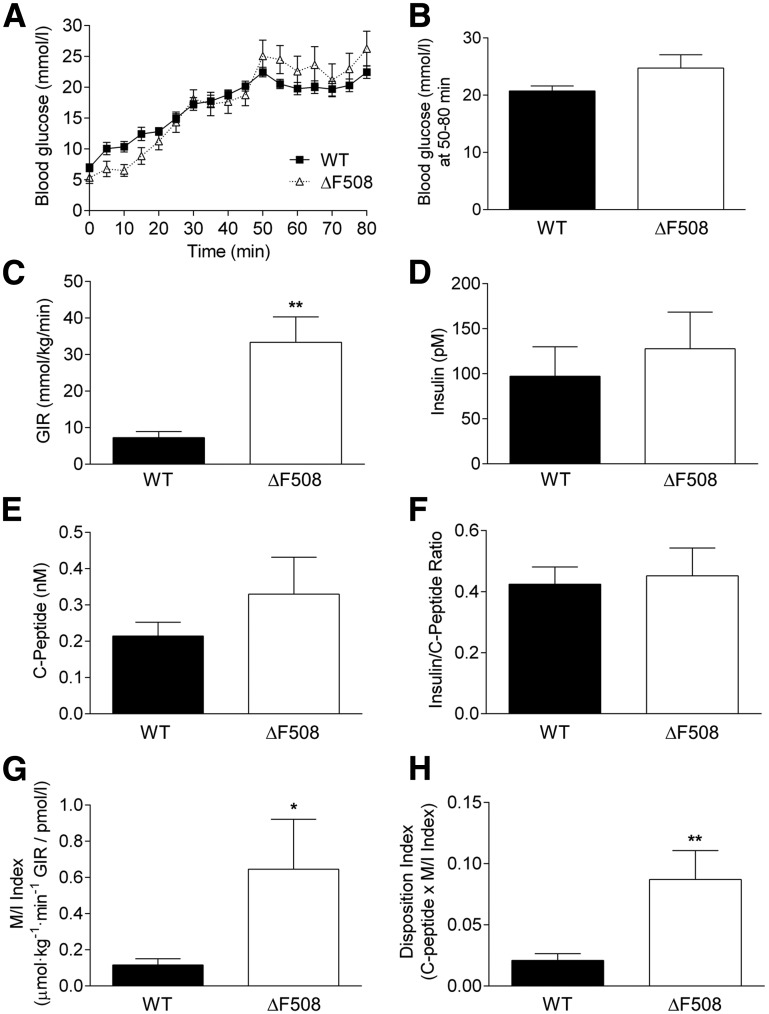
Fed and fasting blood glucose levels were lower in 10- to 13-week old male (Fig. 3A) but not female (Fig. 3B) ΔF508 mutant mice. Both male and female ΔF508 mutant mice had improved oral glucose tolerance (Fig. 3C and D), as shown by a significantly lower area under the glucose curve during the OGTT (Fig. 3E and F). Intraperitoneal insulin tolerance test performed at 12 weeks of age showed a trend toward increased insulin sensitivity in male (Fig. 3G), but not female (Fig. 3H), ΔF508 homo- and heterozygous mutant mice. There was no significant difference in blood glucose levels or glucose tolerance in HET versus WT animals (Fig. 3). Circulating glucagon and insulin levels were not significantly different between 14-week-old ΔF508 and WT mice in males (Table 1) and females (data not shown). TG and total cholesterol levels were lower in male (Table 1) but not female (data not shown) ΔF508 versus WT mice. Given their more severe phenotype, subsequent experiments were performed in male animals only. For assessment of insulin secretion in vivo, we performed one-step hyperglycemic clamps in 12- and 18-week-old male ΔF508 and WT mice (Figs. 4 and 5). At 12 weeks of age, although blood glucose levels during the clamp tended to be lower in ΔF508 mice (Fig. 4A), the difference was not statistically significant (Fig. 4B). The average glucose infusion rate (GIR) was significantly higher (despite slightly lower blood glucose levels) in ΔF508 mice (Fig. 4C), while the insulin levels during the second half of the clamp were lower (Fig. 4D). C-peptide levels during the steady state were unchanged (Fig. 4E), and as a result the insulin–to–C-peptide ratio was lower in ΔF508 mice (Fig. 4F), suggestive of increased insulin clearance. Accordingly, M/I was higher in ΔF508 mice (Fig. 4G). The DI, which takes into account the degree of insulin sensitivity, was also higher in ΔF508 mice (Fig. 4H). Results from hyperglycemic clamps in 18-week-old male ΔF508 and WT mice revealed that although blood glucose (Fig. 5A and B), insulin (Fig. 5D), and C-peptide (Fig. 5E) levels and the insulin–to–C-peptide ratio (Fig. 5F) were not significantly different, the average GIR (Fig. 5C), M/I index (Fig. 5G), and DI (Fig. 5H) were higher in ΔF508 mice. Taken together, these data indicate that 12- and 18-week-old ΔF508 mutant mice are more insulin sensitive than their WT littermates and have normal β-cell function in vivo.
Figure 3.
Glucose and insulin tolerance in young ΔF508 mutant mice. A and B: Fed and fasting blood glucose levels were measured in 10- to 13-week-old male (A) and female (B) WT, HET, and ΔF508 mice. Data are expressed as mean ± SEM of 9–32 mice per group. *P < 0.05, **P < 0.01 vs. WT. C–F: OGTTs were performed in 10-week-old male (C) and female (D) WT, HET, and ΔF508 mice. The area under the curve of blood glucose levels was calculated during OGTT in male (E) and female (F) mice. **P < 0.01 vs. WT. G and H: Intraperitoneal insulin tolerance tests were performed in 12-week-old male (G) and female (H) WT, HET, and ΔF508 mice. C–H: Data are mean ± SEM of 8–18 mice per group.
Table 1.
Metabolic parameters of 14- and 24-week-old male WT and ΔF508 mutant mice
| 14 weeks old |
24 weeks old |
|||
|---|---|---|---|---|
| WT | ΔF508 | WT | ΔF508 | |
| Insulin (pmol/L) | 141 ± 27 | 88 ± 24 | 205 ± 26 | 134 ± 21 |
| Glucagon (ng/L) | 315 ± 32 | 398 ± 30 | 340 ± 32 | 268 ± 19 |
| TG (mmol/L) | 11.9 ± 1.9 | 5.1 ± 0.7*** | 9.6 ± 1.5 | 7.5 ± 1.3 |
| Total cholesterol (mmol/L) | 1.84 ± 0.23 | 1.11 ± 0.28* | 2.10 ± 0.15 | 2.05 ± 0.80 |
Data are means ± SEM of 4–17 mice.
*P < 0.05,
***P < 0.001 vs. WT mice.
Figure 4.
Insulin secretion and sensitivity in vivo in 12-week-old male ΔF508 mutant mice. Hyperglycemic clamps were performed in 12-week-old male WT and ΔF508 mice. A: Blood glucose levels during the glucose clamp. B: Average blood glucose levels during the steady-state between 30 and 60 min. C: GIR. D: Plasma insulin levels. E: Plasma C-peptide levels. F: Insulin–to–C-peptide ratio. G: M/I. H: DI. Data are mean ± SEM of 10 mice per group. *P < 0.05, **P < 0.01 vs. WT.
Figure 5.
Insulin secretion and sensitivity in vivo in 18-week-old male ΔF508 mutant mice. Hyperglycemic clamps were performed in 18-week-old male WT and ΔF508 mice. A: Blood glucose levels during the glucose clamp. B: Average blood glucose levels during the steady-state between 30 and 60 min. C: GIR. D: Plasma insulin levels. E: Plasma C-peptide levels. F: Insulin–to–C-peptide ratio. G: M/I. H: DI. Data are mean ± SEM of 7–9 mice per group. *P < 0.05, **P < 0.01 vs. WT.
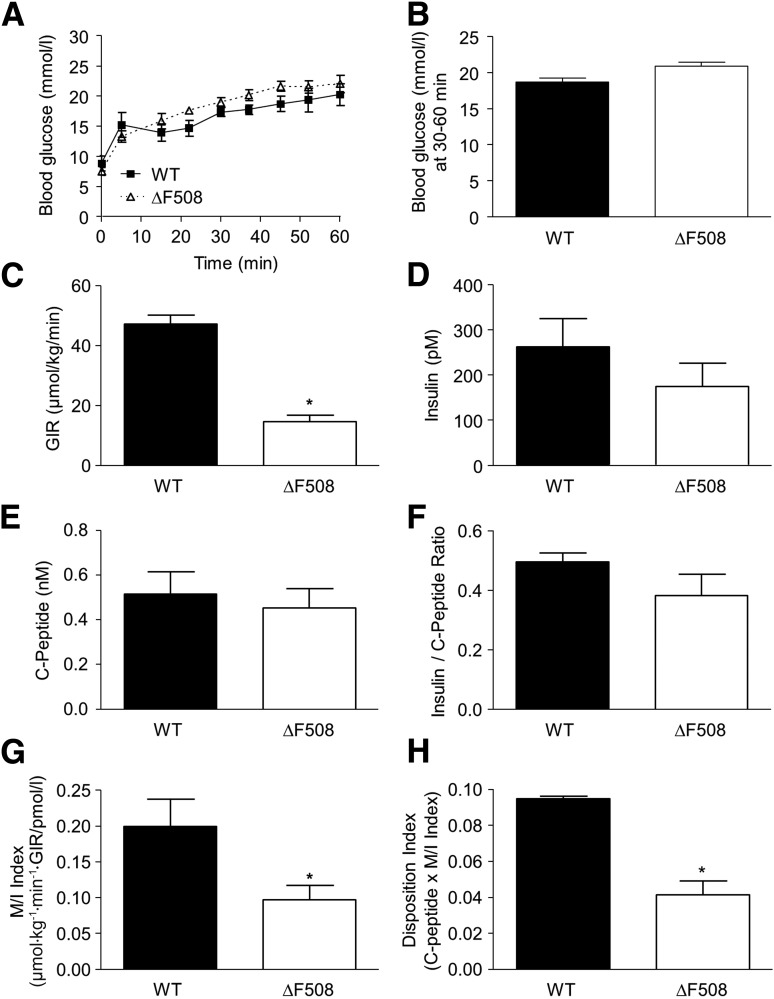
The prevalence of CFRD increases with age, suggesting that a silent β-cell defect might become apparent with age-related insulin resistance. To test this possibility in the ΔF508 mouse model, we examined glucose homeostasis in 24-week-old males. Fasting blood glucose levels were lower in 24-week-old male ΔF508 mice (ΔF508 3.7 ± 0.2 mmol/L vs. WT 4.6 ± 0.1 mmol/L; n = 10, P < 0.01). Circulating insulin, glucagon, TG, and total cholesterol levels were not significantly different between 24-week-old ΔF508 and WT male mice (Table 1). In hyperglycemic clamps, blood glucose levels were similar in ΔF508 and WT mice (Fig. 6A and B), but in contrast to our observations in 12- and 18-week-old animals (Figs. 4 and 5), the GIR was significantly lower in ΔF508 mutant than in WT mice (Fig. 6C). Insulin (Fig. 6D) and C-peptide (Fig. 6E) levels were not statistically different between ΔF508 and WT mice. Accordingly, the insulin–to–C-peptide ratio was unchanged (Fig. 6F). The M/I index was significantly lower in ΔF508 mutant mice (Fig. 6G), indicative of insulin resistance. The DI was also lower in ΔF508 mutant mice, suggestive of impaired β-cell function in the context of insulin resistance (Fig. 6H). These data suggest that the ΔF508 mutation is associated with an increase in insulin resistance with age that is not compensated for by an increase in β-cell function.
Figure 6.
Insulin secretion and sensitivity in 24-week-old male ΔF508 mutant mice. Hyperglycemic clamps were performed in 24-week-old male WT and ΔF508 mice. A: Blood glucose levels during the glucose clamp. B: Average blood glucose levels during the steady state between 30 and 60 min. C: GIR. D: Plasma insulin levels. E: Plasma C-peptide levels. F: Insulin–to–C-peptide ratio. G: M/I. H: DI. Data are mean ± SEM of 3–4 mice per group. *P < 0.05.
β-Cell Mass and Pancreas Morphology in ΔF508 Mutant Mice
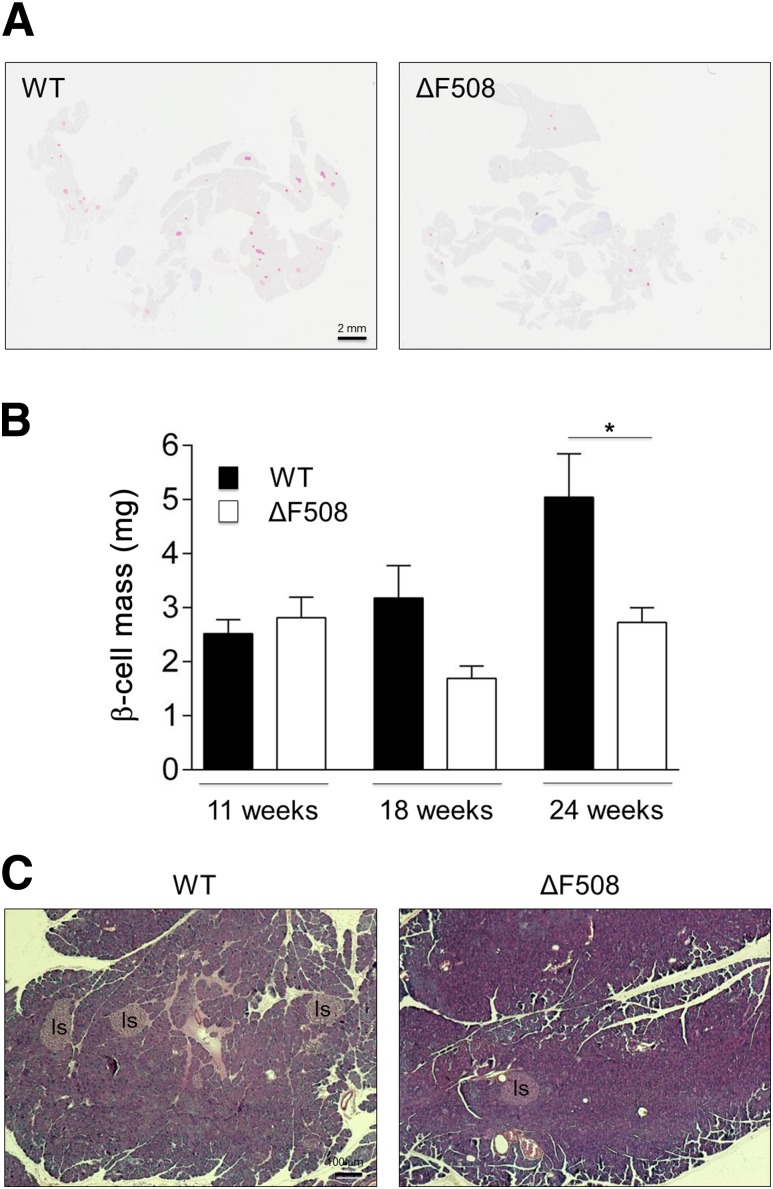
Since we did not observe any intrinsic defect in insulin secretion in isolated islets (Fig. 2), the decrease in DI in 24-week-old ΔF508 mutants (Fig. 6H) prompted us to measure β-cell mass in 11-, 18- and 24-week-old animals. Although β-cell mass was similar in both groups at 11 weeks, it was significantly lower in ΔF508 mutants at 24 weeks (Fig. 7A and B). We then asked whether the exocrine pancreas is affected in ΔF508 mutant mice as has been described previously in CF humans (20,21) and animal models (27,40). HPS staining of pancreatic sections of 24-week-old ΔF508 mutants revealed no fibrosis, inflammation, or other pathological findings (Fig. 7C). There was no increase in circulating inflammatory cytokine levels in 18- and 24-week-old ΔF508 mutants (data not shown). Overall, these data suggest that the β-cell defect observed in 24-week-old ΔF508 mutant mice is due to a deficit in β-cell mass that is not a secondary consequence of overt inflammation or exocrine pancreatic disease.
Figure 7.
β-Cell mass and pancreatic histology in ΔF508 mutant mice. Pancreata were harvested from 11-, 18- and 24-week-old male ΔF508 mutant and WT mice, and β-cell mass was measured by morphometric analysis after insulin immunostaining as described in research design and methods. A: Representative image from a WT (left) and ΔF508 mutant (right) mouse at 24 weeks of age. B: Mean ± SEM β-cell mass at the ages indicated of 4–7 animals in each group. *P < 0.05. C: Representative HPS-stained pancreatic section from a WT (left) and ΔF508 mutant (right) mouse at 24 weeks of age (n = 7). Is, islets.
Discussion
The objective of this study was to test the hypothesis that the ΔF508 cftr mutation alters glucose homeostasis in an age-dependent manner in mice. Systematic evaluation of β-cell function and insulin sensitivity revealed that 1) islets from young ΔF508 mutants have a mild secretory defect ex vivo that can be accounted for by the decreased insulin content but are not more susceptible to proinflammatory stress, 2) the ΔF508 mutation in 12- to 18-week-old mice is unexpectedly associated with improved glucose tolerance and higher insulin sensitivity, 3) 12- to 18-week-old ΔF508 mutants have adequate β-cell function in vivo given their level of insulin sensitivity, and 4) as ΔF508 mutant mice age, they become insulin resistant and have reduced β-cell function in vivo associated with a marked decrease in β-cell mass. Our interpretation of these findings is that the ΔF508 mutation does not lead to a bona fide insulin secretory defect but is associated with insulin resistance and a β-cell mass deficit in aging mutants.
Male ΔF508 mutant mice had lower body weight from the beginning of the study, which was associated with a lower fat mass and reduced locomotor activity but no significant differences in weight gain or energy expenditure (Fig. 1 and Supplementary Fig. 2). Total food intake was not different, although since the ΔF508 mutant mice weigh less, their food consumption per unit of body weight was increased. This, together with lower locomotor activity, would be predicted to lead to a higher weight gain that was not observed. We suspect that this discrepancy is explained by reduced intestinal absorption in ΔF508 mutant mice (41). Unlike male mice, ΔF508 mutant females did not show a significant difference in body weight or fat mass compared with WT controls. Sex-specific modifier loci that have been shown to influence body weight in cftr-mutant mice (42) may be responsible for these differences.
Fed and fasted blood glucose was reduced and glucose tolerance was greatly improved in young ΔF508 mutant mice, particularly in males (Fig. 3). This can be due to reduced intestinal absorption of glucose (43), improved insulin secretion, and/or enhanced insulin sensitivity. Insulin tolerance tests (Fig. 3) and hyperglycemic clamps (Figs. 4 and 5) confirmed that 12- and 18-week-old ΔF508 mutant mice were more insulin sensitive. We acknowledge that hyperglycemic clamps are primarily designed to assess β-cell function and that insulin sensitivity is best measured using euglycemic-hyperinsulinemic clamps. However, given that it is essentially impossible to perform two separate clamps in the same mouse, the M/I index during a hyperglycemic clamp is an acceptable estimate of insulin sensitivity in rodents (36). In addition, the results of the insulin tolerance tests corroborate those of the clamps and show a trend for greater insulin sensitivity in male ΔF508 mutants. Accordingly, insulin clearance was increased in ΔF508 mutant mice (Fig. 4), as also observed in CF patients (44). The reasons for the increased insulin sensitivity in ΔF508 mutant mice are unknown, but it has been suggested in humans to represent an adaptive process to the exocrine deficiency and energy deficit. In our model, it could also be due in part to the reduced fat mass of male ΔF508 mutants. Strikingly, however, the difference between ΔF508 and WT mice was reversed at 24 weeks of age, with ΔF508 mutants showing insulin resistance as assessed by a lower M/I index (Fig. 6). The underlying cause of insulin resistance in aging ΔF508 mutants is unknown but could be due to expression of the mutant protein in peripheral tissues. Clinically, conflicting results have been obtained regarding insulin sensitivity in CF patients, which has been reported as increased (14,15,45), unchanged (9,10,12,44), or decreased (13,17). Our results in mice suggest that these discrepancies might be due, in part, to the age at which insulin sensitivity is measured. In addition, a study in CF patients using hyperinsulinemic-euglycemic clamps revealed a complex pattern with increased hepatic glucose production (i.e., liver insulin resistance) but enhanced peripheral insulin sensitivity (14).
Hyperglycemic clamps in 12- and 18-week-old ΔF508 mutant mice failed to reveal any β-cell secretory defect (Figs. 4 and 5). C-peptide levels were unchanged, and the lower circulating insulin levels at 12 weeks of age were likely due to increased insulin clearance, consistent with enhanced insulin sensitivity. DI, an index of β-cell function that takes into account the prevailing level of insulin sensitivity, was actually higher in ΔF508 mutants. The similar C-peptide levels between ΔF508 mutant and WT mice suggest that β-cell function is not reduced in mutants and is in fact high in light of the prevailing increase in insulin sensitivity. This possibly explains the increase in glucose tolerance in young ΔF508 mutants. Nonetheless, we can conclude from these experiments that there is no β-cell defect in vivo in 12- and 18-week-old ΔF508 mutant mice. This conclusion is also supported by the results of static incubations in isolated islet (Fig. 2).
The absence of a marked insulin secretory defect in young ΔF508 mutant mice in this study contrasts with reports of β-cell dysfunction in newborn CF pigs (40) and cftr-null ferrets (27). In the latter study, CF kits demonstrated impaired insulin secretion prior to the occurrence of overt pancreatic lesions. These differences can be explained by several factors. First, the impact of CFTR deficiency varies significantly between species (46). In that regard, our results are consistent with the normal glucose tolerance in 11- to 13-week-old cftr-null mice (26), although islet function was not examined in that study. Second, since the ΔF508 mutant CFTR retains partial expression and function (47), the functional consequences of complete CFTR deletion in β-cells might be more severe. Nevertheless, recent studies suggest that CFTR in β-cells is implicated in glucose-dependent electrical activity (28) and the potentiating effect of cAMP (29). We acknowledge that the hyperglycemic clamps and 1-h static incubations used to measure insulin secretion may have masked a minor defect; however, our findings indicate that there is no major secretory defect in ΔF508 mutant mouse islets.
In 24-week-old ΔF508 mutants, we observed a significant decrease in DI (Fig. 6), indicating insufficient β-cell function relative to the level of insulin sensitivity. Although β-cell mass was normal in 11-week-old male ΔF508 mutant mice, it was significantly reduced at 24 weeks (Fig. 7). In CF humans, the defect in β-cell mass is thought to be secondary to exocrine pancreatic disease (20,21). Similarly, newborn cftr-null ferrets have smaller islets, and β-cell area decreases with time and correlates with increased severity of pancreatic pathology (27). In contrast to these studies, we did not observe any overt pathology in ΔF508 mutant pancreata at 24 weeks of age (Fig. 7).
In conclusion, our results show that the ΔF508 CFTR mutation is associated with increased insulin sensitivity and normal insulin secretion in vivo in young mice. As the mice age, however, they develop a deficit in β-cell mass combined with insulin resistance relative to their WT counterparts. These results are consistent with the notion that an underlying β-cell defect related to the ΔF508 mutation remains silent under normal conditions but becomes apparent in situations of increased secretory demand. This possibility is supported by the clinical observation that the prevalence of CFRD dramatically increases with age (1) and suggests that alleviation of insulin resistance might be beneficial in preserving glucose homeostasis in CF patients.
Article Information
Acknowledgments. The authors are grateful to B.L. Scholte (Erasmus University, Rotterdam, the Netherlands) for providing ΔF508 mutant mice. The authors thank G. Fergusson and M. Éthier of the Rodent Metabolic Phenotyping core facility of the University of Montreal Hospital Research Centre (CRCHUM) for performing the clamp studies; M. Guévremont, J. Morin, and the Cellular Physiology Service core of the CRCHUM for quantification of β-cell mass; and C. Tremblay (CRCHUM) for valuable technical assistance.
Funding. This study was supported by a Small Team Grant from Cystic Fibrosis Canada. V.P. holds the Canada Research Chair in Diabetes and Pancreatic Beta-Cell Function.
Duality of Interest. B.Z. was supported by a postdoctoral fellowship from Eli Lilly Canada. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.F. and J.G. performed the study, researched data, analyzed the results, and wrote the manuscript. I.B. and B.Z. performed the study, researched data, analyzed the results, and reviewed the manuscript. D.T. analyzed the results. Y.B. conceived the study and reviewed the manuscript. V.P. conceived the study, analyzed the results, and wrote the manuscript. V.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-0810/-/DC1.
G.F. is currently affiliated with the Department of Microbiology and Immunology, McGill University, Montreal, Quebec, Canada, and Y.B. is currently affiliated with Institut de recherches cliniques de Montreal, Montreal, Quebec, Canada.
References
- 1.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 2009;32:1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrington M, Krueger LJ, Holsclaw DS Jr, Iannuzzi MC, Dean M, Mann D. Cystic fibrosis-related diabetes is associated with HLA DQB1 alleles encoding Asp-57- molecules. J Clin Immunol 1994;14:353–358 [DOI] [PubMed] [Google Scholar]
- 3.Blackman SM, Hsu S, Ritter SE, et al. A susceptibility gene for type 2 diabetes confers substantial risk for diabetes complicating cystic fibrosis. Diabetologia 2009;52:1858–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran A, Brunzell C, Cohen RC, et al.; CFRD Guidelines Committee . Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33:2697–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa M, Potvin S, Hammana I, et al. Increased glucose excursion in cystic fibrosis and its association with a worse clinical status. J Cyst Fibros 2007;6:376–383 [DOI] [PubMed] [Google Scholar]
- 6.Nathan BM, Laguna T, Moran A. Recent trends in cystic fibrosis-related diabetes. Curr Opin Endocrinol Diabetes Obes 2010;17:335–341 [DOI] [PubMed] [Google Scholar]
- 7.Dobson L, Sheldon CD, Hattersley AT. Understanding cystic-fibrosis-related diabetes: best thought of as insulin deficiency? J R Soc Med 2004;97(Suppl. 44):26–35 [PMC free article] [PubMed] [Google Scholar]
- 8.Lombardo F, De Luca F, Rosano M, et al. Natural history of glucose tolerance, beta-cell function and peripheral insulin sensitivity in cystic fibrosis patients with fasting euglycemia. Eur J Endocrinol 2003;149:53–59 [DOI] [PubMed] [Google Scholar]
- 9.Cucinotta D, De Luca F, Gigante A, et al. No changes of insulin sensitivity in cystic fibrosis patients with different degrees of glucose tolerance: an epidemiological and longitudinal study. Eur J Endocrinol 1994;130:253–258 [DOI] [PubMed] [Google Scholar]
- 10.Mohan K, Miller H, Dyce P, et al. Mechanisms of glucose intolerance in cystic fibrosis. Diabet Med 2009;26:582–588 [DOI] [PubMed] [Google Scholar]
- 11.Preumont V, Hermans MP, Lebecque P, Buysschaert M. Glucose homeostasis and genotype-phenotype interplay in cystic fibrosis patients with CFTR gene deltaF508 mutation. Diabetes Care 2007;30:1187–1192 [DOI] [PubMed] [Google Scholar]
- 12.Yung B, Noormohamed FH, Kemp M, Hooper J, Lant AF, Hodson ME. Cystic fibrosis-related diabetes: the role of peripheral insulin resistance and beta-cell dysfunction. Diabet Med 2002;19:221–226 [DOI] [PubMed] [Google Scholar]
- 13.Hardin DS, LeBlanc A, Lukenbough S, Seilheimer DK. Insulin resistance is associated with decreased clinical status in cystic fibrosis. J Pediatr 1997;130:948–956 [DOI] [PubMed] [Google Scholar]
- 14.Moran A, Pyzdrowski KL, Weinreb J, et al. Insulin sensitivity in cystic fibrosis. Diabetes 1994;43:1020–1026 [DOI] [PubMed] [Google Scholar]
- 15.Tofe S, Moreno JC, Maiz L, Alonso M, Escobar H, Barrio R. Insulin-secretion abnormalities and clinical deterioration related to impaired glucose tolerance in cystic fibrosis. Eur J Endocrinol 2005;152:241–247 [DOI] [PubMed] [Google Scholar]
- 16.Hardin DS, Ahn C, Rice J, Rice M, Rosenblatt R. Elevated gluconeogenesis and lack of suppression by insulin contribute to cystic fibrosis-related diabetes. J Investig Med 2008;56:567–573 [DOI] [PubMed] [Google Scholar]
- 17.Hardin DS, LeBlanc A, Para L, Seilheimer DK. Hepatic insulin resistance and defects in substrate utilization in cystic fibrosis. Diabetes 1999;48:1082–1087 [DOI] [PubMed] [Google Scholar]
- 18.Austin A, Kalhan SC, Orenstein D, Nixon P, Arslanian S. Roles of insulin resistance and beta-cell dysfunction in the pathogenesis of glucose intolerance in cystic fibrosis. J Clin Endocrinol Metab 1994;79:80–85 [DOI] [PubMed] [Google Scholar]
- 19.Boom A, Lybaert P, Pollet JF, et al. Expression and localization of cystic fibrosis transmembrane conductance regulator in the rat endocrine pancreas. Endocrine 2007;32:197–205 [DOI] [PubMed] [Google Scholar]
- 20.Löhr M, Goertchen P, Nizze H, et al. Cystic fibrosis associated islet changes may provide a basis for diabetes. An immunocytochemical and morphometrical study. Virchows Arch A Pathol Anat Histopathol 1989;414:179–185 [DOI] [PubMed] [Google Scholar]
- 21.Iannucci A, Mukai K, Johnson D, Burke B. Endocrine pancreas in cystic fibrosis: an immunohistochemical study. Hum Pathol 1984;15:278–284 [DOI] [PubMed] [Google Scholar]
- 22.Couce M, O’Brien TD, Moran A, Roche PC, Butler PC. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. J Clin Endocrinol Metab 1996;81:1267–1272 [DOI] [PubMed] [Google Scholar]
- 23.Soejima K, Landing BH. Pancreatic islets in older patients with cystic fibrosis with and without diabetes mellitus: morphometric and immunocytologic studies. Pediatr Pathol 1986;6:25–46 [DOI] [PubMed] [Google Scholar]
- 24.Wooldridge JL, Szczesniak RD, Fenchel MC, Elder DA. Insulin secretion abnormalities in exocrine pancreatic sufficient cystic fibrosis patients. J Cyst Fibros. 5 March 2015 [Epub ahead of print]. DOI: 10.1016/j.jcf.2015.1002.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ode KL, Frohnert B, Laguna T, et al. Oral glucose tolerance testing in children with cystic fibrosis. Pediatr Diabetes 2010;11:487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stalvey MS, Muller C, Schatz DA, et al. Cystic fibrosis transmembrane conductance regulator deficiency exacerbates islet cell dysfunction after beta-cell injury. Diabetes 2006;55:1939–1945 [DOI] [PubMed] [Google Scholar]
- 27.Olivier AK, Yi Y, Sun X, et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest 2012;122:3755–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo JH, Chen H, Ruan YC, et al. Glucose-induced electrical activities and insulin secretion in pancreatic islet β-cells are modulated by CFTR. Nat Commun 2014;5:4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edlund A, Esguerra JL, Wendt A, Flodström-Tullberg M, Eliasson L. CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med 2014;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng SH, Gregory RJ, Marshall J, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 1990;63:827–834 [DOI] [PubMed] [Google Scholar]
- 31.Knorre A, Wagner M, Schaefer HE, Colledge WH, Pahl HL. DeltaF508-CFTR causes constitutive NF-kappaB activation through an ER-overload response in cystic fibrosis lungs. Biol Chem 2002;383:271–282 [DOI] [PubMed] [Google Scholar]
- 32.Ali BR. Is cystic fibrosis-related diabetes an apoptotic consequence of ER stress in pancreatic cells? Med Hypotheses 2009;72:55–57 [DOI] [PubMed] [Google Scholar]
- 33.van Doorninck JH, French PJ, Verbeek E, et al. A mouse model for the cystic fibrosis delta F508 mutation. EMBO J 1995;14:4403–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latour MG, Alquier T, Oseid E, et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes 2007;56:1087–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 36.Goh TT, Mason TM, Gupta N, et al. Lipid-induced beta-cell dysfunction in vivo in models of progressive beta-cell failure. Am J Physiol Endocrinol Metab 2007;292:E549–E560 [DOI] [PubMed] [Google Scholar]
- 37.Jetton TL, Lausier J, LaRock K, et al. Mechanisms of compensatory beta-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes 2005;54:2294–2304 [DOI] [PubMed] [Google Scholar]
- 38.Kaur S, Norkina O, Ziemer D, Samuelson LC, De Lisle RC. Acidic duodenal pH alters gene expression in the cystic fibrosis mouse pancreas. Am J Physiol Gastrointest Liver Physiol 2004;287:G480–G490 [DOI] [PubMed] [Google Scholar]
- 39.Dimagno MJ, Lee SH, Hao Y, Zhou SY, McKenna BJ, Owyang C. A proinflammatory, antiapoptotic phenotype underlies the susceptibility to acute pancreatitis in cystic fibrosis transmembrane regulator (-/-) mice. Gastroenterology 2005;129:665–681 [DOI] [PubMed] [Google Scholar]
- 40.Uc A, Olivier AK, Griffin MA, et al. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond) 2015;128:131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bijvelds MJ, Bronsveld I, Havinga R, Sinaasappel M, de Jonge HR, Verkade HJ. Fat absorption in cystic fibrosis mice is impeded by defective lipolysis and post-lipolytic events. Am J Physiol Gastrointest Liver Physiol 2005;288:G646–G653 [DOI] [PubMed] [Google Scholar]
- 42.Haston CK, Corey M, Tsui LC. Mapping of genetic factors influencing the weight of cystic fibrosis knockout mice. Mamm Genome 2002;13:614–618 [DOI] [PubMed] [Google Scholar]
- 43.Hardcastle J, Harwood MD, Taylor CJ. Small intestinal glucose absorption in cystic fibrosis: a study in human and transgenic DeltaF508 cystic fibrosis mouse tissues. J Pharm Pharmacol 2004;56:329–338 [DOI] [PubMed] [Google Scholar]
- 44.Lanng S, Thorsteinsson B, Røder ME, Nerup J, Koch C. Insulin sensitivity and insulin clearance in cystic fibrosis patients with normal and diabetic glucose tolerance. Clin Endocrinol (Oxf) 1994;41:217–223 [DOI] [PubMed] [Google Scholar]
- 45.Ahmad T, Nelson R, Taylor R. Insulin sensitivity and metabolic clearance rate of insulin in cystic fibrosis. Metabolism 1994;43:163–167 [DOI] [PubMed] [Google Scholar]
- 46.Keiser NW, Engelhardt JF. New animal models of cystic fibrosis: what are they teaching us? Curr Opin Pulm Med 2011;17:478–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 1992;358:761–764 [DOI] [PubMed] [Google Scholar]