Summary
Systemic anaplastic large cell lymphomas (sALCLs) comprise a heterogeneous group of relatively rare T-cell non-Hodgkin lymphomas characterized by CD30 expression and other unifying pathologic features. ALK fusions are present in about 50% of cases. Pathological diagnosis can be challenging, particularly in ALK-negative cases. Though ALK-positive and ALK-negative sALCL are similar morphologically and immunophenotypically, they are separate entities with different genetics, clinical behavior, and outcomes. Evidence-based data evaluating treatment regimens are limited as randomized controlled trials are lacking and most prospective studies are too small to draw definitive conclusions. However, recent advances in molecular biology are bringing forth much needed knowledge in this field, and are likely to guide further targeted therapeutic development.
Keywords: Anaplastic large cell lymphoma, ALK translocation, NPM-ALK fusion, DUSP22 rearrangements, T-cell non-Hodgkin lymphoma, brentuximab vedotin, crizotinib
INTRODUCTION
It is estimated that in 2015 close to 72,000 new non-Hodgkin lymphoma (NHL) cases will be diagnosed in the United States and approximately 20,000 will die from their disease [1]. About 15% of these NHLs are of mature (peripheral) T-cell origin and are known as peripheral T-cell lymphomas (PTCLs). PTCLs are a heterogeneous group of lymphomas with a worse prognosis for most subtypes compared with the majority of their B-cell counterparts. The International T-cell Lymphoma Project evaluated a cohort of over 1100 PTCLs and natural killer/T-cell lymphomas. PTCL, not otherwise specified (NOS) was the most common subgroup (25.9%), followed by angioimmunoblastic lymphoma (18.5%) and anaplastic large cell lymphomas (ALCLs; 13.8%) [2]. ALCLs share several pathological features, including the presence of large pleomorphic neoplastic cells and strong expression of CD30 [3–6]. Systemic ALCLs (sALCLs) must be distinguished from primary cutaneous ALCLs, which tend to be localized and have a more indolent clinical course, often not requiring systemic therapy [7]. Additional localized forms of ALCL also have emerged recently, including seroma-associated ALCL linked to breast implants and primary mucosal cases [8–11]. This review will focus only on sALCL.
The 2008 World Health Organization (WHO) classification divides sALCL into two distinct entities based on ALK positivity: ALK+ ALCL, and ALK− ALCL [3,4]. ALK− ALCL was listed as a provisional entity in the 2008 classification system and is anticipated to become a definite entity in a pending WHO update. Although both subtypes can be indistinguishable morphologically, the consideration of ALK+ and ALK− ALCL as two separate entities reflects data that ALK translocations affect the biology of the disease and that ALK+ and ALK− cases have different clinical behavior and outcomes [12]. ALK+ ALCL carries the best clinical outcome of all systemic PTCLs, with a 5-year overall survival (OS) rate of about 70%, while ALK− ALCL has an intermediate prognosis and PTCL, NOS a poor prognosis, with 5-year OS rates of about 50% and 32%, respectively [2,12].
Although sALCLs have a prognosis somewhat better than other systemic PTCLs, their overall rarity among NHLs, poor understanding of the ALK− subtype, and lack of randomized controlled trials indicate an unmet need for further clarification of the biology and management of these tumors. Recent advances in molecular biology are bringing forth much needed knowledge in this field. This review will discuss the clinical characteristics of sALCL, its pathobiology and diagnostic recommendations, prognosis, and best therapeutic approaches in adults.
CLINICAL FEATURES
sALCL is primarily a nodal disease, though extranodal involvement is seen in ~20% of cases, especially in skin, soft tissues, liver, bone, and bone marrow [13,14]. Male predominance (60%) is seen in both ALK+ and ALK− subtypes. Most patients are symptomatic with advanced stage at presentation (stage III/IV in ~ 60% of cases) and B symptoms. Patients are younger in ALK+ ALCL (typically children and adults in their 30s) than in ALK− ALCL (peak incidence in the late 50s) [2,12].
Patients presenting with skin lesions ought to be staged appropriately to differentiate primary cutaneous ALCL from sALCL secondarily involving the skin, as primary cutaneous ALCL has a more indolent course than sALCL, with a 5-year OS rate in the range of 90%, and management needs to be tailored accordingly [7,15].
PATHOBIOLOGY
Morphologic Features
ALK+ and ALK− sALCL share similar morphologic features and diagnosis cannot be established based on morphologic evaluation alone (Figs.1,2). Though features can vary from case to case, all cases display large cells known as “hallmark” cells, which exhibit large, kidney- or horseshoe-shaped eccentric nuclei with perinuclear eosinophilic clearing probably representing the Golgi zone [16]. Small, basophilic nucleoli can be seen and the cytoplasm tends to be abundant. These cells typically form sheets of neoplastic cells effacing the nodal architecture, mimicking at times solid tumor metastases. A sinusoidal infiltrative pattern is commonly seen [17].
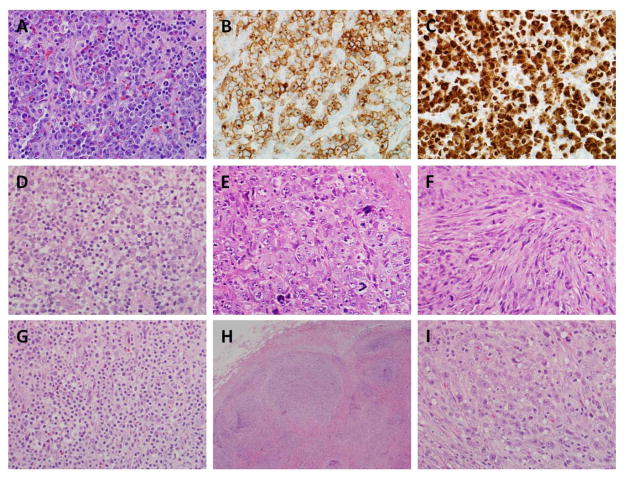
Figure 1. Pathological features of ALK-positive ALCL.
(A) Photomicrograph of typical ALK-positive ALCL, common pattern, showing sheets of large atypical lymphocytes (“hallmark” cells). (B) Immunohistochemical staining for CD30 in the same case shows the tumor cells to be positive, with a membranous and Golgi pattern of staining. (C) The tumor cells are positive for ALK. The presence of both nuclear and cytoplasmic staining indicates the ALK fusion partner is likely to be NPM. (D) Lymphohistiocytic pattern, showing tumor cells interspersed in a background of small lymphocytes and histiocytes. (E) This example shows a predominance of very large, markedly pleomorphic cells. (F) Case with so-called “sarcomatoid” features, showing marked spindling of the tumor cells. (G) Small cell variant, showing mostly small tumor cells with abundant pale cytoplasm. (H) Low-power image of case with “Hodgkin-like” features, including tumor nodules separated by bands of sclerosis. (I) Despite the Hodgkin-like appearance at low power, a higher power image shows sheets of hallmark cells typical of ALCL.
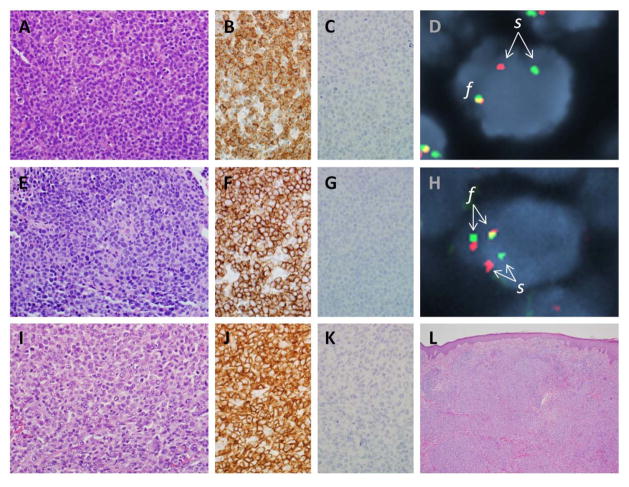
Figure 2. Pathological features of ALK-negative ALCL.
(A) Photomicrograph of ALK-negative ALCL with DUSP22 rearrangement (see text), showing sheets of tumor cells. (B) Tumor cells from the same case are positive for CD30 by immunohistochemistry. (C) Tumor cells are negative for ALK. (D) Fluorescence in situ hybridization (FISH) using a breakapart probe to the DUSP22-IRF4 locus on 6p25.3 shows a tumor cell nucleus (blue counterstain) with a single normal fusion signal (f) on one allele and abnormal separation (s) of the red and green portions of the probe on the other allele, indicating the presence of a rearrangement. (E) ALK-negative ALCL with TP63 rearrangement (see text). (F) Tumor cells are positive for CD30. (G) Tumor cells are negative for ALK. (H) FISH using a dual-fusion probe to the TBL1XR1 and TP63 loci on 3q show normal separation (s) of the two loci on one allele, and TBL1XR1-TP63 fusion (f) on the other allele. (I) Primary cutaneous ALCL, showing histological features similar to those of both ALK-positive and ALK-negative systemic ALCL. (J) Tumor cells also show a similar pattern of staining for CD30. (K) Tumor cells are negative for ALK. (L) A low-power image shows the relationship of the tumor cell infiltrate to the overlying epidermis.
Five morphologic patterns have been described in ALK+ ALCL: a common pattern (60% of cases; Fig. 1A), lymphohistiocytic pattern (10%; Fig. 1D), small cell pattern (5–10%; Fig. 1G), Hodgkin-like pattern (3%; Fig. 1H), and composite pattern with more than one of the above patterns (15%) [16]. Differential diagnosis based on morphologic findings includes classical Hodgkin lymphoma (cHL; especially in the Hodgkin-like pattern); solid malignancy metastases; and PTCL, NOS. Of note, the small cell pattern has not been described in the ALK− subset of sALCL [18].
Immunophenotypic Studies
Immunohistochemistry (IHC) is essential in the diagnosis and subclassification of ALCL. Strong CD30 antigen expression in the neoplastic cells is distinctive of ALCL, bearing in mind that CD30 expression is not specific for ALCL (Figs. 1A; 2B, F, and J). The CD30 staining in ALCL (all subtypes) tends to be uniformly strong on the cell membrane and perinuclear Golgi area of all neoplastic cells (though there may be variability of staining in the small cell pattern of ALK+ ALCL). This strong and uniform staining is helpful in distinguishing ALCL from other CD30-positive lymphomas, particularly PTCL, NOS. Because of the morphological spectrum seen in ALCL, it is reasonable to perform CD30 immunostaining in all PTCLs, and to stain cases that are positive for CD30 for ALK. While the presence of an ALK translocation can be confirmed by molecular studies, this is not necessary in routine diagnostic practice. ALK immunostaining tends to be localized in both the cytoplasm and the nucleus in cases with the most common NPM1-ALK fusion (Fig. 1C), but varies in cases with other gene partners. Neither a positive ALK stain nor the presence of an ALK translocation is specific for ALK+ ALCL, and other diagnoses should be considered, including ALK+ large B-cell lymphoma, inflammatory myofibroblastic tumor, and a variety of solid tumors including lung carcinoma [6,16,19]. It also should be noted that rare cases of primary cutaneous ALCL can be positive for ALK [6,19,20]; thus positive staining for ALK in a skin biopsy should not be considered sufficient for a diagnosis of sALCL, and staging studies should be performed.
The distinction between sALCL and cHL can be challenging at times, particularly for ALK− ALCL. Immunostaining for PAX5 (a B-cell transcription factor) can be valuable in this setting as it is almost always positive in cHL; however, rare cases of sALCL may stain weakly for PAX5, similar to cHL. PAX5-positive cases of ALCL have been shown to have additional copies of the PAX5 gene locus by fluorescence in situ hybridization (FISH) [21].
The neoplastic cells in both ALK+ and ALK− sALCL often lack expression of one or more pan-T-cell antigens, including CD3, CD5, CD7, and CD8. CD2 and CD4 are the T-cell antigens most often expressed [17,22]. A “null” phenotype without T-cell antigen expression is more commonly seen in sALCL (particularly ALK+) than in PTCL, NOS, and can be helpful but not diagnostic in distinguishing the two [12]. T-cell receptor (TCR) gene rearrangement studies often are positive for clonality even in these “null-cell” cases, and may be helpful to prove the T-cell origin of the neoplastic cells.
The majority of sALCLs stain positively for the cytotoxic markers TIA1, granzyme B, and perforin, though these may be absent in ALK− ALCLs with DUSP22 rearrangements (see below) [23,24]. About 80% of ALK+ ALCLs stain positively for epithelial membrane antigen (EMA), compared to half that in ALK− cases [12].
Molecular Genetics
The involvement of ALK in translocations was described in sALCL two decades ago [25]. The translocation between chromosomes 2 and 5 [t(2;5)(p23;q35)] leads to a chimeric 80kDa nucleophosmin- (NPM-) ALK fusion protein with subsequent constitutive activation of the ALK kinase. Since then, ALK translocations with different gene partners leading to other chimeric proteins were described both in lymphomas and solid malignancies [4,26–32]. In ALK+ ALCL, the NPM-ALK fusion protein is present in 80% of the cases [33,34].
ALK is a receptor tyrosine kinase with an extracellular-binding domain, a transmembrane domain, and an intracellular tyrosine kinase with homology to the family of insulin receptor kinases [34]. ALK protein is absent in normal human tissues but is expressed in embryonal neuronal tissues, and is believed to have a critical role in normal neuronal embryogenesis. The ligand to its receptor remains unknown in mammals, though recently heparin [35] and FAM150A and FAM150B [36] were reported as possible activating ligands for ALK kinase.
Downregulation or inhibition of ALK is associated with anti-tumor effects both in vitro and in vivo [34]. ALK’s role in oncogenesis remains incompletely understood, partly due to the fact that ALK translocations with different fusion partners, activating point mutations, and amplifications influence ALK signaling in different ways. It is clear, though, that ALK acts as an oncogenic driver in various malignancies, promoting proliferation and survival through several pathways including RAS-MAPK, PI3K-AKT-mTOR, and JAK-STAT. It also appears to affect the mitochondrial anti-apoptotic pathway through Bcl-2. Further understanding of how ALK leads to lymphomagenesis is of utmost importance, as many of the above pathways are potentially targetable.
As both the diagnosis and management of ALK− ALCL can be a challenge, recent efforts have focused on understanding the genetics and identifying a characteristic molecular signature.[32,37] Next generation sequencing and other techniques have identified two recurrent rearrangements in ALK− sALCL: one involving the DUSP22/IRF4 locus on chromosome 6p25.3 (30% of cases), and the other involving TP63 on 3q28 (8%) [23,38–40]. These rearrangements were mutually exclusive and were not found in any of the ALK+ cases. Furthermore, these translocations were of prognostic value. When present, ALK− ALCLs with DUSP22 rearrangements had OS rates similar to ALK+ ALCLs, while TP63 rearrangements carried a worse prognosis; these findings were independent of the intensity of treatment received [23]. While the relative rarity of TP63 rearrangements make this group of patients difficult to characterize definitively, DUSP22-rearranged ALCLs have consistent morphologic and phenotypic features in addition to their favorable prognostic features, suggesting the possibility that these cases might represent a distinct clinicopathologic entity [23,24]. The pathogenetic role of DUSP22 rearrangements remains to be demonstrated. In addition to elucidating their biology, understanding the molecular consequences of these rearrangements also may lead to new clinical tests at the RNA and/or protein level to identify this group of patients.
Recently, through the use of cancer outlier profile analysis (COPA) of gene expression profiling (GEP) data, ERBB4 and COL29A1 were found to be exclusively co-expressed in 8 out of 24 cases of ALK− ALCL with a specific gene signature [41]. While the significance of these findings for clinical outcomes are unclear, experimental data suggest that pharmacologic inhibition of ERBB4 retards cell growth and tumor progression.
Recurrent chromosomal copy number abnormalities (CNAs) have been described in both ALK+ and ALK− ALCL, highlighting both similarities and differences between both entities as well as differences from other PTCLs. Gains of chromosome 7 and losses of 6q, 11q and 13q are common to both ALK+ and ALK− ALCL. Gains of 17p and losses of chromosome 4 are recurrent in ALK+ ALCL, and only rarely seen in ALK− ALCL and other PTCLs. Losses of 5q and 9p, seen in ~ 30% of the cases in one series, were not found in the ALK− cases. Recurrent CNAs in ALK− ALCL include gains of 7p, 7q, 17q, 5q, 6p, 8q, 12q, and 1q, and losses of 4q, 11q, 6q, and 13q, with frequencies ranging from 15 to 35% [42]. Additional studies have examined PTCLs by GEP and identified molecular signatures that distinguished between ALK− ALCLs and other PTCLs [43–47]. This may be most critical for the distinction between ALK− ALCL and PTCL, NOS, since this differential diagnosis carries prognostic implications and diagnostic criteria remain imprecise [12]. Of note, GEP data suggest that expression of only 3 genes – TNFRSF8, BATF, and TMOD1 – could differentiate these entities, showing promise for the development of clinical molecular classifiers [46]. Moreover, GEP data may provide further prognostic stratification of PTCLs [45,47]. Finally, although comprehensive profiling of recurrent somatic mutations in ALCL requires further effort, recent data indicate recurrent abnormalities targeting the JAK-STAT pathway, particularly point mutations of JAK1 and STAT3 but also including novel fusion genes [48–50]. These findings suggest a potential target for novel therapeutic approaches in at least some ALCLs.
DIAGNOSIS: PITFALLS AND CHALLENGES
The diagnosis of sALCL can be challenging. The importance of correlation of pathologic findings with staging and other clinical data cannot be overemphasized. As discussed above, both ALK+ and ALK− sALCL can secondarily involve the skin and primary cutaneous ALCL can secondarily involve locoregional lymph nodes. While not comprehensively addressed in the current WHO classification system, ALK+ cases with localized involvement of the skin and ALK− cases associated with breast implants or with localized involvement of mucosal sites should be considered [8–11,20]. The major pitfall for the diagnosis of ALK+ ALCL is the failure to recognize morphologic variants as potential ALCL, especially the small cell pattern and other rare variants such as cases with a sarcomatoid appearance. Once the diagnosis is suspected, immunostaining for CD30, T-cell antigens, and ALK, with or without additional molecular studies, generally lead to the correct diagnosis without further difficulty. The diagnosis of ALK− ALCL can be challenging due to similarities in morphologic findings to other entities, the variable T-cell phenotype and aberrant expression of antigens more commonly associated with other lineages, and the absence of a molecular marker such as ALK to aid in diagnosis. The main differential diagnosis is with PTCL, NOS, which is a diagnosis of exclusion. In the International PTCL working group study where over 1000 cases were reviewed, there was a consensus agreement among hematopathologists in 97% of ALK+ ALCLs; 75% of PTCLs, NOS; and 74% of ALK− ALCLs [2]. In a study of PTCLs led by the Nordic Lymphoma Group, 13% of cases were reassigned a different diagnosis upon central review [51]. New tools such as gene expression profiling have demonstrated promise for refining diagnostic classification further, but have not yet been validated for routine clinical use [44–47].
Recommendations for Diagnosis
In the appropriate clinical setting, and when morphology is suggestive of PTCL, a tiered approach to the use of immunohistochemical stains and genetic studies can be followed to help with diagnosis [22]. Cases should be evaluated for T- or B-cell origin using stains for CD3 and CD20, respectively. Additional T-lineage immunostains, including CD2, CD4, CD5, CD7, and CD8, are useful to support T-cell lineage when CD3 is absent and to identify aberrant antigenic loss when CD3 is expressed. When lineage is uncertain (“null-cell” phenotype or occasionally cases that express both T- and B-cell antigens), molecular studies to detect clonal T-cell receptor and/or immunoglobulin gene rearrangements can be performed. Cytotoxic marker expression is a useful feature, though often negative in ALK− cases with DUSP22 rearrangements. EMA may be helpful when positive; CD56 is expressed infrequently is less often helpful [12,22]. Additional immunostains may be indicated in specific clinical scenarios or when certain PTCL subtypes are suspected, including stains for T-cell receptor-β and/or -γ/δ, TCL1, FOXP3 and T-follicular helper- TFH-) associated markers such as PD-1. In situ hybridization (or immunostains) for Epstein-Barr virus also should be considered. Because of the marked morphologic variation seen in ALCL, we would advocate staining for CD30 in all PTCLs, and staining for ALK in all CD30+ cases, even if the CD30 expression is only partial. If the differential diagnosis includes cHL, stains for PAX5, CD15, and CD45 are recommended. If identification of TP63 rearrangements is to be considered, immunohistochemistry for p63 can be performed at the same time as the ALK stain. While not all p63+ PTCLs have a TP63 rearrangement, p63 immunohistochemistry can be a useful screening test to identify positive cases for genetic evaluation by FISH [40]. DUSP22 rearrangements can be identified by FISH in cases meeting criteria for ALK− ALCL, in which it has been reported to have prognostic significance [23]. However, the presence or absence of a DUSP22 rearrangement should not be used as a diagnostic criterion for ALK− ALCL in the absence of further studies to validate its use for this purpose. Particularly, it should be remembered that DUSP22 rearrangements are identified at similar frequencies in systemic ALK− ALCL (30%) and primary cutaneous ALCL (28%), and clinical staging, not genetics, is paramount in distinguishing these entities [23,52]. FISH for ALK can be used to confirm a rearrangement in ALK+ ALCL, but is unnecessary in the majority of cases that are ALK+ by immunohistochemistry.
PROGNOSIS
sALCL remains a moderately aggressive lymphoma with worse outcomes than most but not all B-cell lymphomas, and significantly better survival than other PTCLs, with outcomes in ALK+ cases more favorable than in ALK− cases [53]. In data reported by the International Peripheral T-Cell Lymphoma Project, ALK+ ALCL had the best prognosis of all PTCLs, with a 5-year OS rate of 70%, while ALK− ALCL had a 5-year OS rate of 49% versus 32% in PTCL, NOS [12].
Clinical factors impact the prognosis of ALCL, similar to other lymphomas. While the International Prognostic Index (IPI) was developed for prognostication in B-cell lymphomas, it holds its value in PTCLs. The more risk factors, the worse the prognosis, with a 5-year OS rate ranging from 90% for low IPI scores to as low as 13% for the high IPI group when 4 to 5 risk factors were present [12]. Once 3 or more risk factors were present, the prognosis remained worse despite the presence of an ALK rearrangement, which emphasizes the value of clinical factors. Similarly, patients with ALK− ALCL and low IPI score had almost the same survival rate as the ALK+ group. In a study lead by the French Groupe d’Etude des Lymphomes de l’Adulte (GELA), age and β2 microglobulin, but not ALK rearrangement status, significantly affected survival with an 8-year OS rate of 84% in patients younger than 40 years who had β2 microglobulin <3mg/L, compared to 22% in patients older than 40 years with β2 microglobulin ≥3mg/L [53].
More recently, novel rearrangements were identified that not only may help to better characterize ALK− ALCLs but also may have prognostic significance [39,40]. Parrilla Castellar et al have reported that ALK-ALCLs that had DUSP22 rearrangements had a prognosis similar to ALK+ ALCLs with a 5-year OS rate of 90%, while ALK− ALCLs with TP63 rearrangements had a dismal prognosis (5-year OS rate of 17%) [23]. ALCLs without rearrangement of ALK, DUSP22, or TP63 (“triple-negative” ALCLs) had an intermediate prognosis with a 5-year OS rate of 42%.
Assessment of minimal residual disease (MRD) to predict prognosis and risk of relapse of ALK+ ALCL has been explored in the pediatric population. Damm-Welk et al evaluated 52 patients for MRD via RT-PCR in peripheral blood and/or bone marrow early in the treatment course (prior to the 2nd course of chemotherapy) [54]. The presence of MRD predicted higher risk of relapse, with a cumulative incidence of relapse of 81% (versus 31% in the absence of MRD) and a significantly worse 5-year OS of 65% (versus 92% in the absence of MRD). This concept could potentially be used in adults.
It is important to bear in mind that the studies reporting sALCL outcomes are retrospective, and non-randomized, with relatively small sample sizes. Also, as newer targeted therapies are more commonly used/approved in clinical practice, outcomes are likely to improve. Moreover, we anticipate that as more sophisticated molecular studies become standard of care as part of the evaluation of more challenging cases, therapy will be increasingly tailored to each individual case, potentially leading to better outcomes. However, this is yet to be proven.
MANAGEMENT
sALCL is clearly not one disease entity, and the “one size fit all” strategy is not ideal, especially in the era of individualized medicine. Currently, due to the lack of phase III randomized clinical trials, it is not clear what is the best treatment strategy for this disease, especially for the ALK− ALCL subset. Whenever possible, patients ought to be enrolled in clinical trials (Tables 1,2). sALCL is sensitive to cytotoxic chemotherapy both in the 1st line and relapsed settings; however, duration of response tends to be short-lived, particularly for ALK− ALCL with 5-year failure-free survival of only 36% [2].
Table 1.
Ongoing Therapeutic Clinical Trials in Adult sALCL in the Relapsed/Refractory Setting
| Diseases Studied | Brief Description | Study Design |
Study Drugs | Primary Endpoint |
Recruitment Status |
Location | NCT Number |
|---|---|---|---|---|---|---|---|
| CD30+ HL, ALCL | Combination of BV and Bendamustine in Relapsed or Refractory HL or ALCL | Phase 1/2; Open-label | BV + Bendamustine | Safety/Efficacy | Recruiting | Multicenter, Canada | NCT01657331 |
| ALK+ ALCL | BV and Imatinib in Patients With RR ALK+ ALCL or Patients Ineligible for Chemotherapy | Open-label; pilot study | BV + Imatinib | Safety/Efficacy | Not open yet | Austria | NCT02462538 |
| CD30 ALCL | A Phase 2 Study of BV in Treatment of Patients With RR sALCL | A Phase 2; Open-label | BV | Best clinical response (ORR) | Active, not recruiting | International | NCT00866047 |
| sALCL | Study of BV in Patients With RR sALCL | Phase 4; Open-label | BV | ORR | Recruiting | Europe | NCT01909934 |
| CD30+ Lymphoma | A Pilot Study of Weekly BV in Patients With CD30+ Malignancies Refractory to Every 3 Week BV | Pilot; Open-label | BV weekly | ORR | Recruiting | United States | NCT01703949 |
| CD30+ HL, ALCL | BV Maintenance After AlloSCT in High Risk CD30+ Lymphoma | Phase 2; Open-label | BV | - Graft Failure Incidence - Safety |
Recruiting | United States | NCT02169505 |
| CD30+ HL, ALCL | BV (Recombinant) for IV Infusion - Special Drug Use Surveillance in RR CD30+ HL or ALCL | Observational; Prospective cohort | BV (recombinant) | Frequency of adverse events | Recruiting | Japan | NCT02139592 |
| ALK+ ALCL; Solid tumors with ALK or MEK pathway alterations | Cross-tumoral Phase 2 Clinical Trial Exploring Crizotinib in Patients With Advanced Tumors Induced by Causal Alterations of ALK and/or MET “CREATE” | Phase 2; Open-label | Crizotinib | Anti-tumor activity | Recruiting | Multicenter, Europe (EORTC) | NCT01524926 |
| NSCLC or any other tumor with ALK rearrangement | A Phase 1/2 Study of the Oral ALK/EGFR Inhibitor AP26113 | Phase 1/2; Open-label | ALK/EGFR Inhibitor AP26113 | Phase 2 dose finding; ORR | Active, Not Recruiting | United States, Spain | NCT01449461 |
| PTCL | Alisertib in Treating Patients With RR PTCL | Phase 2; Open-label | Alisertib | Aurora Kinase expression; ORR | Active, Not Recruiting | United States, Canada | NCT01466881 |
| HL; B-Cell NHL; PTCL | Alisertib in Combination With Vorinostat in Treating Patients With RR HL, B-Cell NHL, or PTCL | Phase 1 | Alisertib + Vorinostat | MTD; Toxicities | Active, Not Recruiting | United States | NCT01567709 |
| T-cell NHL; HL | CPI-613 and Bendamustine in Treating Patients With RR T-Cell NHL or HL | Phase 1 | CPI-613 + Bendamustine | MTD | Recruiting | United States | NCT02168140 |
| T-Cell Lymphoma | Carfilzomib in Treating Patients With RR T-Cell Lymphoma | Phase 1 | Carfilzomib | MTD | Active, Not Recruiting | United States | NCT01336920 |
| Lymphoid Malignancies | Panobinostat in Treating Patients With RR NHL | Phase 2; Open-label | Panobinostat | ORR | Recruiting | United States | NCT01261247 |
| Lymphoid Malignancies | Study of Akt Inhibitor MK2206 in Patients With Relapsed Lymphoma | Phase 2; Open-label | Akt Inhibitor MK2206 | ORR | Recruiting | United States | NCT01258998 |
| Lymphoid Malignancies | Monoclonal Antibody Therapy Before SCT in Patients With RR Lymphoid Malignancies | Phase 1 | yttrium-90 anti-CD45 mAb BC8 | MTD | Recruiting | United States | NCT01678443 |
| CD30+ Lymphomas | Safety and Efficacy of 4th Generation CAR T-Cells Targeting RR CD30+ Lymphomas | Phase 1/2; Open-label | CAR T-cells | Safety/Efficacy | Recruiting | United States, China | NCT02274584 |
Abbreviations. ALCL: anaplastic large cell lymphoma; AlloSCT: allogeneic stem cell transplant; BV: brentuximab vedotin; FL: follicular lymphoma; HL: Hodgkin lymphoma; MCL mantle cell lymphoma; MTD: maximum tolerated dose; NHL: non-Hodgkin lymphoma; NSCLC: non-small cell lung carcinoma; ORR: objective response rate; PFS: progression-free survival; PTCL: peripheral T-cell lymphoma; RR: relapsed/refractory. Data from ClinicalTrials.gov (accessed September 8, 2015).
Table 2.
Ongoing Therapeutic Clinical Trials in Adult sALCL in the First Line Setting
| Diseases Studied | Brief Description | Study Design | Study Drugs | Primary Endpoint | Recruitment Status | Location | NCT Number |
|---|---|---|---|---|---|---|---|
| PTCL | Combination Chemotherapy and Pralatrexate as First-Line Therapy in Patients With PTCL | Phase 2; Open-label | CEOP Alternating Pralatrexate | CR rate | Active, Not Recruiting | United States | NCT01336933 |
| CD30+ mature T-cell and NK-cell lymphomas | BV Given Sequentially and Combined With Multi-Agent Chemotherapy for CD30 + Mature T-Cell and NK-Cell Neoplasms | Phase 1; Open-label | BV/CH-P; BV→CHOP | Safety | Active, Not Recruiting | United States, United Kingdom | NCT01309789 |
| CD30+ mature T-cell lymphoma | BV and CHP (A+CHP) Versus CHOP in the Frontline Treatment of Patients With CD30-positive Mature T- cell Lymphomas | Phase 3; Double-blind | BV/CHP CHOP | PFS | Recruiting | International | NCT01777152 |
| DLBCL, gray zone lymphoma, FL grade IIIB, PTCL, Burkitt lymphoma. | Dose-Adjusted EPOCH and Rituximab in Adults and Children With Previously Untreated Aggressive NHL | Phase 2; Open-label | DA-EPOCH + Rituximab | Overall response and PFS | Recruiting | United States | NCT00001337 |
| PTCL; PTCL, NOS; AITL; ALK− ALCL | Romidepsin in Combination With CHOEP as First Line Treatment Before HSCT in Young Patients With Nodal PTCL | Phase 1/2; Open-label | Romidepsin + CHOEP | MTD, PFS | Recruiting | Italy | NCT02223208 |
| All PTCL | Romidepsin and Lenalidomide in Treating Patients With Previously Untreated PTCL | Phase 2; Open-label | Romidepsin + Lenalidomide | ORR | Recruiting | United States | NCT02232516 |
| ALK+ ALCL | Efficacy and safety of crizotinib combined with CHOP chemotherapy for patients with ALK+ ALCL. | Open-label, NR | Crizotinib + CHOP | CR rate Safety/Efficacy | Recruiting | Multicenter, China | NCT02487316 |
Abbreviations. AITL: angioimmunoblastic T-cell lymphoma; BV: brentuximab vedotin; CEOP: cyclophosphamide, etoposide, vincristine, and prednisone; CR: complete remission; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; MCL: mantle cell lymphoma; MTD: maximum tolerated dose; NOS: not otherwise specified; NR: non-randomized; ORR: objective response rate; PFS: progression-free survival; PTCL: peripheral T-cell lymphoma; RR: relapsed/refractory. Data from ClinicalTrials.gov (accessed September 8, 2015).
First-Line Therapy
Localized Disease
Data are scarce in this setting. Most recommendations are based on the experience and data from diffuse large B-cell lymphoma. A retrospective study at MD Anderson Cancer Center reviewed data between 1985 and 1998 in 39 patients with early stage PTCLs treated with doxorubicin-based chemotherapy with and without radiation [55]. Twenty patients had ALCL, including 5 with primary cutaneous ALCL. The 5-year PFS and OS were 74 and 79%, respectively. No difference in outcomes (5-year local control, PFS, or OS) was observed based on ALK status or with the addition of involved field radiation after chemotherapy. However, due to the retrospective nature of this study and the small sample size, these results ought to be interpreted with caution. Also, primary cutaneous ALCL cases were included in this analysis, which can affect outcomes favorably as they have a very good prognosis with a survival at 5-years exceeding 90% [15]. A more recent retrospective study showed similar outcomes in limited stage PTCLs, i.e. that addition of radiotherapy did not improve outcomes in patients who had chemosensitive PTCLs [56]; however, outcomes in the sALCL cohort (n=35 with 40% ALK+) were not studied separately. The number of chemotherapy cycles is also open for debate and remains an area in need of further investigation.
Advanced Disease
Anthracycline-based chemotherapy remains the standard of care for advanced sALCL though not based on randomized data. In a retrospective analysis by MD Anderson Cancer Center of 135 untreated cases with T-cell lymphomas (all histologies except for mycosis fungoides), 40 patients had ALCL, 60% of which received CHOP (Cyclophosphamide, Adriamycin, Vincristine, Prednisone) chemotherapy [57]. ALK status was known for 31 cases; 61% had ALK− ALCL. The estimated 3-year OS was 66% and 100% for ALK− ALCL (excluding primary cutaneous ALCL) and ALK+ ALCL, respectively, compared to 43% for other T-cell lymphomas. Outcomes of patients treated with more intensive regimens (i.e. HyperCHOP, HyperCVAD, or early stem cell transplantation) were not superior to those obtained with CHOP therapy; however, the retrospective nature of the study and the small sample size preclude definite conclusions.
The GELA group performed a subset analysis from three prospective trials of aggressive lymphomas [53]. Out of 138 patients with ALCL, 54% were ALK− ALCL. Anthracycline-based combination chemotherapy was used in all but 1 patient. Intensive chemotherapy combinations (doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone) were not associated with improved outcomes as compared to the CHOP regimen. Estimated 8-year PFS for ALK+ and ALK− subsets were 72% and 39%, respectively; OS rates were 82% and 49%, respectively. Younger patients (<40 years) had better outcomes independent of ALK status.
In an analysis of T-cell lymphoma outcomes in trials led by the German High-Grade NHL Study Group (DSHNHL) evaluated 78 and 113 patients with ALK+ and ALK− ALCL, respectively [58]. CHOP or CHOEP (with addition of etoposide on a 2 to 3 week basis) were used. Three-year event-free survival (EFS) and OS were estimated at 75.8% and 89.8%, respectively, for ALK+ tumors, and 45.7% and 62.1%, respectively for ALK− ALCL. Interestingly, in patients 60 years or older with any T-cell histology, no benefit was seen in EFS or OS with shorter treatment intervals (14 versus 21 day cycles), more cycles (6 versus 8 cycles), or addition of etoposide to the treatment regimen (CHOP vs CHOEP). In patients younger than 60 years and low tumor burden as evidenced by low lactate dehydrogenase levels with any T-cell histology, a statistically significant benefit was seen in 3-year EFS (but not OS) when etoposide was added to CHOP (75.4 vs 51.0%; p=0.03). This benefit in 3-year EFS was even more pronounced in the ALK+ ALCL cohort (91.2% vs. 57.1%, P=0.012), though again there was no benefit in OS. When the ALK+ ALCL subset was excluded, the 3-year EFS was improved with the addition of etoposide, but lost its statistical significance (60.7% vs. 48.3%, P= 0.057). These results may still be clinically relevant, as addition of etoposide was associated with an increase in overall response rate (ORR) in all subgroups, which may allow eligible patients (histologies other than ALK+ ALCL) a chance to receive stem cell transplant.
A small prospective study led by the National Cancer Institute exploring the efficacy of 6 cycles of dose-adjusted EPOCH included 22 patients with sALCL (15 ALK+, 7 ALK−) [59]. Neither 5-year PFS (80% vs 71%, p=0.82) nor 5-year OS (86% for both) was significantly different between ALK+ and ALK− subsets, with outcomes seen in the ALK− group higher than anticipated from other studies. Though this study was prospective, the sample size was relatively small and selection biases could have played a role; specifically, the ALK− ALCL group had lower risk IPI scores (43% with IPI ≥2 compared to 60% in the ALK+ subset).
More recently, and based on success in the relapsed setting, the focus has shifted towards introduction of targeted therapies earlier in the therapeutic course in combination with cytotoxic chemotherapy. Brentuximab vedotin, a CD30 antibody-drug conjugate, was recently studied in the 1st line setting in a multicenter phase 1 trial given sequentially with CHOP or concomitantly with vincristine omission [60]. Nineteen patients with sALCL (16/19 ALK−) were included. An objective response was seen in all patients with CR in 88% and a reasonable safety profile. This study led to the ECHELON-2 trial, an ongoing phase 3 randomized international study conducted in mature T-cell lymphomas (NCT01777152); 75% of the patients in this study will have a diagnosis of sALCL (Table 2).
Autologous Stem Cell Transplant at 1st Complete Remission for sALCL: What is the Evidence?
A non-randomized prospective multi-institutional study led by the Nordic Lymphoma Group (NLG-T-01) in treatment naïve PTCLs evaluated the value of consolidative high dose chemotherapy (HDC) followed by stem cell rescue [51]. In this study, patients received a dose dense induction regimen with biweekly CHOEP-14 for 6 cycles. Patients with complete response (CR) or partial response (PR) then received HDC and autologous stem cell (ASCT) rescue. 31 patients (19%) had ALK− ALCL. ALK+ ALCL patients were excluded. The 5-year OS and PFS were 70% and 61%, respectively, for ALK− ALCL, which was significantly superior to all other subtypes when grouped together. Though non-randomized, this is the largest study done in the upfront transplant setting in PTCLs (n=160). These findings suggest a potential benefit for HDC and ASCT in ALK− ALCL. Other smaller non-randomized studies suggest similar outcomes [61,62].
It is important to note that ALK+ ALCL cases have been excluded from all transplant trials. As discussed before, patients with high-risk IPI ALK+ ALCL have a worse prognosis, and could benefit from approaches other than the standard CHOP chemotherapy. Will these patients benefit from HDC and ASCT? Furthermore, it is clear that ALK− ALCL is a heterogeneous group with a subset having a similar prognosis as ALK+ ALCL (i.e the tumors with DUSP22 rearrangements). Is this group of patients better off with CHOP chemotherapy, and can they be spared the unnecessary toxicity of HDC? These questions remain unanswered and would require further study.
Treatment Recommendations in the 1st Line Setting
Since the evidence is limited in this disease, enrollment in clinical trials when available is the best option for patients. In limited stage disease, 4 to 6 cycles of CHOP-like chemotherapy is recommended. For bulky disease, addition of involved field radiotherapy is recommended. Clinical factors and IPI score should be taken into account and more aggressive treatment should be considered accordingly. For advanced stage ALK+ ALCL, we recommend 6 cycles of CHOP. Etoposide could be added to CHOP in younger patients with higher IPI scores. For advanced stage ALK− ALCL, we recommend 6 cycles of CHOEP, followed by HDC and ASCT in responding transplant eligible patients. Chemotherapy dose intensification during induction is not recommended, as it is associated with higher toxicity and no improvement in outcomes [53,57]. These recommendations may change in the future based on the ability to stratify this subgroup of patients further with molecular studies and introduction of targeted therapies [37].
Relapsed/Refractory Disease
Patients with sALCL who progress or relapse after initial chemotherapy have a dismal prognosis. An analysis of the Lymphoid Cancer database by The British Columbia Cancer Agency identified 191 relapsed/refractory PTCLs between 1976 and 2010 [63]. Only 38 patients received transplant, while 153 were deemed unfit for transplant. The study included 36 sALCLs (11 ALK+ and 25 ALK−). Interestingly, outcomes of sALCL did not differ from other PTCLs; median OS and PFS for all PTCLs were only 5.5 months and 3.1 months, respectively. Patients who received chemotherapy did not have improved survival with median OS and PFS of only 6.5 and 3.7 months, respectively. Given the retrospective nature of this study, selection bias may have played a role. Most patients were not deemed transplant candidates, which could have been related to chemotherapy resistance or clinical factors such as lower performance status (PS). Median OS and PFS in patients who received chemotherapy with PS of 0 to 1 (13.7 and 5.0 months, respectively) were significantly higher than when PS was 2 or more. Patients who did not achieve CR had worse outcomes. Median OS and 3-year OS rates were 18 months and 35%, respectively, for those with CR versus 9.6 months and 17%, respectively, for patients with PR only. Of note, novel therapies such as brentuximab vedotin (BV) were not part of the treatment armamentarium.
Brentuximab Vedotin
BV is a CD30 antibody-drug conjugate incorporating monomethyl auristatin E, a potent anti-microtubule agent, that was FDA approved in 2011 for the treatment of sALCL in the relapsed setting after failure of at least one line of multi-agent chemotherapy. The approval was based on a single-arm multicenter clinical trial in 58 patients (42 ALK−) that showed unprecedented activity in this disease with an ORR of 86% [64]. CR was achieved in 57% with a median duration of response of 12.6 months. This is particularly impressive given that 62% of these patients were deemed refractory to 1st line chemotherapy, and 26% were post-ASCT. Since its approval, BV has become standard of care in relapsed patients and often is used as a bridge to stem transplant. It also has been studied in the frontline setting in combination with cytotoxic multi-agent chemotherapy. A systematic review conducted after approval of BV in patients with relapsed/refractory HL and ALCL to assess real-practice experience included 28 ALCLs [65]. The ORR and CR rates were 75 and 74% respectively. Grade 3/4 toxicity incidence was 6% for peripheral neuropathy and 12% for hematologic toxicity (thrombocytopenia). Only 5% of patients discontinued therapy because of toxicity. A small phase 2 trial has evaluated the efficacy and safety of retreatment with BV in patients with relapsed/refractory HL and ALCL, including 8 patients with sALCL (3 ALK+, 5 ALK−) [66]. ORR and CR rates were 88% and 63%, respectively. Median PFS was 12.9 months (median OS not reached). Peripheral motor neuropathy was the most significant grade 3 side effect, observed in 48% of patients, which was somewhat expected as it is a cumulative toxicity. Though the sample size was small, this was a proof of concept that re-treatment with BV is relatively safe and active.
Is There a Role for Allogeneic Stem Cell Transplant (AlloSCT) in sALCL?
The Center for International Blood and Marrow Transplant Research (CIBMTR) recently analyzed from the registry hematopoietic stem cell transplant data in T-cell lymphomas [67]. A total of 112 cases of sALCL were found; unfortunately, the ALK status was not reported. Fifty-one patients received AlloSCT. Outcomes were better after ASCT than after AlloSCT, including both PFS (55% vs 35%) and OS (68% vs 41%). Worse outcomes were observed in heavily pretreated patients. AlloSCT did not overcome chemotherapy refractory disease, and was associated with higher non-relapse mortality. A retrospective study led by La Societé Française de Greffe de Moëlle et de Therapie Cellulaire included 77 patients with aggressive T-cell lymphomas, including 27 sALCLs (8 ALK+, 6 ALK−, 13 unknown ALK status) [68]. Five-year OS and EFS for all patients were 57% and 53%, respectively. Five-year OS for sALCL patients only was estimated at 55%, with 19 patients achieving a CR after transplant. Similar to the CIBMTR study, chemoresistant disease prior to transplant predicted worse prognosis. The 5-year OS in patients with CR or PR at time of transplant was 69%, compared to a dismal 29% in patients with stable, progressive, or refractory disease. Also, grade 3–4 acute graft-versus host disease was associated with worse outcomes. Taken together, these data suggest that AlloSCT may be beneficial in patients with chemosensitive disease and that some patients may be cured. However, the benefits of AlloSCT may be greater in the early course of therapy while the disease is still chemosensitive. Novel targeted therapies could be used as a bridge to increase response and achieve a deeper response prior to proceeding to AlloSCT. Prospective clinical trials in this area are highly needed.
Novel Therapies
Due to the lack of therapeutic options in the relapsed setting and poor outcomes in chemorefractory disease, there has been an emphasis on investigation of novel therapies for ALCL. As biological and molecular knowledge advances, drugs targeting specific molecular aberrations are sought with hopes of increasing efficacy and limiting toxicity.
Crizotinib is the first ALK inhibitor approved as a single agent for the treatment of metastatic ALK+ non-small cell lung carcinoma in the first line setting, and has been associated with an impressive increase in response rate and PFS compared to standard combination chemotherapy in this disease [69]. Clinical trials of ALK inhibitors in sALCL are ongoing (Table 1). An open-label phase-1 dose escalation study in the pediatric population was reported recently where crizotinib was used in different relapsed pediatric tumors with ALK alterations, including 9 sALCLs [70]. There was an 88% ORR (8/9) with 7 CRs, 1 PR, and 1 patient with stable disease (SD). Interestingly, the patient with SD remained on protocol for more than 30 cycles. Crizotinib was relatively well tolerated with mostly grade 1–2 toxicities. Clinical trials are underway in adults (Table 1).
In the last few years, three new therapies have been approved for relapsed PTCLs after failure of frontline therapy. Pralatrexate, an anti-folate agent, was approved in 2009, followed by two histone deacetylase inhibitors: romidepsin, approved in 2011, and belinostat, approved in 2014. All appear to have similar efficacy with an ORR in the 25–30% range, but different toxicities. Immunotherapeutic approaches with anti-PD1 and anti-PDL1 inhibitors alone or in combinations with CTLA4 inhibitors are exciting new approaches to therapy of lymphoma, and currently are under study (Table 1).
Treatment Recommendations in the Relapsed Setting
Outcome after relapse of ALCL is poor, but a subset of patients can be salvaged and achieve potential cure. The goal of treatment should be CR or PR and consolidation with either HDC/ASCT or AlloSCT in transplant eligible patients. BV could be used in this setting as ORR and CRs are quite high. In transplant ineligible or chemotherapy refractory patients, the sequence of therapies depends on clinical characteristics and potential side effects.
Expert Commentary
sALCL is a heterogeneous group of T-cell non-Hodgkin lymphomas with aggressive behavior. Progress in improving outcomes has been slow due to the rarity of the disease and lack of randomized clinical trials. However, due to significant advances in molecular technologies and development of newer targeted therapies, we are at the dawn of witnessing significant changes in the management of this disease. Inclusion in clinical trials has never been more important.
Five-Year View
We anticipate that in the next five years, management of sALCL in the 1st line setting will likely include combinations of cytotoxic chemotherapy and targeted therapy such as brentuximab vedotin. Consolidation with high dose chemotherapy and stem cell rescue in patients with high risk ALK− ALCL will still be standard of care. The new focus of management will be to better stratify patients with both ALK+ and ALK− ALCL into high and low risks groups using new molecular techniques, including next generation sequencing, and to tailor therapy accordingly. As the evolving genetic heterogeneity of ALK− ALCL is clarified further, we anticipate both incorporation of new molecular findings into the diagnosis and subclassification of ALCL as well as biologic studies that identify candidate therapeutic targets for new genetic subtypes. For ALK+ ALCLs, ALK inhibitors will be added to the armamentarium first in the relapsed setting then potentially as 1st line agents. Evaluation of MRD could also guide therapy, as some patients may benefit from consolidation/maintenance strategies and others may not; determining MRD status may help limit not only toxicity, but also cost to both the patient and society. Immune checkpoint inhibitors are under study and are likely to be introduced in the relapsed and/or consolidation/maintenance settings.
Key Issues.
sALCL represents a rare and heterogeneous group of T-cell non-Hodgkin lymphomas with generally aggressive clinical behavior and poor outcomes in some patients.
ALK fusion proteins resulting from translocations of the ALK gene are present in close to half of the cases.
The diagnosis of ALK-negative ALCL can be challenging and expert pathology review may be required.
New genetic subgroups of ALK-negative ALCL have prognostic significance and can be identified using clinically available FISH assays.
The lack of randomized controlled trials has hindered development of improved treatment strategies for ALCL, and entry of patients on clinical trials should be encouraged.
ALCL is a chemosensitive disease, but responses can be short lived – especially for ALK− disease – and relapses are common.
Newer targeted therapies like brentuximab vedotin are likely to change the outcomes of ALCL patients and the way we approach this disease.
Acknowledgments
ALF is supported by Award Number R01 CA177734 from the National Cancer Institute.
Footnotes
Financial Disclosure
The authors have no competing financial interests to disclose.
Contributor Information
Nabila Bennani-Baiti, Email: bennani.nabila@mayo.edu, Division of Hematology, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, Phone: 507-284-2511, Fax: 507-266-4972.
Stephen Ansell, Email: ansell.stephen@mayo.edu, Division of Hematology, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, Phone: 507-538-1592, Fax: 507-266-4972.
Andrew L. Feldman, Email: feldman.andrew@mayo.edu, Department of Laboratory Medicine and Pathology, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, Phone: 507-284-4939, Fax: 507-284-5115.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 3.Mason DY, Harris NL, Delsol G, et al. Anaplastic large cell lymphoma, ALK-negative. In: Swerdlow S, Campo E, Harris N, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; Lyon: 2008. pp. 317–319. [Google Scholar]
- 4.Delsol G, Falini B, Muller-Hermelink HK, et al. Anaplastic large cell lymphoma, ALK-positive. In: Swerdlow S, Campo E, Harris N, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; Lyon: 2008. pp. 312–316. [Google Scholar]
- 5.Jaffe ES. Anaplastic large cell lymphoma: the shifting sands of diagnostic hematopathology. Mod Pathol. 2001;14(3):219–228. doi: 10.1038/modpathol.3880289. [DOI] [PubMed] [Google Scholar]
- 6.Xing X, Feldman AL. Anaplastic large cell lymphomas: ALK positive, ALK negative, and primary cutaneous. Adv Anat Pathol. 2015;22(1):29–49. doi: 10.1097/PAP.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 7.Ralfkiaer E, Willemze R, Paulli M, Kadin ME. Primary cutaneous CD30-positive T-cell lymphoproliferative disorders. In: Swerdlow S, Campo E, Harris N, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; Lyon: 2008. pp. 300–301. [Google Scholar]
- 8.Roden AC, Macon WR, Keeney GL, Myers JL, Feldman AL, Dogan A. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: an indolent T-cell lymphoproliferative disorder. Mod Pathol. 2008;21(4):455–463. doi: 10.1038/modpathol.3801024. [DOI] [PubMed] [Google Scholar]
- 9.Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32(2):114–120. doi: 10.1200/JCO.2013.52.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sciallis AP, Law ME, Inwards DJ, et al. Mucosal CD30-positive T-cell lymphoproliferations of the head and neck show a clinicopathologic spectrum similar to cutaneous CD30-positive T-cell lymphoproliferative disorders. Mod Pathol. 2012 doi: 10.1038/modpathol.2012.38. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Cai Y, Sheng W, Lu H, Li X. The spectrum of primary mucosal CD30-positive T-cell lymphoproliferative disorders of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(1):96–104. doi: 10.1016/j.oooo.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Savage KJ, Harris NL, Vose JM, et al. ALK− anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 13.Falini B, Pileri S, Zinzani PL, et al. ALK+ lymphoma: clinico-pathological findings and outcome. Blood. 1999;93(8):2697–2706. [PubMed] [Google Scholar]
- 14.Gascoyne RD, Aoun P, Wu D, et al. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood. 1999;93(11):3913–3921. [PubMed] [Google Scholar]
- 15.Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118(15):4024–4035. doi: 10.1182/blood-2011-05-351346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benharroch D, Meguerian-Bedoyan Z, Lamant L, et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood. 1998;91(6):2076–2084. [PubMed] [Google Scholar]
- 17.Jaffe ES, Nicolae A, Pittaluga S. Peripheral T-cell and NK-cell lymphomas in the WHO classification: pearls and pitfalls. Mod Pathol. 2013;26(Suppl 1):S71–87. doi: 10.1038/modpathol.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreri AJ, Govi S, Pileri SA, Savage KJ. Anaplastic large cell lymphoma, ALK-negative. Crit Rev Oncol Hematol. 2013;85(2):206–215. doi: 10.1016/j.critrevonc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Attygalle AD, Cabecadas J, Gaulard P, et al. Peripheral T-cell and NK-cell lymphomas and their mimics; taking a step forward - report on the lymphoma workshop of the XVIth meeting of the European Association for Haematopathology and the Society for Hematopathology. Histopathology. 2014;64(2):171–199. doi: 10.1111/his.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oschlies I, Lisfeld J, Lamant L, et al. ALK-positive anaplastic large cell lymphoma limited to the skin: clinical, histopathological and molecular analysis of 6 pediatric cases. A report from the ALCL99 study. Haematologica. 2013;98(1):50–56. doi: 10.3324/haematol.2012.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman AL, Law ME, Inwards DJ, Dogan A, McClure RF, Macon WR. PAX5-positive T-cell anaplastic large cell lymphomas associated with extra copies of the PAX5 gene locus. Mod Pathol. 2010;23(4):593–602. doi: 10.1038/modpathol.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsi ED, Said J, Macon WR, et al. Diagnostic accuracy of a defined immunophenotypic and molecular genetic approach for peripheral T/NK-cell lymphomas. A North American PTCL study group project. Am J Surg Pathol. 2014;38(6):768–775. doi: 10.1097/PAS.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124(9):1473–1480. doi: 10.1182/blood-2014-04-571091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King RL, Dao L, McPhail E, et al. Morphology of ALK-negative anaplastic large cell lymphomas with DUSP22 rearrangements. Am J Surg Pathol. doi: 10.1097/PAS.0000000000000500. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263(5151):1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 26.Lamant L, Dastugue N, Pulford K, Delsol G, Mariame B. A new fusion gene TPM3-ALK in anaplastic large cell lymphoma created by a (1;2)(q25;p23) translocation. Blood. 1999;93(9):3088–3095. [PubMed] [Google Scholar]
- 27.Touriol C, Greenland C, Lamant L, et al. Further demonstration of the diversity of chromosomal changes involving 2p23 in ALK-positive lymphoma: 2 cases expressing ALK kinase fused to CLTCL (clathrin chain polypeptide-like) Blood. 2000;95(10):3204–3207. [PubMed] [Google Scholar]
- 28.Tort F, Pinyol M, Pulford K, et al. Molecular characterization of a new ALK translocation involving moesin (MSN-ALK) in anaplastic large cell lymphoma. Lab Invest. 2001;81(3):419–426. doi: 10.1038/labinvest.3780249. [DOI] [PubMed] [Google Scholar]
- 29.Damm-Welk C, Klapper W, Oschlies I, et al. Distribution of NPM1-ALK and X-ALK fusion transcripts in paediatric anaplastic large cell lymphoma: a molecular-histological correlation. Br J Haematol. 2009;146(3):306–309. doi: 10.1111/j.1365-2141.2009.07754.x. [DOI] [PubMed] [Google Scholar]
- 30.Feldman AL, Vasmatzis G, Asmann YW, et al. Novel TRAF1-ALK fusion identified by deep RNA sequencing of anaplastic large cell lymphoma. Genes Chromosomes Cancer. 2013;52(11):1097–1102. doi: 10.1002/gcc.22104. [DOI] [PubMed] [Google Scholar]
- 31.Pulford K, Lamant L, Espinos E, et al. The emerging normal and disease-related roles of anaplastic lymphoma kinase. Cell Mol Life Sci. 2004;61(23):2939–2953. doi: 10.1007/s00018-004-4275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng Y, Feldman AL. Genetics of anaplastic large cell lymphoma. Leuk Lymphoma. 2015:1–7. doi: 10.3109/10428194.2015.1064530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccaluga PP, Gazzola A, Mannu C, et al. Pathobiology of anaplastic large cell lymphoma. Adv Hematol. 2010:345053. doi: 10.1155/2010/345053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13(10):685–700. doi: 10.1038/nrc3580. [DOI] [PubMed] [Google Scholar]
- 35.Murray PB, Lax I, Reshetnyak A, et al. Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal. 2015;8(360):ra6. doi: 10.1126/scisignal.2005916. [DOI] [PubMed] [Google Scholar]
- 36.Guan J, Umapathy G, Yamazaki Y, et al. FAM150A and FAM150B are activating ligands for Anaplastic Lymphoma Kinase. Elife. 2015:4. doi: 10.7554/eLife.09811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boddicker RL, Feldman AL. Progress in the identification of subgroups in ALK-negative anaplastic large-cell lymphoma. Biomark Med. 2015;9(8):719–722. doi: 10.2217/BMM.15.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldman AL, Law M, Remstein ED, et al. Recurrent translocations involving the IRF4 oncogene locus in peripheral T-cell lymphomas. Leukemia. 2009;23(3):574–580. doi: 10.1038/leu.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldman AL, Dogan A, Smith DI, et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively-parallel genomic sequencing. Blood. 2011;117(3):915–919. doi: 10.1182/blood-2010-08-303305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasmatzis G, Johnson SH, Knudson RA, et al. Genome-wide analysis reveals recurrent structural abnormalities of TP63 and other p53-related genes in peripheral T-cell lymphomas. Blood. 2012;120:2280–2289. doi: 10.1182/blood-2012-03-419937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scarfo I, Pellegrino E, Mereu E, et al. Identification of a new subclass of ALK negative ALCL expressing aberrant levels of ERBB4 transcripts. Blood. 2015 doi: 10.1182/blood-2014-12-614503. [DOI] [PubMed] [Google Scholar]
- 42.Salaverria I, Bea S, Lopez-Guillermo A, et al. Genomic profiling reveals different genetic aberrations in systemic ALK-positive and ALK-negative anaplastic large cell lymphomas. Br J Haematol. 2008;140(5):516–526. doi: 10.1111/j.1365-2141.2007.06924.x. [DOI] [PubMed] [Google Scholar]
- 43.Ballester B, Ramuz O, Gisselbrecht C, et al. Gene expression profiling identifies molecular subgroups among nodal peripheral T-cell lymphomas. Oncogene. 2006;25(10):1560–1570. doi: 10.1038/sj.onc.1209178. [DOI] [PubMed] [Google Scholar]
- 44.Piva R, Agnelli L, Pellegrino E, et al. Gene expression profiling uncovers molecular classifiers for the recognition of anaplastic large-cell lymphoma within peripheral T-cell neoplasms. J Clin Oncol. 2010;28(9):1583–1590. doi: 10.1200/JCO.2008.20.9759. [DOI] [PubMed] [Google Scholar]
- 45.Piccaluga PP, Fuligni F, De Leo A, et al. Molecular profiling improves classification and prognostication of nodal peripheral T-cell lymphomas: results of a phase III diagnostic accuracy study. J Clin Oncol. 2013;31(24):3019–3025. doi: 10.1200/JCO.2012.42.5611. [DOI] [PubMed] [Google Scholar]
- 46.Agnelli L, Mereu E, Pellegrino E, et al. Identification of a 3-gene model as a powerful diagnostic tool for the recognition of ALK-negative anaplastic large-cell lymphoma. Blood. 2012;120(6):1274–1281. doi: 10.1182/blood-2012-01-405555. [DOI] [PubMed] [Google Scholar]
- 47.Iqbal J, Wright G, Wang C, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123(19):2915–2923. doi: 10.1182/blood-2013-11-536359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehrentraut S, Nagel S, Scherr ME, et al. t(8;9)(p22;p24)/PCM1-JAK2 activates SOCS2 and SOCS3 via STAT5. PLoS ONE. 2013;8(1):e53767. doi: 10.1371/journal.pone.0053767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohgami RS, Ma L, Merker JD, Martinez B, Zehnder JL, Arber DA. STAT3 mutations are frequent in CD30+ T-cell lymphomas and T-cell large granular lymphocytic leukemia. Leukemia. 2013;27(11):2244–2247. doi: 10.1038/leu.2013.104. [DOI] [PubMed] [Google Scholar]
- 50.Crescenzo R, Abate F, Lasorsa E, et al. Convergent Mutations and Kinase Fusions Lead to Oncogenic STAT3 Activation in Anaplastic Large Cell Lymphoma. Cancer Cell. 2015;27(4):516–532. doi: 10.1016/j.ccell.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.d’Amore F, Relander T, Lauritzsen GF, et al. Up-Front Autologous Stem-Cell Transplantation in Peripheral T-Cell Lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093–3099. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- 52.Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24(4):596–605. doi: 10.1038/modpathol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sibon D, Fournier M, Briere J, et al. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte trials. J Clin Oncol. 2012;30(32):3939–3946. doi: 10.1200/JCO.2012.42.2345. [DOI] [PubMed] [Google Scholar]
- 54.Damm-Welk C, Mussolin L, Zimmermann M, et al. Early assessment of minimal residual disease identifies patients at very high relapse risk in NPM-ALK-positive anaplastic large-cell lymphoma. Blood. 2014;123(3):334–337. doi: 10.1182/blood-2013-09-526202. [DOI] [PubMed] [Google Scholar]
- 55.Lee HK, Wilder RB, Jones D, et al. Outcomes using doxorubicin-based chemotherapy with or without radiotherapy for early-stage peripheral T-cell lymphomas. Leuk Lymphoma. 2002;43(9):1769–1775. doi: 10.1080/1042819021000006277. [DOI] [PubMed] [Google Scholar]
- 56.Briski R, Feldman AL, Bailey NG, et al. Survival in patients with limited-stage peripheral T-cell lymphomas. Leuk Lymphoma. 2015;56(6):1665–1670. doi: 10.3109/10428194.2014.963078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Escalon MP, Liu NS, Yang Y, et al. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: the M. D. Anderson Cancer Center experience. Cancer. 2005;103(10):2091–2098. doi: 10.1002/cncr.20999. [DOI] [PubMed] [Google Scholar]
- 58.Schmitz N, Trumper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- 59.Dunleavy K, Shovlin M, Pittaluga S, et al. DA-EPOCH Chemotherapy Is Highly Effective in ALK-Positive and ALK-Negative ALCL: Results of a Prospective Study of PTCL Subtypes in Adults. Blood. 2011;118 Abstract 1618. [Google Scholar]
- 60.Fanale MA, Shustov AR, Forero-Torres A, et al. Brentuximab Vedotin Administered Concurrently with Multi-Agent Chemotherapy As Frontline Treatment of ALCL and Other CD30-Positive Mature T-Cell and NK-Cell Lymphomas. Blood. 2012;120 Abstract 60. [Google Scholar]
- 61.Rodriguez J, Conde E, Gutierrez A, et al. Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: a prospective study from The Gel-Tamo Study Group. Eur J Haematol. 2007;79(1):32–38. doi: 10.1111/j.1600-0609.2007.00856.x. [DOI] [PubMed] [Google Scholar]
- 62.Reimer P, Rudiger T, Geissinger E, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27(1):106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- 63.Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31(16):1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]
- 64.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 65.Zinzani PL, Sasse S, Radford J, Shonukan O, Bonthapally V. Experience of brentuximab vedotin in relapsed/refractory Hodgkin lymphoma and relapsed/refractory systemic anaplastic large-cell lymphoma in the Named Patient Program: Review of the literature. Crit Rev Oncol Hematol. 2015;95(3):359–369. doi: 10.1016/j.critrevonc.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 66.Bartlett NL, Chen R, Fanale MA, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014;7:24. doi: 10.1186/1756-8722-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith SM, Burns LJ, van Besien K, et al. Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol. 2013;31(25):3100–3109. doi: 10.1200/JCO.2012.46.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le Gouill S, Milpied N, Buzyn A, et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol. 2008;26(14):2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- 69.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 70.Mosse YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14(6):472–480. doi: 10.1016/S1470-2045(13)70095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]