Abstract
Objectives
Although brief interventions (BIs) have shown some success for smoking cessation and alcohol misuse, it is not known if they can be applied in the emergency department (ED) to drug use and misuse. The objectives of this investigation were to assess the 3-month efficacy of a BI to reduce drug use and misuse, increase drug treatment services utilization among adult ED patients, and identify subgroups more likely to benefit from the BI.
Methods
This randomized, controlled trial enrolled 18- to 64-year-old English- or Spanish-speaking patients from two urban, academic EDs whose responses to the Alcohol, Smoking, and Substance Involvement Screening Test indicated a need for a brief or intensive intervention. Treatment participants received a tailored BI, while control participants only completed the study questionnaires. At the 3-month follow-up, each participant’s past 3-month drug use and misuse and treatment utilization were compared to his or her baseline enrollment data. Regression modeling was used to identify subgroups of patients (per demographic and clinical factors) more likely to stop or reduce their drug use or misuse or engage in drug treatment by the 3-month follow-up assessment.
Results
Of the 1,030 participants, the median age was 30 years (interquartile range = 24 to 42 years), and 46% were female; 57% were white/non-Hispanic, 24.9% were black/non-Hispanic, and 15% were Hispanic. The most commonly misused drugs were marijuana, prescription opioids, cocaine/crack, and benzodiazepines. Although at follow-up the proportions of participants reporting any past 3-month drug misuse had decreased in both study arms (control 84% vs. treatment 78%), the decreases were similar between the two study arms (Δ−6.3%; 95% confidence interval [CI] = −13.0% to 0.0). In addition, at follow-up there were no differences between study arms in those who were currently receiving drug treatment (Δ1.8; 95% CI = −3.5 to 6.8), who had received treatment during the past 3 months (Δ−2.0; 95% CI = −6.5 to 2.4), or who at least contacted a treatment program (Δ 1.7; 95% CI = −2.4 to 6.1). Those whose baseline screening indicated the need for a brief instead of a more intensive intervention, and those currently engaged in drug treatment at the 3-month follow-up, were generally more likely to stop or decrease their drug use/misuse.
Conclusions
The BI employed in this study did not reduce drug use and misuse or increase treatment utilization more than the control condition over a 3-month period. Future research should help determine what role, if any, BIs should play in affecting drug use and misuse among ED patients.
Illicit and prescription drug use and misuse is a tremendous problem in the United States, with serious negative economic, financial, health, and social consequences.1,2 People who use or misuse drugs often receive medical care from U.S. emergency departments (EDs),3–9 frequently due to illnesses and injuries linked to their drug use or misuse.10–13 Yet, screening for drug use or misuse and assessment for treatment needs is not standard practice in U.S. EDs.14 Furthermore, studies have demonstrated that many ED patients who need substance abuse treatment do not receive it.14 Linking patients to appropriate treatment services at the time of the ED visits might decrease ongoing and future misuse, and consequentially more expensive health care.15
Some screening, brief intervention (BI), and referral to treatment (SBIRT) models have been shown in some studies to be effective in reducing alcohol misuse, risk-taking behaviors, and negative consequences of alcohol misuse16–21 and marijuana use22,23 among some ED patients. However, it is not yet been demonstrated that SBIRT can be applied successfully in the ED to other illicit or prescription drug misuse. If SBIRT is effective in the ED for drug misuse by decreasing use and increasing access to appropriate treatment resources, it might help reduce the societal and individual burdens of this public health problem.
The primary aim of this investigation was to determine if a tailored BI aimed to reduce illicit or prescription drug misuse among adult ED patients requiring brief or more intensive intervention was more efficacious than no BI (study questionnaires only) in the short term (3 months after ED enrollment). The secondary aim was to determine if this BI was more efficacious than no BI in increasing use of drug treatment services in the same time period. The tertiary aim was to determine if there were subgroups of patients (identified by their demographic and clinical factors) more likely to benefit from the BI.
METHODS
Study Design
The Brief Intervention for Drug Misuse in the Emergency Department (BIDMED) was a randomized, controlled trial that enrolled participants from July 2010 through December 2012. The hospital institutional review board approved the study. We obtained a certificate of confidentiality from the National Institutes of Health due to the sensitive nature of the drug use and misuse questions and informed study participants about its potential protections and limitations.
Study Setting and Population
The study location was two urban EDs affiliated with a medical school in Providence, Rhode Island. One ED is a Level I trauma center with an annual patient volume of >100,000 adult visits, and the other is a community hospital ED with an annual patient volume of >55,000 adult visits. Rhode Island consistently ranks as having one of the highest percentage of its citizens reporting illicit drug use, and one of the highest reported prevalences of drug dependency in the United States (9% to 13%).24 In 2010–2011, 14.6% of those 18 years or older in the general population of the state reported that they used illicit drugs, 66.2% had consumed alcohol, and 29.7% used tobacco products within the past month.25
A random sample of patients present in each ED during study collection periods was approached and evaluated for study inclusion through random selection of their patient care rooms. If the ED electronic medical record indicated that a patient potentially was eligible, a research assistant (RA) confirmed study eligibility through a brief interview. Data collection for the study was performed from 8:00 AM to midnight 7 days a week when bilingual (English- and Spanish-speaking) RAs were available to conduct the study.
Patients were potentially eligible if they were 18 to 64 years old; English- or Spanish-speaking; not critically ill or injured; not prison inmates, under arrest, nor undergoing home confinement; not presenting for acute psychiatric illness; not requesting treatment for substance use or misuse; not intoxicated; and having no physical or mental impairments preventing them from providing consent or participating in the study. The inclusion and exclusion criteria attempted to mirror the general adult ED population who would be included in an SBIRT program, i.e., excluding those presenting for evaluation of their substance misuse, those acutely intoxicated, and those undergoing formal substance misuse or psychiatric evaluations. ED staff members were not permitted to encourage or refer patients to be in the study. Participants received gift cards to a local pharmacy for completing the baseline and 3-month follow-up.
Study Protocol
Study Questionnaire Content and Administration
Before randomization assignment, the RAs queried ED patients who met initial study eligibility criteria about their demographic characteristics. Patients next completed a confidential, audio computer self-administered interview (ACASI)-based assessment of their substance use and misuse using the Alcohol, Smoking and Substance Involvement Screening Test, Version 3 (ASSIST V.3).26 The adaptation, pilot testing, psychometric assessments, and reading level of this and the other study instruments have been described previously.27 Per World Health Organization recommendations, ED patients with ASSIST scores of at least four points for any drug category or who had ever injected drugs (signifying a need for at least a BI) were invited to enroll in BIDMED.26 ASSIST scores over 26 indicate the need for more intensive treatment, although only a BI was delivered as part of this study protocol.
Following enrollment, participants were randomly assigned 1:1 into the two study arms (treatment vs. control) using block randomization with a block size of 6. Afterward, participants completed a drug misuse frequency questionnaire and the Treatment Services Review questionnaire, which was adapted for this study28 (see Data Supplement S1, available as supporting information in the online version of this paper, for English-language copies of study questionnaires). At 3 months after ED enrollment, participants completed the follow-up questionnaires via the Internet using the ACASI. Participants completed the baseline questionnaires in approximately 10 to 15 minutes. The RAs were blinded to the participant questionnaire responses.
Description of the BI
The primary goal of the BI was to motivate participants to reduce their drug misuse and seek appropriate treatment. A brief outline of the BI content is in Data Supplement S2 (available as supporting information in the online version of this paper). The BI sessions were approximately 20 to 30 minutes in duration and were based on two theoretically driven approaches to behavior change: motivational interviewing29 and the health beliefs model.30 During the BI the RAs took on the role of a health educator and used motivational interviewing techniques (e.g., decisional balance, discussing goals and values)31 to facilitate a discussion about behavior changes. Each BI participant was contacted via telephone for a booster session by the interventionist 2 to 4 weeks after ED enrollment and discussed progress, if any, with reaching the goals outlined during the initial BI session. The RAs met with the study investigators throughout the study to discuss clinical and procedural issues arising from the delivery of the BI. To ensure fidelity to the BI, RAs voice recorded their BI sessions and these were reviewed by the psychologist research team members and discussed with the RAs. Deviations from the BI protocol were addressed, and suggestions for improvement were provided at these review sessions.
Data Analysis
Study eligibility assessments and enrollment were summarized using the recommended Consolidated Standards of Reporting Trials (CONSORT) approach for randomized, controlled trials.32 Participant demographic characteristics were compared by study arm for those enrolled and those who completed the 3-month follow-up. Commensurate with findings from prior drug misuse reduction intervention studies,33–35 we based the sample size needed for BIDMED on the primary aim of a hypothesized 25% greater decrease in drug use and misuse in the treatment versus the control arm by 3-month follow-up. Our estimated sample size was 550 per study arm for an 80% power and a two-sided Type I error rate of 0.05 under this hypothesis. Drug misuse was compared by study arm as follows: 1) differences in proportions of participants reporting any drug use in the past 3 months at 3-month follow-up versus baseline, with corresponding 95% confidence intervals (CIs); 2) changes in drug use/misuse frequency in the past 3 months at 3-month follow-up versus baseline on a continuous scale, using differences in means with corresponding 95% CIs; 3) differences in the distributions of drug use/misuse frequency in the past 3 months at 3-month follow-up on a categorical scale, using Pearson’s chi-square or Fisher’s exact test; and 4) differences in means of total days of drug use, most number of drugs used/day, and typical number of drugs used/ day in the past 3 months at 3-month follow versus baseline, with corresponding 95% CIs. For the secondary outcome of drug treatment utilization, we compared study arms by the differences in proportions (3 months vs. baseline) with corresponding 95% CIs of those who received treatment in the prior 3 months, were currently in treatment, or who sought but did not receive treatment. All 95% CIs were calculated using the bootstrap method with 1,000 resamples to avoid the normality assumption, which is not always feasible in comparing differences in proportions and frequencies of drug use when data are sparse or skewed. For the tertiary aim, we constructed multivariable models to examine demographic, drug use and misuse, and treatment factors associated with no drug use at 3-month follow-up (logistic regression); decrease in total days of drug use; most number of drugs used/day; and typical number of drugs used/day (3-month follow-up vs. baseline; linear regression); and receipt of treatment within the prior 3 months (logistic regression). Separate models were created for all drugs, marijuana only, and all drugs except marijuana for the drug frequency analyses. Variables with theoretical reasons for being associated with the outcome (e.g., treatment arm, need for a BI vs. intensive intervention, usual source of medical care) and other factors from the univariable analyses related to the outcome were included in the multivariable models. Hosmer-Lemeshow testing was used to confirm multivariable logistic model fitness. All analyses were conducted using STATA 13.1.
RESULTS
Participant Enrollment and Characteristics
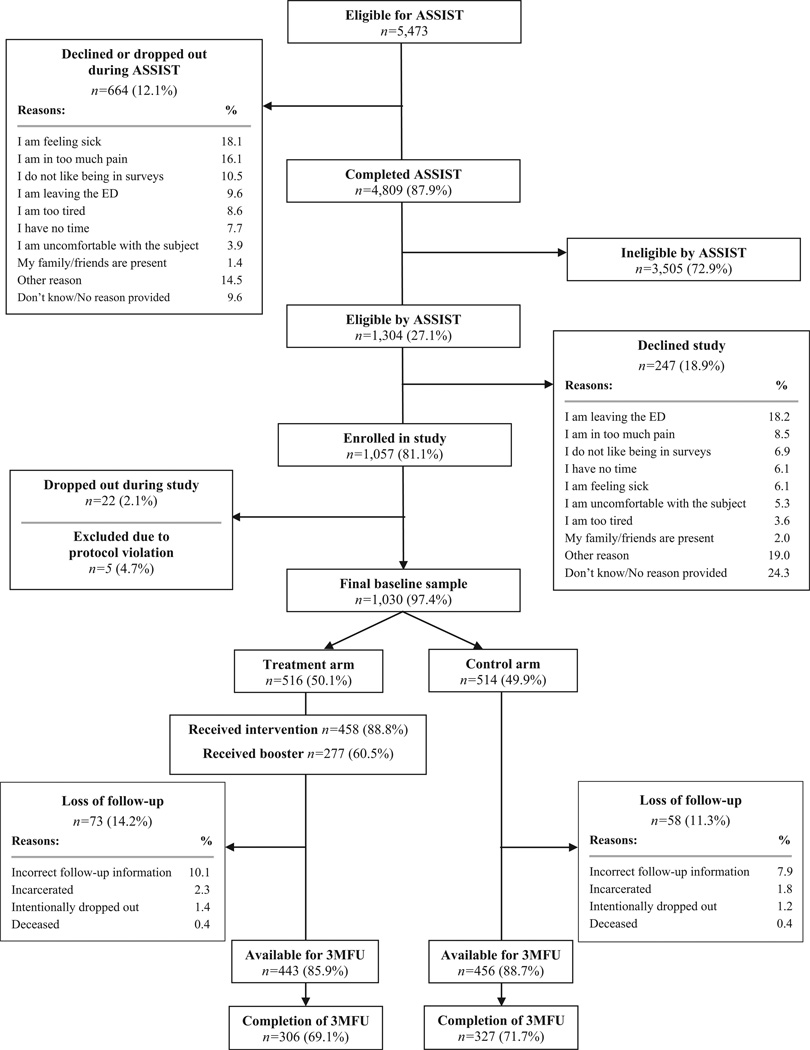
Participant enrollment and retention are depicted in Figure 1. The demographic characteristics of those who enrolled in the study (baseline) and completed the 3-month follow-up were similar (Data Supplement S3, available as supporting information in the online version of this paper). Baseline substance misuse and frequency of substance use or misuse by category also were similar by study arm (Data Supplement S4, available as supporting information in the online version of this paper). The baseline proportions of participants who ever had received drug misuse treatment also were similar (35.5% control vs. 36.6% treatment; Δ−1.1%, 95% CI = −6.0% to 8.7%).
Figure 1.
Eligibility assessment and enrollment flow diagram. ASSIST = The Alcohol, Smoking and Substance Involvement Screening Test; 3MFU = Three Month Follow-Up.
Change in Drug Use/Misuse
For most of the 12 drug categories, drug use or misuse did decrease from baseline by the 3-month follow-up mark in both study arms (Table 1). However, there were no differences between the two study arms in total drug use or misuse from baseline to 3-month follow-up, whether including or excluding marijuana in the total drug use or misuse calculations. Whether measured on a continuous scale (Table 2) or categorical scale (Data Supplement S5, available as supporting information in the online version of this paper), total drug use and misuse frequency also did not differ by study arm (including or excluding marijuana) by the 3-month follow-up. For all drugs including or excluding marijuana, total days of drug use and misuse, number of times typically using or misusing drugs per day, and most number of times using or misusing drugs in a day (Data Supplement S6, available as supporting information in the online version of this paper) did not differ by study arm at 3-month follow-up. As shown in the multivariable regression analyses, for all drugs including marijuana, those who qualified for brief instead of a more intensive intervention according to their ASSIST scores at baseline and those currently engaged in drug treatment at the 3-month follow-up were generally more likely to stop or decrease their drug use or misuse (Data Supplement S7, available as supporting information in the online version of this paper). Those requiring brief instead of intensive intervention (as indicated by baseline ASSIST scores) and those who had had received treatment within the past 3 months had reductions in the number of times using drugs and most times using per day. For all drugs excluding marijuana, the results were similar, except those randomly assigned to the treatment arm were marginally more likely to stop their drug use or misuse, as were those who were not white or Hispanic (Data Supplement S8, available as supporting information in the online version of this paper). Data Supplement S9 (available as supporting information in the online version of this paper) portrays the subgroups whose marijuana use/misuse differed at 3-month follow-up. Univariable analysis results are provided in Data Supplement S10 (available as supporting information in the online version of this paper).
Table 1.
Differences in any Drug Use/Misuse by Study Arm to Baseline Versus 3-Month Follow-up
| Control Arm (n = 327) |
Treatment Arm (n = 306) |
||||||
|---|---|---|---|---|---|---|---|
| Substance categories | Baseline, % |
3-Month Follow-up, % |
Baseline -3-Month Follow-up, Δ1% (95% CI) |
Baseline, % |
3-Month Follow-up % |
Baseline -3-Month Follow-up, Δ2% (95% CI) |
Control Arm - Treatment Arm, Δ1% - Δ2% (95% CI) |
| Marijuana | 82.9 | 69.0 | 13.9 (9.0 to 18.0) | 81.1 | 63.7 | 17.4 (12.0 to 22.0) | −3.5 (−10.0 to 3.0) |
| Cocaine or crack | 22.7 | 13.8 | 8.9 (5.0 to 13.0) | 21.3 | 14.1 | 7.2 (3.0 to 11.0) | 1.7 (−4.0 to 8.0) |
| Methamphetamines | 5.9 | 3.7 | 2.2 (−1.0 to 5.0) | 4.3 | 2.3 | 2.0 (0.0 to 4.0) | 0.2 (−3.0 to 4.0) |
| Inhalants | 3.4 | 2.2 | 1.2 (0.0 to 3.0) | 2.6 | 2.0 | 0.6 (−1.0 to 3.0) | 0.6 (−2.0 to 3.0) |
| Hallucinogens | 5.6 | 4.6 | 1.0 (−2.0 to 4.0) | 3.3 | 2.3 | 1.0 (−1.0 to 3.0) | 0.0 (−3.0 to 3.0) |
| Illicit opioids | 10.5 | 6.2 | 4.3 (1.0 to 8.0) | 8.9 | 8.9 | 0.0 (−3.0 to 3.0) | 4.3 (0.0 to 9.0) |
| Gamma hydroxybutyrate (GHB) | 0.9 | 0.6 | 0.3 (−1.0 to 1.0) | 0.3 | 0.7 | −0.4 (−1.0 to 0.0) | −0.1 (−1.0 to 2.0) |
| Amphetamines | 6.2 | 5.3 | 0.9 (−2.0 to 4.0) | 6.6 | 3.0 | 3.6 (1.0 to 6.0) | −2.7 (−7.0 to 1.0) |
| Benzodiazepines | 16.3 | 14.0 | 2.3 (−2.0 to 7.0) | 13.1 | 8.9 | 4.2 (0.0 to 8.0) | −1.9 (−8.0 to 4.0) |
| Barbiturates | 1.5 | 0.6 | 0.9 (−1.0 to 3.0) | 0.7 | 0.7 | 0.0 (−1.0 to 1.0) | 0.9 (−1.0 to 3.0) |
| Methadone | 5.5 | 4.7 | 0.8 (−2.0 to 4.0) | 5.9 | 4.9 | 1.0 (−2.0 to 4.0) | −0.2 (−4.0 to 4.0) |
| Prescription opioids | 24.6 | 20.5 | 4.1 (−1.0 to 9.0) | 21.7 | 15.5 | 6.2 (2.0 to 10.0) | −2.1 (−9.0 to 4.0) |
| Total drugs including marijuana | 100.0 | 84.1 | 15.9 (12.0 to 20.0) | 100.0 | 77.8 | 22.2 (18.0 to 27.0) | −6.3 (−13.0 to 0.0) |
| Total drugs excluding marijuana | 48.6 | 40.1 | 8.5 (3.0 to 14.2) | 43.5 | 30.1 | 13.4 (8.4 to 18.2) | −4.9 (−12.5 to 2.8) |
Table 2.
Differences in Past 3-Month Drug Use/Misuse Frequency on a Continuous Scale by Study Group to Baseline Versus 3-Month Follow-up
| Control Group (n = 327) |
Treatment Group (n = 306) |
||||||
|---|---|---|---|---|---|---|---|
| Substance Categories | Baseline, x̄(SE) |
3-Month Follow-up, x̄(SE) |
Baseline −3-Month Follow-up Δ1x̄ (95% CI) |
Baseline, x̄(SE) |
3-Month Follow-up, x̄(SE) |
Baseline −3-Month Follow-up, Δ2x̄ (95% CI) |
Control Group - Treatment Group, Δ1x̄ - Δ2x̄ (95% CI) |
| Marijuana | 3.56 (0.12) | 3.09 (0.13) | 0.47 (0.28 to 0.68) | 3.64 (0.12) | 2.88 (0.14) | 0.76 (0.54 to 0.99) | −0.29 (−0.59 to 0.02) |
| Cocaine or crack | 0.69 (0.08) | 0.44 (0.07) | 0.25 (0.12 to 0.38) | 0.74 (0.09) | 0.46 (0.07) | 0.28 (0.13 to 0.44) | −0.03 (−0.24 to 0.16) |
| Methamphetamines | 0.17 (0.04) | 0.10 (0.03) | 0.07 (−0.02 to 0.16) | 0.13 (0.04) | 0.08 (0.03) | 0.05 (−0.01 to 0.13) | 0.02 (−0.11 to 0.12) |
| Inhalants | 0.11 (0.03) | 0.08 (0.03) | 0.03 (−0.03 to 0.09) | 0.10 (0.04) | 0.06 (0.02) | 0.04 (−0.02 to 0.12) | −0.01 (−0.11 to 0.08) |
| Hallucinogens | 0.15 (0.04) | 0.13 (0.04) | 0.02 (−0.08 to 0.12) | 0.09 (0.03) | 0.06 (0.02) | 0.03 (−0.02 to 0.08) | −0.01 (−0.12 to 0.10) |
| Illicit opioids | 0.37 (0.07) | 0.23 (0.05) | 0.14 (0.00 to 0.27) | 0.32 (0.06) | 0.38 (0.08) | −0.06 (−0.18 to 0.05) | 0.20 (0.01 to 0.37) |
| Gamma hydroxy butyrate (GHB) | 0.02 (0.01) | 0.03 (0.02) | −0.01 (−0.05 to 0.03) | 0.01 (0.01) | 0.03 (0.02) | −0.02 (−0.06 to 0.00) | 0.01 (−0.04 to 0.07) |
| Amphetamines | 0.19 (0.04) | 0.17 (0.04) | 0.02 (−0.09 to 0.12) | 0.12 (0.05) | 0.11 (0.04) | 0.01 (0.01 to 0.17) | 0.01 (−0.21 to 0.06) |
| Benzodiazepines | 0.56 (0.08) | 0.47 (0.07) | 0.09 (−0.05 to 0.25) | 0.44 (0.07) | 0.32 (0.06) | 0.12 (−0.03 to 0.27) | −0.03 (−0.24 to 0.20) |
| Barbiturates | 0.06 (0.03) | 0.03 (0.02) | 0.03 (−0.04 to 0.10) | 0.02 (0.01) | 0.03 (0.02) | −0.01 (−0.06 to 0.02) | 0.04 (−0.05 to 0.12) |
| Methadone | 0.20 (0.05) | 0.18 (0.05) | 0.02 (−0.08 to 0.12) | 0.20 (0.05) | 0.22 (0.06) | −0.02 (−0.12 to 0.10) | 0.04 (−0.12 to 0.18) |
| Prescription opioids | 0.87 (0.09) | 0.70 (0.08) | 0.17 (−0.02 to 0.37) | 0.75 (0.09) | 0.60 (0.09) | 0.15 (0.00 to 0.30) | 0.02 (−0.22 to 0.27) |
| Total drugs including marijuana | 6.94 (0.32) | 5.63 (0.31) | 1.31 (0.57 to 2.11) | 6.63 (0.28) | 5.20 (0.34) | 1.43 (0.83 to 2.04) | −0.10 (−1.12 to 0.87) |
| Total drugs excluding marijuana | 3.37 (0.32) | 2.55 (0.28) | 0.82 (0.18 to 1.51) | 2.98 (0.28) | 2.32 (0.32) | 0.66 (0.14 to 1.15) | 0.16 (−0.65 to 1.02) |
x̄ = mean; SE = standard error.
Change in Drug Treatment Utilization
At 3-month follow-up, the difference in proportions of participants (past 3 months vs. baseline) currently receiving treatment, who received treatment in the past 3 months, contacted treatment programs only, or did not receive treatment was similar between study arms (Table 3). As shown in the multivariable regression analyses (Data Supplement S11, available as supporting information in the online version of this paper), participants who qualified for intensive intervention by their ASSIST scores at baseline and those 30 years old and older generally were more likely to currently be in or have received treatment in the past 3 months by the 3-month follow-up mark. This outcome did not differ between treatment arms.
Table 3.
Differences in Drug Treatment Utilization at 3-Month Follow-up by Study Group, Baseline Versus 3-Month Follow-up (N = 633)
| Control Group (n = 327) |
Treatment Group (n = 306) |
||||||
|---|---|---|---|---|---|---|---|
| Baseline, % |
3-Month Follow-up, % |
Baseline – 3-month Follow-up, Δ1% (95% CI) |
Baseline, % |
3-Month Follow-up, % |
Baseline – 3-Month Follow-up, Δ2% (95% CI) |
Control Group – Treatment Group, Δ1% – Δ2% (95% CI) |
|
| Currently receiving treatment |
8.6 | 11.9 | −3.4 (−6.7 to 0.3) | 8.5 | 13.7 | −5.2 (−8.8 to −1.3) | 1.8 (−3.5 to 6.8) |
| Received treatment in the past 3 months, but not currently receiving treatment |
5.5 | 5.5 | 0.0 (−3.1 to 3.1) | 6.5 | 4.6 | 2.0 (−1.6 to 5.6) | −2.0 (−6.5 to 2.4) |
| Contacted treatment only in the past 3 months |
6.4 | 2.5 | 4.0 (1.0 to 7.3) | 4.6 | 2.3 | 2.3 (−0.3 to 4.9) | 1.7 (−2.4 to 6.1) |
| No contact, no treatment in past 3 months |
79.5 | 80.1 | −0.6 (−4.6 to 3.7) | 80.4 | 79.4 | 1.0 (−4.2 to 6.2) | −1.6 (−8.3 to 5.5) |
Note: Participants who responded with “don’t know” or “refuse to answer” were considered to be in the “No contact, no treatment” category at 3 months. The sum of categories at 3-month follow-up should then equal 100% by study arm (e.g., currently receiving treatment, received treatment past 3 months, contacted only, and no contact or treatment in past 3 months. All CIs are calculated using the bootstrap method with 1,000 resamples.
DISCUSSION
The results of this randomized, controlled trial are disappointing, especially given the need for drug misuse interventions, as shown by the observed high prevalence of illicit and prescription drug use/misuse by adult ED patients in the United States.3–9 Saitz et al.36 and Roy-Byrne et al.37 recently reported similar null findings of a BI designed to affect drug misuse among outpatient clinic patients in Boston, Massachusetts, and Washington State, respectively, as did Woodruff et al.38 for ED patients in San Diego, California. Our results also appear to support the conclusions reached in a recent commentary by Hingson and Compton39 who voiced a need to “return to the drawing board” for BIs and drug misuse.
Although we recognize that the findings from these studies in tandem indicate that the BIs employed in these studies were not efficacious, it might be premature to ring the death knell of this type of intervention. It should be noted that all of these studies, including this current study we report on, involved successful screening for drug misuse and high participation rates. Accordingly, patients in these settings demonstrated strong interest in having their problems recognized and addressed and showed at least an initial willingness to engage in intervention. Further, drug misuse decreased in the treatment and control arms in all studies. Although the decrease might be explained by social desirability and regression to the mean, it might be due to the intensity of the screening and evaluation instruments, which could have served as an intervention by sparking self-recognition and motivation to change.
In this study in particular, engagement in treatment programs after ED enrollment was poor, and higher treatment utilization might have led to better outcomes. The stark reality is that drug treatment programs are few in number and are difficult to gain access to because of limitations in capacity and restrictions of insurance status.40 Because drug misuse, lower socioeconomic status, and insurance status are strongly related, most of those who need or want drug treatment have great difficulty in accessing it.
Nevertheless, BIs as employed in BIDMED and recently published studies did not confer an advantage over screening and evaluation only. What are the next steps given the results of these studies? Just as pharmacologic therapy for other mental health conditions is paired with behavioral/cognitive approaches, future BIs for drug misuse in these settings might consider the potential synergy of BI with appropriate pharmacologic treatment. Also, more aggressive linkage to care steps along with greater contact with patients after the initial encounter might improve outcomes, given the finding that those who received treatment within the past 3 months were generally more likely to stop or decrease their drug use or misuse. Further, although the results should be interpreted with caution, our study findings suggest more of a benefit for the BI for those needing brief instead of intensive intervention. This interpretation mirrors the conclusions of a systematic review of 16 randomized controlled trials on the effects of BI on reducing alcohol use among adult primary care patients.41 Saitz41 concluded that while there was evidence to support the benefit of BI for unhealthy alcohol use, the effects on dependent alcohol users had not been demonstrated. Future randomized controlled trials that focus on these aspects might indicate if an appropriate BI is more efficacious for these two populations. In addition, addressing the myriad social, economic, and psychological factors that potentiate drug use and misuse in a social work approach concurrently with these other approaches might also be more successful than focusing on drug use or misuse and treatment-seeking.
LIMITATIONS
Patients excluded from the study, and patients at EDs in other locales, might have a dissimilar spectrum of substance use or misuse and socioeconomic and demographic characteristics, and therefore might have responded differently to the BI. Even though confidentiality and fidelity of responses likely was enhanced by the use of the ACASI approach,42–46 and perhaps by the mention of the certificate of confidentiality, we cannot be assured that all participants answered the questions truthfully. As would be expected, other screening strategies and substance misuse evaluation instruments might yield different results. The study interpretation is also limited by the inability to follow up with all participants. The disadvantaged socioeconomic existence these patients live gives further evidence to the problems of their drug misuse and challenges of conducting studies such as these. However, we believe that had we limited enrollment to those who could have been more readily tracked (e.g., having a permanent and confirmed telephone number or address), external validity of the findings would have suffered.
CONCLUSIONS
The brief intervention employed in this randomized controlled trial did not decrease overall drug use or misuse and increase drug treatment services utilization among adult ED patients requiring at least a BI for drug misuse more than screening and evaluation only by 3 months postenrollment. However, the screening and evaluation process with or without the BI might have positively affected these two outcomes, although this cannot be determined definitely by the results of this study. Future research should help determine what role, if any, brief interventions should play in affecting drug use and misuse among ED patients.
The research team gratefully acknowledges the assistance of Ms. Vera Bernardino for preparing the data for analysis and publication, the research assistants who assessed patients for the study and helped coordinate the study (Naira Arellano, Vera Bernardino, Rosalie Berrios-Candelaria, Vianella Burgos, Ian Donaghy, Dora Estrela, Cindy Gonzalez, Alyssa Hozey, Michelle Leveillee, Stefanie Paolino, Ayanaris Reyes, and Becca Rose), and the support of the staff and patients at our two hospitals. The authors also are thankful for the assistance of Michael J. Mello, MD, MPH, and Ted Nirenberg, PhD.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse (R01 DA026066) and the Lifespan/Tufts/Brown Centers for AIDS Research (P30 AI042853). ClinicalTrials.gov identifier: NCT01124591. Dr. Merchant, an associate editor at this journal, had no role in the peer review process or publication decision for this paper.
Footnotes
Presented at the Society for Academic Emergency Medicine annual meeting, San Diego, CA, May 2015.
The authors have no potential conflicts to disclose.
Supporting Information
The following supporting information is available in the online version of this paper:
Data Supplement S1. Demographics questionnaire.
Data Supplement S2. ED BI session outline themes and discussion topics.
Data Supplement S3. Demographics characteristics at baseline and 3 months.
Data Supplement S4. Past 3-month drug use/misuse frequency at baseline by study group among participants who completed the 3-month follow-up.
Data Supplement S5. Past 3-month drug use/misuse at 3-month follow-up on a categorical scale by study group.
Data Supplement S6. Differences in total days of drug use/misuse in the past 3 months.
Data Supplement S7. Multivariable regression analysis of differences in all drug use/misuse including marijuana at 3-month follow-up.
Data Supplement S8. Multivariable regression analysis of differences in all drug use/misuse excluding marijuana at 3-month follow-up.
Data Supplement S9. Multivariable regression analysis of differences in marijuana use/misuse at 3-month follow-up.
Data Supplement S10. Results of univariate analysis – including marijuana, excluding marijuana, and marijuana only.
Data Supplement S11. Univariable and multivariable regression analysis of differences in drug treatment utilization at 3-month follow-up.
References
- 1.Miller TR, Levy DT, Cohen MA, Cox KL. Costs of alcohol and drug-involved crime. Prev Sci. 2006;7:333–342. doi: 10.1007/s11121-006-0041-6. [DOI] [PubMed] [Google Scholar]
- 2.Harwood H, Fountain F, Livermore G. The economic cost of alcohol and drug abuse in the United States, 1992. [Accessed Jul 26, 2015];National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism. 1998 Available at: https://www.ncjrs.gov/App/Publications/abstract.aspx?ID=182086.
- 3.Substance Abuse and Mental Health Services Administration. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. [Google Scholar]
- 4.Blow FC, Walton MA, Barry KL, et al. Alcohol and drug use among patients presenting to an inner-city emergency department: a latent class analysis. Addict Behav. 2011;36:793–800. doi: 10.1016/j.addbeh.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu LT, Swartz MS, Wu Z, Mannelli P, Yang C, Blazer DG. Alcohol and drug use disorders among adults in emergency department settings in the United States. Ann Emerg Med. 2012;60:172–180. doi: 10.1016/j.annemergmed.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson JA, Woychek A, Vaughan D, Seale JP. Screening for at-risk alcohol use and drug use in an emergency department: integration of screening questions into electronic triage forms achieves high screening rates. Ann Emerg Med. 2013;62:262–266. doi: 10.1016/j.annemergmed.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Hankin A, Daugherty M, Bethea A, Haley L. The emergency department as a prevention site: a demographic analysis of substance use among ED patients. Drug Alcohol Depend. 2013;130:230–233. doi: 10.1016/j.drugalcdep.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Sanjuan PM, Rice SL, Witkiewitz K, Mandler RN, Crandall C, Bogenschutz MP. Alcohol, tobacco, and drug use among emergency department patients. Drug Alcohol Depend. 2014;138:32–38. doi: 10.1016/j.drugalcdep.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstantopoulos WL, Dreifuss JA, McDermott KA, et al. Identifying patients with problematic drug use in the emergency department: results of a multisite study. Ann Emerg Med. 2014;64:516–525. doi: 10.1016/j.annemergmed.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivara FP, Mueller BA, Fligner CL, et al. Drug use in trauma victims. J Trauma. 1989;29:462–470. doi: 10.1097/00005373-198904000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Soderstrom CA, Smith GS, Dischinger PC, et al. Psychoactive substance use disorders among seriously injured trauma center patients. JAMA. 1997;277:1769–1774. [PubMed] [Google Scholar]
- 12.Hollander JE, Todd KH, Green G, et al. Chest pain associated with cocaine: an assessment of prevalence in suburban and urban emergency departments. Ann Emerg Med. 1995;26:671–676. doi: 10.1016/s0196-0644(95)70035-8. [DOI] [PubMed] [Google Scholar]
- 13.Hendrickson RG, Cloutier R, McConnell KJ. Methamphetamine-related emergency department utilization and cost. Acad Emerg Med. 2008;15:23–31. doi: 10.1111/j.1553-2712.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 14.Rockett IR, Putnam SL, Jia H, Smith GS. Assessing substance abuse treatment need: a statewide hospital emergency department study. Ann Emerg Med. 2003;41:802–813. doi: 10.1067/mem.2003.189. [DOI] [PubMed] [Google Scholar]
- 15.Friedmann PD, Hendrickson JC, Gerstein DR, Zhang Z, Stein MD. Do mechanisms that link addiction treatment patients to primary care influence subsequent utilization of emergency and hospital care? Med Care. 2006;44:8–15. doi: 10.1097/01.mlr.0000188913.50489.77. [DOI] [PubMed] [Google Scholar]
- 16.Academic EDSBIRT, Collaborative Research. The impact of screening, brief intervention and referral for treatment in emergency department patients’ alcohol use: a 3-, 6- and 12-month follow-up. Alcohol Alcohol. 2010;45:514–519. doi: 10.1093/alcalc/agq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mello MJ, Baird J, Nirenberg TD, Lee C, Woolard R, Longabaugh R. DIAL: a randomised trial of a telephone brief intervention for alcohol. Inj Prev. 2013;19:44–48. doi: 10.1136/injuryprev-2012-040334. [DOI] [PubMed] [Google Scholar]
- 18.Longabaugh R, Woolard RE, Nirenberg TD, et al. Evaluating the effects of a brief motivational intervention for injured drinkers in the emergency department. J Stud Alcohol. 2001;62:806–816. doi: 10.15288/jsa.2001.62.806. [DOI] [PubMed] [Google Scholar]
- 19.D’Onofrio G, Fiellin DA, Pantalon MV, et al. A brief intervention reduces hazardous and harmful drinking in emergency department patients. Ann Emerg Med. 2012;60:181–192. doi: 10.1016/j.annemergmed.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazargan-Hejazi S, Bing E, Bazargan M, et al. Evaluation of a brief intervention in an inner-city emergency department. Ann Emerg Med. 2005;46:67–76. doi: 10.1016/j.annemergmed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein J, Heeren T, Edward E, et al. A brief motivational interview in a pediatric emergency department, plus 10-day telephone follow-up, increases attempts to quit drinking among youth and young adults who screen positive for problematic drinking. Acad Emerg Med. 2010;17:890–902. doi: 10.1111/j.1553-2712.2010.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woolard R, Baird J, Longabaugh R, et al. Project reduce: reducing alcohol and marijuana misuse: effects of a brief intervention in the emergency department. Addict Behav. 2013;38:1732–1739. doi: 10.1016/j.addbeh.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernstein E, Edwards E, Dorfman D, Heeren T, Bliss C, Bernstein J. Screening and brief intervention to reduce marijuana use among youth and young adults in a pediatric emergency department. Acad Emerg Med. 2009;16:1174–1185. doi: 10.1111/j.1553-2712.2009.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Results from the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07-4293) Rockville, MD: Substance Abuse and Mental Health Services Administration; 2007. Substance Abuse and Mental Health Services Administration. [Google Scholar]
- 25.National Survey on Drug Use and Health, 2010 and 2011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration (SAMHSA) [Google Scholar]
- 26.Humeniuk R, Ali R. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) and Pilot Brief Intervention: A Technical Report of Phase II Findings of the WHO ASSIST Project. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 27.Merchant RC, Baird JR, Liu T, Taylor LE, Montague BT, Nirenberg TD. Brief intervention to increase emergency department uptake of combined rapid human immunodeficiency virus and hepatitis C screening among a drug misusing population. Acad Emerg Med. 2014;21:752–767. doi: 10.1111/acem.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien CP. A new measure of substance abuse treatment. Initial studies of the treatment services review. J Nerv Ment Dis. 1992;180:101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd. New York, NY: Guilford Press; 2002. [Google Scholar]
- 30.Rosenstock IM. Historical origins of the health belief model. Health Educ Monographs. 1974;2:328–335. [Google Scholar]
- 31.Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychologist. 2009;64:527–537. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–1194. [PubMed] [Google Scholar]
- 33.Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction. 2001;96:1725–1742. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- 34.Baker A, Lee NK, Claire M, et al. Brief cognitive behavioural interventions for regular amphetamine users: a step in the right direction. Addiction. 2005;100:367–378. doi: 10.1111/j.1360-0443.2005.01002.x. [DOI] [PubMed] [Google Scholar]
- 35.Bashir K, King M, Ashworth M. Controlled evaluation of brief intervention by general practitioners to reduce chronic use of benzodiazepines. Br J Gen Pract. 1994;44:408–412. [PMC free article] [PubMed] [Google Scholar]
- 36.Saitz R, Palfai TP, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312:502–513. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy-Byrne P, Bumgardner K, Krupski A, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312:492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodruff SI, Clapp JD, Eisenberg K, et al. Randomized clinical trial of the effects of screening and brief intervention for illicit drug use: the Life Shift/Shift Gears study. Addiction Sci Clin Pract. 2014;9:8. doi: 10.1186/1940-0640-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hingson R, Compton WM. Screening and brief intervention and referral to treatment for drug use in primary care: back to the drawing board. JAMA. 2014;312:488–489. doi: 10.1001/jama.2014.7863. [DOI] [PubMed] [Google Scholar]
- 40.Williams CT, Latkin CA. Neighborhood socioeconomic status, personal network attributes, and use of heroin and cocaine. Am J Prev Med. 2007;32(6 Suppl):S203–S210. doi: 10.1016/j.amepre.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saitz R. Alcohol screening and brief intervention in primary care: absence of evidence for efficacy in people with dependence or very heavy drinking. Drug Alcohol Rev. 2010;29:631–640. doi: 10.1111/j.1465-3362.2010.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perlis TE, Des Jarlais DC, Friedman SR, Arasteh K, Turner CF. Audio-computerized self-interviewing versus face-to-face interviewing for research data collection at drug abuse treatment programs. Addiction. 2004;99:885–896. doi: 10.1111/j.1360-0443.2004.00740.x. [DOI] [PubMed] [Google Scholar]
- 43.Caldwell DH, Jan G. Computerized assessment facilitates disclosure of sensitive HIV risk behaviors among African Americans entering substance abuse treatment. Am J Drug Alcohol Abuse. 2012;38:365–369. doi: 10.3109/00952990.2012.673663. [DOI] [PubMed] [Google Scholar]
- 44.Islam MM, Topp L, Conigrave KM, et al. The reliability of sensitive information provided by injecting drug users in a clinical setting: clinician-administered versus audio computer-assisted self-interviewing (ACASI) AIDS Care. 2012;24:1496–1503. doi: 10.1080/09540121.2012.663886. [DOI] [PubMed] [Google Scholar]
- 45.Newman JC, Des Jarlais DC, Turner CF, Gribble J, Cooley P, Paone D. The differential effects of face-to-face and computer interview modes. Am J Public Health. 2002;92:294–297. doi: 10.2105/ajph.92.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner CF, Villarroel MA, Rogers SM, et al. Reducing bias in telephone survey estimates of the prevalence of drug use: a randomized trial of telephone audio-CASI. Addiction. 2005;100:1432–1444. doi: 10.1111/j.1360-0443.2005.01196.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.