Abstract
Early after stroke, there is loss of intracortical facilitation (ICF) and increase in short-interval intracortical inhibition (SICI) in the primary motor cortex (M1) contralateral to a cerebellar infarct. Our goal was to investigate intracortical M1 function in the chronic stage following cerebellar infarcts (>4 months). We measured resting motor threshold (rMT), SICI, ICF, and ratios between motor-evoked potential amplitudes (MEP) and supramaximal M response amplitudes (MEP/M; %), after transcranial magnetic stimulation was applied to the M1 contralateral (M1contralesional) and ipsilateral (M1ipsilesional) to the cerebellar infarct in patients and to both M1s of healthy age-matched volunteers. SICI was decreased in M1contralesional compared to M1ipsilesional in the patient group in the absence of side-to-side differences in controls. There were no significant interhemispheric or between-group differences in rMT, ICF, or MEP/M (%). Our results document disinhibition of M1contralesional in the chronic phase after cerebellar stroke.
Keywords: Transcranial magnetic stimulation, Paired pulse, Cerebellar disease, Stroke
Introduction
Cerebellar projections modulate activity in the contralateral primary motor cortex (M1contralesional) through dentothalamocortical projections [1, 2]. Patients with limb ataxia due to cerebellar lesions often present with impairment in dexterity due to grip-load force in coordination [3, 4], prediction of sensory outcome of motor commands, correction of motor commands through internal feedback, and motor learning [5–8]. Acute ischemic damage of deep cerebellar nuclei is known to result in ataxia and loss of excitatory input in M1contralesional. The influence of chronic cerebellar lesions on intracortical motor function has not been investigated.
Transcranial magnetic stimulation (TMS) can be used to evaluate intracortical excitability. A suprathreshold test stimulus given over M1 preceded by a subthreshold stimulus (conditioning stimulus) results in suppression (short-interval intracortical inhibition, SICI) or facilitation (intracortical facilitation, ICF) of test responses [9]. SICI and ICF likely reflect intracortical excitability in separate excitatory and inhibitory neurons, with a relative contribution of spinal mechanisms to ICF [10]. Intra- and intersubject variability is higher for SICI and ICF than for other measures of excitability to TMS such as motor threshold. However, the difference in SICI and ICF between the right and left hemispheres is minimal, making interhemispheric asymmetry a stable neurophysiological marker [11, 12].
Patients with acute cerebellar infarcts and decreased SICI or increased ICF in M1contralesional have poor performance in dexterity tests, while patients without these abnormalities have a performance within the normal range [1]. Interhemispheric asymmetry in SICI and ICF tends to normalize 5 to 6 weeks later in parallel with clinical improvement [1]. Considering that the “lesioned” cerebellum modulates intracortical excitability of M1contralesional [2], we hypothesized that (1) differences in intracortical excitability between M1contralesional and M1ipsilesional to a cerebellar infarct would exist in the chronic phase after a unilateral cerebellar infarct and (2) decreased SICI and increased ICF in M1contralesional would correlate with hand dexterity.
Materials and Methods
Subjects
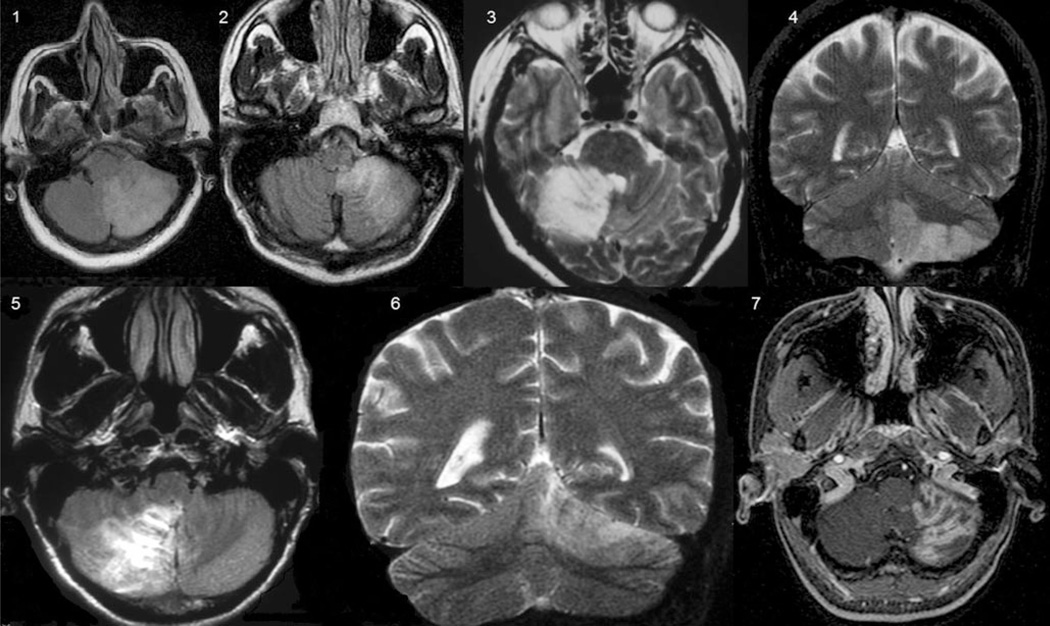
Seven patients (age, 45.8±9.0 years) with chronic (26.7± 34.3 months; range, 4–108 months) cerebellar unilateral infarcts (Fig. 1) and seven age-matched healthy volunteers (47.1±15.6 years) participated in the study. National Institutes of Health Stroke Scale (NIHSS) [13], Jebsen–Taylor test (JTT) [14], and the scale for the assessment and rating of ataxia (SARA) [15], scores are shown in Table 1. NIHSS is a widely clinical scale that provides a quantitative measure of stroke-related neurologic deficit [13]. JTT is an objective and standardized dexterity test designed to assess motor hand functions that reflect daily living activities. It consists of seven unilateral subtests, including writing, turning over cards, picking up small common objects, simulated feeding, stacking checkers, picking up large light objects, and picking up large heavy objects. The score reflects time to complete the tasks [14]. SARA is an eight-item reliable and valid clinical scale used to assess ataxia; scores range from 0 (no ataxia) to 40 (most severe ataxia) [15].
Fig. 1.
Cerebellar infarcts in the territories supplied by the posterior inferior cerebellar artery (1, 2, 4, 5, and 7) and the superior cerebellar artery (3 and 6)
Table 1.
Characteristics and results of transcranial magnetic stimulation in patients and healthy volunteers in the motor cortex ipsilesional or contralesional to cerebellar infarcts
| Age (years) | Sex | Territory | SARA | NIHSS | JTT (s) | rMT contral. | rMT ipsil. | SICI contral. | SICI ipsil. | ICF contral. | ICF ipsil. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | ||||||||||||
| 1 | 63 | F | PICA | 1 | 0 | 108.2 | 45 | 50 | 92.4 | 40.0 | 104.8 | 78.3 |
| 2 | 49 | M | PICA | 6 | 2 | 110.8 | 45 | 47 | 57.3 | 31.9 | 127.7 | 104.5 |
| 3 | 39 | F | SUCA | 11 | 4 | 351.3 | 45 | 51 | 51.1 | 11.8 | 165.4 | 179.0 |
| 4 | 56 | M | PICA | 1 | 0 | 86.3 | 81 | 61 | 88.4 | 62.6 | 162.7 | 96.4 |
| 5 | 41 | M | PICA | 0 | 0 | 76.6 | 50 | 52 | 117.6 | 50.9 | 177.9 | 211.3 |
| 6 | 49 | M | SUCA | 2 | 2 | 77.9 | 47 | 49 | 109.2 | 47.9 | 229.5 | 218.8 |
| 7 | 21 | F | PICA | 1 | 0 | 113.6 | 60 | 48 | 71.8 | 57.1 | 266.1 | 156.4 |
| Healthy volunteers | ||||||||||||
| 1 | 68 | F | 51 | 58 | 113.3 | 139.0 | 141.9 | 363.6 | ||||
| 2 | 45 | M | 76 | 70 | 116.3 | 138.6 | 127.6 | 315.4 | ||||
| 3 | 43 | F | 54 | 51 | 59.6 | 59.7 | 141.7 | 156.4 | ||||
| 4 | 60 | M | 68 | 64 | 44.8 | 233.9 | 277.3 | 299.6 | ||||
| 5 | 38 | M | 48 | 45 | 48.2 | 58.2 | 90.8 | 124.3 | ||||
| 6 | 55 | M | 49 | 49 | 117.5 | 47.2 | 134.7 | 95.3 | ||||
| 7 | 21 | F | 41 | 41 | 70.1 | 35.3 | 185.8 | 185.8 | ||||
SARA scale for the assessment and rating of ataxia, NIHSS National Institutes of Health stroke scale, JTT (s) Jebsen–Taylor test in seconds, rMT resting motor threshold (% of stimulator’s output), contral. contralesional hemisphere, ipsil. ipsilesional hemisphere, rMT contral. resting motor threshold in the contralesional hemisphere, rMT ipsil. resting motor threshold in the ipsilesional hemisphere, SICI short-interval intracortical inhibition (%), ICF intracortical facilitation (%), SICI contral. short-interval intracortical inhibition (%) in the contralesional hemisphere, SICI ipsil. short-interval intracortical inhibition (%) in the ipsilesional hemisphere, ICF contral. intracortical facilitation (%) in the contralesional hemisphere, ICF ipsil. intracortical facilitation (%) in the ipsilesional hemisphere, PICA posterior cerebellar inferior artery, SUCA superior cerebellar artery
A neuroradiologist, blind to clinical and neurophysiological data, classified ischemic lesions according to location and arterial supply (Fig. 1). Patient 2 had a lesion in the posterior medulla, not involving motor tracts. All subjects provided written informed consent, and the protocol was approved by the ethics committee of Hospital das Clínicas/São Paulo University, Brazil.
Experimental Paradigm
TMS was delivered through a figure of eight coil (outside diameter 70 mm, maximum rate of change 22.5 × 103T/s) connected to two 2002 Magstim stimulators through a Bistim2 module (The Magstim Company, Dyfed, UK). TMS measurements were obtained after identification of the hot spot of the abductor pollicis brevis (APB) muscle. Motor-evoked potential (MEP) amplitudes were recorded in the APB contralateral to the stimulated cerebral hemisphere with surface electrodes. MEPs were preamplified and band-pass filtered (2 Hz to 2 kHz).
Resting motor thresholds (rMT) is a measure of corticomotor excitability defined as the minimum TMS intensity required to elicit at least three out of six MEP≥50 µV in consecutive trials at rest. TMS stimulus intensities were expressed relative to rMT measured from the APB [16].
SICI and ICF were determined with a classical protocol using paired-pulse TMS [9]. The intensity of the test stimulus was that required to evoke MEPs (MEPTS) of approximately 0.5–1 mV. The intensity of the conditioning stimulus was 80% of the APB rMT. The order of presentation of inhibitory (2 ms) and facilitatory (10 ms) trials as well as test stimuli alone was randomized. Sixteen trials were recorded for each ISI.
MEPs were recorded at rMT, 130% rMT, and 100% stimulator’s output intensities. M responses were obtained by supramaximal stimulation of the median nerve at the wrist, and results were expressed relative to the maximal peripheral M response peak-to-peak amplitudes (MEP/M, %). This measurement allows controlling for differences in muscle bulk and electrode position across subjects [17] reflecting the extent of activation of the spinal motor neuron pool of a target muscle by a single TMS pulse at a given stimulus intensity [18]. Ten MEP were recorded at each stimulus intensity.
Interhemispheric asymmetry for each TMS measurement was defined in patients as the ratio between results obtained in the two cerebral hemispheres: M1 contralateral to the cerebellar infarct (M1contralesional)/M1 ipsilateral to the cerebellar infarct (Mipsilesional).
The right hemisphere of age-matched healthy volunteers was used as a control for the right hemisphere of patients, and the same procedure was used for the left hemisphere. So, for an age-matched control of a patient with a right cerebellar lesion, the absolute difference was calculated as:
All waveforms were evaluated “off-line” with a playback program written in LabVIEW graphical programming language [19].
Statistical Analysis
We quantified interhemispheric differences in TMS measurements within subject (right and left hemispheres) and across groups (patients and controls) using the Mann–Whitney test. Differences were considered statistically significant if p<0.05.
Distribution of the data was checked with the Shapiro–Wilk test. Correlations between dexterity, evaluated with the Jebsen–Taylor test and TMS measurements, were checked with Spearman’s rho for data not normally distributed. Correlations between time from stroke and interhemispheric asymmetry for SICI and ICF were calculated with Pearson’s r.
Results
Except for patient 3 (SARA score, 11; Jebsen–Taylor score, 351.3), patients were well recovered and did not present severe ataxia. TMS results are shown in Tables 1, 2, and 3 for each individual chronic cerebellar stroke patient and healthy volunteer. There were no significant differences between patients and control subjects in rMT, SICI, ICF, or MEP/M (%) of either M1ipsilesional or M1contralesional. On the other hand, interhemispheric asymmetry in SICI was significantly different between patients and control subjects (p=0.048). In all patients, SICI was decreased in M1contralesional compared to M1ipsilesional (Fig. 2). There were no significant differences between patients and controls regarding interhemispheric asymmetry for rMT, ICF, or MEP/M (%).
Table 2.
Ratios between MEP amplitudes and M amplitudes (MEP/M, %) at different stimulus intensities in motor cortex ipsilesional (ipsil.) or contralesional (contral.) to cerebellar infarcts and in healthy volunteers
| MEP/M (%)rMT contral |
MEP/M (%)rMT ipsil. |
MEP/M (%)130%rMT contral |
MEP/M (%)130% ipsil. |
MEP/M (%)100%contral |
MEP/M (%)100% ipsil. |
|
|---|---|---|---|---|---|---|
| Patients | ||||||
| 1 | 0.5 | 1.2 | 16.2 | 28.6 | 22.8 | 36.1 |
| 2 | 2.3 | 2.5 | 27.4 | 22.3 | 71.1 | 52.2 |
| 4 | 2.2 | 1.0 | 10.4 | 10.2 | 10.4 | 36.2 |
| 5 | 1.0 | 0.1 | 1.5 | 4.9 | 4.4 | 6.3 |
| 6 | 0.1 | 0.5 | 2.4 | 3.4 | 4.5 | 10.1 |
| 7 | 0.5 | 1.4 | 6.8 | 11.9 | 19.7 | 40.9 |
| Healthy volunteers | ||||||
| 1 | 1.9 | 1.5 | 67.5 | 22.1 | 161.3 | 35.0 |
| 2 | 1.8 | 1.9 | 9.4 | 18.8 | 7.8 | 19.6 |
| 3 | 3.6 | 18.6 | 30.9 | 182.7 | 58.6 | 246.3 |
| 4 | 1.1 | 0.7 | 7.4 | 5.9 | 4.7 | 7.8 |
| 5 | 0.3 | 3.1 | 7.7 | 24.2 | 24.8 | 38.2 |
| 6 | 0.4 | 0.4 | 6.5 | 5.2 | 11.5 | 20.7 |
| 7 | 1.6 | 0.8 | 20.3 | 13.3 | 35.3 | 43.0 |
MEP/M (%) relation between motor evoked potential amplitude (MEP) and supramaximal M responses in IPSH and CH, at stimulus intensities corresponding to resting motor threshold (rMT), 130% rMT, and 100% of stimulator’s output, CH contralesional hemisphere, IPSH ipsilesional hemisphere
Table 3.
Ratios between measures of corticomotor excitability in the motor cortex contralesional (contral.)/ipsilesional (ipsil.) to cerebellar infarcts and in healthy volunteers
| rMT ratio | SICI ratio | ICF ratio | MEP/M (%) ratio at rMT |
MEP/M (%) ratio at 130% rMT |
MEP/M (%) ratio at 100% stimulator’s output |
|
|---|---|---|---|---|---|---|
| Patients | ||||||
| 1 | 0.9 | 2.3 | 1.3 | 0.4 | 0.6 | 0.6 |
| 2 | 1 | 1.8 | 1.2 | 0.9 | 1.2 | 1.4 |
| 3 | 0.9 | 4.3 | 0.9 | 7.9 | 7.5 | 1.9 |
| 4 | 1.3 | 1.4 | 1.7 | 2.2 | 1.0 | 0.3 |
| 5 | 1 | 2.3 | 0.8 | 10.0 | 0.3 | 0.7 |
| 6 | 1 | 2.3 | 1.0 | 0.2 | 0.7 | 0.4 |
| 7 | 1.3 | 1.3 | 1.7 | 0.4 | 0.6 | 0.5 |
| Healthy volunteers | ||||||
| 1 | 0.9 | 0.8 | 0.4 | 1.3 | 3.1 | 4.6 |
| 2 | 1.1 | 0.8 | 0.4 | 0.9 | 0.5 | 0.4 |
| 3 | 1.1 | 1.0 | 0.9 | 0.2 | 0.2 | 0.2 |
| 4 | 1.1 | 0.2 | 0.9 | 1.6 | 1.3 | 0.6 |
| 5 | 1.1 | 0.8 | 0.7 | 0.1 | 0.3 | 0.6 |
| 6 | 1.0 | 2.5 | 1.4 | 1.0 | 1.3 | 0.6 |
| 7 | 1.0 | 2.0 | 1.0 | 2.0 | 1.5 | 0.8 |
rMT resting motor threshold, SICI short-interval intracortical inhibition (%), ICF intracortical facilitation (%), MEP/M (%) relation between motor-evoked potential amplitude (MEP) and supramaximal M responses in M1ipsilesional (ipsil.) and M1contralesional (contral.), at stimulus intensities corresponding to resting motor threshold (rMT), 130%rMT, and 100% of stimulator’s output
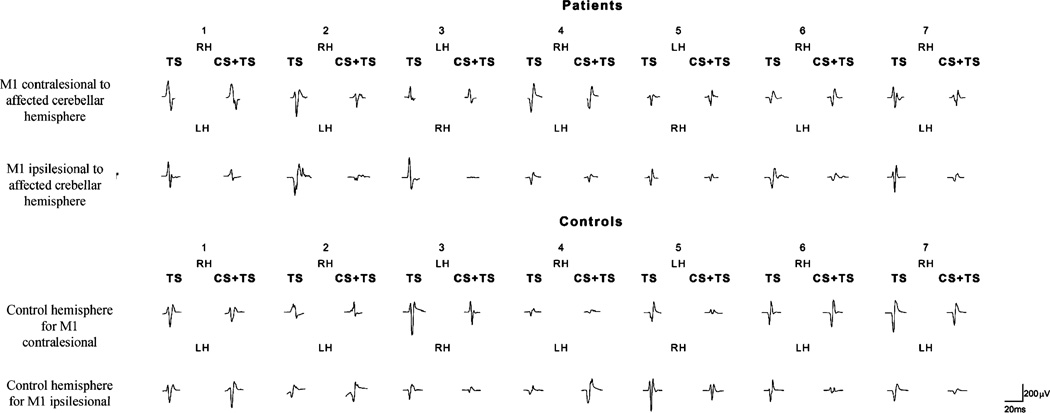
Fig. 2.
Raw data, motor-evoked potentials after administration of test stimulus (TS) or paired pulses (conditioning stimulus, CS, followed by test stimuli, TS) in the right (RH) and left (LH) hemispheres in patients and controls. In patients, decreased inhibition of test responses is noticed in cerebral hemispheres contralateral to cerebellar infarcts compared to hemispheres ipsilateral to cerebellar infarcts. In healthy controls, inhibition of test responses were similar in the two cerebral hemispheres
There was a significant correlation between time from stroke and interhemispheric asymmetry in SICI (r=0.91, p= 0.004) but not in ICF (r=−0.49, p=0.26). Better dexterity, reflected in lower scores in the Jebsen–Taylor test, correlated significantly with decreased SICI inM1contralesional (rho=−0.93; p=0.003). Patient 3 was an outlier, with worse performance in the JTT than the others. However, the significance of the correlation remained present even if this patient was excluded from analysis (rho=−0.89; p=0.019).
Discussion
The main finding of our study was that intracortical inhibition was decreased in M1contralesional compared to M1ipsilesional in all patients with chronic cerebellar infarcts. This effect was greater in patients studied at later stages after stroke onset.
In primates and rodents, deep cerebellar nuclei exert a primarily facilitatory effect on excitability in the opposite primary motor cortex [2, 20]. In monkeys, microstimulation of deep cerebellar nuclei often increases the likelihood of discharge of contralateral M1 neurons [2]. In rats, hemicerebellectomy decreases ICF [21] and blocks facilitatory effects of somatosensory stimulation on M1 excitability [22]. The latter phenomenon is also observed after inhibition of the interpositus nucleus [23, 24]. In humans, TMS or electrical stimulation of the cerebellum, given 5–7 ms before a TMS pulse is administered to the contralateral M1, result in M1 inhibition reflected in decreased MEP amplitudes. This is attributed to preferential excitation of Purkinje cells [25] by TMS or electrical stimulation. Purkinje cells are located more superficially compared to deep nuclei and inhibit the dentate nucleus. Inhibition of this nucleus in turn leads to loss of motor cortical excitation through dentatothalamocortical projections. Cerebellar infarcts in patients, on the other hand, often affect the cerebellar cortex as well as deep cerebellar nuclei, leading acutely to increased contralateral inhibition of M1.
It is then possible that, in the acute phase after a cerebellar stroke, net increase in intracortical inhibition in the contralesional M1 [1] relates to the interruption of these dentate-cortical facilitatory connections [26] and possibly to the documented reduction in blood flow in the hemisphere contralesional to the cerebellar infarct (crossed hemispheric diaschisis). Our findings now indicate that in the chronic stage after a cerebellar infarct, there is a decrease in intracortical inhibition in the contralesional M1 (Fig. 3). These neurophysiologic changes in M1 function seem to impact motor behavior since levels of intracortical inhibition correlated with dexterity in acute patients previously reported [1] and in our cohort of chronic patients in this study. A less likely explanation for our findings in chronic cerebellar patients is that at this stage, the lesion affected differentially dentate–cortical excitatory connections and Purkinje–dentate inhibitory connections, a possibility that we cannot discern with our present data (see Holdefer [2]).
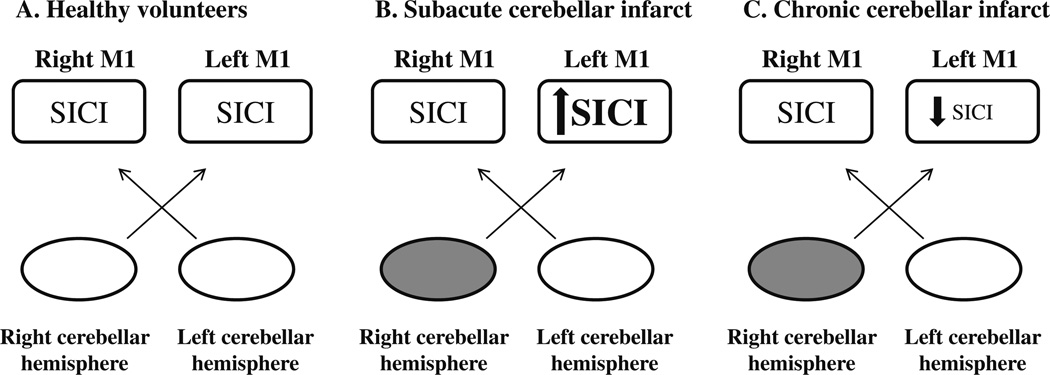
Fig. 3.
Schematic representation of short-interval intracortical inhibition (SICI) in healthy volunteers and patients with stroke. In healthy volunteers, there is symmetry in SICI between M1 in the right and left cerebral hemispheres (A). An imbalance in SICI favors a relative increase in SICI in M1 contralesional to the cerebellar infarct in subacute cerebellar infarcts (B) and a relative decrease in SICI in M1 contralesional to the cerebellar infarcts in the chronic phase (C)
Overall, our results point to a progression of changes in intracortical motor function over time following a contralateral cerebellar stroke leading toward progressive disinhibition of the primary motor cortex (Fig. 3). In other paradigms, disinhibition of M1 has been a marker of intracortical plastic changes following motor learning, practice [27, 28], somatosensory stimulation [29], M1 anodal transcranial direct current stimulation [28], and in pathological conditions such as cerebral hemispheric stroke (for review, see Talelli [30]) and dystonia [31]. In that sense, finding of progressively more intracortical GABAergically mediated [32] disinhibition in M1 that correlated with dexterity may reflect adaptation to the motor control deficits evident in the acute phase post-infarct [1], a role that has been previously assigned to the cerebellum [33, 34]. We cannot rule out the possibility that resolution of diaschisis and plasticity in unaffected cerebellar tissue, thalamus, or motor cortex may contribute to changes in SICI, issues beyond our experimental design. In line with findings in subtypes of cerebellar degeneration [35] and acute infarcts [1] in previous reports, we found no interhemispheric asymmetries in corticomotor excitability or ICF in our patients consistent with a prominent influence of cerebellar efferents on modulation of intracortical function in the opposite primary motor cortex [21].
Conclusion
The modulatory role exerted by the cerebellum on contralateral M1 is crucial to smooth motor performance. Each cerebellar hemisphere compares afferent information during ongoing motor acts and provides the contralateral M1 with appropriate feedback, allowing for adjustments in motor output and control [20] as well as error correction [5, 6, 20]. SICI provides information on M1 intracortical inhibitory circuits, predominantly GABAergic [9], and is influenced by manipulation of sensory input [36], motor training, learning, cerebral hemisphere strokes, and also by cerebellar input. In healthy volunteers, SICI is symmetric. After a unilateral cerebellar infarct, there is an imbalance in output between the lesioned and the unlesioned cerebellar hemispheres: M1contralesional experiences differential changes in SICI (increase in the subacute period [1] and decrease in the chronic period, our results; Fig. 3) compared with M1ipsilesional. We conclude that intracortical M1 function experiences substantial plasticity at different stages following a contralateral cerebellar infarct and that these changes are likely to contribute to resolution of motor impairments present immediately after the ictal event.
Acknowledgments
Dr. Suzete N. Farias received a research scholarship from Fundação Faculdade de Medicina, Clinics Hospital/São Paulo University. We thank Michael Dimyan for helpful comments and suggestions.
Footnotes
Conflict of Interest The authors have no conflicts of interest.
Contributor Information
Suzete Nascimento Farias da Guarda, Email: suzetefarias@terra.com.br, Department of Neurology, Clinics Hospital/São Paulo University, São Paulo, Brazil; Neurostimulation Laboratory, Clinics Hospital/São Paulo University, São Paulo, Brazil; Rua Oito de Dezembro, n 291 ap 1202, Graça, Salvador, Bahia, BrazilCEP 40 150 000.
Leonardo G. Cohen, Human Cortical Physiology and Stroke Neurorehabilitation Section, NINDS, NIH, Bethesda, MD, USA
Marco da Cunha Pinho, Department of Radiology, Clinics Hospital/São Paulo University, São Paulo, Brazil.
Fábio Iuji Yamamoto, Department of Neurology, Clinics Hospital/São Paulo University, São Paulo, Brazil.
Paulo Eurípedes Marchiori, Department of Neurology, Clinics Hospital/São Paulo University, São Paulo, Brazil.
Milberto Scaff, Department of Neurology, Clinics Hospital/São Paulo University, São Paulo, Brazil.
Adriana Bastos Conforto, Department of Neurology, Clinics Hospital/São Paulo University, São Paulo, Brazil; Neurostimulation Laboratory, Clinics Hospital/São Paulo University, São Paulo, Brazil; Instituto Israelita de Ensino e Pesquisa Albert Einstein, São Paulo, Brazil.
References
- 1.Liepert J, Kucinski T, Tüscher O, Pawlas F, Bäumer T, Weiller C. Motor cortex excitability after cerebellar infarction. Stroke. 2004;35:2484–2488. doi: 10.1161/01.STR.0000143152.45801.ca. [DOI] [PubMed] [Google Scholar]
- 2.Holdefer RN, Miller LE, Chen LL, Houk JC. Functional connectivity between cerebellum and primary motor cortex in the awake monkey. J Neurophysiol. 2000;84:585–590. doi: 10.1152/jn.2000.84.1.585. [DOI] [PubMed] [Google Scholar]
- 3.Nowak DA, Topka H, Timmann D, Boecker H, Hermsdörfer J. The role of the cerebellum for predictive control of grasping. Cerebellum. 2007;6:7–17. doi: 10.1080/14734220600776379. [DOI] [PubMed] [Google Scholar]
- 4.Wiesendanger M, Serrien DJ. Toward a physiological understanding of human dexterity. News Physiol Sci. 2001;16:228–233. doi: 10.1152/physiologyonline.2001.16.5.228. [DOI] [PubMed] [Google Scholar]
- 5.Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saywell N, Taylor D. The role of the cerebellum in procedural learning—are there implications for physiotherapists’ clinical practice? Physiother Theory Pract. 2008;24(5):321–328. doi: 10.1080/09593980701884832. [DOI] [PubMed] [Google Scholar]
- 7.Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, et al. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review, Cortex. 2009 doi: 10.1016/j.cortex.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Mandolesi L, Foti F, Cutuli D, Laricchiuta D, Gelfo F, De Bartolo P, et al. Features of sequential learning in hemicerebellectomized rats. J Neurosci Res. 2010;88:478–486. doi: 10.1002/jnr.22220. [DOI] [PubMed] [Google Scholar]
- 9.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticortical inhibition in human motor cortex. J Physiol(Lond) 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, et al. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- 11.Cicinelli P, Traversa R, Oliveri M, Palmieri MG, Filippi MM, Pasqualetti P, et al. Intracortical excitatory and inhibitory phenomena to paired transcranial magnetic stimulation in healthy human subjects: differences between the right and left hemisphere. Neurosci Lett. 2000;288:171–174. doi: 10.1016/s0304-3940(00)01216-7. [DOI] [PubMed] [Google Scholar]
- 12.Cahn SD, Herzog AG, Pascual-Leone A. Paired-pulse transcranial magnetic stimulation: effects of hemispheric laterality, gender, and handedness in normal controls. J Clin Neurophysiol. 2003;20:371–374. doi: 10.1097/00004691-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 14.Jebsen R, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- 15.Schmitz-Hübsch T, Tezenas du Montcel S, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia. Development of a new clinical scale. Neurology. 2006;66:1717–1720. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 16.Hanajima R, Wang R, Nakatani-Enomoto S, et al. Comparison of different methods for estimating motor threshold with transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:2120–2122. doi: 10.1016/j.clinph.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 17.Weber M, Eisen AA. Magnetic stimulation of the central and peripheral nervous systems. Muscle Nerve. 2002;25:160–175. doi: 10.1002/mus.10038. [DOI] [PubMed] [Google Scholar]
- 18.Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaelin-Lang A, Cohen LG. Enhancing the quality of studies using transcranial magnetic and electrical stimulation with a new computer-controlled system. J Neurosci Methods. 2000;102:81–89. doi: 10.1016/s0165-0270(00)00284-3. [DOI] [PubMed] [Google Scholar]
- 20.Evarts EV, Thach WT. Motor mechanisms of the CNS: cerebrocerebellar interrelations. Annu Rev Physiol. 1969;31:451–498. doi: 10.1146/annurev.ph.31.030169.002315. [DOI] [PubMed] [Google Scholar]
- 21.Taib NOB, Manto M. Effects of trains of high-frequency stimulation of the premotor/supplementary motor area on conditioned corticomotor responses in hemicerebellectomized rats. Exp Neurol. 2008;212:157–165. doi: 10.1016/j.expneurol.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Taib NOB, Manto M, Massimo P, Brotchi J. Hemicerebellectomy blocks the enhancement of cortical motor output associated with repetitive somatosensory stimulation in the rat. J Physiol. 2005;567(1):293–300. doi: 10.1113/jphysiol.2005.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luft AR, Manto MU, Taib NOB. Modulation of motor cortex excitability by sustained peripheral stimulation: the interaction between the motor cortex and the cerebellum. Cerebellum. 2005;4:90–96. doi: 10.1080/14734220410019084. [DOI] [PubMed] [Google Scholar]
- 24.Taib NOB, Manto M, Laute MA, Brotchi J. The cerebellum modulates rodent cortical motor output after repetitive somatosensory stimulation. Neurosurgery. 2005;56:811–820. doi: 10.1227/01.neu.0000156616.94446.00. [DOI] [PubMed] [Google Scholar]
- 25.Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clin Neurophysiol. 2002;19(4):322–343. doi: 10.1097/00004691-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Sönmezoğlu K, Sperling B, Henriksen T, Tfelt-Hansen P, Lassen NA. Reduced contralateral hemispheric flow measured by SPECT in cerebellar lesions: crossed cerebral diaschisis. Acta Neurol Scand. 1993;87:275–280. doi: 10.1111/j.1600-0404.1993.tb05507.x. [DOI] [PubMed] [Google Scholar]
- 27.Camus M, Ragert P, Vandermeeren Y, Cohen LG. Mechanisms controlling motor output to a transfer hand after learning a sequential pinch force skill with the opposite hand. Clin Neurophysiol. 2009;120:1859–1865. doi: 10.1016/j.clinph.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards DJ, Krebs HI, Rykman A, Zipse J, Thickbroom GW, Mastaglia FL, et al. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restor Neurol Neurosci. 2009;27(3):199–207. doi: 10.3233/RNN-2009-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celnik P, Hummel F, Harris-Love M, Wolk R, Cohen LG. Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch Phys Med Rehabil. 2007;88:1369–1376. doi: 10.1016/j.apmr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006;117:1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Quartarone A, Rizzo V, Terranova C, Morgante F, Schneider S, Ibrahim N. Abnormal sensorimotor plasticity in organic but not in psychogenic dystonia. Brain. 2009;132:2871–2877. doi: 10.1093/brain/awp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziemann U, Lõnnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- 33.Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol. 2008;21(6):628–633. doi: 10.1097/WCO.0b013e328315a293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29(1):58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamburin S, Fiaschia A, Andreolib A, Marania S, Manganottia P, Zanette G. Stimulus-response properties of motor system in patients with cerebellar ataxia. Clin Neurophysiol. 2004;115:348–355. doi: 10.1016/s1388-2457(03)00357-2. [DOI] [PubMed] [Google Scholar]
- 36.Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]