Abstract
Background
By understanding the global inflammatory effects on distant myopathies, surgeons can better guide the rehabilitative process for burn patients. The authors tested the systemic effect of burn injury on distant injured muscle and native bone using immunohistochemistry and validated a new morphometric analytic modality to reproducibly quantify muscle atrophy using computed tomographic imaging.
Methods
In vivo studies were performed on C57/BL6 mice using an Achilles tenotomy with concurrent burn injury model. Total muscle and bone (tibia and fibula) volume/density were quantified near the site of Achilles tenotomy using micro–computed tomography at 5, 7, and 9 to 12 weeks after surgery. The impact of burn injury on the inflammatory cascade [nuclear factor (NF)-κB, p-NF-κB] and the interconnected protein catabolism signaling pathway (Atrogin-1) was assessed by immunohistochemistry.
Results
Muscle volume and density at the site of Achilles tenotomy in burned mice were significantly diminished compared with nonburned mice at 5 weeks and 9 to 12 weeks. Similar decreases in muscle volume and density were observed when comparing tenotomy to no tenotomy. Cortical bone health remained stable in burn/tenotomy mice compared with tenotomy. Muscle atrophy was associated with up-regulation of p-NF-κB, NF-κB, and Atrogin-1 assessed by immunohistochemistry.
Conclusions
Burn injury significantly decreases muscle volume and density. Increased muscle atrophy using our computed tomographic morphometric analysis correlated with a significant increase in intramuscular inflammatory markers and proteolysis enzymes. This study demonstrates a unique characterization of how burn injuries may worsen local myopathy. Moreover, it provides a novel approach for quantifying muscle atrophy over an expanded period.
Trauma and soft-tissue injury play a significant role in the management of burn patients. Severe burns lead to a sustained hypermetabolic state contributing to a systemic response involving the breakdown of both local and distant soft tissue.1 The breakdown of skeletal muscle leads to mass and functional deficits that may persist for months to years after the original burn insult, thereby impairing recovery.1,2 Muscle wasting is associated with increased risk of septic complications, poor wound healing, and reduced capacity for full rehabilitation after injury.3–5 Moreover, additional soft-tissue trauma (i.e., fractured bones, tendon damage, or muscle contusion) may further exacerbate the recovery process by impeding rehabilitation efforts. In general, the morbidity of burn patients is proportional to the magnitude of the catabolic response and associated with long-term patient outcomes.2,6 Despite advancement in burn, dietary, and surgical protocols, an optimal method of inhibiting muscle atrophy, especially near secondary injuries, has not been demonstrated.
Muscle content and mass are maintained in a delicate balance between protein synthesis and breakdown. The activation of multiple atrophy-related genes, diminished muscle fiber size, and absence in up-regulation of protein synthesis enzymes suggest that proteolysis is driving muscle atrophy in the acute-phase postburn and soft-tissue injury.7 The Ub-proteasome pathway has been regarded as the major mechanism for inducing atrophy after burn and following stress-induced states. MAFbx/Atrogin-1 is a Ub-proteasome E3 ligase that is specific for skeletal and muscle tissue and directs the polyubiquitination of proteins to target them for proteolysis by the 26S proteasome.8–10 Atrogin-1 levels are up-regulated in various muscle wasting conditions, including sepsis, renal failure, diabetes, cancer, muscle unloading, and burn injury.11–16 Furthermore, transgenic mice with null alleles to Atrogin-1 display resistance to muscle wasting in denervated mice, demonstrating the principal role proteasome signaling has in muscle atrophy.8
Trauma also induces muscle atrophy by immobilization-induced unloading. Recent studies using an Achilles tenotomy model demonstrate a significant increase in regional myopathy following initial trauma.17 Regulation of genes involved in the autophagy-lysosomal pathway were found to play a distinct role in muscle atrophy following trauma.17 Autophagy-related signaling is dependent on FOXO3 transcription factor, which also stimulates transcription of Fbxo32/atrogin-1 and Trim63/Murf-1.2,18
Current methods of assessing muscle and bone atrophy states are often arduous, restrictive in the region being quantified, rely on histology, and do not allow for multiple measurements over various time points. We demonstrate a novel approach to measuring total muscle changes caused by burn and trauma using morphometric analysis to quantify both muscle and bone changes over various time points using micro–computed tomography.
Despite our increased understanding of the acute and reconstructive treatment of burn patients, there is a gap in the literature assessing the role of burn injury on distant tissues, including muscle and bone, after concomitant trauma. A significant number of patients being treated for burn wounds have secondary injuries involving both bones and soft tissue (tendons). By understanding the global inflammatory and remote catabolic effects of burn injury, surgeons can better guide the rehabilitative processes. In this article, we examine the combined effect of burn and tenotomy injury on local muscle and bone characteristics. More specifically, we compare changes in (1) muscle volume and composition and (2) bone composition and volume after tenotomy in mice with second-degree burn and those without (sham/nonburn). We also present a novel morphometric tool that may help future studies quantify both muscle and bone changes. Furthermore, we assess the impact of burn injury on the inflammatory cascade [nuclear factor (NF)- κB, p-NF-κB) and the interconnected protein catabolism-signaling pathway (Atrogin-1) to understand the pathogenesis behind this myopathy.
MATERIALS AND METHODS
Animals
C57/BL6 mice from The Jackson Laboratory (Bar Harbor, Me.) were used for all experiments. Animal procedures were conducted under the guidelines provided by the Guide for the Use and Care of Laboratory Animals: Eighth Edition from the Institute for Laboratory Animal Research. We received approval by the Institutional Animal Care and Use Committee of the University of Michigan (PRO0001553).
Burn Injury and Tenotomy Procedure
To assess the systemic effects of burn on local myopathy, we used the modified burned mouse model as described previously19,20 Dorsal hair was removed using clippers under isoflurane anesthesia to expose approximately 30 percent of the total body surface area. The exposed dorsal skin was immersed in a water bath (60°C; n = 16) for 18 seconds to produce a partial-thickness dermal burn. The same procedure was performed on sham/nonburn animals, with the exception of the water bath being room temperature (30°C; n = 13). Buprenorphine (0.01 mg/kg) (Buprenex; Reckitt Benckiser Pharmaceuticals, Inc., Richmond, Va.) was administered every 12 hours for the initial 72 hours after burn injury.
All mice underwent an Achilles tenotomy at the tendon midpoint on the left leg after burn or sham/nonburn injury as described previously19 to represent a model of muscle unloading. Specifically, the Achilles tenotomy was performed using a 1-cm incision on the lateral aspect of the Achilles tendon with subsequent exposure of tendon from its origin to insertion. The Achilles tendon was divided at its midpoint and was left in discontinuity. The incision was then sutured closed. Mouse activity and posture were documented at 9 weeks after tenotomy in comparison with nontenotomy mice. (See Video, Supplemental Digital Content 1, which demonstrates a 9-week posttenotomy injury displaying posture and activity level. Video displays mouse walking on a moving treadmill. The measuring stick in the background was used to assess maximum height of mouse’s injured ankle, http://links.lww.com/PRS/B427. See Video, Supplemental Digital Content 2, which demonstrates a nontenotomy mouse displaying posture and activity level. Video displays mouse walking on a moving treadmill. The measuring stick in the background was used to assess maximum height of the mouse’s injured ankle, http://links.lww.com/PRS/B428.) Activity was assessed using a treadmill exercise session on a flat treadmill at 8 m/minute. Total steps were counted during 1 minute of ambulation. Leg movement was also measured by assessing height of the injured leg during the swing phase or midstance of ambulation.
Micro–Computed Tomography
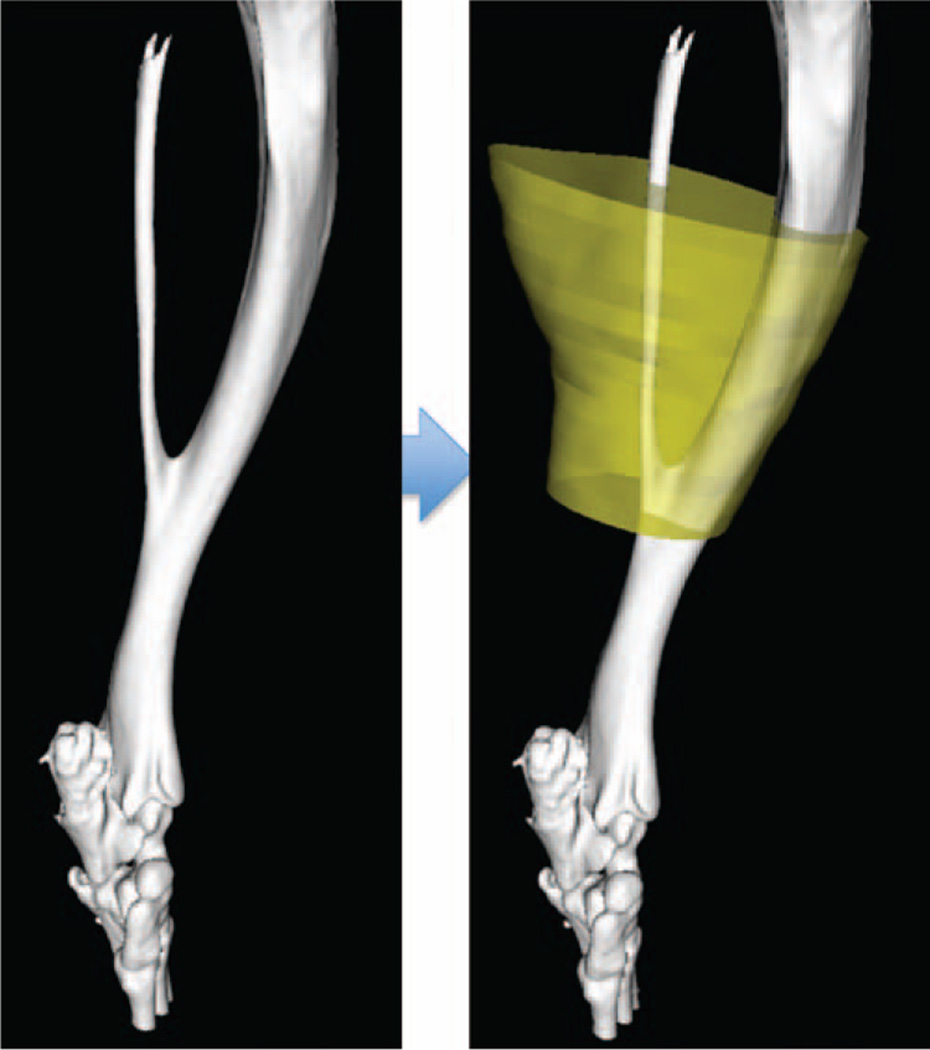
Longitudinal micro-computed tomographic (GE eXplore Locus; GE Healthcare, Little Chalfont, United Kingdom) imaging was performed at multiple time points (5 weeks, 7 weeks, and 9 to 12 weeks) to quantify extent of muscle atrophy and cortical bone changes (Fig. 1). A calibrated imaging protocol using variation in Hounsfield units was used to reconstruct muscle and bone parameters.21 Tibia and fibula fuse point was used as an anatomical landmark to measure 100 slices superior and 30 slices inferior as our region of interest (Fig. 2). Individual computed tomographic slices were splined to define both cortical bone and total muscle volume. Total muscle was quantified using the lowest threshold of bone as our upper exclusion and air as our lower exclusion value. Both total muscle volume (in cubic millimeters) and muscle density (in Hounsfield units) were measured. A similar region of interest and protocol was used to measure total cortical bone. Total bone volume, bone mineral density, and bone mineral content were quantified at each time point. A phantom with calibrated bone, water, and air density was used at the time of micro-computed tomographic imaging of all for standardization.
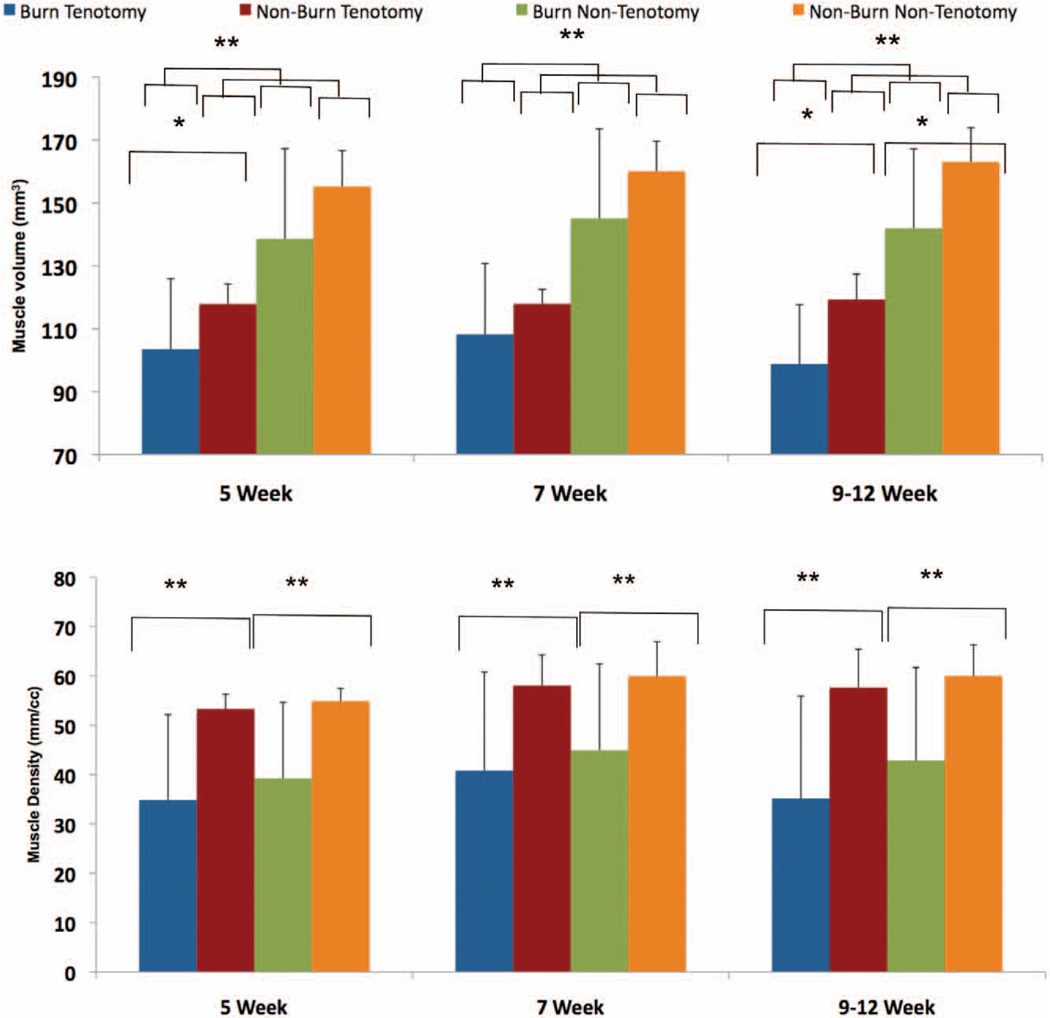
Fig. 1.
Burn injury promotes muscular atrophy. Micro–computed tomographic imaging was used to quantify muscle volume (above) and muscle density (below) at 5-, 7-, and 9- to 12-week time points (*p < 0.05; **p < 0.01).
Fig. 2.
The defined three-dimensional region of interest overlaid in yellow dictates the boundaries of muscle used in micro-computed tomographic analysis for muscle volume and density.
Immunohistochemistry Staining
NF-κB (Santa Cruz Biotechnology, Santa Cruz, Calif.), p-NF-κB (Santa Cruz Biotechnology), and Atrogin-1 (ECM Biosciences, Versailles, Ky.) staining was performed on four slides each from the same region of interest as noted above in burn plus tenotomy and tenotomy alone (sham/ nonburn) mice. Paraffin was removed and rehydrated using xylenes and ethanol. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide. Five percent goat serum in phosphate-buffered saline was used to block individual slides, which were incubated in a 1:100 dilution of primary antibody for 1 hour. The appropriate biotinylated secondary antibody was used in 1:300 dilution for 30 minutes (Vector Laboratories, Burlingame, Calif). The Vectastain ABC system (Vector Laboratories) was used according to the manufacturer’s instructions. Visualization was obtained with diaminobenzidine solution (Zymed Laboratories, South San Francisco, Calif). Microscopy was performed. Slides were visualized at 2× and 40× magnification; p-NF-κB-stained nuclei were quantified by 40× magnification using individuals who were blinded to treatment.
Statistical Analysis
Means and standard deviations were calculated from numerical data as presented in text and figures. Bar graphs represent the means, whereas error bars refer to standard error. Statistical analysis was performed using a two-tailed t test for significance. A value of p < 0.05 was considered statistically significant.
RESULTS
Burn and Tenotomy Injury Lead to Significant Muscle Atrophy and Decreased Density Measured by Micro–Computed Tomography
Prior studies have shown that burn injury and Achilles tenotomy lead to significant muscle atrophy.12,22 These studies used direct weight measurements to quantify the extent of atrophy, which involves killing the animal. We set out to demonstrate a novel approach to measuring muscle volume and density that enables multiple measurements over a defined period. We confirmed previous results, demonstrating a significant decrease in muscle volume in both a burn (nontenotomy 9 to 12 week: burn versus sham, 155 mm3 versus 138 mm3, p = 0.013) and Achilles tenotomy model (tenotomy versus nontenotomy: 5 weeks, 103 mm3 versus 138 mm3, p = 0.0001; 7 weeks, 108 mm3 versus 145 mm3, p = 0.0000002; 9 to 12 weeks, 142 mm3 versus 103 mm3, p = 0.0001) can be quantified using micro–computed tomographic analysis (Fig. 1, above). Interestingly, when assessing the global effect of burn injury on the nontenotomy leg, muscle volume was found to be only significantly impacted at the late time point (9 to 12 weeks, 163 mm3 versus 142 mm3, p = 0.01). Muscle density was significantly diminished in burn mice in both tenotomy and nontenotomy mice. However, tenotomy injury alone did not appear to have a significant impact on muscle density (Fig. 1, below).
Burn Injury Increases Muscle Atrophy and Decreases Muscle Density Near the Site of Tenotomy
We found a significant and sustained decrease in muscle volume at both early (5 week) (burn versus nonburn, 103 mm3 versus 117 mm3, p = 0.049) and late time points (9 to 12 weeks) (burn versus nonburn, 103 mm3 versus 117 mm3, p = 0.019) following burn plus tenotomy compared with tenotomy alone. Similarly, muscle density was significantly decreased at all time points (burn versus nonburn: 5 weeks, 34.9 HU versus 53.3 HU, p = 0.002; 7 weeks, 40.8 HU versus 58.0 HU, p = 0.005; 9 to 12 weeks, 39.9 HU versus 59.6 HU, p = 0.002) in burn mice compared with nonburn mice near the tenotomy site (Fig. 1, above). Interestingly, tenotomy alone did not have a significant effect on muscle density. However, burn injury appears to be the major mediator of decreased muscle density in both tenotomy and nontenotomy legs. Our model suggests that burn injury has an additive effect on muscle unloading by inducing both muscle atrophy and density changes.
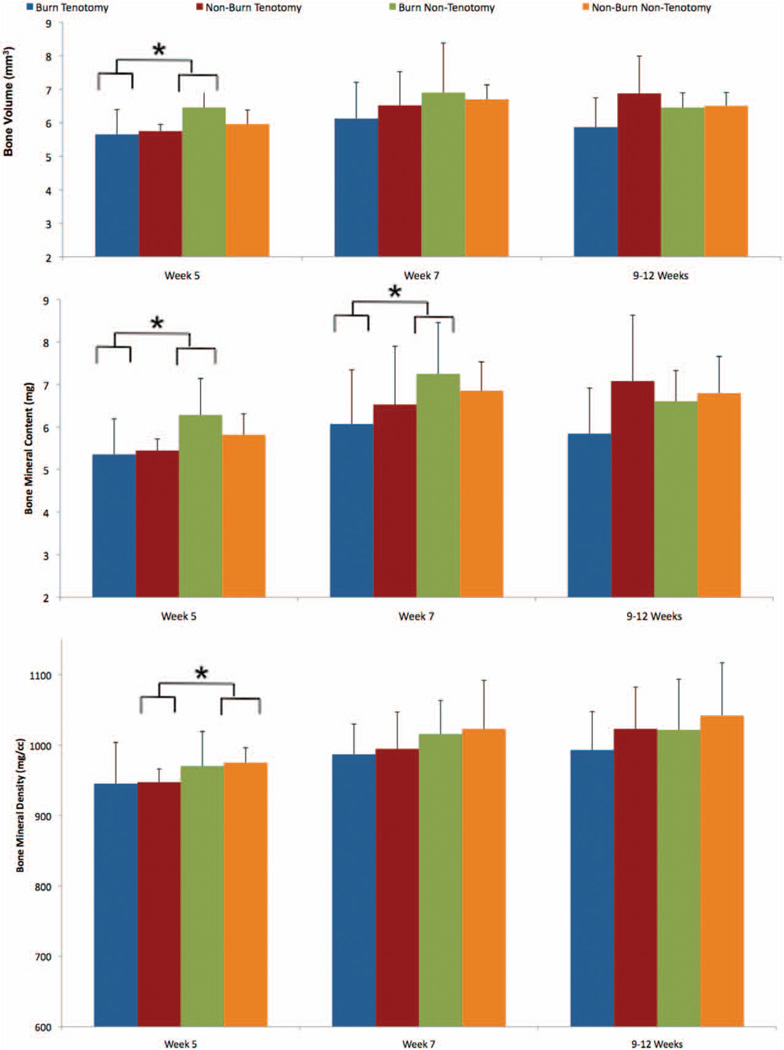
Burn plus Tenotomy Injury Has No Effect on Bone Volume, Bone Mineral Content, or Bone Mineral Density Near the Trauma Site
Studies assessing the impact of burn injury and muscle unloading have demonstrated a direct impact on bone metabolism. Contrary to these previous studies, tenotomy plus concomitant burn injury results in no significant decrease in bone volume, bone mineral content, or bone mineral density compared to tenotomy alone (Figs. 3 and 4). This, however, may be because the burn size differs in previous studies.23,24 When examining the effect of tenotomy, bone volume at 5 weeks (burn/tenotomy versus burn/nontenotomy, 5.65 mm3 versus 6.46 mm3, p = 0.01) and bone mineral content at 5 and 7 weeks (burn versus nonburn: 5 weeks, 5.35 mg versus 6.28 mg, p = 0.012; 7 weeks, 6.07 mg versus 7.26 mg, p = 0.019) were significantly decreased with burn injury (Fig. 3, above and center). In addition, bone mineral density was decreased in nonburn mice with tenotomy compared with nontenotomy at 5 weeks (sham/tenotomy versus sham/nontenotomy, 947 mg/cc versus 974 mg/cc, p = 0.037) (Fig. 3, below).
Fig. 3.
Bone response remains stable despite tenotomy and/or burn insults. Micro–computed tomography was used to quantify bone volume (above), bone mineral content (center), and bone mineral density (below) at 5-, 7-, and 9- to 12-week time points (*p < 0.05; **p < 0.01).
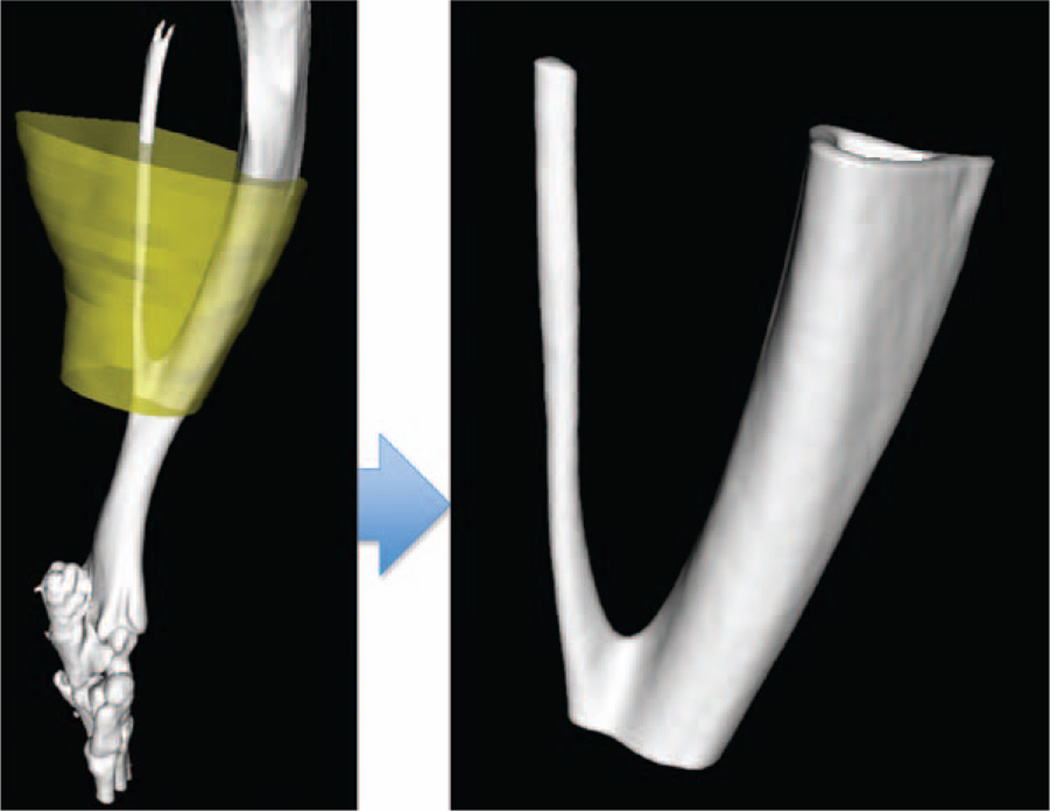
Fig. 4.
The yellow overlay in the left pane (left) delineates the region of interest that defined the borders of the section of analyzed bone. A representative section of analyzed bone is shown (right).
Ambulation Pattern but Not Distance Is Altered by Tenotomy
Given the fact that our mice had a burn injury and tenotomy, we sought to assess whether the tenotomy injury altered mouse ambulation pattern and distance. Interestingly, we found that mice with and without a tenotomy took the same number of steps per minute (Table 1) (see Videos, Supplemental Digital Content 1 and 2, http://links.lww.com/PRS/B427 and http://links.lww.com/PRS/B428, respectively). When looking at their gait, however, the mice with the tenotomy had decreased height of their leg during the swing phase (midstance) of gait (Table 1) (see Videos, Supplemental Digital Content 1 and 2, http://links.lww.com/PRS/B427 and http://links.lww.com/PRS/B428, respectively).
Table 1.
Comparison of Posture and Activity Levels between Tenotomy and Nontenotomy Mice
| Posture/Activity Measurements | Tenotomy following Injury (9 wk) | Nontenotomy |
|---|---|---|
| Frequency of steps taken by tenotomy leg in 1 min | 135 | 126 |
| Maximum height reached by ankle near site of Achilles tenotomy, cm | 0.60 | 1.20 |
Burn plus Tenotomy Injury Is Associated with a Subsequent Rise in Intramuscular Inflammatory Signaling
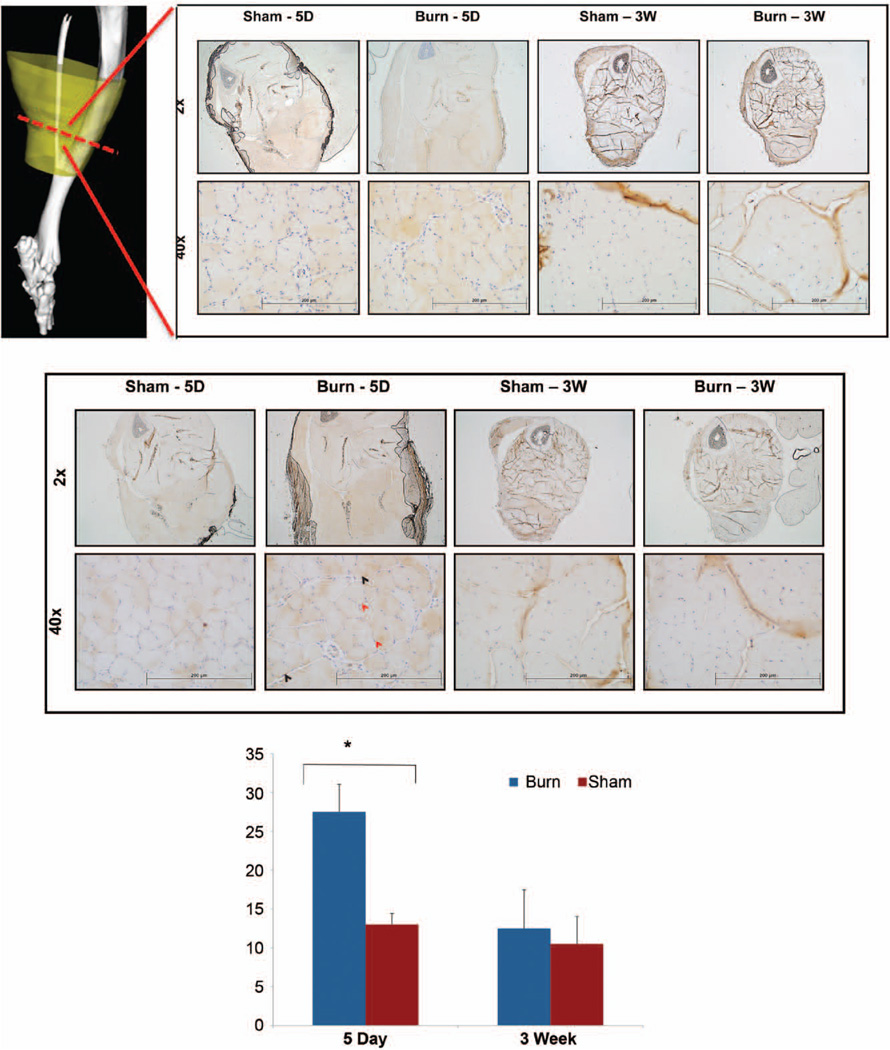
To better understand the mechanism behind the significant increase in muscle atrophy observed following burn plus tenotomy injury, we examined the NF-κB inflammatory signaling cascade because of its link with both burn and muscle unloading atrophy.22,25,26 Qualitative analysis demonstrated an increase in cytoplasmic staining of both NF-κB and p-NF-κB in burn plus tenotomy versus tenotomy alone at 5 days after injury (Fig. 5, above and center). Similarly, relative quantitative analysis of p-NF-κB-stained nuclei demonstrated a significant increase in burn plus tenotomy mice at 5 days after injury compared with nonburn mice (p = 0.037) (Fig. 5, below). Interestingly, a sustained rise in both NF-κB and p-NF-κB is observed at 3 weeks following tenotomy in both burn and nonburn mice, suggesting that NF-κB signaling playing an important role in sustaining muscle atrophy after tenotomy. Qualitative observation shows NF-κB and p-NF-κB signaling to affect the posterior muscle compartment nearest the tenotomy injury. Similar results were seen in a burn atrophy model.12
Fig. 5.
NF-κB and p-NF-κB indicate an elevated expression of p-NF-κB associated with burn status. Micrographs of NF-κB staining shown for burn and sham mice across 5-day and 3-week time points at 2× and 40× magnification levels. Red hashed line indicates the plane of cross-section used in tissue samples (above). Scale bar = 200 µm. Micrographs of p-NF-κB staining shown for burn and sham mice across 5-day and 3-week time points at 2× and 40× magnification (center). Representative stained nuclei are indicated by black arrow. Representative nonstained nuclei are indicated by red arrow. Scale bar = 200 µm. Quantification of nuclei of the gastrocnemius muscle from p-NF-κB slides at 40× are shown graphically for both burn and sham mice (below).
Burn plus Tenotomy Injury Is Associated with Early Up-Regulation of the Ub-Proteasome Pathway
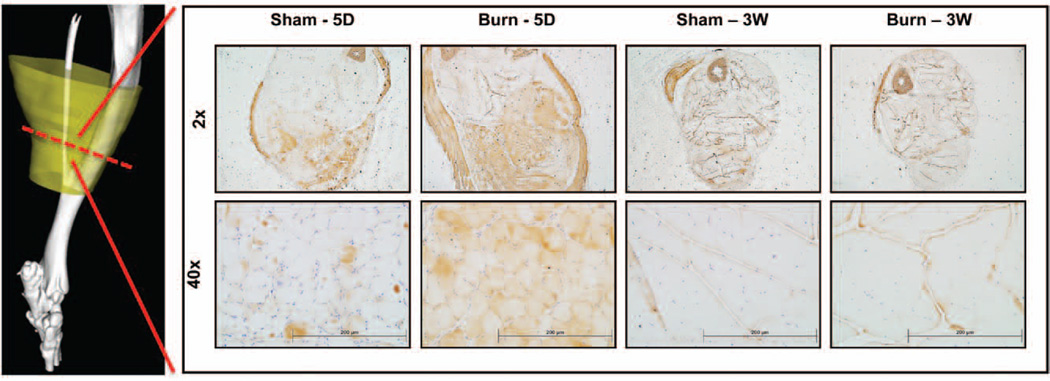
Ub-proteasome expression was examined because of its previously observed function in proteolysis following burn injury.12 We chose to primarily look at Atrogin-1 because of muscle ring finger 1 (MuRF1) mRNA levels being previously shown to return to control levels by 5 days after burn injury, suggesting that MuRF1 does not play a significant role in attenuating sustained muscle breakdown following injury.12 In concordance with prior studies, our results demonstrate a significant acute up-regulation in Atrogin-1 following burn plus tenotomy injury compared with tenotomy alone at 5 days following injury. This up-regulation is observed primarily in the posterior compartment of hind limb with staining involving gastrocnemius, soleus, flexor hallucis, and peroneus longus. However, Atrogin-1 appears to return to control levels at 3 weeks after injury (Fig. 6). Interestingly, Achilles tenotomy is not associated with up-regulation of Atrogin-1, suggesting that tenotomy-induced atrophy stimulates greater NF-κB signaling.
Fig. 6.
Atrogin-1 staining shows stark increases in cytoplasmic expression in early burn. Micrographs of Atrogin-1 staining shown for burn and sham mice across 5-day and 3-week time points at 2× and 40× magnification. Red hashed line indicates the plane of cross-section used in tissue samples. Scale bar = 200 µm.
DISCUSSION
Plastic surgeons play a crucial role in the acute and reconstructive treatment of burn and trauma patients. Surgical treatment often focuses on the direct injury of the burn on skin and soft tissues; however, severe burns and trauma precipitate a global inflammatory response, leading to both local and distant tissue changes.2,27 Loss of muscle and bone mass has significant clinical implications leading to diminished strength and reduced capacity for full rehabilitation after injury.28 By understanding the global inflammatory effects on distant myopathies, surgeons can better guide the rehabilitative process for burn patients. We validated the systemic effect of burn injury on distant injured muscle and native bone using a novel morphometric analysis approach.
This study demonstrates a unique characterization of how burn injuries may worsen local myopathy. We used an Achilles tenotomy with concurrent burn injury model to simulate the effect of burn in trauma patients. Local muscle volume and density near the site of the Achilles tenotomy was significantly reduced in mice that underwent simultaneous burn compared with nonburn mice. The difference in muscle volume and density was quantified using micro–computed tomography. Differences in muscle volume were seen as early as 5 weeks after injury and were sustained up to 12 weeks. Interestingly, changes observed in muscle characteristics vary by mechanism of injury. Burn injury leads to a significant decrease in muscle density compared with tenotomy alone, suggesting that burn injury is a major mediator of diminished muscle density. In addition, burn injury in the nontenotomy leg imparts a significant reduction in muscle volume at late time points. Finally, muscle unloading by tenotomy leads to significant muscle atrophy and overall decreased muscle volume.
As demonstrated in the current study, burn injury has systemic effects that markedly affect the expression of multiple genes that regulate protein metabolism and muscle mass. MuRF1 and muscle atrophy F-box/Atrogin-1 have been shown to be significantly up-regulated in multiple models of skeletal muscle atrophy, including skeletal muscle denervation, immobilization, glucocorticoid treatment, sepsis, cancer, renal failure, and burn injury.8,16,22,29–32 In concordance with these works, Atrogin-1 was significantly up-regulated at 5 days in our burn tenotomy model compared with tenotomy alone, and diminished significantly 3 weeks following injury.
Interestingly, the up-regulation of Atrogin-1 is visualized primarily in the posterior compartments of the tenotomized leg, with predominance toward the gastrocnemius, soleus, flexor hallucis, and peroneus longus. Prior research showed that burn-induced protein degradation is primarily detected in fast-twitch skeletal muscle (gastrocnemius) compared with slow-twitch (soleus).12,33,34 Our findings suggest a direct role for trauma (tenotomy) and concomitant burn injury in inducing local muscle expression of Atrogin-1 and subsequent muscle atrophy. Rates of proteolysis were not directly quantified in our study; however, we present qualitative and physiologic evidence for the important role of Atrogin-1 in mediating burn-induced atrophy.
Burn injury produces a rapid and sustained elevation in circulating inflammatory mediators, including tumor necrosis factor (TNF)-α and interleukin-6.7,35,36 NF-κB has been linked to a systemic rise in TNF-α following burn injury.25,26 Similarly, NF-κB mediates protein loss induced by TNF-α in differentiated skeletal muscle myotubes, and is up-regulated in multiple models of atrophy, which include sepsis, burn, malignancy, and skeletal muscle unloading.22 Transgenic mice using knockout of the p105/p50 NF-κB1 gene demonstrated decreased muscle atrophy in NF-κB (−/−) mice compared with wild type.26 Our laboratory has also demonstrated that trauma induced by Achilles tenotomy produces local elevation in NF-κB.19 Our present results demonstrate similar findings, with significantly increased expression of NF-κB and resultant activation and nuclear translocation of p-NF-κB in burn plus tenotomy mice compared with tenotomy alone. Furthermore, the activation of NF-κB is sustained in both burn plus tenotomy and tenotomy at 3 weeks following injury, suggesting the prominent role NF-κB signaling has in unloading atrophy. Activation of the NF-κB pathway has been previously shown to be sufficient to induce significant atrophy in isolated muscles.
The elevation in the inflammatory cascade mirrors the subsequent rise observed by Atrogin-1 following burn/tenotomy injury. Interestingly, previous research demonstrated that Atrogin-1 expression was not disturbed on NF-κB activation, suggesting that Atrogin-1 up-regulation is not required for muscle atrophy.32,37 Consequently, a separate pathway for Atrogin-1 activation has been proposed through the activation of the p38 MAPK signaling cascade by TNF-α. Based on our findings, increased muscle atrophy observed in burn plus tenotomy appears to be mediated through a combined inflammatory and proteasome mechanism.
Elevated systemic inflammatory markers have also been shown to affect bone composition.38 Continuous TNF-α release has been shown to cause osteopenia, decrease bone volume, and reduce bone mechanical strength through induction of osteoblasts by receptor activator of NF-κB ligand.38,39 Burn injury in combination with muscle unloading was also found to negatively impact cortical bone health. Rats receiving a 40 percent total body surface area burn demonstrated decreased total bone volume, bone mineral density, and bone mineral content.23 Alternatively, mice receiving nonsevere burn injury (15 percent total body surface area) had no significant decrease in cortical bone parameters.24 Similarly, our results demonstrate no significant trend in the difference in bone volume, bone mineral content, and bone mineral density at the site of tenotomy in burn mice compared with nonburn mice. It would be interesting to note whether cortical bone fracture with concomitant burn injury would negatively impact native bone health. Further studies examining this hypothesis need to be examined.
The results in the present study show a sustained up-regulation in the inflammatory cascade and associated rise in proteasome pathway 5 days following burn plus tenotomy injury. Prior studies, as noted above, demonstrate a distinct separation in Atrogin-1 versus NF-κB signaling in promoting muscle atrophy.37 Based on our findings, dualpathway activation stemming from the burn plus tenotomy injury leads to an overall additive effect on muscle atrophy. Clinically, our results suggest that coupled pathway blockade may promote the greatest inhibition of muscle breakdown. Both in vitro and in vivo studies using various proteasome inhibitors, insulin-like growth factor-1, and glucocorticoid receptor antagonist significantly attenuated the accelerated proteolysis produced in burn and sepsis models through inhibition of Atrogin-1.12,34,40,41 Moreover, inhibition of NF-κB activation by eicosapentaenoic acid and antiinflammatory agents (ibuprofen and thalidomide) diminished weight loss among cancer and human immunodeficiency virus–infected patients.42–44 Preclinical studies using curcumin in a sepsis rat model also attenuated muscle loss through NF-κB blockade.45
This study has notable limitations. Most notably, we focus on a single burn/trauma model. In addition, although we observe changes in specific signaling cascades, we have not manipulated the system with inhibitors of this pathway, although these studies are underway. Furthermore, the size of our burn may not be large enough to elicit the changes in the bony skeleton seen in other studies.24
In light of our current results, burn injury appears to exacerbate muscle wasting observed in tenotomy-induced trauma. The ensuing atrophy appears to be mediated through a combined inflammatory and proteasome mechanism. Future studies examining the impact of various pharmaceuticals on muscle wasting conditions can use our methods reported here to assess bone and soft-tissue changes over large regions of interest and multiple time points. Furthermore, bone health appears to remain unchanged as a result of 30 percent total body surface area burn or tenotomy injury, suggesting no beneficial influence of bone-modulating medications.
CONCLUSIONS
Burn injury significantly decreases muscle volume and density in mice with burn injury and preserves native cortical bone. We present a novel methodology of assessing muscle and bone changes in an atrophy model over multiple time points. Muscle atrophy appears to be mediated through a combined inflammatory and proteasome mechanism. This study demonstrates a unique characterization of how burn injuries may worsen local myopathy. Based on our findings, potential inflammatory pathways may be targeted to decrease muscle atrophy at distant sites of secondary injury, thereby improving the rehabilitation process. Furthermore, early tendon repair in burn patients may promote sustained muscle composition and improved rehabilitation potential.
Supplementary Material
Video 1. Supplemental Digital Content 1 demonstrates a 9-week posttenotomy injury displaying posture and activity level. Video displays mouse walking on a moving treadmill. The measuring stick in the background was used to assess maximum height of mouse’s injured ankle, http://links.lww.com/PRS/B427.
Video 2. Supplemental Digital Content 2 demonstrates a nontenotomy mouse displaying posture and activity level. Video displays mouse walking on a moving treadmill. The measuring stick in the background was used to assess maximum height of the mouse’s injured ankle, http://links.lww.com/PRS/B428.
Acknowledgments
Dr. Levy received funding from NIH/NIGMS grant K08GM109105-0, Plastic Surgery Foundation National Endowment Award, the Association for Academic Surgery Roslyn Award and the Research & Education Foundation Scholarship, American Association for the Surgery of Trauma Research & Education Foundation Scholarship, DOD: W81XWH-14-DMRDP-CRMRP- NMSIRA, and American Association of Plastic Surgeons Research Fellowship. We would like to thank Jon Li and Ryan Selley for their assistance.
This work was supported by THE PLASTIC SURGERY FOUNDATION.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the text; simply type the URL address into any Web browser to access this content. Clickable links to the material are provided in the HTML text of this article on the Journal’s Web site (www.PRSJournal.com).
REFERENCES
- 1.Merritt EK, Thalacker-Mercer A, Cross JM, Windham ST, Thomas SJ, Bamman MM. Increased expression of atrogenes and TWEAK family members after severe burn injury in nonburned human skeletal muscle. J Burn Care Res. 2013;34:e297–e304. doi: 10.1097/BCR.0b013e31827a2a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 3.Chandra RK. Nutrition and immunology: From the clinic to cellular biology and back again. Proc Nutr Soc. 1999;58:681–683. doi: 10.1017/s0029665199000890. [DOI] [PubMed] [Google Scholar]
- 4.Wilmore DW. Catabolic illness: Strategies for enhancing recovery. N Engl J Med. 1991;325:695–702. doi: 10.1056/NEJM199109053251005. [DOI] [PubMed] [Google Scholar]
- 5.Ibebunjo C, Martyn J. Disparate dysfunction of skeletal muscles located near and distant from burn site in the rat. Muscle Nerve. 2001;24:1283–1294. doi: 10.1002/mus.1146. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths RD, Jones C, Palmer TE. Outcome of nutrition therapies in the intensive care unit. Nutrition. 1995;11:224–228. [PubMed] [Google Scholar]
- 7.Merritt EK, Cross JM, Bamman MM. Inflammatory and protein metabolism signaling responses in human skeletal muscle after burn injury. J Burn Care Res. 2012;33:291–297. doi: 10.1097/BCR.0b013e3182331e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 9.Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307:E469–E484. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foletta VC, White LJ, Larsen AE, Léger B, Russell AP. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch. 2011;461:325–335. doi: 10.1007/s00424-010-0919-9. [DOI] [PubMed] [Google Scholar]
- 11.Gustafsson T, Osterlund T, Flanagan JN, et al. Effects of 3 days unloading on molecular regulators of muscle size in humans. J Appl Physiol (1985) 2010;109:721–727. doi: 10.1152/japplphysiol.00110.2009. [DOI] [PubMed] [Google Scholar]
- 12.Lang CH, Huber D, Frost RA. Burn-induced increase in atrogin-1 and MuRF-1 in skeletal muscle is glucocorticoid independent but downregulated by IGF-I. Am J Physiol Regul Integr Comp Physiol. 2007;292:R328–R336. doi: 10.1152/ajpregu.00561.2006. [DOI] [PubMed] [Google Scholar]
- 13.Hasselgren P-O. Protein Metabolism in Sepsis. Austin: RG Landes; 1993. [Google Scholar]
- 14.Lang CH, Fan J, Cooney R, Vary TC. IL-1 receptor antagonist attenuates sepsis-induced alterations in the IGF system and protein synthesis. Am J Physiol. 1996;270:E430–E437. doi: 10.1152/ajpendo.1996.270.3.E430. [DOI] [PubMed] [Google Scholar]
- 15.Lecker SH, Jagoe RT, Gilbert A, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 16.Dehoux MJ, van Beneden RP, Fernández-Celemín L, Lause PL, Thissen JP. Induction of MafBx and Murf ubiquitin ligase mRNAs in rat skeletal muscle after LPS injection. FEBS Lett. 2003;544:214–217. doi: 10.1016/s0014-5793(03)00505-2. [DOI] [PubMed] [Google Scholar]
- 17.Bialek P, Morris C, Parkington J, et al. Distinct protein degradation profiles are induced by different disuse models of skeletal muscle atrophy. Physiol Genomics. 2011;43:1075–1086. doi: 10.1152/physiolgenomics.00247.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson JR, Eboda ON, Brownley RC, et al. Effects of aging on osteogenic response and heterotopic ossification following burn injury in mice. Stem Cells Dev. 2015;24:205–213. doi: 10.1089/scd.2014.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ipaktchi K, Mattar A, Niederbichler AD, et al. Topical p38 MAPK inhibition reduces bacterial growth in an in vivo burn wound model. Surgery. 2007;142:86–93. doi: 10.1016/j.surg.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson JR, De La Rosa S, Eboda O, et al. Treatment of heterotopic ossification through remote ATP hydrolysis. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3008810. 255ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Baer LA, Wu X, Tou JC, Johnson E, Wolf SE, Wade CE. Contributions of severe burn and disuse to bone structure and strength in rats. Bone. 2013;52:644–650. doi: 10.1016/j.bone.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Halloran E, Kular J, Xu J, Wood F, Fear M. Non-severe burn injury leads to depletion of bone volume that can be ameliorated by inhibiting TNF-α. Burns. 2015;41:556–564. doi: 10.1016/j.burns.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura H, Aoki K, Masuda W, et al. Disruption of NF-κB1 prevents bone loss caused by mechanical unloading. J Bone Miner Res. 2013;28:1457–1467. doi: 10.1002/jbmr.1866. [DOI] [PubMed] [Google Scholar]
- 26.Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Windsor JA, Hill GL. Weight loss with physiologic impairment: A basic indicator of surgical risk. Ann Surg. 1988;207:290–296. doi: 10.1097/00000658-198803000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecker SH, Solomon V, Price SR, Kwon YT, Mitch WE, Goldberg AL. Ubiquitin conjugation by the N-end rule pathway and mRNAs for its components increase in muscles of diabetic rats. J Clin Invest. 1999;104:1411–1420. doi: 10.1172/JCI7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tawa NE, Jr, Odessey R, Goldberg AL. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J Clin Invest. 1997;100:197–203. doi: 10.1172/JCI119513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li YP, Chen Y, John J, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai J, Wu Y, Sheng Z. The relationship between skeletal muscle proteolysis and ubiquitin-proteasome proteolytic pathway in burned rats. Burns. 2002;28:527–533. doi: 10.1016/s0305-4179(02)00049-9. [DOI] [PubMed] [Google Scholar]
- 34.Fang CH, Li BG, Wang JJ, Fischer JE, Hasselgren PO. Insulinlike growth factor 1 stimulates protein synthesis and inhibits protein breakdown in muscle from burned rats. JPEN J Parenter Enteral Nutr. 1997;21:245–251. doi: 10.1177/0148607197021005245. [DOI] [PubMed] [Google Scholar]
- 35.Finnerty CC, Herndon DN, Przkora R, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 36.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985) 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 37.Cai D, Frantz JD, Tawa NE, Jr, et al. IKKbeta/NF-κappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 38.Głuszko P. Effects of biologic antirheumatic treatments on bone metabolism in rheumatoid arthritis and ankylosing spondylitis (in Polish) Endokrynol Pol. 2009;60:115–121. [PubMed] [Google Scholar]
- 39.Barnabe C, Hanley DA. Effect of tumor necrosis factor alpha inhibition on bone density and turnover markers in patients with rheumatoid arthritis and spondyloarthropathy. Semin Arthritis Rheum. 2009;39:116–122. doi: 10.1016/j.semarthrit.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Fang CH, Li BG, Wray CJ, Hasselgren PO. Insulin-like growth factor-I inhibits lysosomal and proteasome-dependent proteolysis in skeletal muscle after burn injury. J Burn Care Rehabil. 2002;23:318–325. doi: 10.1097/00004630-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Fang CH, Li BG, Wang JJ, Fischer JE, Hasselgren PO. Treatment of burned rats with insulin-like growth factor I inhibits the catabolic response in skeletal muscle. Am J Physiol. 1998;275:R1091–R1098. doi: 10.1152/ajpregu.1998.275.4.R1091. [DOI] [PubMed] [Google Scholar]
- 42.Whitehouse AS, Tisdale MJ. Increased expression of the ubiquitin-proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-κappaB. Br J Cancer. 2003;89:1116–1122. doi: 10.1038/sj.bjc.6601132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMillan DC, Wigmore SJ, Fearon KC, O’Gorman P, Wright CE, McArdle CS. A prospective randomized study of megestrol acetate and ibuprofen in gastrointestinal cancer patients with weight loss. Br J Cancer. 1999;79:495–500. doi: 10.1038/sj.bjc.6690077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS., Jr Inhibition of NF-κappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem. 2001;276:22382–22387. doi: 10.1074/jbc.M100938200. [DOI] [PubMed] [Google Scholar]
- 45.Wyke SM, Russell ST, Tisdale MJ. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-κappaB activation. Br J Cancer. 2004;91:1742–1750. doi: 10.1038/sj.bjc.6602165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Supplemental Digital Content 1 demonstrates a 9-week posttenotomy injury displaying posture and activity level. Video displays mouse walking on a moving treadmill. The measuring stick in the background was used to assess maximum height of mouse’s injured ankle, http://links.lww.com/PRS/B427.
Video 2. Supplemental Digital Content 2 demonstrates a nontenotomy mouse displaying posture and activity level. Video displays mouse walking on a moving treadmill. The measuring stick in the background was used to assess maximum height of the mouse’s injured ankle, http://links.lww.com/PRS/B428.