Abstract
The central complex of the insect brain is a system of midline neuropils involved in transforming sensory information into behavioral outputs. Genetic studies focusing on nerve cells supplying the central complex from the protocerebrum propose that such neurons play key roles in circuits involved in learning the distinction of visual cues during operant conditioning. To better identify the possible sites of such circuits we used Bodian and anti-synapsin staining to resolve divisions of the superior protocerebrum into discrete neuropils. Here we show that in the fly Neobellieria bullata, the superior protocerebrum is composed of at least five clearly defined regions that correspond to those identified in Drosophila melanogaster. Intracellular dye fills and Golgi impregnations resolve “tangential neurons” that have intricate systems of branches in two of these regions. The branches are elaborate, decorated with specializations indicative of pre- and postsynaptic sites. The tangentially arranged terminals of these neurons extend across characteristic levels of the central complex’s fan-shaped body. In this and another blowfly species, we identify an asymmetric pair of neuropils situated deep in the fan-shaped body, called the asymmetric bodies because of their likely homology with similar elements in Drosophila. One of the pair of bodies receives collaterals from symmetric arrangements of tangential neuron terminals. Cobalt injections reveal that the superior protocerebrum is richly supplied with local interneurons that are likely participants in microcircuitry associated with the distal processes of tangential neurons. Understanding the morphologies and arrangements of these and other neurons is essential for correctly interpreting functional attributes of the central complex.
INDEXING TERMS: neuroanatomy, insect brain, protocerebrum, asymmetry, central complex, Neobellieria bullata
The central complex of the insect brain has been shown to be involved both in sensory integration and the control of behavioral actions (Strauss et al., 1992; Liu et al., 2006; Ridgel et al., 2007; Heinze and Homberg, 2007; Ritzmann et al., 2008). It comprises four interconnected midline neuropils. These are the protocerebral bridge, the fan-shaped body, the ellipsoid body, and the paired noduli. A number of satellite neuropils, such as the pair of lateral accessory lobes and their subdomains (Müller et al., 1997), receive connections from the central complex and are critical in the expression of motor actions (Iwano et al., 2010).
The components of the central complex are notable for the precise organization of their constituent neurons (Williams, 1975). In many insect species, neuronal processes projecting within and among central complex neuropils reveal a repeat modular organization (Strausfeld, 1976, 1999; Hanesch, 1989; Heinze and Homberg, 2008). Other descriptions stress the presence of tangential processes that intersect these modules, and which derive from neuropils that are lateral to the central complex (Hanesch et al., 1989; Pereanu et al., 2011). Almost unrecognized, other than in a few accounts (Liu et al., 2006; Ito and Awasaki, 2008; Young and Armstrong, 2010; Li et al., 2010), are inputs to the fan-shaped body that originate from the brain’s superior protocerebrum, a neuropil that is essentially terra incognita, having received very little attention because of its tangled appearance and lack of connections to primary sensory neuropils. These neuropils, which constitute the larger volume of the midbrain, have been referred to as “unstructured” (Liu et al., 2006).
However, the superior protocerebrum is indeed structured (Strausfeld, 1976, 2012; Ito et al., 2012), and its importance in determining behavioral actions is suggested by the observation that many output neurons from the mushroom body terminate in the medial and lateral protocerebrum (Li and Strausfeld, 1997, 1999). Together these pathways imply that a crucial system of protocerebral relays are likely involved in linking the mushroom body indirectly to the central complex (Strausfeld, 2012).
Notably, the central complex does not receive inputs directly from primary sensory neuropils of the brain, nor from ascending sensory relays from thoracic and abdominal ganglia. Mass dye-fills into the ventral nerve cords never show processes in the central complex. Nevertheless, neurons responsive to sensory parameters project to the central complex (Ritzmann et al., 2008), not from primary sensory neuropils but from areas of the brain that receive relays from sensory processing neuropils. For example, in grasshoppers and monarch butterflies neurons sensitive to the e-vector of polarized light project into the protocerebral bridge in a highly organized manner (Heinze and Homburg, 2009; Heinze and Reppert, 2011). These neurons have their dendrites in neuropils lateral to the central complex, in a region called the posterior optic tubercle supplied by outputs from the accessory medulla of the ipsilateral optic lobe (Heinze and Homberg, 2007). Other visual inputs to the bridge are as indirect, such as those originating from the optic glomerular complex (Phillips-Portillo, 2012). The protocerebral bridge, in turn, sends modular relay neurons into the central body (Williams, 1975; Hanesch et al., 1989; Vitzthum and Homberg, 1998; Boyan et al., 2008).
Experimental evidence supports the proposition, made by several authors, that the central complex plays a cardinal role in motor actions, particularly those that involve jointed appendages (Huber, 1960; Strausfeld, 1999; Heinrich et al., 2001; Poeck et al., 2008; Kahsai et al., 2010; Bender et al., 2010). Developmental studies of the mealworm Tenebrio molitor have shown that the elaboration and differentiation of central body architecture matches the development of the legs (Wegerhoff and Breidbach, 1992; Wegerhoff et al., 1996) and that taxa having the most finely controlled leg movements possess fan-shaped body neuropils with exaggerated modular organization (Strausfeld, 1999, 2012).
Genetic manipulations of Drosophila have shown that an intact central complex is also necessary for proper coordination of the limbs during asymmetrical movements such as turning, as evidenced by the mutant nobridge (nob), in which the protocerebral bridge is incompletely formed, disrupts turning (Strauss et al., 1992; Strauss and Heisenberg, 1993; Strauss, 2002). Ridgel et al. (2007) found that lesions to the central complex in the cockroach Blaberus discoidalis similarly result in animals that either made continuous turning movements during walking, or made inappropriate turns when presented with an obstacle that necessitated a change of course.
The central complex has also been suggested to be important in executing actions arising from visual discrimination and learning (Liu et al., 2006; Pan et al., 2009; Triphan et al., 2010). Liu et al. (2006) used a tethered flight preparation (see Wolf and Heisenberg, 1990) to test the ability of wildtype and mutant Drosophila to learn to distinguish between two similar visual patterns. Wildtype animals readily learn to avoid flying toward punished patterns, whereas animals that express a mutant form of the adenylyl cyclase rutabaga protein required for learning (Levin et al., 1992; Zars et al., 2000) perform no better than chance. These studies have drawn special attention to neurons, called “tangential neurons,” that connect the superior protocerebrum with the fan-shaped body where their terminals extend across its extent (Liu et al., 2006). It was proposed that different types of tangential neurons carry information about the discrimination of different pattern parameters — the height of the pattern’s center of mass (elevation), or pattern inclination (contour orientation). Driving expression of rutabaga in these neurons against a mutant rutabaga- deficient background rescues the learned ability to discriminate elevation or orientation (Liu et al., 2006; Wang et al., 2008).
As a step to understand how the central complex relates to the neuronal organization of the superior protocerebrum, we here describe results using reduced silver and anti-synapsin-stained brains that identify distinct anatomical domains of the protocerebrum. Into these have been mapped the arborizations of dye-filled neurons that provide tangential endings across levels of the fanshaped body. We show that, whereas the processes of many of these neurons superficially appear identical in the fan-shaped body, on close examination they constitute distinctive cell types that invade overlapping strata. Their distal arborizations reveal very different morphologies, each relating to a characteristic domain of the superior protocerebrum. These domains, which are associated with systems of local interneurons, feedback pathways, and inputs from other brain area, are likely candidates for the location of integrative circuits between inputs to the dorsal protocerebrum and outputs from it to the central complex.
MATERIALS AND METHODS
Animals
Neobellieria bullata pupae were acquired commercially from Carolina Biological Supply (Burlington, NC) and raised in the laboratory. Adult flies were maintained in mesh cages in environmental chambers and provided with powdered milk, sugar, and water ad libitum. Mainly male adults between 1 week and 1 month after eclosion were used for this study. Calliphora erythrocephala were raised in a laboratory colony. Males of 5–10 days old were used for cobalt-silver staining. Recordings and dye fills were performed on individual animals, as were other tracer methods. Golgi-impregnations were performed on 30 individuals.
Intracellular fills
The dye-filled neurons described here were filled with neurobiotin tracer (Vector Laboratories, Burlingame, CA) during the course of intracellular recordings. For these recordings, animals were cold-immobilized and mounted to a support that stabilized the head. The head-capsule was opened from behind and the perineural sheath removed. Electrodes were drawn from thick-walled borosilicate glass (World Precision Instruments, Sarasota, FL) on a Sutter P-2000 laser puller (Sutter instruments, Novato, CA). Electrode tips were filled with 4% neurobiotin and backfilled with 2M potassium acetate. With these solutions, electrodes had tip resistances between 60–100 MΩ. Neurobiotin was driven into impaled neurons with a series of positive current pulses of 1 nA at a frequency of 1 Hz. Electrodes were aimed at a position estimated to be just lateral and above the superior margin of the fan-shaped body (see Nomenclature, below).
Tissue processing and immunohistology
After injection with neurobiotin, brains were removed from the head capsule while immersed in 4% formaldehyde in phosphate-buffered saline (PBS) adjusted to pH 7.4. Brains were then microwaved at 4°C and allowed to fix overnight again at 4°C. Fixed brains were washed with PBS, dehydrated through ascending concentrations of alcohol, permeabilized with propylene oxide, and then rehydrated. Brains were embedded in agarose (type 1A, Sigma-Aldrich, St. Louis, MO) and the resulting blocks were vibratome-sectioned at 60 µm. The sections were washed in PBS with 0.5% Triton X-100 (EMS, Hatfield, PA) (PBST) and then blocked in 5% normal goat serum (NGS) and PBST. Sections were incubated overnight with an antiserum raised against synapsin (anti-SYNORF1 (mAb3C11) as described by Klagges et al. (1996), obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA), at a concentration of 1:100 in 5% NGS in PBST. Afterwards, sections were again washed and blocked before secondary incubation with fluorescent markers (Cy2 antimouse, and Cy3 conjugated streptavidin, both from Jackson ImmunoResearch, West Grove, PA). Sections were then thoroughly washed in PBS and mounted using elvanol (Rodriguez and Deinhardt, 1960).
Antibody characterization
The anti-SYNORF1 (mAb3C11) antiserum acquired from the Developmental Studies Hybridoma Bank was raised against the 5′ sequence of Drosophila synapsins (Klagges et al., 1996). This antiserum recognizes synapsins from many invertebrate species (anti-SYNORF1 data sheet, Developmental Studies Hybridoma Bank) and the staining pattern in N. bullata is consistent with staining for other synaptic proteins in the brain of D. melanogaster (Young and Armstrong, 2010).
Confocal microscopy and reconstructions
Processed sections were imaged at 40× using a Zeiss LSM 5 confocal microscope (Carl Zeiss, Jena, Germany). Stacks of optical sections were collected through each agarose slice and then combined in Adobe Photoshop (Adobe Systems, San Jose, CA) for reconstruction. Neurons were reconstructed either by projecting imaged stacks onto paper and then tracing or tracing optical sections displayed using Adobe Photoshop. Some examples of the confocal sections are shown in the figures. For these, brightness, contrast, and levels were adjusted for clarity using Adobe Photoshop.
Silver staining and Golgi impregnations
Brains were fixed in AAF (16 ml 80% ethanol, 1 ml glacial acetic acid, 3 ml 37% formaldehyde). After dehydration and clearing in terpineol, brains were embedding in Paraplast plus (Sherwood Medical, St. Louis, MO) and then Bodian-stained using a procedure similar to that described by Gregory (1980), as described in Paulk et al. (2008). Golgi-impregnations were produced using the mixed Colonnier-rapid technique (Li and Strausfeld, 1997). Opened heads were immersed for 4 days at 4°C in 2.5% aqueous potassium dichromate and 25% E.M. grade glutaraldehyde (5:1), then washed in 2.5% potassium dichromate before immersion in 2.5% potassium dichromate and 1% osmium tetroxide (99:1), also at 4°C. After 3 days, heads were swirled in distilled water (less than 3 seconds), and then placed in a 0.75% silver nitrate solution for 3 days. After washing in distilled water, heads were dehydrated, embedded in epoxy resin, and serial sectioned.
For cobalt silver staining, a blunt 3–8-µm tip diameter electrode, filled with 1% cobalt nitrate, was inserted into the exposed protocerebrum of a living but immobilized animal. Cobalt was allowed to diffuse out of the electrode for 30 minutes. Afterwards the head was cut from the body and immersed in distilled 5% sucrose solution through which was bubbled hydrogen sulfide. After 1 minute the head was removed and immersed in AAF fixative. After overnight fixation the brains were removed and treated by silver intensification, as described in Strausfeld and Okamura (2007).
Golgi and cobalt preparations were photographed using a Zeiss Axioimager Z2 with a computer-controlled stage that allowed automatic montaging of segmented sections. Individual images were processed with Zeiss’s software into large stacks taken at different depths through each section. Stacks were then flattened into a single focused image using helicon focus (Helicon Soft, Ukraine). Brightness and contrast were adjusted using Adobe Photoshop.
Nomenclature
Throughout this account, neurons and brain structures are viewed in frontal sections. The terms anterior/posterior and superior/inferior are used to describe relative positions with respect to the body axis. Rostral/caudal and dorsal/ventral are occasionally used to refer to the neuraxis. A frontal section is one cut perpendicular to the body axis, but parallel with the neuraxis.
The term “module” refers to divisions across the central complex that are imposed by small neurons linking the protocerebral bridge and central body. The fanshaped body is composed of an upper and lower division, each of which is divided into a succession of strata. The most superior stratum (rostral according to the neuraxis, or dorsal according to the body axis) is called the “superior arch” and its swollen flanks are termed the flanges (FLA). The most lateral portions of strata 5–7 that flank the posterior part of the ellipsoid body are called the “lateral protuberances” (Strausfeld, 1976). Divisions of the superior protocerebrum and other regions are named according to determined nomenclature for the Drosophila brain (Ito et al., 2012).
RESULTS
Organization of the N. bullata fan-shaped body
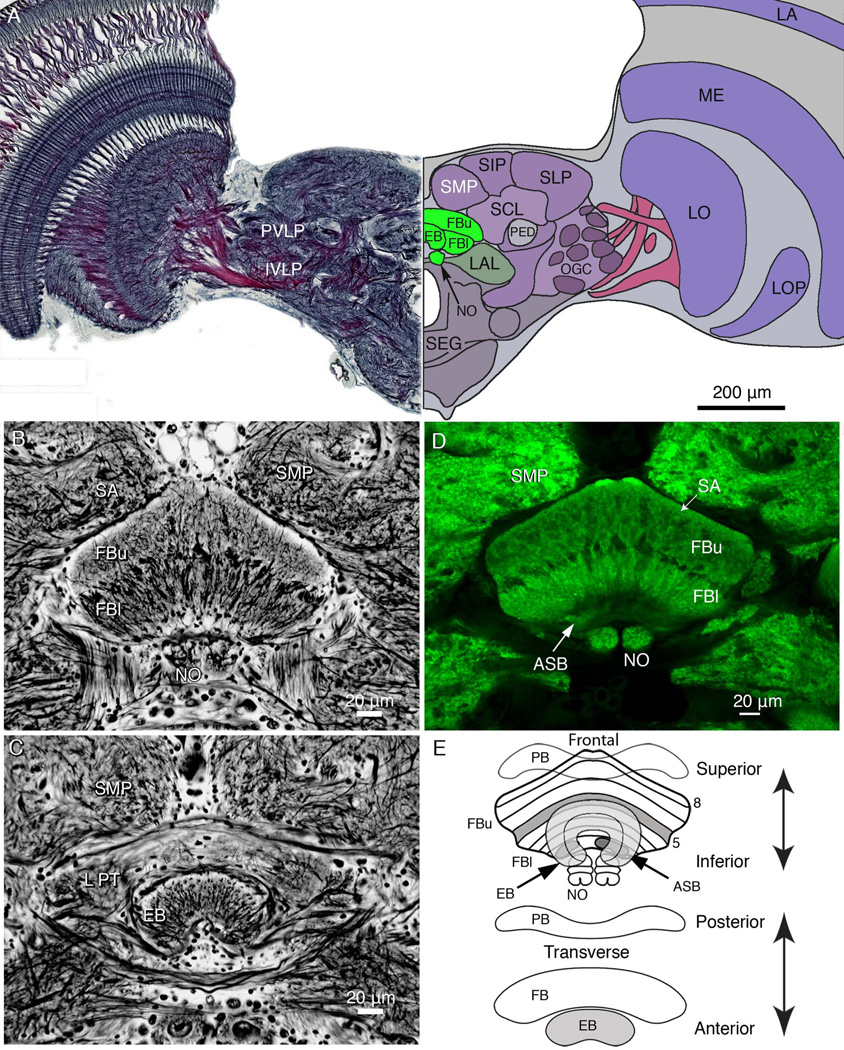
As its name suggests, the central complex lies in the brain centrally, at its midline (Fig. 1A), almost enclosed by surrounding neuropils. Although the constituent neuropils of the central complex are small compared to many much larger regions, their shapes and cellular arrangements are characteristic and unmistakable in almost all insects. The fan-shaped body is the largest component of the central complex, which also comprises the protocerebral bridge, ellipsoid body, and the noduli (Fig. 1B–D). The fan-shaped body is divided into a superior (upper: FBu) and inferior (lower: FBl) division, which are referred to by Hanesch et al. (1989) as, respectively, the dorsal and ventral parts. These two levels of neuropil are clearly demarcated by a band of anteroposteriorly projecting fibers (Fig. 1B,D). As shown in the schematic of Figure 1E, the two divisions are further divided into eight strata by the tangentially directed terminals of neurons supplying the central body from the protocerebrum and, as demonstrated for D. melanogaster, by the levels of termination of fan-shaped and tangential processes of neurons carrying neuroactive substances (Kahsai and Winther, 2011). An earlier study by Hanesch et al. (1989), using Golgi impregnations, divided the whole fan-shaped body of D. melanogaster into six layers, whereas Kahsai and Winther (2011) demonstrate seven to eight layers. A similar organization typifies Neobellieria and Calliphora, and is likely to be common across the Drosophillidea and Calliphoridae. In Neobellieria, reduced silver staining demonstrates eight strata spanning the width of the fanshaped body. We adopt Kahsai and Winther’s (2011) designation of stratum 1 as the deepest layer (ventralmost according to the body axis) and stratum 8 as the most shallow (dorsalmost). The prominent band of anteroposteriorly projecting fibers that divide the fanshaped body into upper and lower divisions define the fifth stratum. Here we describe neurons, the terminals of which span the width of the fan-shaped body exclusively within its upper division.
Figure 1.
Overview of the brain and central body architecture. A: Frontal view of a section cut across the brain, at the level of the ellipsoid body (EB) and showing the relative proportions of neuropils at this level. The left side of the panel shows the Holmes-Blest silver-stained hemibrain. The right side of the panel shows a schematic view of the hemibrain indicating some of its salient neuropils. Neuropils of the central complex are shown green, indicating the upper and lower divisions of the fan-shaped body (FBu, FBl), the EB, and noduli (NO). The lateral accessory lobe, a major region receiving outputs from the central complex fan-shaped lies to one side of it (LAL). The superior protocerebrum is divided into the superior medial, superior intermediate, and superior lateral lobes (SMP, SIP, and SLP). These lie above the superior clamp (SCL), which partly enfolds the mushroom body pedunculus (PED). The posterior ventrolateral and inferior ventrolateral protocerebrum (labels to the left, PVLP, IVLP) contain numerous glomeruli supplied from the optic lobes. These contribute to the more extensive optic glomerular complex (OGC). The optic lobes are composed of the lamina (LA), medulla (ME), lobula (LO), and lobula plate (LP). The subesophageal ganglion is indicated as SEG. B: Frontal Bodian-stained section through the fan-shaped body (FB) showing its division into two layers: the upper division (FBu), and lower division of (FBl). The NO are located beneath the FBl. The FB lies beneath the two inward bulging lobes of the superior medial protocerebra (SMP), separated by the pars intercerebralis (PI), which contains cell bodies of neuromodulatory cells. C: More anteriorly, a section from the same preparation as that provided in A shows the lateral protuberances (LPT) of the FB. These are the most lateral parts of the upper division of the fan-shaped body that extend anteriorly alongside the EB. D: Scanning confocal image of an anti-synapsin-labeled section taken at the same level as that in B, showing the same structures. Strata of the FB are numbered 1–8, the eighth stratum referred to as the superior arch (SA), which is defined by its characteristic homogenous neuropil extending over stratum 7. Elsewhere, the FB is clearly divided across its extent by modular subunits. Stratum 1 contains the asymmetric bodies (ASB, one visible). E: Schematic showing the central complex, as seen from the front and from above. The EB, which is organized into concentric layers, lies in front of the FB, which is divided into eight strata. The asymmetric paired ASB reside in stratum 1. The paired noduli, which are connected to the FB, lie beneath and somewhat behind the FB and are composed of four nodules. The protocerebral bridge (PB) lies posterior to the FB.
Organization of the superior protocerebrum of N. bullata
Comparisons of Bodian and anti-synapsin-stained sections cut frontally through the brain reveal several clear and separate domains of the superior protocerebrum. These are summarized in Figure 1A and further illustrated in Figure 2, which shows a series of consecutive sections that demonstrate the anatomical divisions of the superior protocerebrum relative to the central complex. The regions considered here reach to the midline, where the inner borders of the superior medial protocerebrum’s lobes flank the pars intercerebralis (PI) and approximately cover the fan-shaped body. Laterally, the superior protocerebrum extends as far as the optic peduncle, which links the central brain and the optic lobes.
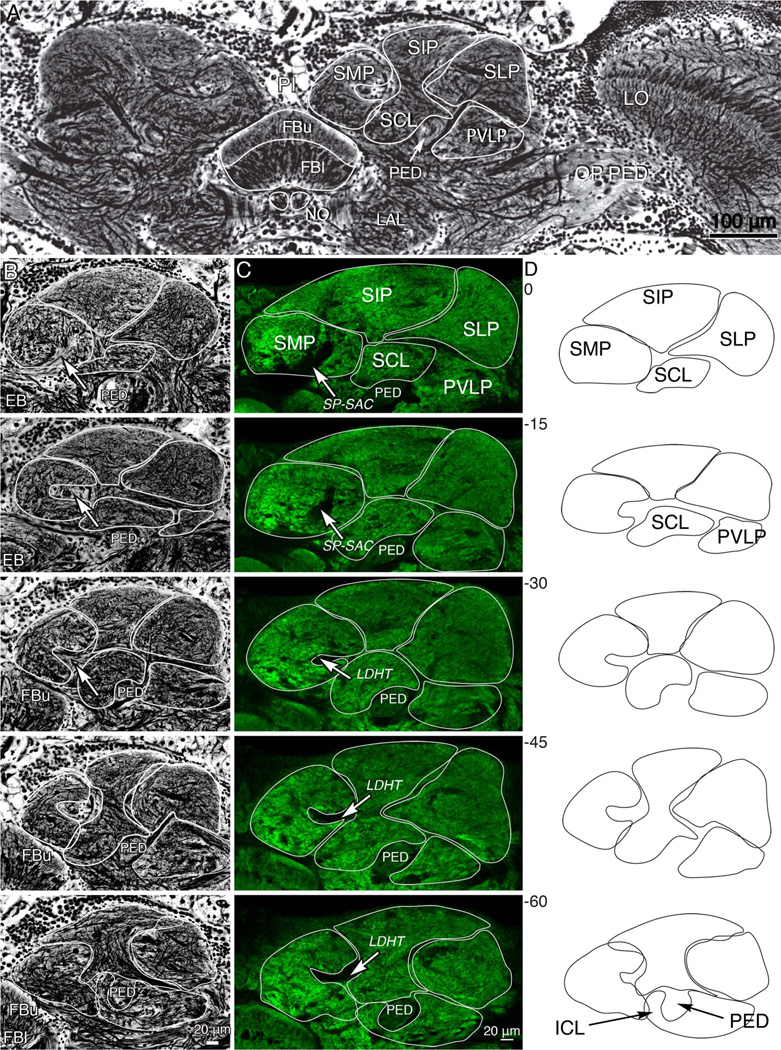
Figure 2.
Divisions of the superior protocerebrum. A: Part of a frontal section stained by the Bodian method showing the location of the fan-shaped body (FBu, FBl) and volumes of the superior protocerebrum. Shown are the superior median, superior intermediate, and superior lateral protocerebrum (SMP, SIP, SLP). These lie above the mushroom body pedunculus (PED) here seen in cross-section. The underside of the PED is flanked by the inferior clamp neuropil (ICL, lower image, panel D). The fan-shaped body lies beneath the pars intercerebralis, and central to the paired lateral accessory lobes (LAL). The optic lobe’s lobula (LO) is shown to the right. Axons from the optic lobe reach the central brain via the optic pedunculus (OP PED). B: Five panels showing a sequence of 15-µm-thick frontal sections (0 µm, the most frontal of the series to −60 µm, the most posterior) through the superior protocerebrum to illustrate gradual changes of protocerebral volumes through a depth of 60 µm. The anatomical divisions of the superior protocerebrum are outlined and are shown separately in panel D. C: A corresponding series of vibratome sections labeled with an antiserum raised against synapsin. In both B,C, the white arrows indicate a tract of axons occupied by neurons connecting the superior protocerebrum with the fan-shaped body (superior protocerebrum–superior arch commissure SP-SAC: axons shown in Figs. 3–7). The laterodorsal horizontal tract (LDHT) is shown at level −30 to −60 in panel C. D: Outlines of divisions of the superior protocerebrum, obtained by averaging traces from B,C, showing the arrangements among the discussed neuropil regions. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Sections through the superior protocerebrum, beginning at the level of the ellipsoid body and progressing posteriorly through to the rear face of the fan-shaped body, are shown in Figure 2B–D. Figure 2B shows serial 15-µm-thick Bodian-stained sections to illustrate the fibroarchitecture of the superior protocerebrum and provide information about morphological change in depth. Figure 2C shows a series of equivalent optical sections labeled with an antibody raised against synapsin (see Materials and Methods). Based on cytoarchitecture and synaptic densities, five regions of the superior protocerebrum are identified. These are outlined in Figure 2B,C and shown as averages in Figure 2D. Although in Neobellieria the volumes and shapes differ somewhat from those in Drosophila — for example, the superior intermediate protocerebrum being far larger than in the fruit fly — homologous domains are recognizable and have here been given the same names as those used for Drosophila (Ito et al., 2012). From medial to lateral, the domains of the Neobellieria superior protocerebrum are: the superior medial protocerebrum (SMP), the superior intermediate protocerebrum (SIP), and the superior clamp (SCL), which together with the inferior clamp (ICL) enclose the mushroom body pedunculus (PED), the superior lateral protocerebrum (SLP), and the posterior ventrolateral protocerebrum (PVLP). “Posterior” distinguishes these neuropils from as distinctive but more anterior volumes of the protocerebrum. “Superior” (rostral, according to the neuraxis, dorsal according to the body axis) denotes neuropils at and above the level of the central complex.
The superior medial protocerebrum is closest to the midline and contains the superior protocerebrum-superior arch commissure, which is visible in the first sections of Figure 2C (arrowed SP-SAC at level 0 to −15) as a striated bundle of fibers in Bodian preparations and as a dark region devoid of anti-synapsin immunoreactivity in the anti-synapsin preparations. This commissure carries the axons of tangential neurons from their branches in the superior protocerebrum to the upper division of the fan-shaped body. More posteriorly, the lateral surface of the superior medial protocerebrum takes on a concave shape because its synaptic regions are divided by the laterodorsal horizontal tract (LDHT), which is morphologically distinct from the surrounding tissue in Bodian-stained material and appears as another volume devoid of immunoreactivity in the anti-synapsin-labeled brains (arrows at levels −30 to −60). These commissures and tracts serve as additional landmarks in mapping the arborization patterns of neurons in these regions.
The superior intermediate protocerebrum extends above and lateral to the superior medial protocerebrum. This domain contains the most dorsal (neuraxis rostral) extent of the protocerebrum and is most easily distinguished in Bodian preparations by the thinner appearance of its most superficial fibers belonging to dendrites and terminals associated with the median bundle (Strausfeld, 1976). Thicker fibers are visible in the more inferior and posterior portions of the superior intermediate protocerebrum.
In anterior sections (levels 0 to −30), the superior intermediate protocerebrum is bordered beneath by a region that contains a denser network of thick fibers called the superior clamp (SCL), which encloses the upper surface of the mushroom body’s pedunculus. The lateral border of the superior clamp abuts the inner margin of superior lateral protocerebrum. In sections toward the back of the brain (levels −45 to −60) the medial edge of the posterior ventrolateral protocerebrum lies lateral to the pedunculus and is contiguous with the lateral clamp neuropil enclosing the lower margin of the pedunculus.
The posterior ventrolateral protocerebrum is distinct from the superior lateral protocerebrum by virtue of a thick bundle of fibers that projects mediolaterally into the superior lateral protocerebrum. These are seen extending medially from the middle right margin of the brain at level −60 through to level −15 in row B. The posterior ventrolateral protocerebrum (PVLP) is bordered at its inferior margin by larger fibers originating in or projecting to the optic lobes (Figs. 1A, 2A). In anterior sections (levels −15 to −45), the posterior ventrolateral protocerebrum is bordered medially by the inferior clamp.
Representation of the central body in the superior protocerebrum
Dye fills demonstrate that the upper division of the fanshaped body receives terminals from neurons, the distal branches of which occupy domains in the superior medial and superior intermediate protocerebrum (Fig. 3). The terminals extend tangentially across the upper division of the fan-shaped body. All neurons with such endings are termed “tangential neurons.” Tangential neurons link domains of the superior protocerebrum with strata situated at, or immediately beneath, the superior arch.
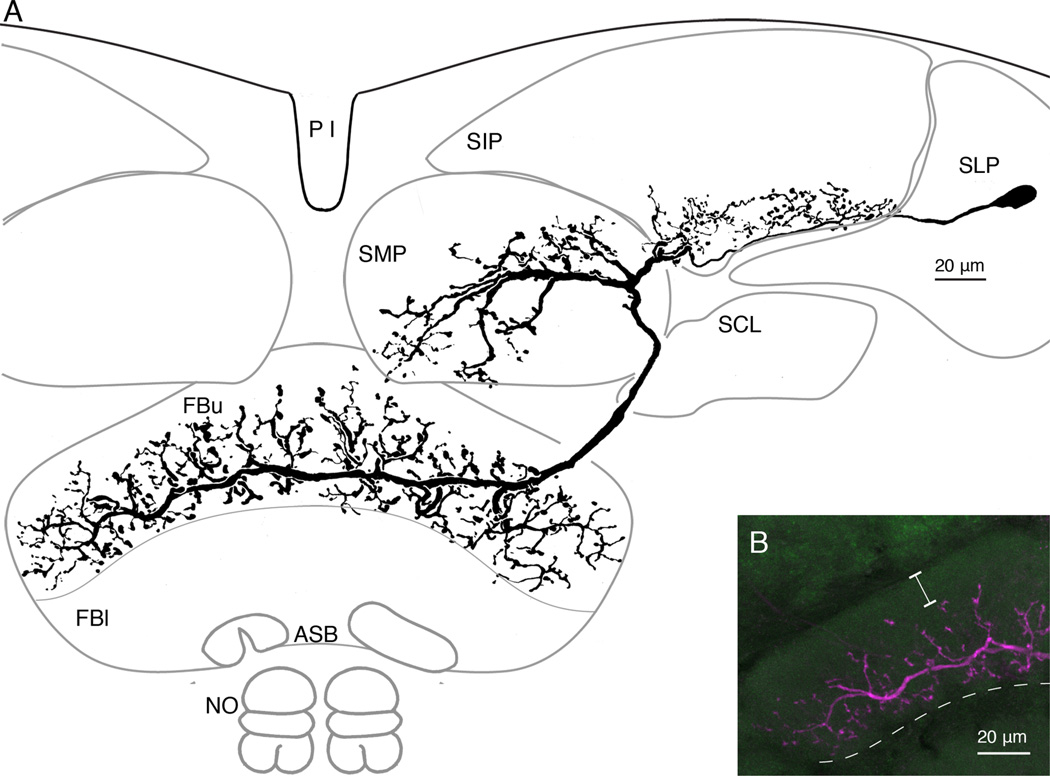
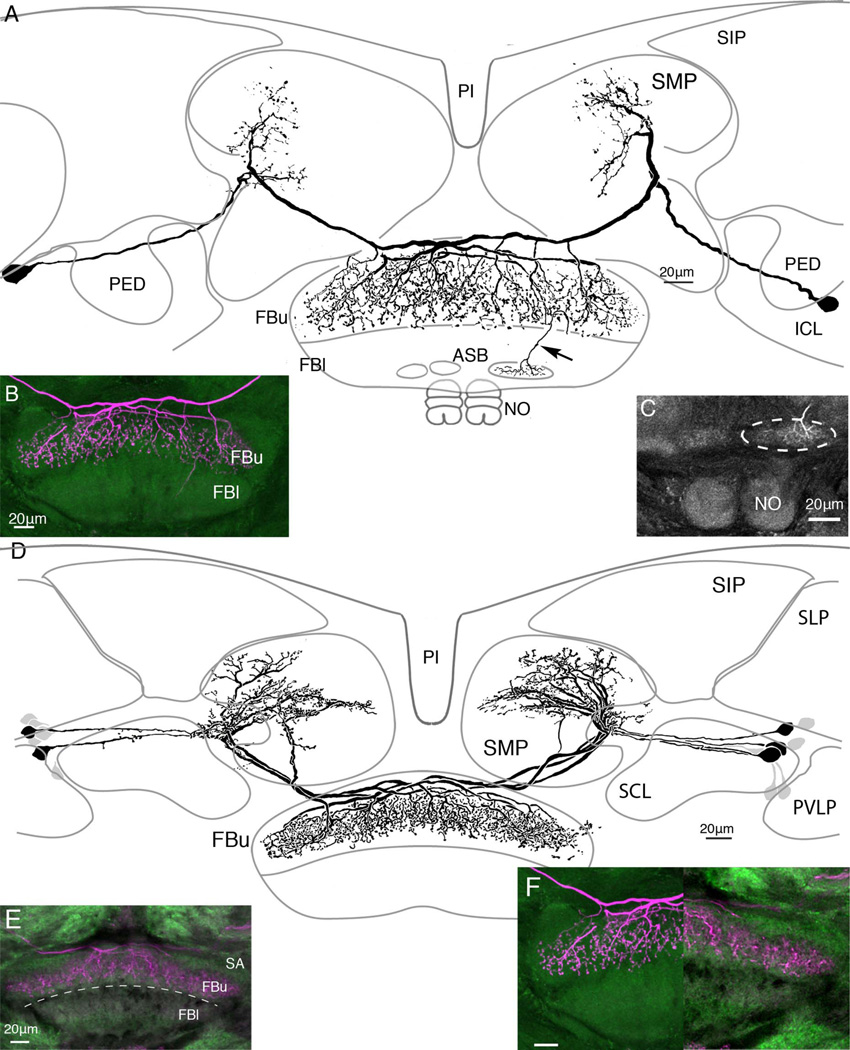
Figure 3.
One morphological type of tangential neuron supplying the upper later (FBu) of the fan-shaped body from two domains in the superior medial and superior intermediate protocerebrum. A: Reconstruction of the neurobiotin-filled neuron showing its varicose branches in the upper fan-shaped body (FBu) extending either side of the large terminal fiber. This derives from bifurcating branches that provide thick processes in a territory of the superior medial protocerebrum (SMP) and more delicate short processes at the lower margin of the superior intermediate protocerebrum (SIP). This neuron has its cell body in lateral cell body rind, behind the superior lateral protocerebrum. B: Confocal stack showing the density and vertical extent of terminal branches in the FBu. Branches do not invade the superior arch, and are restricted to strata 7 and 6, ending just above stratum 5, which lies beneath the dashed line.
Neurons described here are primarily distinguished as different types not by their terminals in the fan-shaped body, but by the patterning and disposition of their branched processes in the superior protocerebrum and the location of their perikarya. Although certain types of neurons have been resolved just once after a single dye fill (e.g., Figs. 3, 4), other types have been resolved in several brains (n = 4 for Fig. 5A,B; n = 2 for Fig. 6A,B). Figure 3 shows an example of a tangential neuron with a terminal in the upper layer of the fan-shaped body and distal processes in the superior medial protocerebrum and the lower margin of the superior intermediate protocerebrum. Its cell body is situated lateral to the calyx (not shown). The reconstruction (Fig. 3A) shows a field of fine processes that extends upward from the cell body neurite into the lower part of the superior intermediate protocerebrum. The volume occupied by the branches of this neuron and other neurons in the superior protocerebrum are summarized in the schematic Figure 9. The cell body neurite bifurcates in the superior medial protocerebrum, one tributary providing a thick branch into this neuropil, the other tributary extending as an axon to the ipsilateral edge of the FBu. It then bends downward to enter the sixth and seventh stratum of the FBu where the neuron’s terminal provides many blebbed processes that extend upward and downward from the contralaterally projecting terminal process. The confocal reconstruction in Figure 3B shows the extent of the terminal processes, which reach as far down as its lower (inferior) margin and upward to just beneath the superior arch.
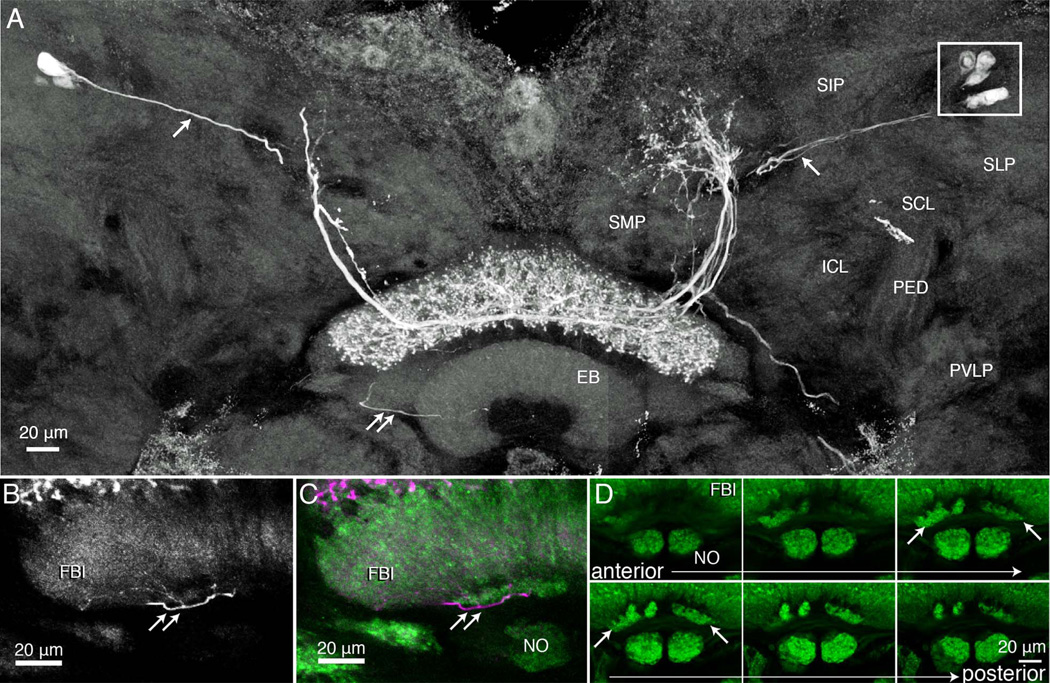
Figure 4.
Tangential neurons with terminal arborizations through all the strata (8–6) of the upper division of the fan-shaped body. A: Composite image, reconstructed from confocal stacks through two consecutive sections at the level of the fan-shaped body and the ellipsoid body (EB). Several neurons have been filled with neurobiotin after an intracellular recording and injection from one neuron. Together, these dye-coupled cells have extensive dendritic branches in an upper middle domain of the superior medial protocerebrum (see schematic, Fig. 9). Axons project from the protocerebral processes to stratum 6 of the upper fan-shaped body where they give rise to varicose and beaded processes extending upward out to, and including, stratum 8. Thin fibers (single white arrows) connect the protocerebral arborizations to cell bodies (boxed, upper right) located dorsal to the superior lateral protocerebrum (SLP). Double arrows indicate one of several thin processes that extend from the terminal arborization downward, behind the ellipsoid body and into one of the two asymmetric bodies. The terminal of one of these collaterals is shown in the enlargement of panel B. C,D: Six consecutive sections through the lower margin of the FBu, showing the noduli, and above them the asymmetric bodies within stratum 1. Other regions shown in A are the superior intermediate protocerebrum (SIP), the superior and inferior clamp (SCL, ICL), both of which enclose the mushroom body’s pedunculus (PED), and part of the posterior ventrolateral protocerebrum (PVLP).
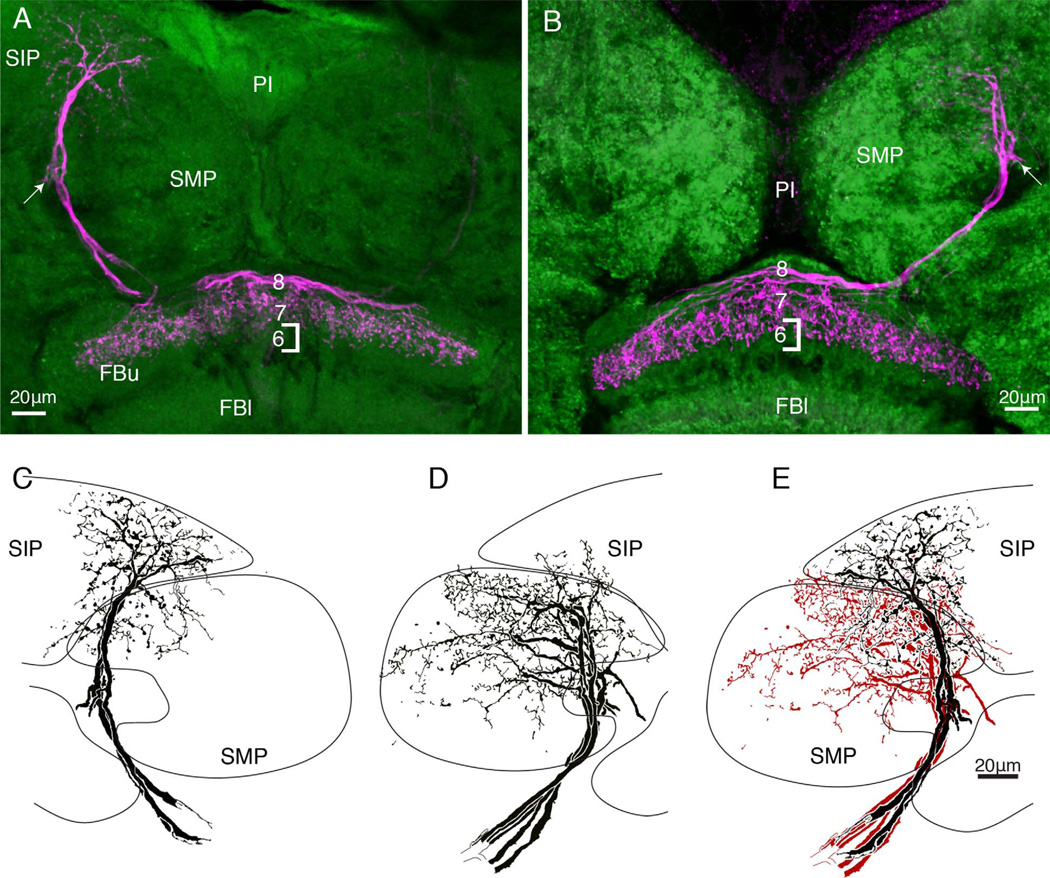
Figure 5.
There are subtle yet clear distinctions of tangential neuron morphologies. A: Paired tangential neurons with their main terminal branches above the superior arch extend long beaded collaterals through strata 8, 7, and 6. B: Confocal stack showing terminal branches that appear to hang down through the upper fan-shaped body (FBu). The distal arborizations of these neurons occupy a vertically arranged domain in the lateral part of the superior medial protocerebrum. One of the two terminals also supplies a collateral to the larger of the two asymmetric bodies (inset C, middle right). D: Reconstruction of neurobiotin-filled neurons, the terminal branches of which extend within and just beneath the superior arch (see also confocal stack, inset E). The branches provide a dense system of fine varicose processes in strata 7 and 6. F: A side-by-side comparison of the neurons shown in A,D (lower right inset, F) demonstrates their difference with respect to their depth of termination in the FBu. Neurons shown in D have their distal arborizations occupying a dorsolateral domain of the superior medial protocerebrum. After filling a single neuron with neurobiotin, a total 15 cell bodies were visible, some more intensely labeled than others.
Figure 6.
A,B: As in Figure 5, here two morphological types of neurons terminate at overlapping but different depths of the upper fanshaped body. The neuron in A occupies a thin domain in stratum 7, whereas the neuron in B has processes reaching into stratum 6. Such differences are subtle, in contrast to their obvious distinctions, which refer to their branches in the superior medial protocerebrum. C,D: Neurons terminating as in panel A have most of their branches in the superior intermediate protocerebrum (SIP). Neurons terminating as in panel B have their branches in the superior medial protocerebrum (SMP). D: These distinctive domains are further accentuated when the two types of arborizations are superimposed.
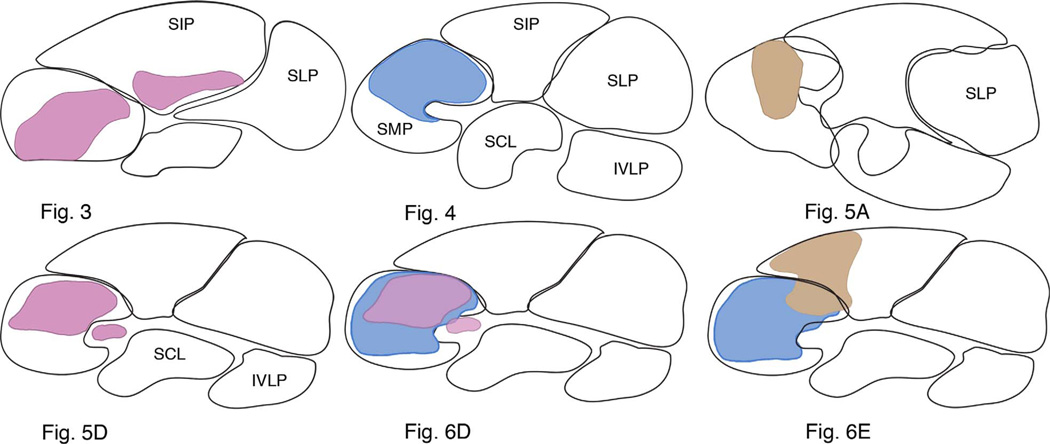
Figure 9.
Summary diagram illustrating that tangential neurons shown in Figures 3–6 occupy distinctive protocerebral domains. Each arborization occupies a broad lateral expanse within one or more protocerebral lobes, but extends through only 15–45 µm through the depth of its domain. Superimposed domains (Figs. 5D+6D, 6D+6E) further demonstrate that the protocerebrum is composed of distinct but partially overlapping territories. Unoccupied volumes in the SIP suggest that there are likely more neurons that are as yet unidentified at this level of the brain.
In several preparations, neurobiotin-filled tangential neurons were resolved as bilaterally symmetric ensembles. For example, as shown from the summed confocal stacks in Figure 4A, as many as 10 cell bodies, five each side of the brain (boxed, Fig. 4A) lying lateral to and in front of the mushroom body calyces (not shown), could sometimes be resolved after biocytin injection. In such instances, when two or more neurons were resolved with equal brightness, it was not possible to distinguishing the arborizations of one from the other. For example, the neurons shown in Figure 4A have their arborizations throughout all three strata of the FBu. The terminal branches mainly extend upward from the axon terminals, thus denoting an unambiguously distinctive terminal morphology to this type of neuron compared with that shown in Figure 3. However, the greatest difference refers to their branches in the superior medial protocerebrum, where arborizations of the cells depicted in Figure 4 occupy a lateral domain of the SMP. The neurons shown in Figure 4 also lack the short collateral processes from the cell body neurites that typify the neuron in Figure 3. The schematics in Figure 9 compare domains occupied by these and other tangential neurons.
These neurons also provide thin processes (one shown, double arrows in Fig. 4A) that extend from just one side of the terminals in the FBu, projecting ventromedially behind the ellipsoid body (EB) to finally end in one of a pair of small synaptically dense structures at the inferior margin of the FBl (Fig. 7B,C). These structures have here been given the name asymmetric bodies, using terminology for neuropils at approximately the same location beneath the fan-shaped body of D. melanogaster (Pascual et al., 2004). Asymmetric bodies have been identified in every SYNORF-labeled brain. One example is shown in a series of optical sections (Fig. 4D) through the lower fanshaped body and noduli of another anti-synapsin-labeled preparation. Labeled asymmetric bodies appear much brighter than the neuropil in which they are embedded, suggesting they are densely packed with synaptic sites. One of these neuropils is always larger than the other, hence their name, and often (n = 9 pairs) the smaller neuropil appears fragmented. We return to these structures in the Discussion, where we argue for their homology with the asymmetric bodies described by Pascual et al. (2004) from observations of D. melanogaster.
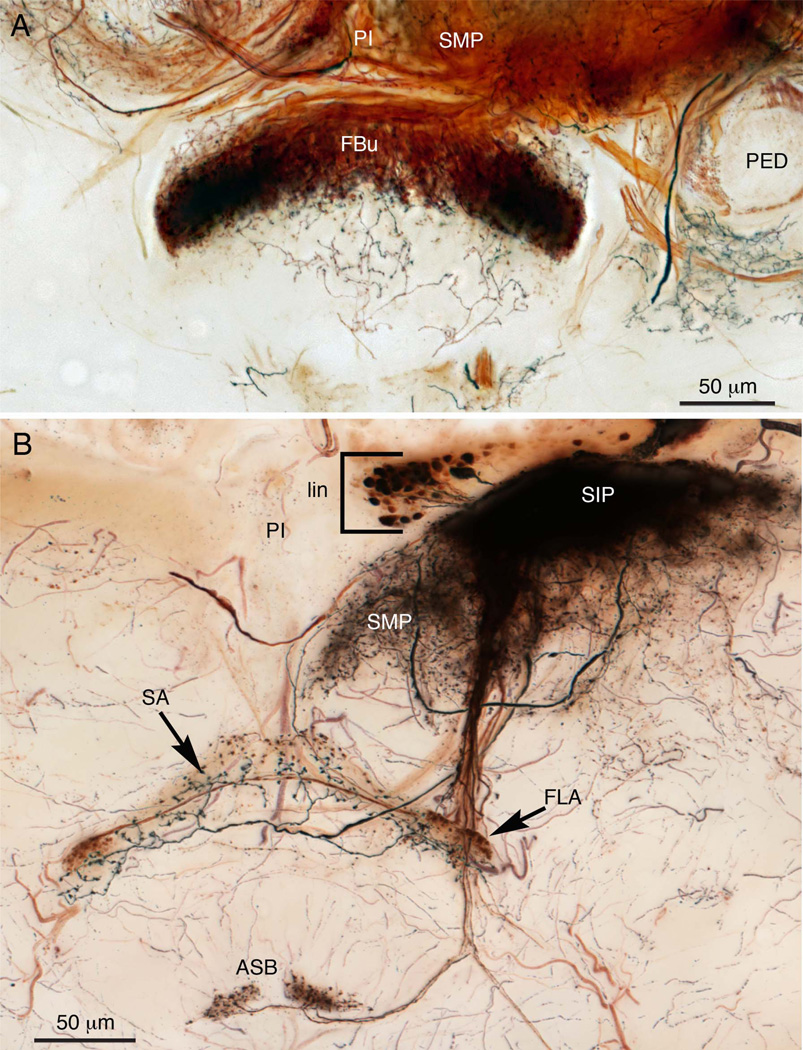
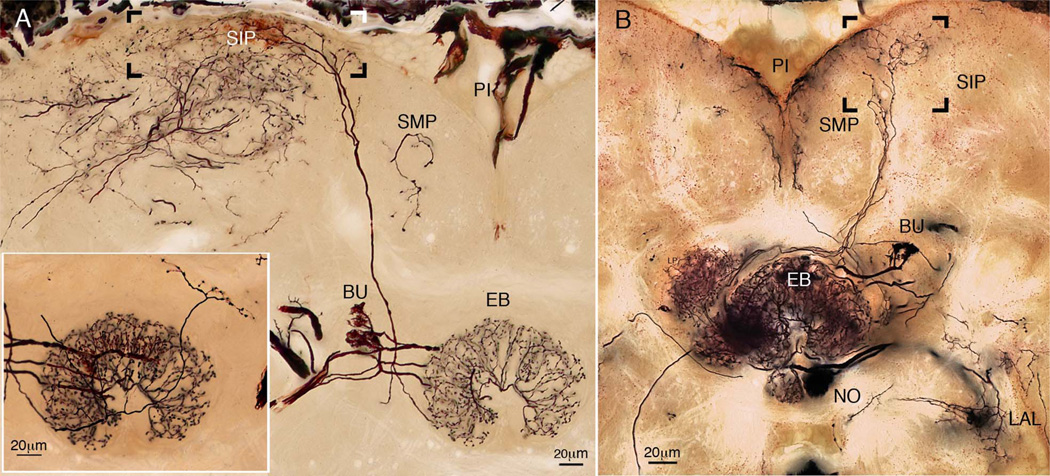
Figure 7.
A: Silver-intensified cobalt injection into one side of the superior protocerebrum resolves an ensemble of about 12–15 identical terminals in the upper layer of the fan-shaped body of Calliphora erythrocephala. This preparation further demonstrates that a palette of different techniques is required to resolve all possible types of neurons invading any part of the brain, including the central complex. B: Silver-intensified cobalt injection into one side of the superior intermediate protocerebrum (SIP) reveals the cell bodies of numerous local interneurons (bracketed, lin). Alone, these 25–30 neurons provide such a dense mass of processes that single arborizations cannot be distinguished. Two tracts connect this mass of fibers with the central complex. One provides two morphological types of terminals to the superior arch (SA) and its lateral swellings (the flanges, FLA). Another fiber projects downward to supply both asymmetric bodies (ASB) with blebbed arborizations.
The type of tangential neuron illustrated in Figure 5A is another example of what are essentially bilaterally symmetric terminals of tangential cells supplying a descending collateral to just the larger of the two asymmetric bodies. Here, the collateral process projects directly downward rather than adopting a curved trajectory as from neurons shown in Figure 4A. This difference denotes another subtle morphological distinction among tangential neurons.
In Figure 5A, two equally bright neurons have been filled after recording from just one neuron. Both neurons have their cell bodies in the lateral rind, situated in a pleat of the cell body rind that extends between the mushroom body’s pedunculus and the posterior ventrolateral protocerebrum. The cell body fibers project medially beneath the pedunculus and then curve upward, where they bifurcate to each give rise to a thick branch that projects vertically into the superior medial protocerebrum. These protocerebral branches are restricted to a lateral domain of its lobe. The second branch, which is the neuron’s axon, projects downward to extend across the fan-shaped body immediately above the superior arch from which it provides diffuse terminal branches that extend downward through strata 8–6.
The resolution of a pair of identical neurons, shown in Figure 5, is probably accidental due to the electrode tip penetrating both axons at their point of crossover at the brain’s midline. However, dye-filling clusters of up to eight neurons from both sides of the brain is less likely to be due to multiple penetration and more likely indicative of dye coupling. Figure 5D illustrates one such example, where eight cell bodies providing bundled neurites are resolved in both sides of the brain. Like the neurons shown in Figure 4, neurons shown in Figure 5D have arborizations reaching a superficial domain of the superior medial protocerebrum. But unlike the neurons shown in Figure 4, the branches of these cells provide a dense and more tightly arranged set of processes restricted to a smaller domain of the SMP. These comparisons are further documented in Figure 9. A further distinction between the neurons shown in Figure 5A,D relates to the depth of termination in the FBu. Their endings are compared side-by-side in Figure 5F, which shows that whereas endings of neurons in Figure 5A extend down as far as stratum 5, those of neurons in Figure 5D reach only part of the way through stratum 6, thus occupying a narrower band of synaptic neuropil.
Discrete domains of the superior protocerebrum are represented in the upper division of the fan-shaped body
Thus far, each morphological type of tangential neuron, as defined by its branches in domains of the superior protocerebrum, provides terminals restricted to a stratified volume of the FBu. This ground pattern is further illustrated by the neurons shown in Figure 6, which again demonstrate how tangential neurons occupy discrete domains in the superior protocerebrum, and how axons from these domains supply distinct, yet overlapping strata in the outer division of the fan-shaped body.
Dye fills into protocerebral branches of tangential neurons reveal that they are decorated with various kinds of specializations: varicosities, swellings, spicules, and spines. The possible significance of these with respect to their involvement in microcircuits is considered further in the Discussion. Here, though, we emphasized that these diverse decorations obviously contrast with those in the FBu, where each tangential neuron has terminal processes that are uniformly equipped with just one type of specialization, be it varicosities, bead-like, or irregular-shaped swellings. Although all the neurons described thus far terminate within the FBu, each type of neuron occupies overlapping yet characteristic levels that correspond to layers 8–6 of the Drosophila fan-shaped body, as defined by labeling with antisera against neuropeptides (Kahsai and Winther, 2011). In summary, cardinal features that distinguish morphological types of tangential cells are their domains and branching morphologies in the protocerebrum, their terminal specializations, and their levels in the FBu.
Superior protocerebrum is integral to central complex function
A third and major contribution to the central complex are neurons that arborize in the superior protocerebrum and extend their terminals across the fan-shaped body. The superior protocerebrum is part of the brain about which little is yet known: neurons described here suggest that it must play crucial roles in computations that occur in the central complex itself. That many more neurons of this type are likely to exist is suggested by Golgi impregnations and cobalt-silver staining, which function particularly well on the relatively large brains of blowflies (C. erythrocephala). Cobalt injections to the superior medial protocerebrum demonstrate that certain types of tangential neurons provide isomorphic ensembles of between 16 and 24 terminals representing just one protocerebral lobe (Fig. 7A). Cobalt injections also reveal other important aspects of this relationship, such as clusters of local interneurons in the superior protocerebrum that form such dense arborizations that single processes cannot be resolved (Fig. 7B). The same injection site reveals that this region of local interneurons provides further types of tangential terminals, two of which are shown here as restricted to the outermost stratum of the central complex (the superior arch), as well as projections directly to the asymmetric bodies. Again, as in Neobellieria, in C. erythrocephala one asymmetric body is not only larger than the other, but is more densely innervated.
However, it is not only the fan-shaped body that receives inputs from the superior protocerebrum. As shown in Figure 8A, the ellipsoid body obtains its inputs both from neuropils lateral to it, such as the bulb (BU in Fig. 8A, inset lower left), and from the superior protocerebrum. Furthermore, the fan-shaped body and ellipsoid body provide ascending projections back to the superior protocerebral lobes (Fig. 8B), suggesting that the central complex provides functional feedback to this part of the forebrain.
Figure 8.
A: Composite image of a Golgi-impregnated ring neuron of the ellipsoid body that receives its axon from a superficial domain of distal arborizations (boxed) in the superior intermediate protocerebrum (SIP). The neuron also provides varicose terminals to the superior arch of the fan-shaped body (inset, lower left). Another system of ring terminals is linked to the lateral bulb (BU, inset lower left). B: Reconstruction through three consecutive sections showing Golgi-impregnated neurons with spine-like arborizations in the fan-shaped body and ellipsoid body with vertically extending axons that end as varicose terminals (boxed area) between the superior medial and superior intermediate protocerebrum (SMP, SIP) and the neighboring portion of the superior intermediate protocerebrum (SIP). Other connections are shown between the ellipsoid body (EB) and bulb (BU), and one linking a nodulus and the ipsilateral accessory lobe (LAL).
DISCUSSION
Where does learned discrimination of visual cues occur?
Most studies on the central complex have concentrated on uniquely identifiable neurons in, or associated with, the modular organization of the protocerebral bridge and central body, with reference to early neuroanatomical studies by Williams (1975), Strausfeld (1976), and Hanesch et al. (1989). Functional studies have drawn particular attention to the representation in these modules of sensory stimuli in space, such as the e-vector of polarized light or the representation of haptic inputs (Ritzmann et al., 2008; Sakura et al., 2008; Heinze et al., 2009; Heinze and Reppert, 2011, Homberg et al., 2011). Others studies have focused on the distribution of neuropeptides in the central complex carried by wide-field arborizations, and the possible roles of these peptides in modulating or constraining the behavioral output (Kahsai et al., 2010; Kahsai and Winther, 2011).
Categorizing neurons on the basis of their branching patterns in the fan-shaped body, without reference to their organization in the superior protocerebrum, has important implications when considering whether visual memory is computed by the fan-shaped body itself or whether it is computed in neuropils of the superior protocerebrum. A case in point refers to genetic experiments that test the ability of wildtype and mutant Drosophila to learn to distinguish between similar visual patterns while attached to a torque meter (Wolf and Heisenberg, 1991). In such experiments, the torque generated by the flying fly is used to control pattern rotation, thus giving the fly control over its flight direction while allowing the experimenter to condition flies using a directed infrared laser beam as punishment (Wolf and Heisenberg, 1991). Wildtype animals readily learn to avoid flying toward a punished pattern, whereas flies that express a mutant form of the adenylyl cyclase rutabaga protein required for learning (see Levin et al., 1992; Zars et al., 2000) perform no better than chance. However, when certain neurons supplying the fan-shaped body from the protocerebrum are engineered to drive expression of wildtype rutabaga in the mutant background, learned visual discrimination is restored. These experiments suggested that different types of tangential neurons in the fan-shaped body learn to discriminate different pattern parameters: the height of the pattern’s center of (elevation), or pattern inclination (contour orientation).
As described by Liu et al. (2006), driving expression of rutabaga in neurons (called by them F5 cells) that extend tangential processes across stratum 5 of the fan-shaped body rescues the fly’s learned ability to discriminate pattern elevation. Expression of rutabaga in neurons (F1 neurons) that extend processes across stratum 1 of the fan-shaped body can rescue the fly’s learned ability to avoid one of two stripes with opposite inclinations. Experiments using controlled expression of a protein kinase called “foraging” (Wang et al., 2008) found that, as with rutabaga, expression of wildtype foraging in F5 neurons in an otherwise mutant background was sufficient to rescue the learned ability to avoid punished patterns based on elevation, but not contour orientation. Thus, evidence suggests that specific types of neurons terminating at different levels in the central body either participate in visual associative memory or, alternatively, carry to the central complex information about such learned associations.
F5 neurons are described as having cell bodies lateral to the mushroom body calyces that provide processes to “undifferentiated” protocerebral neuropil, from which axons supply a superficial stratum of varicose processes in the fan-shaped body (Li et al., 2009). Liu et al. (2006) describe three “F5” neurons ending at two levels in the upper division of the fan-shaped body. However, judging from the different areas of the superior protocerebrum through which their dorsal processes arborize, each of the three “F5” neurons, although supporting the same function, appear to be different cell types. Indeed, a neuroanatomical study of “F5” neurons using flip-out clonal analysis has proposed at least two kinds of F5 neurons based on their distinctive protocerebral arborizations (Li et al., 2009).
The recognition of such differences among what appear to be neurons with similar arrangements in the central complex is crucial for identifying what and where circuits are located that provide specific information to it. That subtle morphological distinctions are important for interpreting function comes from studies of another type of neuron, called “F1,” which supports the learned discrimination of contour orientation. An F1 neuron described in Li et al. (2009, fig. 7a), using the enhancer trap line NP6561, has tangential endings in stratum 1 of the fan-shaped body. So too does the “F1” neuron described by Liu et al. (2006, fig. 5C) from the line NP6510, However, comparison of these two “F1” neurons show them to be morphologically distinct. The “F1” neuron resolved in line NP6510 relates to an arbor in the superior protocerebrum, whereas the “F1” neuron resolved in line NP6561 has a fan-like arborization arising from an axon entering the fan-shaped body through the narrow canal of the ellipsoid body. Thus, although tangential neurons may indeed have nearly identical terminal morphologies in the FB, such similarity can be deceptive.
What the F5-like neurons share in common is having branches in the superior protocerebrum linked by a stout axon to a tangential ending in the fan-shaped body. This generic organization corresponds to the one class of neurons described by Hanesch et al. (1989) from Golgi preparations in Drosophila, and termed by those authors lateral fan-shaped neurons, so named because their axons approach the fan-shaped body from the side, instead of through the ellipsoid body canal. Young and Armstrong (2010) renamed these neurons ExF/2 (for type 2, extrinsic, lateral fan-shaped neurons) and used Gal4-UAS to drive expression of fluorescent pre- and postsynaptic markers in them. From evidence based on the expression of the presynaptic marker nsyb, they proposed that tangential processes of ExF/2 neurons in the fan-shaped body are presynaptic. Indeed, all the tangential neurons described here from Neobellieria have uniformly varicose, blebbed, or beaded terminals, suggesting that they are exclusively presynaptic. However, as shown by Young and Armstrong (2010), sites expressing the postsynaptic marker Dscam on the protocerebral branches of the ExF/2 neuron suggest far fewer postsynaptic specializations than would be expected for such an elaborate branching morphology (compare fig. 13A and 13E in Young and Armstrong, 2010). Li et al. (2010), using a reporter line that selects for presynaptic proteins, unambiguously labeled presynaptic sites on the branches of F5 neurons in the superior protocerebrum.
Thus, taken together, the accounts of Young and Armstrong (2010) and Li et al. (2010) demonstrate that the protocerebral branches of “tangential” neurons are both pre- and postsynaptic; they do not merely receive inputs, but are involved in local computations at that level. As pointed out by Meinertzhagen (2010), there are many electron microscopical analyses showing that purely postsynaptic dendrites in the insect central nervous system are less common than branches having both polarities, and that the coexistence of pre- and postsynaptic sites on the same “dendritic” branch suggests its participation in microcircuits. This general aspect of neuron morphology returns us to the question of exactly where “F5” neurons are most likely participating in circuits that support learned visual discrimination. Their genetic rescue not only reestablishes their participation in circuits within the fan-shaped body, but also reestablishes their participation as pre- and postsynaptic elements in local circuitry within the superior protocerebrum.
Asymmetry of the central complex and memory retention
We have identified a pair of neuropil domains deep in the fan-shaped body, termed the asymmetric bodies (see below), which show high levels of expression of anti-synapsin. Examination of 14 individuals showed all of them to possess asymmetric bodies, where one body is larger than the other, with the smaller of the two often fragmented. This asymmetry is reflected by innervation supplied by collaterals that originate from certain tangential neuron endings in the FBu: even when tangential neurons provide bilaterally symmetrical terminal ensembles in the FBu, only the larger of the two asymmetric bodies receives collateral input. Observations of C. erythrocephala also resolve asymmetric bodies (Fig. 7B), demonstrating their presence in this species as well.
A recent study of the central complex of D. melanogaster (Pascual et al., 2004) was the first to identify left–right asymmetry of a pair of neuropil domains (which they originally termed the “asymmetric bodies”) at the lower margin of the fan-shaped body. Behavioral screens of wildtype Drosophila showed that a small minority of individuals, in which these bodies were aberrantly symmetric, was deficient in long-term memory. This observation finds a counterpart in mice where defects of a natural hippocampus asymmetry, leading to symmetric organization, also result in impaired spatial learning (Goto et al., 2010). In zebrafish, asymmetry of the habenular nuclei have likewise been ascribed to spatial memory functions (see Bianco and Wilson, 2009).
We propose that the asymmetric bodies of N. bullata are homologs of the asymmetric bodies of D. melanogaster described by Pascual et al. (2004). It will be of some importance when further homologs of these asymmetric domains are identified in other insect orders. Furthermore, because the central bodies have been implicated in visual memory (Liu et al., 2006), the role of the asymmetric bodies in memory retention deserves intense study, particularly in the light of their direct supply by tangential neurons originating in the superior medial protocerebrum.
ACKNOWLEDGMENTS
We thank Gabriella Wolff for advice in immunohistology, Patrick Williams for valuable discussions, and David Andrew for scrutiny of the final text.
Grant sponsor: National Institutes of Health (NIH); Grant numbers: NCRR RO1-RR008688, NIGMS T32 Gm08400; Grant sponsor: Research to Prevent Blindness Foundation.
Abbreviations
- ASB
Asymmetric bodies
- CA
Calyx
- EB
Ellipsoid body
- FB
Fan-shaped body
- FLA
Flange of FBu stratum 8
- FBl
Lower Division of the fan-shaped body
- FBu
Upper Division of the fan-shaped body
- IVLP
Inferior ventrolateral protocerebrum
- LA
Lamina
- LAL
Lateral accessory lobe
- LO
Lobula
- LOP
lobula plate
- LP
Lateral protuberance
- ME
Medulla
- OGC
Optic glomerular complex
- SA
Superior arch = stratum 8 of FB
- SEG
Subesophageal ganglion
- SIP
Superior intermediate protocerebrum
- SLP
Superior lateral protocerebrum
- SMP
Superior medial protocerebrum
- SCL
Superior clamp
- ICL
Inferior clamp
- NO
Noduli
- PED
Pedunculus
- PB
Protocerebral bridge
- PI
Pars intercerebralis
- PVLP
Postero-ventrolateral protocerebrum
LITERATURE CITED
- Bender JA, Pollack AJ, Ritzmann RE. Neural activity in the central complex of the insect brain is linked to locomotor changes. Curr Biol. 2010;20:921–926. doi: 10.1016/j.cub.2010.03.054. [DOI] [PubMed] [Google Scholar]
- Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B. 2009;364:1005–1020. doi: 10.1098/rstb.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyan GS, Williams JL, Herbert Z. Fascicle switching generates a chiasmal neuroarchitecture in the embryonic central body of the grasshopper Schistocerca gregaria . Arthropod Struct Dev. 2008;37:539–544. doi: 10.1016/j.asd.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Goto K, Kurashima R, Gokan H, Inoue N, Ito I, Watanabe S. Left-right asymmetry defect in the hippocampal circuitry impairs spatial learning and working memory in mice. PLoS One. 2010;5:e15468. doi: 10.1371/journal.pone.0015468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. The Bodian protargol technique. In: Strausfeld NJ, Miller TA, editors. Neuroanatomical techniques. New York: Springer; 1980. pp. 75–95. [Google Scholar]
- Hanesch U, Fischbach K-F, Heisenberg M. Neuronal architecture of the central complex in Drosophila melanogaster . Cell Tissue Res. 1989;257:343–366. [Google Scholar]
- Heinrich R, Wenzel B, Elsner N. Pharmacological brain stimulation releases elaborate stridulatory behaviour in gomphocerine grasshoppers—conclusions for the organization of the central nervous control. J Comp Physiol A. 2001;187:155–169. doi: 10.1007/s003590100188. [DOI] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Maplike representation of celestial e-vector orientations in the brain of an insect. Science. 2007;315:995–997. doi: 10.1126/science.1135531. [DOI] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Neuroarchitecture of the central complex of the desert locust: intrinsic and columnar neurons. J Comp Neurol. 2008;511:454–478. doi: 10.1002/cne.21842. [DOI] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Linking the input to the output: new sets of neurons complement the polarization vision network in the locust central complex. J Neurosci. 2009;29:4911–4921. doi: 10.1523/JNEUROSCI.0332-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S, Reppert SM. Sun compass integration of skylight cues in migratory monarch butterflies. Neuron. 2011;69:345–358. doi: 10.1016/j.neuron.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Heinze S, Gotthardt S, Homberg U. Transformation of polarized light information in the central complex of the locust. J Neurosci. 2009;29:11783–11793. doi: 10.1523/JNEUROSCI.1870-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg U, Heinze S, Pfeiffer K, Kinoshita M, el Jundi B. Central neural coding of sky polarization in insects. Philos Trans R Soc Lond B. 2011;366:680–687. doi: 10.1098/rstb.2010.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber F. Untersuchungen über die Funktion des Zentralnervensystems und insbesondere des Gehirns bei der Fortbewegung und der Lauterzeugung der Grille. Z Vergl Physiol. 1960;44:60–132. [Google Scholar]
- Ito K, Awasaki T. Clonal unit architecture of the adult fly brain. In: Technau GM, editor. Brain development in Drosophila melanogaster. New York: Landes Bioscience and Springer Science; 2008. pp. 137–158. [DOI] [PubMed] [Google Scholar]
- Ito K, Shinomiya K, Armstrong D, Boyan G, Hartenstein V, Harzsch S, Heisenberg M, Homberg U, Jenett A, Keshishian H, Restifo L, Rössler W, Simpson J, Strausfeld NJ, Strauss R, Vosshall LB. A coordinated nomenclature system for the insect brain. Neuron. 2012 doi: 10.1016/j.neuron.2013.12.017. (in press) [DOI] [PubMed] [Google Scholar]
- Iwano M, Hill ES, Mori A, Mishima T, Mishima T, Ito K, Kanzaki R. Neurons associated with the flip-flop activity in the lateral accessory lobe and ventral protocerebrum of the silkworm moth brain. J Comp Neurol. 2010;518:366–388. doi: 10.1002/cne.22224. [DOI] [PubMed] [Google Scholar]
- Kahsai L, Winther AM. Chemical neuroanatomy of the Drosophila central complex: distribution of multiple neuropeptides in relation to neurotransmitters. J Comp Neurol. 2011;519:290–315. doi: 10.1002/cne.22520. [DOI] [PubMed] [Google Scholar]
- Kahsai L, Martin JR, Winther AM. Neuropeptides in the Drosophila central complex in modulation of locomotor behavior. J Exp Biol. 2010;213:2256–2265. doi: 10.1242/jeb.043190. [DOI] [PubMed] [Google Scholar]
- Klagges BRE, Heimbeck G, Godenschwege TA, Hofbauer A, Pflugfelder GO, Reifegerste R, Reisch D, Schaupp M, Buchner S, Buchner E. Invertebrate synapsins: a single gene codes for several isoforms in Drosophila . J Neurosci. 1996;16:3154–3165. doi: 10.1523/JNEUROSCI.16-10-03154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LR, Han PL, Hwang PM, Feinstein PG, Davis RL, Reed RR. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–89. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- Li Y, Strausfeld NJ. Morphology and sensory modality of mushroom body extrinsic neurons in the brain of the cockroach Periplaneta americana . J Comp Neurol. 1997;387:631–650. [PubMed] [Google Scholar]
- Li Y, Strausfeld NJ. Multimodal efferent and recurrent neurons in the medial lobes of cockroach mushroom bodies. J Comp Neurol. 1999;409:647–663. doi: 10.1002/(sici)1096-9861(19990712)409:4<647::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Li Y, Pan Y, Wang Z, Gong H, Gong Z, Liu L. Morphological characterization of single fan-shaped body neurons in Drosophila melanogaster . Cell Tissue Res. 2009;336:509–519. doi: 10.1007/s00441-009-0781-2. [DOI] [PubMed] [Google Scholar]
- Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, Heisenberg M, Liu L. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;436:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA. The organisation of invertebrate brains: cells, synapses and circuits. Acta Zool (Stockholm) 2010;91:64–71. [Google Scholar]
- Müller M, Homberg U, Kühn A. Neuroarchitecture of the lower division of the central body in the brain of the locust (Schistocerca gregaria) Cell Tissue Res. 1997;288:159–176. doi: 10.1007/s004410050803. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhou Y, Guo C, Gong H, Gong Z, Liu L. Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn Mem. 2009;16:289–295. doi: 10.1101/lm.1331809. [DOI] [PubMed] [Google Scholar]
- Pascual A, Huang K-L, Neveu J, Préat T. Brain asymmetry and long-term memory. Nature. 2004;427:605–606. doi: 10.1038/427605a. [DOI] [PubMed] [Google Scholar]
- Paulk AC, Phillips-Portillo J, Dacks AM, Fellous JM, Gronenberg W. The processing of color, motion, and stimulus timing are anatomically segregated in the bumblebee brain. J Neurosci. 2008;28:6319–6332. doi: 10.1523/JNEUROSCI.1196-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereanu W, Younossi-Hartenstein A, Lovick J, Spindler S, Hartenstein V. Lineage-based analysis of the development of the central complex of the Drosophila brain. J Comp Neurol. 2011;519:661–689. doi: 10.1002/cne.22542. [DOI] [PubMed] [Google Scholar]
- Phillips-Portillo J. The central complex of the flesh fly, Neobellieria bullata: recordings and morphologies of protocerebral inputs and small field neurons. J Comp Neurol. 2012 doi: 10.1002/cne.23134. 000:000-000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeck B, Triphan T, Neuser K, Strauss R. Locomotor control by the central complex in Drosophila: an analysis of the tay bridge mutant. Dev Neurobiol. 2008;68:1046–1058. doi: 10.1002/dneu.20643. [DOI] [PubMed] [Google Scholar]
- Renn SC, Armstrong JD, Yang M, Wang Z, An X, Kaiser K, Taghert PH. Genetic analysis of the Drosophila ellipsoid body neuropil: organization and development of the central complex. J Neurobiol. 1999;41:189–207. [PubMed] [Google Scholar]
- Ridgel AL, Alexander BE, Ritzmann RE. Descending control of turning behavior in the cockroach Blaberus discoidalis . J Comp Physiol A. 2007;193:385–402. doi: 10.1007/s00359-006-0193-7. [DOI] [PubMed] [Google Scholar]
- Ritzmann RE, Ridgel AL, Pollack AJ. Multi-unit recording of antennal mechano-sensitive units in the central complex of the cockroach, Blaberus discoidalis . J Comp Physiol A. 2008;194:341–360. doi: 10.1007/s00359-007-0310-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Deinhardt F. Preparation of a semipermanent mounting medium for fluorescent antibodies studies. Virology. 1960;12:316–317. doi: 10.1016/0042-6822(60)90205-1. [DOI] [PubMed] [Google Scholar]
- Sakura M, Lambrinos D, Labhart T. Polarized skylight navigation in insects: model and electrophysiology of evector coding by neurons in the central complex. J Neurophysiol. 2008;99:667–682. doi: 10.1152/jn.00784.2007. [DOI] [PubMed] [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R, Hanesch U, Kinkelin M, Wolf R, Heisenberg M. No-bridge of Drosophila melanogaster: portrait of a structural brain mutant of the central complex. J Neurogenet. 1992;8:125–155. doi: 10.3109/01677069209083444. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. Atlas of an insect brain. Heidelberg, New York: Springer; 1976. [Google Scholar]
- Strausfeld NJ. A brain region in insects that supervises walking. Prog Brain Res. 1999;123:273–284. doi: 10.1016/s0079-6123(08)62863-0. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. Arthropod brains: evolution, functional elegance, and historical significance. Cambridge, MA: Harvard University Press; 2012. [Google Scholar]
- Strausfeld NJ, Okamura J-Y. Visual system of calliphorid flies: organization of optic glomeruli and their lobula complex efferents. J Comp Neurol. 2007;500:166–188. doi: 10.1002/cne.21196. [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- Triphan T, Poeck B, Neuser K, Strauss R. Visual targeting of motor actions in climbing Drosophila . Curr Biol. 2010;20:663–668. doi: 10.1016/j.cub.2010.02.055. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pan Y, Li W, Jiang H, Chatzimanolis L, Chang J, Gong Z, Liu L. Visual pattern memory requires foraging function in the central complex of Drosophila . Learn Mem. 2008;15:133–142. doi: 10.1101/lm.873008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegerhoff R, Breidbach O. Structure and development of the larval central complex in a holometabolous insect, the beetle Tenebrio molitor . Cell Tissue Res. 1992;268:341–358. [Google Scholar]
- Wegerhoff R, Breidbach O, Lobemeier M. Development of locustatachykinin immunopositive neurons in the central complex of the beetle Tenebrio molitor . J Comp Neurol. 1996;375:157–166. doi: 10.1002/(SICI)1096-9861(19961104)375:1<157::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Williams JLD. Anatomical studies of the insect central nervous system: a ground-plan of the midbrain and an introduction to the central complex in the locust, Schistocerca gregaria (Orthoptera) J Zool (London) 1975;176:67–86. [Google Scholar]
- Wolf R, Heisenberg M. Visual control of straight flight in Drosophila melanogaster . J Comp Physiol A. 1990;167:269–283. doi: 10.1007/BF00188119. [DOI] [PubMed] [Google Scholar]
- Wolf R, Heisenberg M. Basic organization of operant behavior as revealed in Drosophila flight orientation. J Comp Physiol A. 1991;169:699–705. doi: 10.1007/BF00194898. [DOI] [PubMed] [Google Scholar]
- Young JM, Armstrong JD. Structure of the adult central complex in Drosophila: organization of distinct neuronal subsets. J Comp Neurol. 2010;518:1500–1524. doi: 10.1002/cne.22284. [DOI] [PubMed] [Google Scholar]
- Zars T, Wolf R, Davis R, Heisenberg M. Tissue-specific expression of a type 1 adenylyl cyclase rescues the rutabaga mutant memory defect: in search of the engram. Learn Mem. 2000;7:18–31. doi: 10.1101/lm.7.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]