Abstract
Crustaceans are among the most extensively distributed arthropods, occupying many ecologies and manifesting a great variety of compound eye optics; but in comparison with insects, relatively little is known about the organization and neuronal morphologies of their underlying optic neuropils. Most studies, which have been limited to descriptions of the first neuropil - the lamina - suggest that different species have approximately comparable cell types. However, such studies have been limited with regard to the types of neurons they identify and most omit their topographic relationships. It is also uncertain whether similarities, such as they are, are independent of visual ecologies. The present account describes and compares the morphologies and dispositions of monopolar and other efferent neurons as well as the organization of tangential and smaller centrifugal neurons in two grapsoid crabs, one from the S. Atlantic, the other from the N. Pacific. Because these species occupy significantly disparate ecologies we ask whether this might be reflected in differences of cell arrangements within the most peripheral levels of the visual system. The present study identifies such differences with respect to the organization of centrifugal neurons to the lamina. We also identify in both species neurons in the lamina that have hitherto not been identified in crustaceans and we draw specific comparisons between the layered organization of the grapsoid lamina and layered laminas of insects.
Keywords: visual processing, ecological constraints, evolution, Eumalacostraca
INTRODUCTION
Evolutionary relationships within the Arthropoda, particularly between crustaceans and hexapods, are a subject of continuous debate. Osorio and Bacon (1994) proposed that it is the organization of their respective visual systems that suggests close phylogenetic affinities between insects and crustaceans, re-establishing a claim originally made by Hanström in the 1920s. The idea that insects might have arisen from within crustaceans has also been proposed from molecular studies (Regier et al., 2005; Nardi et al., 2003) and genetic and developmental analyses have suggested specific homologies between insects and malacostracan crustaceans (Patel et al., 1992). Retinal commonalties and neuroanatomical analyses of optic lobe morphologies support an origin of the Insecta from within the malacostracan lineage (Dohle, 2001; Strausfeld, 1998, 2005) but others have argued for an entomostracan origin (Regier et al., 2005; Glenner et al., 2006). Whereas the general organization of the optic lobes of higher malacostracans, such as the possession of two successive optic chiasmata and the presence of four nested optic neuropils, has further supported a malacostracan origin for the insects (Strausfeld, 1998, 2005; Sinakevitch et al., 2003; Sztarker et al., 2005) less attention has been paid to neuron-based characters that have been shown to be powerful for high-level phylogenetic analyses (Buschbeck and Strausfeld, 1996, Buschbeck, 2000). These include neuron morphologies, layer relationships through successive neuropils, and the planar organization of networks that extend across the retinotopic mosaic.
The optic lobe’s lamina is the simplest and the most studied of the four nested retinotopic neuropils in tetraconeate arthropods (eumalacostracans + insects) and is thus likely to provide neuroanatomical data from which to evaluate relationships within and between eumalacostracans and insects. Although greatly outnumbered by studies on insect optic lobes, species used for describing the lamina of malacostracans include the crayfish (Hafner, 1973, 1974; Nässel, 1976, 1977; Elofsson et al., 1977; Wang-Bennett et al., 1989; Glantz et al., 2000), species of prawn (Nässel, 1975), crabs (Stowe et al., 1977), and stomatopods (Strausfeld and Nässel, 1980; Kleinlogel et al., 2003). Three studies have focused on branchiopod laminas (Retzius, 1906; Nässel et al., 1978; Strausfeld, 2005).
Taxa used for these surveys have been predominantly aquatic and it is likely that vision is an important, if not the most important, sensory modality. Stomatopod crustaceans are notoriously visual; crayfish live in turbid water and are active in dim light; investigated species of prawns either live in deep water, where adaptations of the compound eye to increase light capture may be at the expense of visual acuity, or are pelagic in conditions where high-level vision may not confer any advantage. It is unclear at what level optic lobe organization might relate to different behaviors and ecologies. Might such correlates, if they exist, be already apparent in the most distal level of the system? Or would ecology- and behavior-related differences pertain to deeper computational levels as is evident from dipterous insects (Buschbeck and Strausfeld 1996)? Decapod crustaceans are a good group on which to address such questions. They can be found in almost every possible niche, including land, shallow and deep sea, fresh water, and even deserts (Diesel et al., 2000). There exist a number of reports about anatomical and behavioral adaptations of their visual systems to different habitats (see, Nunnemacher, 1966; Zeil et al., 1986; Nalbach and Nalbach, 1987; Tomsic et al., 1993). For instance, the eyestalk length, eye separation, and acute zones for vertical resolving power have been shown to correlate with the structure of specific environments (Zeil et al., 1986). Accordingly, the vertical distribution of optokinetic sensitivity varies significantly within different habitats (Nalbach and Nalbach, 1987). The brains of malacostracans can show major differences. For example, whereas most crabs and shrimps have eyestalks, isopod compound eyes are integrated as part of the head cuticle, as they are in insects. Optic lobes of littoral species are equipped with large lobula plates equipped with giant tangential cells that approach the size of those of dipterous insects (Sinakevitch et al., 2003). In addition, the number of ommatidia also shows wide variation across the Malacostraca, from less than 200 in the ghost shrimp Callianassa (NJS, personal observations) to around 18000 in land crabs such as Grapsus grapsus (Nunnemacher, 1966).
Such ecological constraints or gross differences in brain anatomy do not limit, indeed they amplify, the identification of general principles of organization that might apply across the Arthropoda; particularly those taxa whose behavior is largely driven by visual cues, such as grapsoid crabs, salticid spiders, and numerous orders of fast flying insects. Here we focus on two species of grapsoids that have large and elaborate deep optic lobe neuropils, and in which vision is a dominant sensory modality eliciting reactive and active visual behaviors. Crabs exhibit visually guided homing, courtship, and defensive behaviors (Nalbach, 1990; Cannicci et al., 2000; Backwell et al., 2000; Zeil and Hoffmann, 2001). The estuarine South Atlantic species Chasmagnathus granulatus is a semiterrestrial crab that lives in colonies, socially interacts, and is subject to attack by aerial predators (Bachmann and Martinez, 1999; Berón, 2003). At low tide it is a terrestrial forager, viewing in clear air whereas during intermediate and high tide it lives in turbid water. The North Pacific species, Hemigrapsus oregonensis, is likewise an active visual forager, uses vision for place recognition and homing, is highly territorial, and is non-social. It lives mostly submerged and occupies usually clear waters irrespective of tide.
Amongst insects, related species can show differences of neuronal morphologies and disposition, even at distal levels of the optic lobes. Such differences do not necessarily reflect major ecological differences, as for example in nocturnal or diurnal foraging bees (Greiner et al., 2005), but are apparent even amongst species that are sympatric, such as certain syrphid and calliphorid flies (Strausfeld, 1970). Here we determine whether related species of grapsoid crabs, which are vastly separated geographically, and which occupy similar but by no means identical habitats, share common or distinct neural arrangements in their optic lobe laminas.
MATERIAL AND METHODS
Animals
Adult male Chasmagnathus granulatus, measuring 1.8–3.0 cm across the carapace, were collected in narrow coastal inlets (rías) of San Clemente del Tuyú, Argentina. Crabs were maintained in the laboratory in plastic tanks (35 × 48 × 27 cm) filled to 2 cm depth with diluted marine water prepared using hw-Marinex (Winex, Hamburg, Germany), having a salinity of 10–14 % and a pH of 7.4–7.6 and maintained at 22–24°C and on a 12 hours light/dark cycle. Adult male Hemigrapsus oregonensis (carapace width 1.5–2.5 cm) were collected from rock pools tidal mills at designated collection sites belonging the University of Washington’s marine reserve on San Juan Island. Crabs were maintained in running seawater tanks at the University of Washington’s Friday Harbor Marine Laboratories.
Coordinates of the optic lobe and planes of sectioning
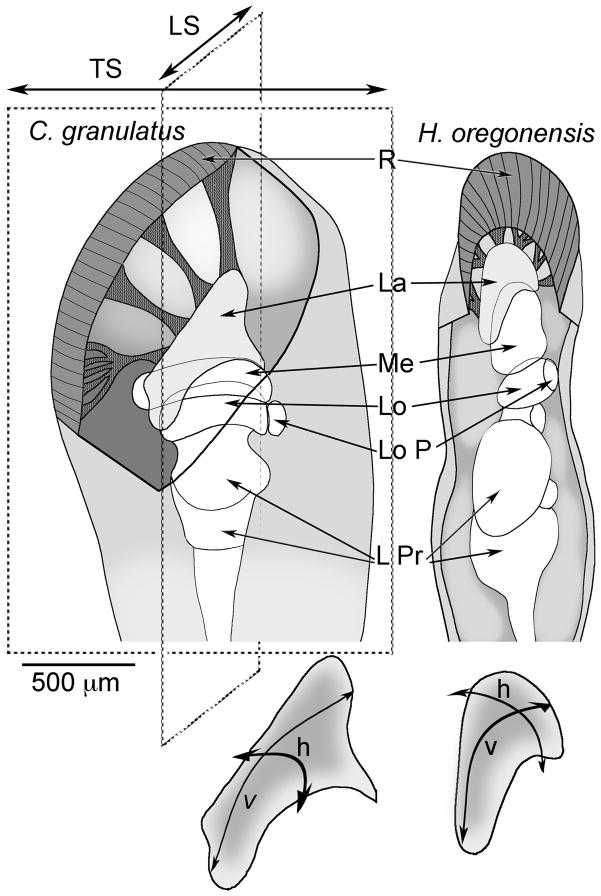
The eyestalks of C. granulatus and H. oregonensis are oriented at about 45°–70° from horizontal. Descriptions here are from longitudinal and transverse sections cut parallel to the antero-posterior or latero-medial axes respectively (see Fig. 1). Due to the curvature of the lamina, the first sections of a longitudinal or transverse series offer tangential (top-down) views of the neuropil.
Fig. 1.
Schematic diagrams of the eyestalks and optic lobes of C. granulatus and H. oregonensis. The anteroposterior and dorsoventral axes of the retina are equivalent to the horizontal (h) and vertical (v) axis of the retinotopic mosaic of the lamina, each of which is parallel to the transverse (TS) and longitudinal (LS) plane of sections. Abbreviations: R, retina; La, lamina; Me, medulla; Lo, lobula; LoP, lobula plate; LPr, lateral protocerebrum. Scale bar: 500 μm.
Histology and anatomical reconstructions
Both species are amenable to reduced-silver techniques and selective impregnation by the Golgi method. Optic lobes were impregnated using the combined Golgi Colonnier-Golgi rapid method (Li and Strausfeld, 1997). Eyestalks were removed and under fixative the optic lobes were partially exposed beneath the overlying muscles and cuticle. Tissue was fixed for 1–3 hours in 2.5% potassium dichromate (with 10% w/v sucrose) and 25% glutaraldehyde (5 volumes to 1 volume). Some C. granulatus were alternatively fixed in 2.5% potassium dichromate (with 10% w/v sucrose), 25% glutaraldehyde and 37% formaldehyde (5:1:1 vols). After about 3 hours fixation, eyestalk neuropil (optic lobes and the lateral protocerebrum) was cleaned of cuticle, surrounding muscles and connective tissue. Neural tissue was fixed for another 3–4 days in the dark at room temperature, then rinsed in several changes of 2.5% potassium dichromate and placed in 99 volumes of 2.5% potassium dichromate with one volume of 1% osmium tetroxide for 3 days, omitting sucrose. Next, under 2.5% potassium dichromate, areas of retina were exposed and parts of the surface of optic lobe neuropils were scraped free of the overlying perineural sheath to allow entry of silver nitrate. This was done by swirling the tissue in several changes of 0.75% silver nitrate and then leaving it in 0.75% silver nitrate for 3 days, in the dark. H. oregonensis were subjected to a second chromation and silver impregnation cycle. After the first silver impregnation, tissue was briefly washed in distilled water and returned to the dichromate-osmium admixture. After 3 days, tissue was again swirled in 0.75% silver nitrate and incubated in this for another 2 days. Finally, tissue was rinsed in distilled water, dehydrated, transferred to propylene oxide and embedded in Durcupan plastic (Fluka, Heidelberg, Germany). Tissue was serially sectioned in the longitudinal or transverse planes at 35–40 μm on a sliding microtome. Sections were mounted under coverslips in Permount (Electron Microscopy Sciences, Ft. Washington, PA). Drawings were made at x1000 magnification using a camera lucida attachment to an Olympus or Leitz Dialux microscope. Except for some tangential and amacrine elements that had to be traced through 2–3 sections, reconstructions were made from elements within a single section. Photographs of C. granulatus laminas were made using an Infinity 2 CCD digital camera (Lumenera Corp., Canada) linked to a computer equipped with graphics software. Dendritic trees and terminals of Golgi-impregnated neurons in H. oregonensis were reconstructed from stacked optical sections captured with a Sony DKC 5000 CCD digital camera linked to an Apple G4 computer equipped with graphics software. For both species, images were captured at an initial magnification of x600, using planapochromat oil immersion objectives. For any 35 μm thick section, between 5 and 30 optical images were captured and layered in register. These were made transparent by using the Photoshop darkening function. Shadows were removed, and the images then flattened. For larger areas, between two and four such reconstructions were made separately and then seamlessly montaged. These procedures result in images of Golgi-impregnated neurons that show each process in focus throughout a maximum depth of 40 μm. In some cases, the contrast of images was enhanced using the image adjust tools from the same softwere. Due to the distance and trajectory of the first optic chiasma of C. granulatus, medulla components could not be confidently matched to lamina elements simply by following the axon across the chiasma. However, medulla counterparts could sometimes be identified on the basis of a corresponding axon diameter or by reverse matching the number and sequence of elements viewed separately in the lamina and medulla. The smaller optic lobes of H. oregonensis, in which axons could be more readily followed through successive sections, provided confirmatory data regarding medullary components of monopolar cells and centrifugal neurons.
Reduced silver preparations
Bodian’s original 1937 method was used to stain neuropils, relying on the reducing properties of cytoskeletal proteins.
Receptor cell tracing
A small pellet of Texas red-conjugated to dextran (7,000 MW) was placed in the living eyes of H. oregonensis. Animals were kept immobilized, half submerged in sea water, overnight at 4°C. Eyestalks were then removed, opened under 4% paraformaldehyde in phosphate buffer (pH. 7.4) containing 10 gm sucrose/100 ml and then fixed for 5 hours. Cuticle and muscle were then removed and the tissue embedded in gelatin for vibratome sectioning at 25 μm. Sections were incubate in antisera raised against rabbit anti-β-tubulin, and then in anti-rabbit conjugated to Cy5, to reveal the retinotopic organization of the neuropil. Sections were scanned using a 3-line Zeiss Pascal confocal.
Terminology
Neurons that originate from cell bodies above the lamina are termed monopolar cells, following the convention used for insects. Neurons that originate from cell bodies above the medulla, and which connect the medulla and lamina are termed centrifugal neurons if their medullary branches are decorate with spines and their lamina arbors appear varicose or beaded. However, at least two cell types originating from cell bodies beneath the lamina have distal processes equipped with spicule- or spine-like specializations, suggestive of postsynaptic sites. The designation “amacrine cells” applies to neurons that lack an axon (Cajal and Sanchez, 1915).
RESULTS
Cytological organization of the lamina
The eyes of both species, C. granulatus and H. oregonensis, surmount movable appendages (called “eye stalks”) that extend obliquely from the anterior margin of the carapace. An eye of an adult C. granulatus typically consists of about 7,000 ommatidia; of H. oregonensis about 5000 ommatidia. The visual field of each eye subtends almost the entire visual panorama. In both species, ommatidia are distributed all around the tip of the eyestalk except for a narrow isthmus of cuticle located towards the medial side of the stalk where, when at rest, it lies within the groove of the carapace.
The general architecture of the optic lobes of C. granulatus (Sztarker et al., 2005) and H. oregonensis is similar, with four retinotopic neuropils arranged in succession distal to the several neuropils of the lateral protocerebrum (Fig. 1). In both species, the lamina’s long axis extends from the anterolateral to the posteromedial part of the eye (Fig. 1). The lamina extends over the medulla, partially covering it, with the long axis of the lamina corresponding to the vertical axis of the retina’s mosaic of facets. Photoreceptor axons leave the base of each ommatidium, collect into bundles and project towards the lamina above which single axons sort out to penetrate into the lamina’s neuropil.
The two species reveal extraordinary precision with regard to the partitioning of lamina neuropil (See Sztarker et al., 2005 and Fig. 2). As in other descriptions of malacostracans, the lamina is composed of primary receptor terminals of retinal photoreceptors, several discrete morphologies of first order relay neurons (monopolar cells) that terminate in the second optic neuropil, the medulla, and T-cells (also first order relays) that carry information from the lamina to the medulla but which originate from cell bodies situated between the lamina and medulla. Wide- and narrow-field neurons that provide terminal arbors in the lamina are interpreted as centrifugal elements that carry centrifugal information peripherally from the medulla.
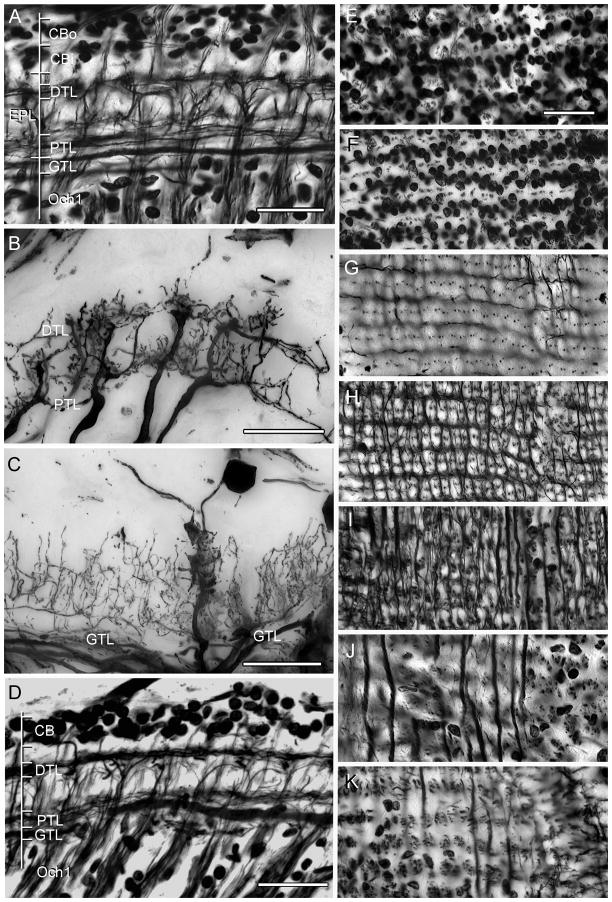
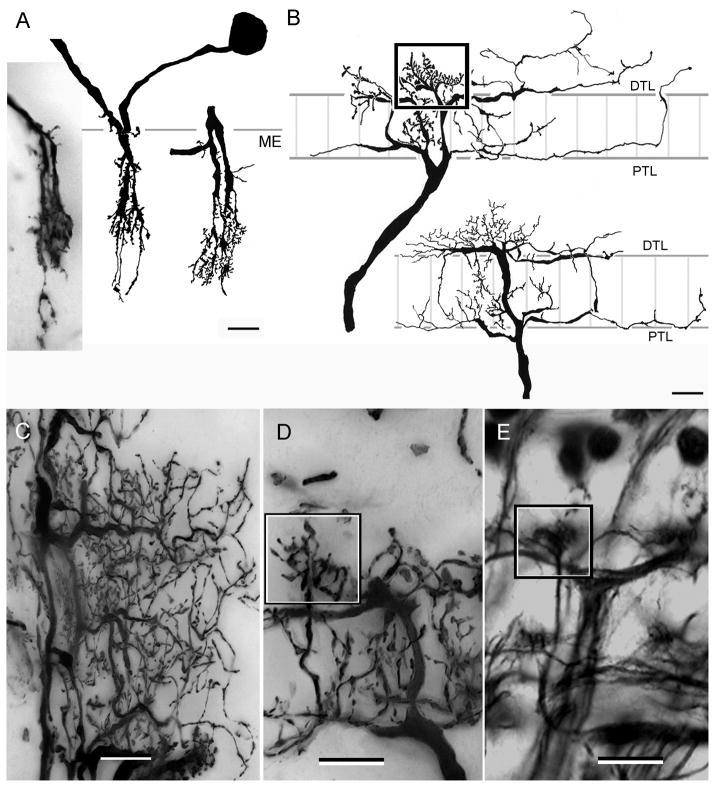
Fig. 2.
Neuroarchitecture of the lamina as revealed by Bodian’s reduced silver with Golgi impregnations to show selective neuron correlates. A. H. oregonensis: Section cut parallel to the vertical axis of the retinotopic mosaic showing levels depicted top-down in panels E–K. An outer cell body layer (CBo, and panel E) provides monopolar cells with long neurites such as the M2 type shown in panel C. The inner layer of cell bodies (CBi, and panel F) provides monopolar cell M1 and M5. A short intermediate zone reveals the clustered neurites of monopolar cells (panel G). The distal tangential layer (DTL, also panel H) receives contributions from the bilayered terminals of the type 1 tangentials (panel B) with the same cell type providing tangential processes of the proximal tangential layer (PTL and panel I). The “giant” tangential layer (GTL) is provided by the primary branches of type 2 tangential endings (panel C and J). The bundled axons from optic cartridges that provide the first optic chiasma (Och1, also panel K) originate just beneath this layer. D. Bodian stained section of the lamina of C. granulatus shows the same layering except for the monopolar cell perikarya, where the two layers are virtually indistinguishable. Scale bars for panels A–C and E–K = 25 μm; for panel D = 50 μm.
The dendrites and terminals of all these elements comprise the lamina’s synaptic plexiform layer. Reduced silver preparations reveal these as a series of discrete strata (Fig. 2A, D). In H. oregonensis (Fig. 2A), two well-separated layers of monopolar cell perikarya lie above a distal stratum that is composed of tangential fibers followed by a tangential-free zone that is succeeded by an inner (proximal) tangential layer. This is followed by a deep stratum of wide-diameter tangentially-directed elements which here refer to as the “giant” tangential layer. All of these tangential components derive from axons that ascend out to the lamina from the medulla. In C. granulatus and H. oregonensis tangential neurons contribute either to both layers (the type 1 tangential) or they provide ascending processes through part or the whole of the plexiform layer. For example, the giant tangential layer provides a delicate filigree of collaterals that ascend and descend through the lamina, constrained to the lamina’s external plexiform layer (Fig. 2C). Despite minor differences in tangential cell morphology (and thus possible functional relationships; see Discussion) the same stratified organization of the lamina is seen in both species, except that in C. granulatus the monopolar cell bodies are not arranged in such quite well defined layers as in H. oregonensis (Fig. 2D). Thus, the laminas of H. oregonensis and C. granulatus show no overt cytoarchitectural differences although certain types of neurons do have subtle differences in the two species, as is evidenced by amacrine cells and certain monopolar cells which will be described in detail below.
As shown in a previous study, top-down views of Bodian preparations of the South Atlantic species reveal a quasi-orthogonal arrangement of tangentially directed processes above and beneath the lamina. The lamina of H. oregonensis reveals exquisite precision layer by layer through the lamina (Fig. 2E–K). As in C. granulatus, the lamina of the North Pacific species is divided into an isomorphic organization of units, each of which represents a facet of the compound eye. Progressing inwards from the base of the ommatidia the observer first encounters two distinct levels of neuronal perikarya (neuron cell bodies). An outer layer (Fig. 2E) is characterized by scattered clusters of 4–5 cell bodies, amongst which project the axons of photoreceptor cells. Immediately beneath this level (Fig. 2F) are neatly arranged horizontal clusters of between 2 and 3 cell bodies, between which projects the now orderly rows of receptor axons (Fig. 2G). Following this level inwards, the distalmost layer of tangential processes provides the beautiful rectilinear network alluded to above (Fig. 2H). At the center of each lozenge are clustered 6–7 argyrophilic profiles that represent the axial fibers of monopolar cells and the through going axon of a long visual fiber, one provided by each ommatidia and ending in the medulla. These lozenge-shaped partitions define the outer ends of discrete units of neuropil called “optic cartridges”, a term that follows the convention used to describe retinotopic columns in the insect lamina.
Deeper still, proximal tangential fibers are organized predominantly along the retinotopic mosaic’s vertical axis with fine collaterals providing the proximal tangential layer’s horizontally directed processes (Fig. 2I). This contrasts to the deep “giant” tangential fibers, which are aligned exclusive along the vertical axis of the lamina (Fig. 2J). Immediately beneath this layer are clusters of argyrophilic profiles, each profile representing an incoming and outgoing axon of an optic cartridge (Figs. 2K, 3).
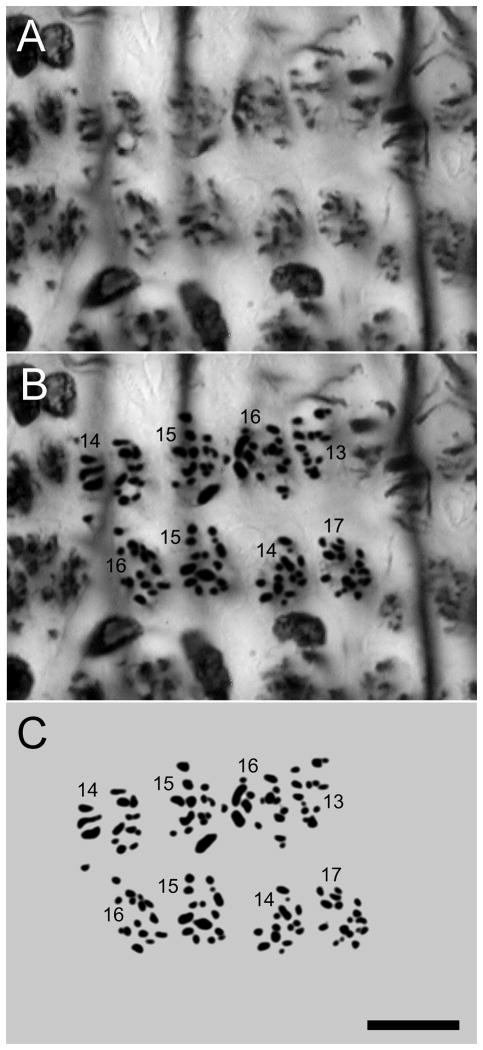
Fig. 3.
Enlargement of panel K, Fig. 2, showing profiles of axons entering and leaving eight adjacent lamina cartridges. A is the original micrograph, B, the enhanced profiles and C the same shown alone, with the profile counts. Scale bar: 10 μm.
Neuron populations of optic cartridges
Golgi impregnations reveal the shapes of neurons that contribute to the structure of an optic cartridge. However, because Golgi impregnations are stochastic they cannot alone provide reliable data about cell populations. Estimates of the number of neurons in each optic cartridge derive from serial sections of Bodian preparations that are cut tangential to the lamina’s surface. As described above, these show cell bodies above the lamina and the argyrophilic cross sections of axons associated with each cartridge through and beneath the lamina. The number of cell bodies above each cartridge is estimated from the way in which perikarya are clustered at their two levels (Fig. 2E, F). Observations of H. oregonensis suggest that a cartridge is parent to at least 7 monopolar cells, a cluster of 5–6 in the outer cell body layer and clusters of 2–3 perikarya in the inner layer. In addition to seven monopolar cells in a cartridge, Golgi impregnations show that in both grapsoid species the distal and proximal tangential layers are supplied by an ascending axon of Tan1 alongside every cartridge. Golgi impregnations also identify at least three types of small field centrifugal cells and two morphologies of neurons equivalent to an efferent T-cell such as described from insects (Strausfeld, 1970). Assuming for the present that each of these cell types, including a long visual fiber, occurs once per optic cartridge, then the axons between 12 and 14 neurons would be expected to be resolved at a level depicted in figure 2K. These numbers correspond to axon cross sections resolved beneath a cartridge. High-resolution micrographs at level K in figure 2 suggest an average of 15 per cartridge (Fig. 3). The Discussion will return to this surprisingly high number of elements, which has important implications with regard to the number of parallel channels that originate from the three levels of photoreceptor terminals, as will be described later.
Neuron morphologies
Retinula cell terminals
Reduced silver preparations of C. granulatus reveal eight photoreceptor axons leaving each ommatidium (Sztarker et al., 2005). As in H. oregonensis, seven of these (from photoreceptors designated as R1–R7) end in the lamina’s plexiform layer whereas the eighth receptor (R8) sends its axon through the lamina to terminate in the medulla.
In both species of grapsoids the R1–R7 terminals are arranged approximately as two tiers of endings (Fig. 4A, B) to which contribute four principal morphological types of terminals. In C. granulatus a stout club-shaped terminals extends through about the one half to two thirds of the plexiform layer’s depth. Receptor terminals initially provide a swollen component, usually within the outer third of the layer, and then taper to provide thinner processes at various depths through the rest of the lamina (Fig. 4C). Two pairs of receptor terminals, one defining the outer tier the other the inner tier in figure 4A, extend either side of these central elements. Typically, tiered terminals have shovel-shaped swellings that are oriented vertically within the retinotopic mosaic (Fig. 4A, C) but are compressed along the horizontal axis (Fig. 4B). In C. granulatus, the tiered terminals typically have one side smoother than the other and give rise to one or several filamentous outgrowths (Fig. 4C). In H. oregonensis R1–R7 terminals are generally smooth or slightly puckered. Similar bi-layered arrangements of receptor endings have been reported from observations of other decapods, including benthic species.
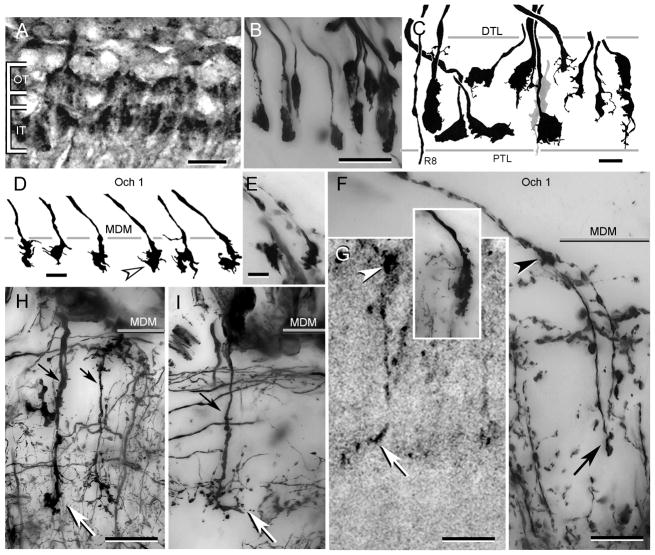
Fig. 4.
Photoreceptor terminals. A. H. oregonensis: Texas red-dextran fills show receptor terminals arranged as two tiers. B. H. oregonensis: horizontal section showing Golgi-impregnated terminals “end-on”. Note that some terminals occupy intermediate positions with respect to the tiers shown in panel A. C. C. granulatus: camera lucida drawings of receptor endings aligned along the vertical axis of the retinotopic mosaic. Spatulate endings (left and center in panel) correspond approximately to the two tiers shown from H. oregonensiswhereas other terminals (leftmost, four to right in panel, and grey terminal center) occupy intermediate positions or extend through the whole depth of the plexiform layer. The axon of a thoroughgoing R8 photoreceptor is shown extreme left. D, E. C. granulatus: swellings of R8 photoreceptors at the medulla surface. F. H. oregonensis: two R8 photoreceptor endings showing their outer swelling (arrowhead) and deep extension ending as a terminal varicosity (at arrow). Inset compares the ending of an M3 monopolar cell. G. H. oregonensis: Texas red-dextran fill of a R8 photoreceptor endings showing the surface swelling (arrowhead) and swollen terminal (at arrow). H, I. H. oregonensis: medulla terminals of M2 and M2d monopolar cells. DTL: distal tangential layer, PTL: proximal tangential layer, Och 1: level of the first optic chiasma lamina, MDM: medulla’s distal margin, OT: outer terminals, IT: inner terminals. Scale bar for panels A, B = 25 μm; panel C, =25 μm; panel D, E = 10 μm; panels F–I = 25 μm.
As in insects, each ommatidium of a decapod compound eye possesses eight photoreceptors. However, unlike insects, only one of these projects an axon to the medulla. The other seven end in the lamina, as described above. In both of the present species, long visual fibers supplying the medulla are extremely slender and in C. granulatus appears to end superficially in the medulla as irregular swellings (Fig. 4D, E). However, Golgi impregnations of H. oregonensis reveal that such swellings give rise to a further extremely slender prolongation that extends as a small varicosity about a third of the distance through the medulla (Fig. 4F), equal to that penetrated by deep monopolar cell endings (Fig. 4H, I). Texas red-dextran fills into the retina of H. oregonensis also occasionally label long visual fibers and show these as ending deep, but with an outer swelling (Fig. 4G) that is distinct from shallow monopolar cell terminals (Fig. 4F, inset).
Neuronal components of the lamina
1. Monopolar cells
Monopolar cells originate from somata above the lamina’s plexiform layer. Each provides a single neurite that extends perpendicularly through the lamina, broadening as a thicker axis fiber within the plexiform layer where it gives rise to its system of dendrites. Monopolar cell axons extend across the first optic chiasma to end at various depths in the medulla (Fig. 4F, H, I).
Whereas some authors have designated each variant of a monopolar cell as a separate type of neuron, rather than splitting obviously similar shapes into many sub-types we have here lumped similar profiles together as a single cell type following Nässel’s classic (1977) description of the crayfish lamina. As in other decapods and in insects, Golgi impregnations of both C. granulatus and H. oregonensis, reveal numerous morphological variations within a class of generally similar looking monopolar cells. Neurons belonging to several different morphological classes can be identified at any location across the lamina. While some of these profiles correspond to monopolar cell morphologies described from other species (Hafner, 1973; Nässel, 1975, 1977; Stowe et al., 1977), five are hitherto undescribed. That they occur in both of the present species suggests their wider distribution, at least in grapsoid crabs.
Lamina monopolar cells are classified according to several criteria: their origin from one of the two layers of perikarya above the lamina’s plexiform layers; the level at which their processes branch; the arrangement of their processes from the axis fiber (radial, lateral, unilateral); the width and orientation of their processes; and morphological features of the processes themselves. These criteria are the same as those adopted for descriptions of monopolar cells in insects (Strausfeld, 1970; Ribi, 1975). Nässel’s designation M, for “monopolar cell”, has been universally used except in Hafner’s account, which classifies lamina monopolar neurons as B1–B11 while granting that many elements are probably variations of the same type. Here we have kept to the classification originally devised by Nässel (1977): M1, M2, etc. However, obvious and consistent varieties of cell morphology require additional descriptors such as, for example, “s” for stratified, “b” for bilateral, “n” for narrow, and so on.
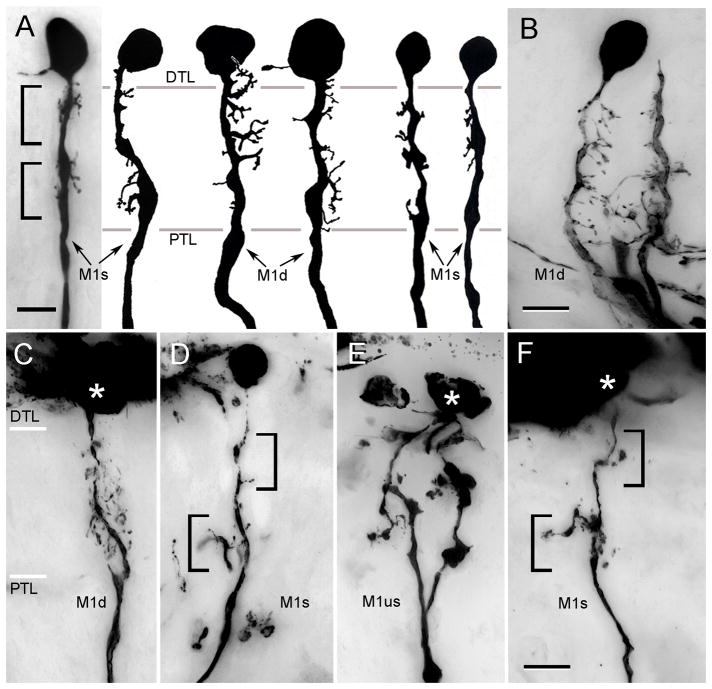
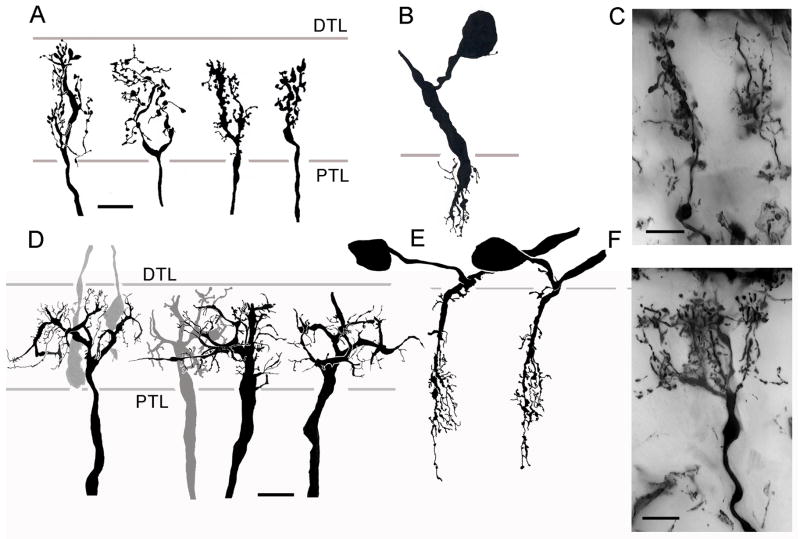
M1 monopolar cells originate from cell bodies that lie directly above the distal tangential layer (Fig. 5). Several characteristic morphologies have been identified. These are bistratified or unistratified M1 cells (M1s, M1us), the branches of which approximately match one or both two tiers of receptor endings (Fig. 5A, D); and diffuse M1 cells (M1d), the branches of which occur through the lamina (Fig. 5A, B, C). Together, these morphologies suggest two distinctive cell types, the stratified M1s, with sparse dendrites, sometimes exceedingly so, and the diffuse M1d, which have numerous dendrites that extend either unilaterally or bilaterally from the axis fiber. The detailed morphology of branches from stratified M1s, at least in H. oregonensis, also suggests differences in their postsynaptic specializations (compare panels B, C, Fig. 5 with panels E, F)
Fig. 5.
M1 monopolar cells. A. C. granulatus: the dendritic disposition of M1 neurons shows considerable morphological variation. However, M1 cells are characterized by their extremely short dendrites and cell bodies that lie immediately about the plexiform layer. Some neurons have stratified arrangements of dendrites others are diffuse (M1s, M1d). B–F. H. oregonensis: panels B, C show diffuse M1 cells (M1d) compared to stratified (panels D, F) and unistratified neurons (panel E). Asterisks indicate the position of cell body. Abbreviations as in Fig. 4. Scale bar for panel A = 5 μm; for panels B–F = 10 μm.
M2 monopolar cells arise from the distal cell body layer. Typically, their axis fibers provide dendrites through the depth of the lamina. Five morphological variants are identified. M2b (broad) have wide dendritic extends, though limited to within the confines of a cartridge (Fig. 6A, C, D). Type M2n (narrow) monopolar cells have compressed dendritic fields about half the width of the first cell type (Fig. 6B, E, G). M2nd (narrow diffuse) cells (Fig. 6G) are similarly constrained, but give rise to about half as many processes as the M2n cells, and thus appear to have diffusely arrangements of dendrites. Two additional broad M2 neurons, shown in figures 6F and 7C, have stratified (M2bs) or diffuse (M2bd) processes restricted to the outer two thirds of the lamina.
Fig. 6.
Monopolar cells M2. A. C. granulatus: The broad variant of M2 (M2b) has a dense arrangement of dendrites through the depth of the plexiform layer. Two inner tier receptor terminals impregnated in the same preparation are shown in grey. B. C. granulatus: narrow field M2 monopolar cells (M2n). These monopolar neurons have short but densely arranged lateral processes distributed along the axis fiber. C. H. oregonensis: A M2b type monopolar shown with its axis fiber next to the dendrites of a second M2b neuron (axis fiber out of section) shown with branches of the type 1 tangential ending, thus demonstrating the extent of branching of this monopolar cell type through the plexiform layer. D. H. oregonensis: several monopolar cells impregnated together compare the relative depths and morphologies of M2b neurons with M3cr and M4. E. H. oregonensis: A narrow variant of the M2 neuron. The consistency of this cell type is further demonstrated in panel G. F. H. oregonensis: two variants of M2 neurons include the broad stratified type, the dendrites of which are clustered at the outer and inner tiers of receptor terminals, and the broad diffuse type. G. H. oregonensis: comparison of the narrow and narrow diffuse variants of M2 neurons. A photoreceptor terminal belonging to the outer tier is shown to the right. Abbreviations as in Fig. 4. Scale bar for A, B = 5 μm; for panels C–G = 10 μm.
Fig. 7.
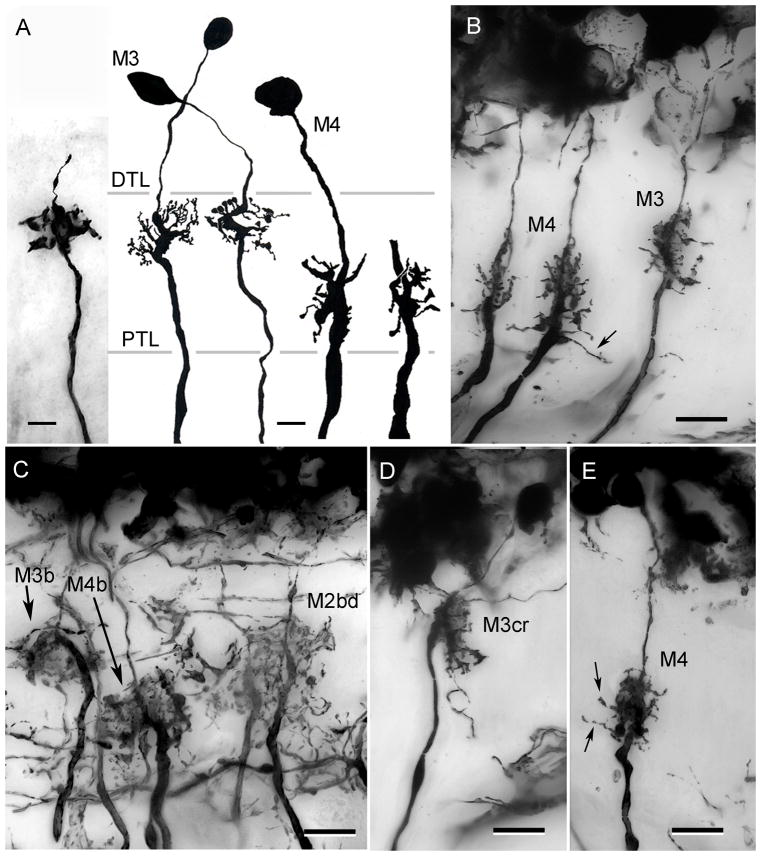
The stratified monopolar cells M3 and M4. A. C. granulatus: The axis fibers of M3 neurons are typically thinner than those of M4, with a local thickening at the level of its dendrites. B. H. oregonensis: M3 monopolar cells. In this species too, the axons of M3 monopolar cells are thinner than those of M4. C, D, E. H. oregonensis: variant of M3 and M4 include neurons with broad dendritic fields (M3b, M4b) and neurons with crook-shaped dendritic trees providing thin M3-like axons (M3cr in panel D). A broad diffuse variant of the M2 neuron (M2bd) is also shown in panel C. E. M4 neurons in H. oregonensis provide slender collaterals at the inner margin of the lamina (arrows in panels B, E). Scale bar for panel A = 5 μm; for panels B–E = 10 μm.
Two kinds of monopolar cells are associated with the two tiers of receptor terminals. These are the shallow and deep M neurons, M3 and M4, respectively. Again, several variants have been identified on the basis of minor differences that probably reflect the corrugation and lateral extends of the receptor terminals with which they make contact. M3 and M4 cells in C. granulatus (Fig. 7A) obviously correspond to their counterparts in H. oregonensis (Fig. 7B). However, in the latter species there are broad variants of both cell types (M3b, M4b; Fig. 7C). A narrow field neuron at the level of the outer receptor tier, which Stowe et al. (1977) identified as a distinct cell type in Scylla (their “M5”) is here ascribed to the M3 monopolar cells, and designated as the crook-neck variant of this cell type (M3cr; Figs. 6D, 7D).
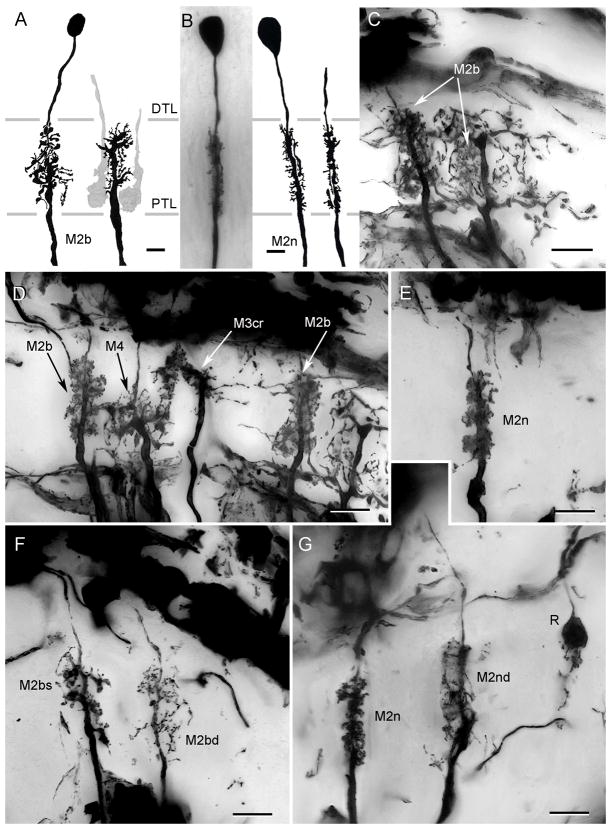
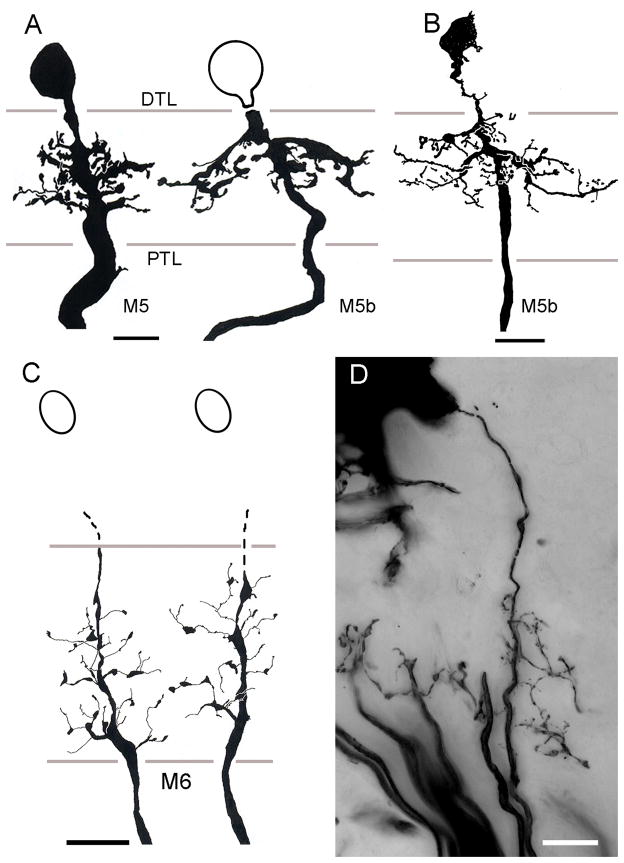
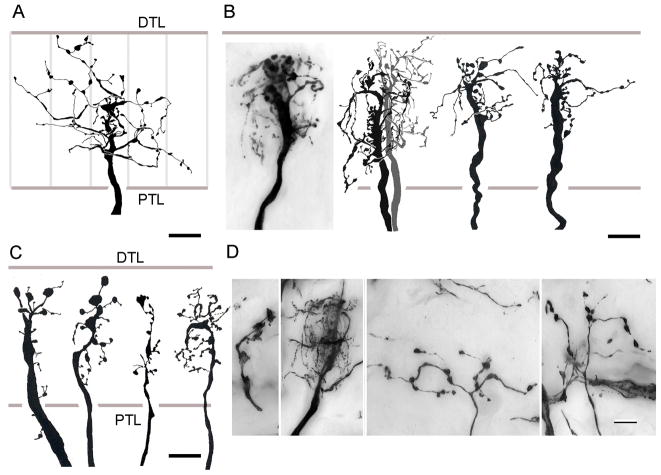
All the monopolar cells thus far described provide dendrites that are either constrained to within their parent optic cartridge, or that extend just beyond its confines. There are, however, two distinctive types of wide-field monopolar cells, again identified in both grapsoid species. One type, designated M5 has a system of robust dendrites that extend laterally outside its parent cartridge to reach the adjacent cartridges along the vertical axis of the lamina (Fig. 8A, B). In C. granulatus, these neurons originate from very large perikarya that lie close to the outer margin of the plexiform layer (Fig. 8A) whereas in H. oregonensis, the cell bodies of these neurons reside more peripherally, with those of M2–M4 neurons (Fig. 8B). M5 monopolar cell branches are restricted to the outer two thirds of the lamina and spread bilaterally into the adjacent cartridges. Some (M5b) have broader fields that extend even further and their branches are situated mainly at the outer tier of photoreceptor cell terminals. Type M6 monopolar cells also have wide-field processes but unlike the stout spiny specializations of M5 cells, the dendrites of M6 neurons are delicate, equipped with occasional pin-head like specializations (Fig. 8C, D). Intact neurons (that is, with their cell bodies) have been impregnated in H. oregonensis (Fig. 8D) but the cell bodies have failed to impregnate in the South Atlantic species (Fig. 8C). Such failure of perikaryal impregnation is not uncommon in tissue that has been cycled only once through silver impregnation. Indeed, in a recent survey of the spotted shrimp Pandalus platyceros Golgi preparations revealed numerous M1–M4 monopolar cells, but invariably without their perikarya impregnated, whereas the cell bodies of amacrines cells were impregnated (NJS, DA, unpublished observations).
Fig. 8.
Wide field monopolar neurons. A. C. granulatus: Dendritic fields of M5 neurons extend through about two cartridges along the vertical axis of the retinotopic mosaic. B. H. oregonensis: the same cell type in the N. Pacific species is typically more slender but has the same distribution in the lamina C. C. granulatus: incomplete impregnations of the M6monopolar cell omit its perikaryon. D. H. oregonensis: complete impregnation reveals the M6 monopolar cell as having sparse dendrites distributed down the length of its axis fiber extending to the adjacent optic cartridge. Abbreviations as in Fig. 4. Scale bars for panels A, B =10 μm; for panel C and D = 10 μm.
2. Centrifugal tangential neurons
Lamina tangential neurons link the medulla to the lamina. In insects, equivalent neurons are distributed one to every several retinotopic columns and provide extensive lateral processes that together supply an isomorphic arrangement of arborizations that extend across the retinotopic mosaic. In the present species, every optic cartridge receives the axon of one type of tangential neuron that is common to both taxa. As a consequence, the tangential supply to the lamina is extraordinarily dense.
Lamina tangential neurons originate from cell bodies located immediately above the medulla (Figs. 9A; 10A–C). In both species, type 1 tangential neurons (Tan 1) originate from a dense cluster of dendrites organized as a narrow cone-shaped dendritic field that penetrates down into the medulla to reach a depth equivalent to that penetrated by the deepest terminals of monopolar cells (Fig. 9A). Each dendritic tree sends an axon to the lamina, there being as many axons as there are optic cartridges. Each axon extends out to the distal tangential layer where it provides a system of secondary and tertiary branches that spread mainly along the vertical axis of the retinotopic mosaic (Fig. 9B, C). The thick distally projecting axon terminal also provides a dense cluster of blebbed processes that protrudes slight above the distal tangential layer such that each cartridge is crowned by one of these small crest-like concentrations of terminal processes (Fig. 9B, D, E). Collateral processes also spread out from the tangential axon where it enters the base of the cartridge. These processes are less dense than those distally, and the number of them decreases with distance along the vertical axis of the mosaic. These collaterals provide secondary branchlets that are set approximately at right angles, thus providing the horizontal component of the rectilinear organization of this proximal layer (Fig. 9C). In H. oregonensis these cells are often impregnated en masse and demonstrate that the secondary tangential processes at the distal and proximal layers give off numerous smaller collaterals that penetrate upwards and downwards through the plexiform layer (Fig. 2B, see also Fig. 9B). In C. granulatus type 1 tangential neurons have large fields that are about ten times broader along the vertical axis than they are wide (along the horizontal axis of the retinotopic mosaic). There is, however, an important difference between the two species, in that whereas in C. granulatus each vertical branch of a Tan 1 extends alongside 10–12 optic cartridges (Sztarker et al., 2005), in H. oregonensis the inner and outer initial branches of Tan 1 intersect only the flanking optic cartridges along the horizontal axis and about 6–8 cartridges vertically. Although these fields are relatively small, their processes provide a rectilinear network as dense as that of C. granulatus (Fig. 9C).
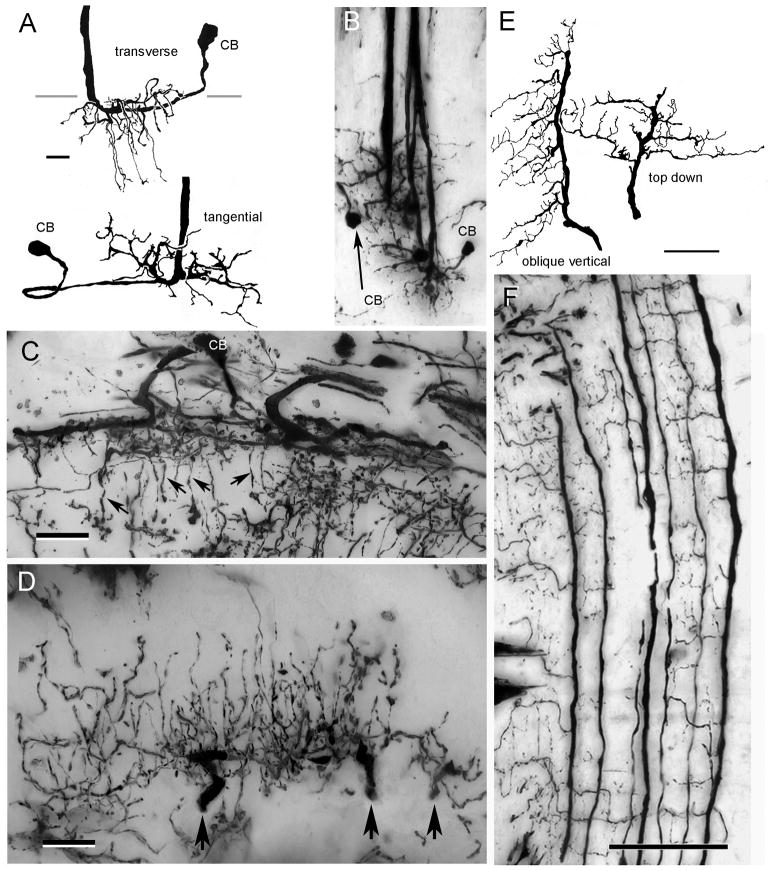
Fig. 9.
Type 1 tangential neurons. A. C. granulatus: The medulla dendrites of Tan 1 consist of a narrow bush-like structure extending through about a quarter of the medulla’s depth. The soma lies above the medulla’s outer surface amongst axons of the first optic chiasma. B. C. granulatus: Camera lucida drawings showing lamina arborizations oriented along the verticalaxis of the lamina. Between 6 and 8 thick processes originate from the axis fiber to provide a bistratified arrangement of branches that further ramify to spread laterally across 10–12 cartridges (vertical gray lines). Note the dense arborizations in the central part of the terminal field (boxed in upper drawing). C. H. oregonensis: Vertical branches of the type 1 tangential (extreme left in panel) show with their horizontally-directed collaterals from which arise smaller vertically oriented processes. Together these provide a rather messy looking rectilinear network. D. H. oregonensis: enlargement of the central component of a Tan 1 terminal showing the peculiar crested component (boxed) at the distal most level of the plexiform layer. E. As shown in reduced silver preparations of H. oregonensis, these crests occur at every optic cartridge. Scale bar =10 μm.
Fig. 10.
Type 2 tangential neurons. A. C. granulatus: the wide field dendrites of this cell type reside superficially in the medulla, some sending long slender branches to deeper levels (upper drawing, transverse view). Top-down views (lower drawing and panel B) show these dendrites extending radially from the axon. Neurites lead from these dendritic fields to elongated cell bodies (CB) near the medulla surface. C. H. oregonensis: transverse section of the medulla showing similar dendritic components from which arise slender processes into medullary columns (arrows). D. H. oregonensis: traverse section of the lamina showing three adjacent terminal branches of one type 2 tangential (at arrows) giving rise to a dense arrangement of varicose processes that ascend through the lamina’s plexiform layer. E. C. granulatus camera lucida drawings showing terminal arbors of two type 2 tangential neurons. F. C. granulatus: vertical section showing en masse impregnated type 2 tangential endings. Adjacent “giant” processes provide orthogonally directed collaterals from which arise ascending processes. Scale bars for A, B=10 μm, C, D = 25 μm, for E, F = 50 μm.
There is also a major difference between the two species with respect to the morphology of elements lying immediately beneath the proximal tangential layer, that we here term the “giant” or type 2 (Tan 2) tangentials (Fig. 2A, D, J). In both C. granulatus and H. oregonensis each of these Tan 2 neurons possesses a thick axon (5–7 μm) that originates from a system of shallow processes in the medulla (Fig. 10A–C). Viewed from above, these extend more or less radially from the axon’s origin (Fig. 10B). In longitudinal and transverse sections, the thick primary dendrites that lie on the surface of the medulla give rise to thinner collaterals that extend deep into the medulla neuropil. Each medulla dendritic tree is connected to a very large elongated cell body located just above the medulla’s outer surface (Fig. 10A–C).
In C. granulatus, axons of these type 2 lamina tangential neurons provide very large primary process located beneath PTL that extend along the vertical axis of the lamina. Golgi impregnations (Fig. 10 E, F), together with Bodian preparations (see Fig. 4D in Sztarker et al., 2005), suggest that these primary processes of adjacent Tan 2 neurons are separated by approximately the distance of one optic cartridge. Because the elongated lamina of Chasmagnathus contains about 100 × 70 rows of cartridges in the vertical and horizontal axes respectively, this suggests that there are approximately 100 Tan 2 neurons per lamina. Each primary tangential process in the lamina provides many long secondary branches that extend approximately at right angles to it. Each secondary process is separated from the next by the width of an optic cartridge (Fig. 10F) so that each Tan 2 neuron provides a system of approximately parallel secondary processes that extend horizontally along rows of optic cartridges. Secondary processes give rise to short tertiary branches that ascend outwards into the lamina’s plexiform layer.
Type 2 tangentials are differently organized in H. oregonensis, however. Whereas the medulla components are almost identical in this species (Fig. 10C), the primary branches of the lamina terminals are separated by 2–3 cartridge widths (Figs. 2J, 10D). Like those of C. granulatus, primary branches extend the length of each vertical row and give off short stout horizontal collaterals. These then provide an extremely dense filigree of ascending processes that form basket-like ensembles around clusters of cartridges (Fig. 2C, 10D).
3. Lamina T-cells
T-neurons are retinotopic cells so named because of the T-like junction between cell body fiber and the functional axon. T-cells connecting the lamina and medulla have their somata located in the optic chiasma, usually closer to the medulla than to the lamina.
Lamina T-cells have been identified in decapods and across many insect orders. Their identification in so many different species has been used to emphasize similarities between insects and crustaceans (Hanström, 1928; Strausfeld and Nässel, 1980). Lamina T-cells are thus considered one of the most conserved elements in the optic lobes of these two groups (Strausfeld et al., 2006). The first description of lamina T-cells, in dipterous insects, showed small distal basket-like arborizations occupying a single lamina cartridge (Cajal and Sanchez, 1915) to which it supplies short spiny processes that are now known to be postsynaptic to amacrine cells and photoreceptor terminals (Campos-Ortega and Strausfeld, 1973). Lamina T-cells are thus efferent neurons and in Diptera are thought to provide for the first stage in non-directional motion computation (Sinakevitch and Strausfeld, 2004; Higgins et al., 2004).
In C. granulatus, type 1 T-cells share some characteristics with insect T1-cells. The type 1 neuron in C. granulatus has a very slender axis fiber that divides soon after entering the plexiform layer. Its two tributaries provide parallel climbing fibers from which multiple small knob-like projections arise (Fig. 11A). The lateral spread of the dendritic field of type 1 T-cells is equal to the width of lamina occupied by the tiered photoreceptor terminals. Intracellular recordings of T-neurons in the crayfish suggest that these cells might receive a photoreceptor input, acting as a functional analogue of a monopolar cell (Wang-Bennett and Glantz, 1987).
Fig. 11.
Small (Type 1) and wide-field (Type 2) T-cells. Four variants of type 1 T-cell dendritic arbors in C. granulatus. Processes originate from two, almost parallel processes in the lamina that arise from a slender axis fiber. These give off many small varicose and pinhead-like specializations. B. The assumed medulla component of these T-cells consist of a swelling from the axon that tapers to provide several short branches within the outer strata of the neuropil. A very short neurite links this component to the soma, which is located just above the medulla. C. Homologous dendritic trees in the lamina of H. oregonensis. D. Four different views of Type 2 T-cell dendrites in C. granulatus. The axon gives rise to several stout V-shaped divisions that provide a system of short spine-like specializations. The dendritic trees extend through several optic cartridges. E. Their medulla components consist of narrow cascades of spiny processes connected by a neurite to somata located just above the medulla. F. Type 2 T-cell dendrites in H. oregonensis give rise to clusters of specializations, each cluster located at an optic cartridge. All scale bars = 10 μm.
Due to the width of first optic chiasma and the slender nature of type 1 T-cell axons, we were unable to follow any type 1 T-cell axon along its entire length. However, narrow arbors extending into the medulla sends axons out towards the lamina. A very short neurite links each axon to a cell body located just above the medulla (Fig. 11B). This organization is reminiscent of the T1 terminals described from dipterous insects and decapods (Hanström, 1928; Strausfeld, 1970; Fischbach and Dittrich, 1989). Golgi impregnations of H. oregonensis show similar morphologies of type 1 T-cells (Fig. 11C). Differences with respect to the branching patterns and varicose specializations suggest considerable variation within this cell type but not several discrete species of neuron.
A second type of T-cell has, however, been consistently resolved across the lamina in both species. In C. granulatus this neuron is characterized by an altogether thicker axis fiber than the type 1 neuron. This penetrates about one third of the depth into the plexiform layer before giving rise to 3–4 V-shaped stout tapering branches that extend laterally from it. These bifurcate into third and fourth order processes (Fig. 11D) that extend through the distal half of the plexiform layer, that is through the outer tier of receptor terminals. The dendritic field of this cell type extends through about 3 cartridges along the vertical axis. The medulla arborizations of this type 2 T-cell has narrower spread, ends relatively shallowly and gives rise to many very thin liana-like processes (Fig. 11E). A short neurite connects the axon to its soma, which is located just distal to the medulla. In H. oregonensis the type 2 T-cell differs from the South Atlantic species with respect to its processes in the lamina (Fig. 11F) but not its medulla component (not shown). In the lamina three clusters of processes are aligned along the vertical axis and supply three adjacent cartridges, ascending through the entire depth of the plexiform layer.
4. Small-field Centrifugal cells
The term centrifugal neuron designates retinotopic elements that, in insects, extend out to the lamina from cell bodies located beneath the inner face of the medulla (Strausfeld, 1970). Their axis fibers in the medulla provide dendritic processes that lead to an axon that crosses optic chiasma to terminate in the lamina’s external plexiform layer (Cajal and Sanchez, 1915; Strausfeld, 1970). Comparable elements have been described from several malacostracan species by Eloffson and Hagberg (1986). In both C. granulatus and H. oregonensis there are a number of elements that can be ascribed to this class of neurons (Fig. 12). These all share the following features. A single axon extending out from the medulla gives rise to one or more ascending processes in the lamina decorated with knob-like projections or with swellings and varicosities. Although the cell bodies and medullary components of these neurons have not yet been confidently identified, nevertheless because none of these elements extend more distally than the outer margin of the plexiform layer, we assume that their somata are located beneath or just above the medulla.
Fig. 12.
Centrifugal endings. A. C1 wide-field terminals in the lamina of C. granulatus consist of thin beaded processes that extend laterally across several optic cartridges (vertical grey lines) from a stout terminal. B. C2 dense centrifugal endings also consist of a stout terminal segment that provide short beaded branches that mainly extend asymmetrically from one side of the axis fiber. A similar ending is in the lamina of H. oregonensis (D, second panel from left). In C. granulatus these processes are densest in the distal levels of plexiform layer whereas in H. oregonensis they are distributed through the whole depth. C. Variations of narrow field centrifugal terminals (C3) in C. granulatus are equipped with sparse varicose processes. D. centrifugal terminals in H. oregonensis. Left panel: narrow field terminal (C3), C2, right hand panels show the two levels of wide-field beaded terminals. Abbreviations as in Fig. 4. Scale bar = 10 μm.
At least three clearly different morphological types of these putative centrifugal cells have been identified in C. granulatus. Four cells have been resolved in H. oregonensis. Two of these are comparable to the cell types in the South Atlantic species. In C. granulatus the C1 centrifugal neuron arborizes diffusely through at least four cartridges of the lamina where it provides thin collaterals equipped with swellings distributed uniformly through the plexiform layer (Fig. 12A). C2 exhibits a predominantly unilateral system of short blebbed branches along most of its length, becoming especially dense nearer the distal tangential layer (Fig. 12B). These terminals also provide some larger branches that extend across the neighboring cartridge. The same cell type has been identified in H. oregonensis (Fig, 12D, second from left). In C. granulatus C3 neurons are narrow, equivalent to the width of an optic cartridge and have short lateral processes equipped with large swellings or irregular varicosities (Fig. 12C). In the North Pacific species, only one narrow field centrifugal has been thus far identified. It has a single ascending fiber from which are arranged a series of unilateral varicosities (Fig. 12D, left panel). Two other centrifugal endings are both wide-field and consist of a sparse arrangement of beaded processes. These endings are arranged at two levels: coincident with the inner tier of receptor endings, or coincident with the outer tier (Fig. 12D, right two panels).
5. Amacrine cells
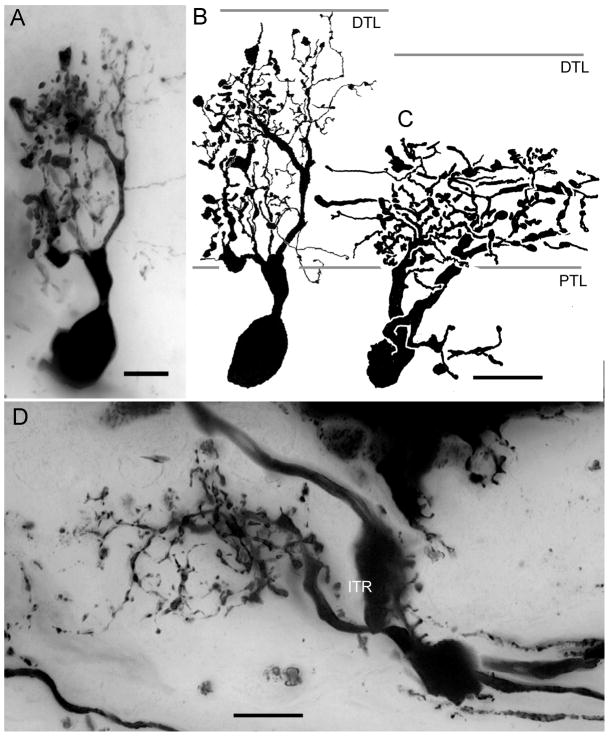
One morphological type of anaxonal or amacrine cell has been found in the lamina of C. granulatus and other in the North Pacific species (Fig. 13). Both are derived from large cell bodies beneath the lamina amongst axons of the first optic chiasma. The general morphology of these amacrine cells are consistent with those described by Glantz et al. (2000) from crayfish and by Strausfeld and Nässel (1980) from Squilla mantis. In C. granulatus, a single thick trunk-like process arises from the cell body and ascends to the plexiform layer where it provides one or more radially arranged basket-like processes equipped with numerous branches. Some of these processes are thin and spiny, other have thickened appearance and are decorated with varicosities and knob-like structures (Fig. 13A, B). The lateral spread of the arbor extends across two cartridges along the horizontal axis and two along the vertical axis of the retinotopic mosaic, thus encompassing an oblong domain that includes at least 4 cartridges. Amacrine cells in H. oregonensis look quite different. Their processes extend mainly through the inner tier of photoreceptor terminals (Fig. 13C, D). However, like amacrines in the lamina of C. granulatus, their arborizations are decorated with both spiny and varicose specializations.
Fig. 13.
The lamina amacrine cells of C. granulatus (A, B) and H. oregonensis (C, D). Amacrine cell bodies are located beneath the lamina and one or two neurites give rise to several densely branched processes. In C. granulatus these ascend through the whole depth of the plexiform layer whereas in H. oregonensis they extend through approximately the inner half, coincident with the deep tier of photoreceptor endings. Amacrine cell processes have features of both dendrites (spines) and terminals (varicosities and beads). ITR: inner receptor tier. Scale bar A, B = 5 μm, C, D = 10 μm.
DISCUSSION
Conserved elements of the decapod lamina
Studies by Nässel (1975, 1976, 1977), Eloffson and Hagberg (1986), and Stowe et al. (1977) agree on the identification of four types of photoreceptor terminals that end at four distinct levels in the visual systems of eucarid crustaceans. The axon of one photoreceptor of each ommatidia ends in the medulla (these are the long visual fibers), while the other seven photoreceptors have axons that end in the lamina. Some of these seven receptors contribute to one of two tiers of endings; others provide club-shaped terminals that penetrate through most or the lamina’s entire plexiform layer. In Pacifastacus, M3 and M4 neurons parse the retinular cell input into two mutually exclusive channels. M3 synapses with four of the retinular terminals in the outer tier, whereas M4 synapses with the other three belonging to the inner tier (Nässel and Waterman, 1977). Although the levels of photoreceptor cells appear less precise in C. granulatus than they do in H. oregonensis or in some of the other described decapods, no significance should be attached to this: the tiered arrangements, even if sloppy, nevertheless correspond to the correspondingly variable levels of the dendritic branches from monopolar cells. Tiered laminas, then, appear to be the rule within the eumalacostracan crustaceans. But they are not exclusive to this group of arthropods. Even though more has been written about the quite simple untiered laminas of flies than the same neuropil in other insects, tiered lamina may be as numerous across the insects as across the malacostraca. Species of Coleoptera, Odonata, and Hemiptera show that the levels of dendritic branches from monopolar cells and other elements coincide with different levels of receptor axon terminals in their laminas (Strausfeld, 1976). For example, in the Backswimmer Notonecta glauca, an aquatic hemipteran, amacrine cell processes coincide with deepest tier of photoreceptor terminals, as do amacrines in H. oregonensis. Like the two grapsoid species described here, tangential neurons in N. glauca distribute to two layers of the plexiform layer and in that insect monopolar cell dendrites correspond to the different levels of photoreceptor endings. Some monopolar cells provide dendrites at one tier; others at a deeper tier and still others have dendrites arranged down the whole length of the axis fiber. The widths of monopolar cell dendrites also vary in insects, even in those species that are diurnal. Again, exceptions to this are the laminas of cyclorrhaphan Diptera which are populated by narrow field monopolar cells, the dendrites of which are restricted to a single cartridge. This exemption, not the rule, provides the limited interests of students of lamina synaptology.
The present study identifies the characteristic decapod arrangement of two types of tiered monopolar cells, the M3 and M4 neurons, and two types of monopolar cells that have dense arrangements of dendrites through the depth of the plexiform layer, the narrow and broad M2 neurons (M2n, M2b). To these are added those neurons whose dendrites are less than half as dense, the diffuse M2 cells (M2d). All these cell types have been observed anywhere across the lamina and Golgi studies show that the smallest distance separating adjacent impregnated neurons is equivalent to the distance between two adjoining cartridges. These monopolar cells, then, provide five clearly defined efferent channels from each cartridge. It is not known if wide-field monopolar cells (M5 and M6 and their variants) are similarly distributed although there is no reason to suggest that they are not. Certainly the midget monopolar cells (variants of M1) occur in every cartridge although variations in the number and stratification of their dendritic specializations suggest that they are unlikely to receive inputs from photoreceptor terminals but may instead receive inputs from tangential neurons, just as the midget L5 monopolar cell does in the dipterous lamina (Strausfeld and Nässel, 1980).
The grapsoid lamina also provides two types of T-cell efferents. These are the narrow field type 1 and wide-field type 2 T-cells. Golgi preparations suggest that both occur in each cartridge because two neurons have been seen so impregnated. Assuming that both types of T-cell are postsynaptic in the lamina, these would provide an additional couple of efferent channels from each cartridge bringing the total to eight output channels if the M1 neurons are included and ten outputs if M5 and M6, the two species of wide-field monopolar cells, are also allowed in this tally. This is twice the number of output channels from an optic cartridge of a fly, but is not unexpected of many insects that have refined color perception and employ linear polarized light for navigation. In C. granulatus, the presence of sustaining and dimming medullary neurons that have orthogonal e-vector sensitivity maxima (Tuthill and Tomsic, unpublished data) similar to those present in Pacifastacus (Glantz and McIsaac, 1998) suggests that crabs also detect linearly polarized light although there is no evidence that they possess a specialized dorsal zone of the retina that serves this function.
Commonalties and differences in two related species
Terminological differences should not confuse an appreciation of the types of monopolar cells common to the Decapoda. That Hafner’s account (1973) annotates these neurons B1–B11 does not change the observation that his description identifies variants of the midget monopolar cell M1, the axial monopolar cell M2, the tiered monopolar cell M3, and the wide-field monopolar cell M5. The appellation as “M5” by Stowe et al. (1977) for a monopolar seen in Scylla serrata with dendrites at the level of the outer tier of receptor terminals is unfortunate because the morphology of this element best corresponds to the crook-neck variant of the M3 (and, in Scylla, M4; loc. cit. Fig. 7G, H) monopolar shown here and the M4b variant in Pacifastacus leniusculus described by Nässel (1977). Therefore, despite some terminological inconsistencies, we can conclude that five principle types of monopolar cells M1–M5 are common across species. Cell types that are probably new, in that they are described here for the first time, are the diffuse and broad diffuse variants of the M2 neurons and the M6 monopolar cell.
Do similarities of the M1–M4 neurons across taxa suggest that cell organization in the decapod lamina is unusually conserved? Here it is necessary to be cautious because, as described here, the morphology and organization of three classes of neuron reveal some important differences between the S. Atlantic and N. Pacific species.
The first difference refers to the type 2 tangential neurons, the processes of which in C. granulatus extend part of the way through the plexiform layer and appear diffusely organized from the main tangential branches whereas in H. oregonensis the homologous cell type provides basket-like clusters of terminal processes that ascend through the whole plexiform layer. Clearly, their synaptic relationships with either photoreceptor terminals and, or, monopolar cells, are likely to be different, at least in number if not in connections. Nevertheless, in both species of grapsoid crabs the dendritic fields of these cells have similar arrangements in the outer layers of the medulla.
A second and surprising difference between the two species relates to their small-field centrifugal neurons. Other authors seldom describe these afferent neurons, but when they have descriptions suggest rather disparate morphologies. Wide-field centrifugal neurons in C. granulatus, such as C1 shown in Fig. 12A have not been identified in H. oregonensis. They could, at a stretch, be compared to centrifugal neuron morphology described by Elofsson and Hagberg (1986) from observation of the anostracans Polyartemia forcipata and Siphonophanes grubei, but because the branchiopods have only two optic lobe neuropils, with the deeper of the two equivalent to the lobula plate (Strausfeld, 2005), such comparisons would be unjustified. The second morphological type of centrifugal terminal, C2, shown in Fig. 12B, has also been identified in H. oregonensis (Fig. 12D, second panel from the left) but a similar morphology has not been described from other species. Further comparisons between even these two related grapsoids break down. In C. granulatus, small-field centrifugal cells are generally constrained to a width of lamina corresponding to one or just slight more than one optic cartridge (Fig. 12C). One variant of a narrow centrifugal cell in C. granulatus, shown in Fig. 12C (right profile), is similar to centrifugal neurons described from P. leniusculus (Nässel, 1977). In H. oregonensis, the closest analogue to this neuron is a terminal in the lamina that is clearly constrained to an extremely narrow field, suggesting its relationship with one or both of the axial monopolar cell M2b or M2n (Fig. 12D, left panel). Other wide-field centrifugal terminals in H. oregonensis branch either in the inner or outer tier of receptor terminals and thus far have no counterpart in C. granulatus
The amacrine neuron supplying the lamina of C. granulatus is consistent with the description of amacrine cells in crayfish based on immunostaining with an antibody against a tachykinin-related peptide (TRP) and by GABA immunocytochemistry (Glantz et al., 2000). These authors showed that although the number of TRP-immunoreactive amacrines is well outnumbered by optic cartridges, the amacrine cell processes contribute to an isomorphic network across the entire retinotopic mosaic and arborize through all levels of the lamina. In contrast, amacrine cells in H. oregonensis do not invade the whole lamina depth but have their processes mainly constrained to the inner half of the plexiform layer, aligned with the inner tier of photoreceptor terminals. In both species, however, the branches of amacrine neurons have features typical of both dendrites and terminals, which is consistent with their suggested functional relationship with photoreceptor terminals (Glantz et al., 2000): namely, that of providing a mechanism for lateral inhibition necessary for both contrast enhancement and gain control.
Ecology and optic lobe organization
How significant are the described similarities and differences between the present grapsoid species? First, we consider their similarities. The organization of their layered photoreceptor terminals and monopolar cells conforms to a highly conserved organization of tiered photoreceptor terminals described not only from other decapods but also from non-decapod malacostracans, such as Squilla mantis and other gonodactyloid stomatopods, and from anostracans (Kleinlogel and Marshall, 2005; Elofsson and Hagberg, 1986). The present identification of “novel” morphologies of monopolar cells is probably less significant that it may appear because this cell type may well be present in other species of Decapoda but so far not resolved by the Golgi method. Where possibly significant differences occur relate to systems of centrifugal cells, not monopolar cells; namely, the type 2 tangential neurons and some of the small and wide-field afferents to the lamina that originate from the medulla. If an argument is to be made that such morphological differences relate to ecological constraints, then at most these differences probably reflect different adaptation mechanisms to a range of luminances experienced by C. granulatus that may be different from those typical of habitats occupied by H. oregonensis. However, on balance, there are more shared features between the laminas of these two species than unshared ones. Such differences should, however, be of interest to physiologists wanting to relate centrifugal and amacrine morphologies with lateral inhibitory mechanisms and light adaptation. But as ascertained from studies across species of one order of insect (Buschbeck and Strausfeld, 1996), ecological constraints probably impose fewer differences of neuronal organization at the level of photoreceptor terminals than at deeper levels of the system that are involved in the higher order computation of pattern and motion.
Acknowledgments
Grant Support: This study was supported by a postdoctoral fellowships from the National Research Council of Argentina (CONICET) to J. Sztarker; research grants from the Universidad de Buenos Aires, grant number X 173, ANPCYT and PICT 12300/02 to D. Tomsic; a grant from the NIH NCRR (2-PO1 NS28495-11) to N. J. Strausfeld; and a Patricia L. Dudley Endowment Scholarship to David Andrew.
References
- Bachmann S, Martinez MM. Feeding tactics of the American oystercatcher (Haematopus palliatus) on Mar Chiquita Coastal lagoon, Argentina. Ornitología Neurotropical. 1999;10:81–84. [Google Scholar]
- Backwell PR, Christy JH, Telford SR, Jennions MD, Passmore NI. Dishonest signaling in a fiddler crab. Proc R Soc Lond B Biol Sci. 2000;267:719–724. doi: 10.1098/rspb.2000.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berón MP. Dieta de juveniles de gaviota cangrejera (Larus atlanticus) en estuarios de la provincia de Buenos Aires. Hornero. 2003;18:113–117. [Google Scholar]
- Bodian D. A new method for staining nerve fibers and nerve endings in mounted paraffin sections. Anatl Record. 1937;69:153–162. [Google Scholar]
- Buschbeck EK. Neurobiological constraints and fly systematics: How different types of neural characters can contribute to a higher level dipteran phylogeny. Evolution. 2000;54:888–898. doi: 10.1111/j.0014-3820.2000.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Buschbeck EK, Strausfeld NJ. Visual motion-detection circuits in flies: small-field retinotopic elements responding to motion are evolutionarily conserved across taxa. J Neurosci. 1996;16:4563–4578. doi: 10.1523/JNEUROSCI.16-15-04563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SR, Sanchez D. Contribucion al conocimiento de los centros nerviosos de los insectos. Parte I. Retina y centros opticos. Trab Lab Invest Biol Univ Madrid. 1915;13:1–168. [Google Scholar]
- Campos-Ortega JA, Strausfeld NJ. Synaptic connections of intrinsic cells and basket arborisations in the external plexiform layer of the fly’s eye. Brain Res. 1973;59:119–136. doi: 10.1016/0006-8993(73)90255-2. [DOI] [PubMed] [Google Scholar]
- Cannicci S, Barelli C, Vannini M. Homing in the swimming crab Thalamita crenata: a mechanism based on underwater landmark memory. Anim Behav. 2000;60:203–210. doi: 10.1006/anbe.2000.1458. [DOI] [PubMed] [Google Scholar]
- Diesel R, Schubart CD, Schuh M. A reconstruction of the invasion of land by Jamaican crabs (Grapsidae: Sesarminae) J Zool. 2000;250:141–160. [Google Scholar]
- Dohle W. Are the insects terrestrial crustaceans? A discussion of some new facts and arguments and the proposal of the proper name ‘Tetraconata’ for the monophyletic unit Crustacea + Hexapoda. Ann Soc Entomol. 2001;37:85–103. [Google Scholar]
- Elofsson R, Hagberg M. Evolutionary aspects of the construction of the first optic neuropil (lamina ganglionaris) in Crustacea. Zoo-Morphology. 1986;106:174–178. [Google Scholar]
- Elofsson R, Nässel D, Myhrberg H. A catecholaminergic neuron connecting the first two optic neuropiles (lamina ganglionaris and medulla externa) of the crayfish Pacifastacus leniusculus. Cell Tiss Res. 1977;182:287–297. doi: 10.1007/BF00219765. [DOI] [PubMed] [Google Scholar]
- Fischbach KF, Dittrich APM. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res. 1989;258:441–475. [Google Scholar]
- Glantz RM, Miller CS, Nässel DR. Tachykinin-related peptide and GABA-mediated presynaptic inhibition of crayfish photoreceptors. J Neurosci. 2000;20:1780–1790. doi: 10.1523/JNEUROSCI.20-05-01780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz RM, Mc Isaac A. Two-channel polarization analyzer in the sustaining fiber-dimming fiber ensemble of crayfish visual system. J Neurophysiol. 1998;80:2571–2583. doi: 10.1152/jn.1998.80.5.2571. [DOI] [PubMed] [Google Scholar]
- Glenner H, Thomsen PF, Hebsgaard MB, Sorensen MV, Willerslev E. The Origin of Insects. Science. 2006;314:1883–1884. doi: 10.1126/science.1129844. [DOI] [PubMed] [Google Scholar]
- Greiner B, Ribi WA, Warrant EJ. A neural network to improve dim-light vision? Dendritic fields of first-order interneurons in the nocturnal bee Megaflops genalis. Cell Tiss Res. 2005;322:313–320. doi: 10.1007/s00441-005-0034-y. [DOI] [PubMed] [Google Scholar]
- Hafner GS. The neural organization of the lamina ganglionaris in the crayfish: a Golgi and EM study. J Comp Neurol. 1973;152:255–280. doi: 10.1002/cne.901520304. [DOI] [PubMed] [Google Scholar]
- Hafner GS. The ultrastructure of retinula cell endings in the compound eye of the crayfish. J Neurocytol. 1974;3:295–311. doi: 10.1007/BF01097915. [DOI] [PubMed] [Google Scholar]
- Hanström B. Vergleichende Anatomie des Nervensystems der wirbellosen Tiere. Berlin: Springer-Verlag; 1928. pp. 1–624. [Google Scholar]
- Higgins CM, Douglass JK, Strausfeld NJ. The computational basis of an identified neuronal circuit for elementary motion detection in dipterous insects. Vis Neurosci. 2004;21:567–586. doi: 10.1017/S0952523804214079. [DOI] [PubMed] [Google Scholar]
- Kleinlogel S, Marshall NJ, Horwood JM, Land MF. Neuroarchitecture of the color and polarization vision system of the stomatopod Haptosquilla. J Comp Neurol. 2003;467:326–342. doi: 10.1002/cne.10922. [DOI] [PubMed] [Google Scholar]
- Kleinlogel S, Marshall NJ. Photoreceptor projection and termination pattern in the lamina of gonodactyloid stomatopods (mantis shrimps) Cell Tissue Res. 2005;321:273–284. doi: 10.1007/s00441-005-1118-4. [DOI] [PubMed] [Google Scholar]
- Li YS, Strausfeld NJ. Morphology and sensory modality of mushroom body extrinsic neurons in the brain of the cockroach, Periplaneta americana. J Comp Neurol. 1997;387:631–650. [PubMed] [Google Scholar]
- Nalbach H-O. Discontinous turning reaction during escape in soldier crabs. J Exp Biol. 1990;148:483–487. [Google Scholar]
- Nalbach H-O, Nalbach G. Distribution of optokinetic sensitivity over the eye of crabs: its relation to habitat and possible role in flow-field analysis. J Comp Physiol A. 1987;160:127–135. [Google Scholar]
- Nardi F, Spinsanti G, Boore JL, Carapelli A, Dallai R, Frati F. Hexapod origins: monophyletic or paraphyletic? Science. 2003;299:1887–1889. doi: 10.1126/science.1078607. [DOI] [PubMed] [Google Scholar]
- Nässel DR. The organization of the lamina ganglionaris of the prawn, Pandalus borealis (Kroyer) Cell Tissue Res. 1975;163:445–464. doi: 10.1007/BF00218491. [DOI] [PubMed] [Google Scholar]
- Nässel DR. The retina and retinal projection on the lamina ganglionaris of the crayfish Pacifastacus leniusculus (Dana) J comp Neurol. 1976;167:341–360. doi: 10.1002/cne.901670305. [DOI] [PubMed] [Google Scholar]
- Nässel DR. Types and arrangements of neurons in the crayfish optic lamina. Cell Tissue Res. 1977;179:45–75. doi: 10.1007/BF00278462. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Elofsson R, Odselius R. Neuronal connectivity patterns in the compound eyes of Artemia salina and Daphnia magna (Crustacea: Branchiopoda) Cell Tissue Res. 1978;190:435–437. doi: 10.1007/BF00219557. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Waterman TH. Golgi EM evidence for visual information channeling in the crayfish lamina ganglionaris. Brain Res. 1977;130:556–563. doi: 10.1016/0006-8993(77)90118-4. [DOI] [PubMed] [Google Scholar]
- Nunnemacher RF. The fine structure of optic tracts of Decapoda. In: Bernhard CG, editor. The functional organisation of the compound eye. Pergamon Press; Oxford: 1966. pp. 363–376. [Google Scholar]
- Osorio D, Bacon JP. A good eye for arthropod evolution. Bioassays. 1994;16:419–424. doi: 10.1002/bies.950160610. [DOI] [PubMed] [Google Scholar]
- Patel NH, Ball EE, Goodman CS. Changing role of even-skipped during the evolution of insect pattern formation. Nature. 1992;357:339–342. doi: 10.1038/357339a0. [DOI] [PubMed] [Google Scholar]
- Regier JC, Shultz JW, Kambic RE. Pancrustacean phylogeny: hexapods are terrestrial crustaceans and maxillopods are not monophyletic. Proc Biol Sci. 2005;272:395–401. doi: 10.1098/rspb.2004.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzius G. Zur Kenntnis des centralen Nervensystems der Daphniden. Biol Untersuch Neue Folge Samson and Whalin, Stockholm. 1906;13:107–116. [Google Scholar]
- Ribi WA. The first optic ganglion of the bee. I. Correlation between visual cell types and their terminals in the lamina and medulla. Cell Tissue Res. 1975;65:103–111. doi: 10.1007/BF00222803. [DOI] [PubMed] [Google Scholar]
- Sinakevitch I, Douglass JK, Scholtz G, Loeser R, Strausfeld NJ. Conserved and convergent organization in the optic lobes of insects and isopods, with reference to other crustacean taxa. J Comp Neurol. 2003;467:150–172. doi: 10.1002/cne.10925. [DOI] [PubMed] [Google Scholar]
- Sinakevitch I, Strausfeld NJ. Chemical neuroanatomy of the fly’s movement detection pathway. J Comp Neurol. 2004;468:6–23. doi: 10.1002/cne.10929. [DOI] [PubMed] [Google Scholar]
- Stowe S, Ribi WA, Sandeman DC. The organization of the lamina ganglionaris of the crabs Scylla serrata and Leptograpsus variegatus. Cell Tissue Res. 1977;178:517–532. doi: 10.1007/BF00219572. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. Golgi studies on insects. Part II. The optic lobes of Diptera. Philos Trans R Soc Lond. 1970;258:135–223. doi: 10.1098/rstb.1970.0033. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. Atlas of an insect brain. Springer; Berlin, Heidelberg, New York: 1976. [Google Scholar]
- Strausfeld NJ. Crustacean-insect relationships: the use of brain characters to derive phylogeny amongst segmented invertebrates. Brain Behav Evol. 1998;52:186–206. doi: 10.1159/000006563. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. The evolution of crustacean and insect optic lobes and the origins of chiasmata. Arthropod Struct Dev. 2005;34:235–256. [Google Scholar]
- Strausfeld NJ, Nässel DR. Neuroarchitecture of brain regions that subserve the compound eyes of crustaceans and insects. In: Autrum H, editor. Handbook of sensory Physiology. VII/6B. Berlin: Springer Verlag; 1980. pp. 1–132. [Google Scholar]
- Strausfeld NJ, Douglass J, Campbell H, Higgins C. Parallel processing in the optic lobes of flies. In: Warrant Eric, Nilsson Dan-Eric., editors. Invertebrate Vision. Cambridge University Press; 2006. pp. 349–398. [Google Scholar]
- Sztarker J, Strausfeld NJ, Tomsic D. Organization of optic lobes that support motion detection in a semiterrestrial crab. J Comp Neurol. 2005;493:396–411. doi: 10.1002/cne.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsic D, Massoni V, Maldonado H. Habituation to a danger stimulus in two semiterrestrial crabs. Ontogenic, ecological and opioid system correlates. J Comp Physiol A. 1993;173:621–633. [Google Scholar]
- Wang-Bennett LT, Pfeiffer C, Arnold J, Glantz RM. Acetylcholine in the crayfish optic lobe: concentration profile and cellular localization. J Neurosci. 1989;9:1864–1871. doi: 10.1523/JNEUROSCI.09-06-01864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeil J, Hoffmann M. Signals from ‘crabworld’: cuticular reflections in a fiddler crab colony. J Exp Biol. 2001;204:2561–2569. doi: 10.1242/jeb.204.14.2561. [DOI] [PubMed] [Google Scholar]
- Zeil J, Nalbach G, Nalbach H-O. Eyes, eyes stalks and the visual world of semi-terrestrial crabs. J Comp Physiol A. 1986;159:801–811. [Google Scholar]