Abstract
Ticks collected in 2011 were screened for the presence of filarial nematode genetic material, and positive samples were sequenced for analysis. Monanema-like filarial nematode DNA was recently discovered in Amblyomma americanum in northern Virginia, marking the first time genetic material from this parasite has been discovered in ticks in the state. Phylogenetic analysis revealed that this material was directly related to a previously discovered filarial nematode in A. americanum populations in Maryland as well as recently identified parasites in Ixodes scapularis from southern Connecticut. Further study is warranted to visually confirm the presence of these nematodes, characterize their distribution, and determine if these ticks are intermediate hosts.
Keywords: Amblyomma americanum, Filarial nematode, Monanema
Introduction
The lone star tick, Amblyomma americanum, is a known vector for numerous pathogens and has a broad distribution throughout the central and eastern United States (Goddard and Varela-Stokes, 2009). This tick species is most commonly known as a vector for the bacteria that cause human ehrlichiosis, such as Ehrlichia chaffeensis, but more recently has also been implicated as vector for Heartland virus and the causative agent of southern tick-associated rash illness (STARI) (Armstrong et al., 1996; Masters et al., 2008; Savage et al., 2013). Its wide distribution, potential to transmit multiple Ehrlichia species, and aggressive feeding behavior all contribute to the recent increasing trends in ehrlichiosis cases in the United States (Childs and Paddock, 2003).
Previous research on Wolbachia endosymbiont infections in A. americanum also revealed the presence of filarial nematodes, a broad family of parasitic, arthropod-transmitted worms that can cause significant human and veterinary infections (Zhang et al., 2011). Although they are most commonly identified in insect vectors, tick species have been implicated in carrying and transmitting nematodes usually associated with veterinary infections (Brianti et al., 2012; Olmeda-Garcia et al., 1993; Otranto et al., 2012). The nematodes identified in the Wolbachia study were most closely related to the genus Acanthocheilonema (Zhang et al., 2011). Additional studies identified a related nematode infecting Ixodes scapularis in Connecticut (Namrata et al., 2014), suggesting a novel clade of filarial nematodes associated with diverse ticks.
In order to better elucidate the prevalence and distribution of filarial nematodes in wild ticks, ticks were collected in Fairfax County, Virginia, and screened to determine the presence of filarial nematodes. The sampling location was selected due to its proximity to a reclaimed landfill that has been the focus of extensive tick investigation in the county. In this study, we identified filarial nematodes in A. americanum from Fairfax County, Virginia, and assessed their relationship with previously reported tick-associated nematodes.
Methods
Tick collections
Ticks were collected from May to August 2011, in central Fairfax County, Virginia, near a site that was the focus of an invasive Amblyomma maculatum population (GPS coordinates: 38.8492, −077.3998) (Fornadel et al., 2011). The sampling location was a park created after the introduction of a row of power lines. The area under the power lines was grass, which was surrounded on both sides by deciduous forest. The intersection of the grass and forest was marked by dense vegetation, which served as the primary sampling location for this study. Ticks were collected using drag collections and carbon dioxide traps (Gladney, 1978).
After collection, all ticks were morphologically identified as A. americanum, A. maculatum, I. scapularis, or Dermacentor variabilis (Keirans and Durden, 1998; Keirans and Litwak, 1989). Following identification, ticks were sorted by sex and life cycle stage and stored at −80 °C until processing.
Sample processing
DNA was extracted from frozen ticks using a modified version of the MasterPure Complete DNA Purification Kit (EPICENTRE Biotechnologies, Madison, WI). Ticks were homogenized in 50 μL of tissue cell lysis solution in a TissueLyser II (Qiagen, Valencia, CA) for 3 min at 30 Hz using a 5-mm stainless steel bead. DNA extraction from the homogenate was subsequently conducted as previously described in Henning et al. (2014).
Molecular and sequence analyses
Tick samples were screened for filarial nematodes by PCR using primers and protocols that amplified specific regions of the filarial 12S mitochondrial rRNA and cytochrome c oxidase subunit I (COI) genes (Casiraghi et al., 2001; Casiraghi et al., 2004). Previously identified positive samples were used for positive controls, and no template controls were used for negative controls. In order to confirm results and determine sequence variability, all samples positive for filarial 12S mitochondrial rRNA gene amplification were purified and directly sequenced using primers 12SF and 12SR. COI positive samples were also confirmed through sequencing using COIintF and COIintR primers. Sequence results using the 12SF primers were analyzed using BLAST (National Center for Biotechnology Information, Bethesda, MD), and alignment and phylogenetic analysis were performed using MEGA version 5.05 (Tamura et al., 2011). Phylogenetic trees were constructed using Maximum likelihood using the general time reversible (GTR) model, and tree robustness was evaluated by 500 bootstrap replications.
Results
Ticks were collected from May 18, 2011, to August 3, 2011, and a total of 1223 A. americanum were processed. Of these, 120 were male, 137 were female, and 966 were nymphs. All ticks were screened for the presence of mitochondrial 12S rRNA and COI genes. A total of 9 of the 1223 (0.74%) tested samples were positive for filarial sequences. None of the A. maculatum (N = 10), D. variabilis (N = 23), or I. scapularis (N = 1) tested positive for filarial nematodes (Table 1).
Table 1.
Infection rates of filarial nematodes in various tick species in Fairfax County, Virginia.
| Total number tested (number positive for 12S rRNA gene) | ||||
|---|---|---|---|---|
| Species | Male | Female | Nymph | Overall infection rate (%) |
| Amblyomma americanum | 120 (2) | 137 (2) | 966 (0) | 0.33 |
| Amblyomma maculatum | 9 (0) | 1 (0) | 0 (0) | 0 |
| Dermacentor variabilis | 11 (0) | 12 (0) | 0 (0) | 0 |
| Ixodes scapularis | 0 (0) | 0 (0) | 1 (0) | 0 |
| Total number tested (number positive for COI gene) | ||||
| Amblyomma americanum | 120 (2) | 137 (6) | 966 (1) | 0.74 |
| Amblyomma maculatum | 9 (0) | 1 (0) | 0 (0) | 0 |
| Dermacentor variabilis | 11 (0) | 12 (0) | 0 (0) | 0 |
| Ixodes scapularis | 0 (0) | 0 (0) | 1 (0) | 0 |
| Total number tested (number positive for filarial genes) | ||||
| Amblyomma americanum | 120 (2) | 137 (6) | 966 (1) | 0.74 |
| Amblyomma maculatum | 9 (0) | 1 (0) | 0 (0) | 0 |
| Dermacentor variabilis | 11 (0) | 12 (0) | 0 (0) | 0 |
| Ixodes scapularis | 0 (0) | 0 (0) | 1 (0) | 0 |
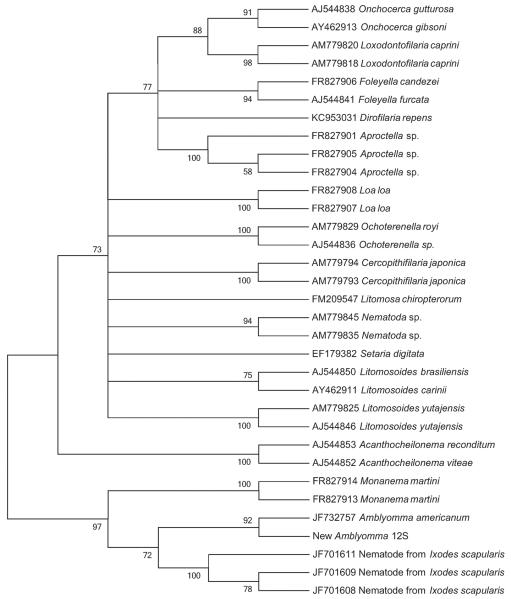
Samples that were PCR positive for filarial nematodes were sequenced to confirm the accuracy of the initial results. 12S mitochondrial rRNA sequences (440 bp) were 96–99% identical to the previously identified filarial nematode species in A. americanum (GenBank accession no: JF732757), and these differences resulted from single substitutions within the target gene (Zhang et al., 2011). The 12S sequence was deposited in GenBank under accession number KR364883. Phylogenetic analysis also showed that nematodes associated with A. americanum formed a monophyletic group with a newly identified nematode species in Connecticut I. scapularis (90% identity) (Fig. 1) (Namrata et al., 2014). This clade of tick-associated nematodes was most closely related to the nematode Monanema martini (Fig. 1).
Fig. 1.
Filarial nematode 12S mitochondrial rRNA gene maximum likelihood 50% consensus unrooted tree.
The COI gene sequence results (640 bp) were most closely related to Dirofilaria repens with 87% identity, with the exception of one sequence, that displayed 85% homology with Onchocerca dewittei japonica. COI gene sequences from this study displayed 96–99% identity with each other. The COI sequences were deposited in Gen-Bank under accession numbers KR364884 and KR364885. Lack of available COI gene sequences from representative taxa in the Gen-Bank database prevented construction of informative phylogenetic trees that were comparable to the 12S analysis.
Discussion
This study provides the first record of Monanema-like filarial nematode DNA in A. americanum ticks in Virginia. The presence of filarial nematode DNA suggests that A. americanum may serve as hosts for the developmental stages of these organisms, but it is important to note that no microscopic visualization was conducted. All samples positive for the 12S mitochondrial rRNA PCR were also positive for the presence of COI genes. Additional samples were positive for the presence of COI genes, but not 12S mitochondrial rRNA genes, which could suggest a lack of sensitivity for the 12S mitochondrial rRNA PCR.
Sequencing results from PCR positive tick samples showed that this nematode species is most closely related to previously described filarial nematodes in A. americanum in Maryland with sequence identity of 99%, and formed a monophyletic group with nematodes identified in I. scapularis in Connecticut. This tick-borne nematode clade was previously reported to be of the genus Acanthocheilonema (Zhang et al., 2011; Namrata et al., 2014), but further analysis using newly available nematode 12S sequences in the Gen-Bank database suggests that these nematodes likely belong to the genus Monanema (Fig. 1).
Having similar sequence identity and phylogenetic relationship to the filarial nematodes discovered in Maryland is not surprising due to the geographic proximity of the sampling areas. The collections from the Zhang et al. (2011) study that yielded positive results for filarial nematode infections were from areas near the Potomac River in Southern Maryland, which is approximately 20 miles from the collection site in this study. It is not implausible to think that ticks could have been transported across the river via birds, which have been documented as being parasitized by A. americanum, or mammalian hosts, thus leading to establishment of infected populations (Scott et al., 2001). Further study into filarial distribution in ticks and elucidation of vertebrate host species will be necessary to confirm any connections between these two populations.
Although there is close identity and a distinct cladistic relationship between the Monanema-like filarial nematodes found in Maryland and Virginia, it is too early to definitively state that these are the same species. Few studies have been done on the levels of intraspecific variation for 12S rRNA gene sequences, but published reports suggest that sequence identity is 97–99% and variation is below 1% (Ferri et al., 2009; McNulty et al., 2013; Otranto et al., 2011; Yatawara et al., 2007). The two nematode sequences referenced in this study fall within this range, but to make a definitive statement that these nematodes are the same species would likely require morphological identification and further phylogenetic analysis.
The low overall infection prevalence in this study is similar to that found in A. americanum ticks in Maryland, but is markedly smaller than that detected in I. scapularis populations in Connecticut (Namrata et al., 2014; Zhang et al., 2011). This phenomenon could reflect that I. scapularis is more favorable to infection and development of nematodes in this clade than A. americanum. It is also possible that differences in host species or the nematode species from this clade may result in higher parasitemia, which could bias the reported infection rates. Continued collections across expanded areas would help to elucidate the true prevalence of infection of Monanema-like nematodes within this clade in local tick populations.
Further investigation into the ecology of the Monanema-like nematodes in A. amercanum is necessary. Monanema nematodes are known to be exclusive parasites of rodents and have had microfilariae isolated from the rodent cutaneous lymphatic system (Bain et al., 1986; Junker et al., 2012). The localization of microfilariae to the cutaneous lymphatic system could allow for uptake by ticks during blood meals, but this suggestion is conjecture without investigation. It is important to note that A. americanum do not traditionally feed on small mammals in nature, making it unlikely that rodents would be the definitive host for these parasites (Childs and Paddock, 2003; Zimmerman et al., 1987). This apparent contradictory ecology needs robust study to determine if there is a biological association between this tick and nematode species or if aberrant feeding behavior led to the results observed in this study.
Conclusions
This study shows the presence of filarial nematode DNA in A. americanum tick populations in Virginia, confirming previously reported results in these ticks in the Mid-Atlantic. This study also provides a phylogenetic analysis of this species, showing its relatedness to a recently discovered nematode in I. scapularis in New England. This nematode clade appears to be related to the Monanema genus of rodent parasites, which ixodid ticks can transmit. Further study is needed to assess the ability of these tick species to propagate infections and what the definitive host species is in nature.
Acknowledgments
We would like to thank Natalie Mendez, Sara Bennett, and Pablo Quiñonez for their contributions towards tick collections, sorting, and identification. This study was supported by a National Institutes of Health Training Grant (T32AI007417) and the A. Ralph and Sylvia E. Barr Fellowship in Vector Biology to Tyler C Henning, support from the National Institutes of Health and National Institute of Allergy and Infectious Diseases (R56AI116636, R01AI067371, R21AI111175, and R21AI070178) to Jason L. Rasgon, and funding from the Fairfax County Health Department. One of the coauthors, Dr Jorge Arias passed away on September 12, 2014. His contribution to the research and writing of this manuscript was significant, and he will be missed.
References
- Armstrong PM, Rich SM, Smith RD, Hartl DL, Spielman A, Telford SR., 3rd A new Borrelia infecting lone star ticks. Lancet. 1996;347:67–68. doi: 10.1016/s0140-6736(96)91604-9. [DOI] [PubMed] [Google Scholar]
- Bain O, Bartlett C, Petit G. Une filaire de muridés africains dans la paroi du colon, Monanema martini n. sp [A filaria of African Muridae in the wall of the colon: Monanema martini n. sp] Ann. Parasitol. Hum. Comp. 1986;61:465–472. doi: 10.1051/parasite/1986614465. [DOI] [PubMed] [Google Scholar]
- Brianti E, Otranto D, Dantas-Torres F, Weigl S, Latrofa MS, Gaglio G, Napoli E, Brucato G, Cauquil L, Giannetto S, Bain O. Rhipicephalus sanguineus (Ixodida, Ixodidae) as intermediate host of a canine neglected filarial species with dermal microfilariae. Vet. Parasitol. 2012;183:330–337. doi: 10.1016/j.vetpar.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Casiraghi M, Anderson TJ, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122(Pt 1):93–103. doi: 10.1017/s0031182000007149. [DOI] [PubMed] [Google Scholar]
- Casiraghi M, Bain O, Guerrero R, Martin C, Pocacqua V, Gardner SL, Franceschi A, Bandi C. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Int. J. Parasitol. 2004;34:191–203. doi: 10.1016/j.ijpara.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- Ferri E, Barbuto M, Bain O, Galimberti A, Uni S, Guerrero R, Ferte H, Bandi C, Martin C, Casiraghi M. Integrated taxonomy: traditional approach and DNA barcoding for the identification of filarioid worms and related parasites (Nematoda) Front. Zool. 2009;6:1. doi: 10.1186/1742-9994-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornadel CM, Zhang X, Smith JD, Paddock CD, Arias JR, Norris DE. High rates of Rickettsia parkeri infection in Gulf Coast ticks (Amblyomma maculatum) and identification of “Candidatus Rickettsia andeanae” from Fairfax County, Virginia. Vector Borne Zoonotic Dis. 2011;11:1535–1539. doi: 10.1089/vbz.2011.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladney WJ. Ticks (Acarina: Argasidae and Ixodidae) In: Bram RA, editor. Surveillance and Collection of Arthropods of Veterinary Importance. Animal and Plant Health Inspection Service. U.S. Department of Agriculture; Washington, DC: 1978. pp. 102–113. [Google Scholar]
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet. Parasitol. 2009;160:1–12. doi: 10.1016/j.vetpar.2008.10.089. [DOI] [PubMed] [Google Scholar]
- Henning TC, Orr JM, Smith JD, Arias JR, Norris DE. Spotted fever group rickettsiae in multiple hard tick species from Fairfax County, Virginia. Vector Borne Zoonotic Dis. 2014;14:482–485. doi: 10.1089/vbz.2013.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker K, Medger K, Lutermann H, Bain O. Monanema joopi n. sp. (Nematoda, Onchocercidae) from Acomys (Acomys) spinosissimus Peters, 1852 (Muridae) in South Africa, with comments on the filarial genus. Parasite. 2012;19:331–340. doi: 10.1051/parasite/2012194331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans JE, Durden LA. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J. Med. Entomol. 1998;35:489–495. doi: 10.1093/jmedent/35.4.489. [DOI] [PubMed] [Google Scholar]
- Keirans JE, Litwak TR. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi River. J. Med. Entomol. 1989;26:435–448. doi: 10.1093/jmedent/26.5.435. [DOI] [PubMed] [Google Scholar]
- Masters EJ, Grigery CN, Masters RW. STARI, or masters disease: lone star tick-vectored lyme-like illness. Infect. Dis. Clin. North Am. 2008;22:361–376. viii. doi: 10.1016/j.idc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- McNulty SN, Mitreva M, Weil GJ, Fischer PU. Inter and intra-specific diversity of parasites that cause lymphatic filariasis. Infect. Genet. Evol. 2013;14:137–146. doi: 10.1016/j.meegid.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namrata P, Miller J, Shilpa M, Reddy P, Bandoski C, Rossi M, Sapi E. Filarial nematode infection in Ixodes scapularis ticks collected from Southern Connecticut. Vet. Sci. 2014;1:5–15. [Google Scholar]
- Olmeda-Garcia AS, Rodriguez-Rodriguez JA, Rojo-Vazquez FA. Experimental transmission of Dipetalonema dracunculoides (Cobbold 1870) by Rhipicephalus sanguineus (Latreille 1806) Vet. Parasitol. 1993;47:339–342. doi: 10.1016/0304-4017(93)90034-k. [DOI] [PubMed] [Google Scholar]
- Otranto D, Brianti E, Latrofa MS, Annoscia G, Weigl S, Lia RP, Gaglio G, Napoli E, Giannetto S, Papadopoulos E, Miro G, Dantas-Torres F, Bain O. On a Cercopithifilaria sp. transmitted by Rhipicephalus sanguineus: a neglected, but widespread filarioid of dogs. Parasites Vectors. 2012;5:1. doi: 10.1186/1756-3305-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D, Diniz DG, Dantas-Torres F, Casiraghi M, de Almeida IN, de Almeida LN, dos Santos JN, Furtado AP, de Almeida Sobrinho EF, Bain O. Human intraocular filariasis caused by Dirofilaria sp. nematode, Brazil. Emerg. Infect. Dis. 2011;17:863–866. doi: 10.3201/eid1705.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HM, Godsey MS, Jr., Lambert A, Panella NA, Burkhalter KL, Harmon JR, Lash RR, Ashley DC, Nicholson WL. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am. J. Trop. Med. Hyg. 2013;89:445–452. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD, Fernando K, Banerjee SN, Durden LA, Byrne SK, Banerjee M, Mann RB, Morshed MG. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J. Med. Entomol. 2001;38:493–500. doi: 10.1603/0022-2585-38.4.493. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatawara L, Wickramasinghe S, Nagataki M, Rajapakse RP, Agatsuma T. Molecular characterization and phylogenetic analysis of Setaria digitata of Sri Lanka based on CO1 and 12S rDNA genes. Vet. Parasitol. 2007;148:161–165. doi: 10.1016/j.vetpar.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Zhang X, Norris DE, Rasgon JL. Distribution and molecular characterization of Wolbachia endosymbionts and filarial nematodes in Maryland populations of the lone star tick (Amblyomma americanum) FEMS Microbiol. Ecol. 2011;77:50–56. doi: 10.1111/j.1574-6941.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RH, McWherter GR, Bloemer SR. Role of small mammals in population dynamics and dissemination of Amblyomma americanum and Dermacentor variabilis (Acari: Ixodidae) at Land Between The Lakes, Tennessee. J. Med. Entomol. 1987;24:370–375. doi: 10.1093/jmedent/24.3.370. [DOI] [PubMed] [Google Scholar]