Abstract
Background
Subthreshold depressive symptoms are common in older adults. The threshold for the clinical significance of such symptoms is unclear. Mechanisms linking depressed mood to increased risk of disability need further investigation.
Methods
Older adults who did not meet criteria for depression were divided into two groups based on the 2-item Patient Health Questionnaire (PHQ-2). Respondents reporting no anhedonia and dysphoria over the past 2 weeks were compared to respondents reporting occasional symptoms on a battery of cognitive, psychomotor, and physical performance tests.
Results
Of 290 community-resident participants without dementia or neurologic disease, 32% (n=93) reported at least one of the two depressive symptoms “several days” in the past 2 weeks. Older adults with mild depressive symptoms did not differ in ADL or IADL disability but reported more physician-diagnosed medical conditions (1.8 vs. 2.2, p < .01) and balance problems (2.9 vs. 1.8 on 0–7 scale, p < .001). Subthreshold depressive symptoms were associated with slowing in gait (p < .01), chair stand time (p < .01) and performance on Trails A (p < .05) and B (p < .001).
Conclusions
Mild depressive symptoms in people who do not meet criteria for depression are associated with slowing across multiple domains.
Keywords: depression, slowing, physical function, cognition, subsyndromal
A growing body of evidence suggests that subthreshold depressive symptoms, that is, symptoms that do not meet criteria for diagnosis of a mood disorder, are clinically significant. Subthreshold depressive symptoms have been associated with an increased risk of incident ADL, IADL, and mobility disability, as well as declines in upper- and lower-extremity function (Gallo et al., 1997; Penninx et al., 1998; Cronin-Stubbs et al., 2000; Lyness et al., 2006; Lyness et al., 2007; Barry et al., 2009; Hybels et al., 2009). Subthreshold depressive symptoms have also been associated with mild cognitive impairment (Bhalla et al., 2009), lower self-ratings of global function (Judd, et al., 2002), and an increase in physician visits and hospital days (Katon et al., 2005).
Subsyndromal depression is also highly prevalent. Across ten European countries, the mean number of depressive symptoms in seniors ranged from 2–3 on the EURO-D, which assesses the presence of 12 affective and motivational symptoms (Castro-Costa et al., 2007). Reports of four or more symptoms indicate a possible depressive disorder. Thus, depressive symptoms are common in older adults cross-nationally, which lends support to claims that subthreshold symptoms are the modal presentation of depressive illness in old age (Judd et al., 2002). Given the disability associated with depressive symptoms, interventions to prevent increases in symptoms (“indicated prevention”) are clearly important (van’t Veer-Tazelaar et al., 2009).
Subthreshold depression is defined in a variety of ways. In studies using the Centers for Epidemiological Studies-Depression scale (CESD), subthreshold depression is defined as scores in the range of 8–15 (Hybels et al., 2009) or 10–19 (Barry et al., 2009). The “no symptom” referent group includes people with scores below this range. One limitation in this approach is the grouping of people without any symptoms (say, scores of 0–2) and people with mild symptoms (scores of 3 to 7 or 9). Mild symptoms in this intermediate range may also be associated with disability. If so, this difference would suggest a need for intervention at much lower levels of depressive symptoms than typically recognized. A second limitation to use of the CESD is the association between somatic symptoms and disability. Participants endorsing fatigue or sleep problems as part of subthreshold depression, for example, may be at greater risk of disability because these represent underlying disease rather than an effect of depressed mood.
We investigated these questions in a simple of older adults who did not meet criteria for depression and who completed the brief Patient Health Questionnaire-2 (Kroenke et al., 2002). We examined the extent to which mild symptoms of depressed mood are associated with physical and cognitive performance to determine ways in which depressive symptoms may lead to disability.
Methods
Participants
Participants in this research were drawn from the Sources of Independence in the Elderly (SITE) project. SITE was designed to investigate risk factors for disability, as well as factors associated with recovery from disability, in people aged 70+. SITE is a tri-ethnic prospective study of white, African-American, and Hispanic seniors likely to experience transitions in disability. People with stroke or other neurological disease (e.g., Parkinson’s disease) or who were living in assisted housing were excluded. Inclusion criteria required that respondents report at least some difficulty with upper and lower extremity function or household activities. About a third met criteria for mild cognitive impairment.
Sampling and recruitment for SITE have been described elsewhere (Albert et al., 2006). Briefly, SITE participants were selected from the larger pool of New York City elders followed in the Washington Heights-Inwood Columbia Aging Project (WHICAP). WHICAP is a population-based survey of Medicare beneficiaries residing in northern Manhattan, New York City. In the parent WHICAP study, 2,165 people were enrolled between 1999 and 2002, with roughly equal numbers of white non-Hispanic, African-American, and Hispanic Americans. People with and without telephones were targeted, and overall 61% of eligible people agreed to participate. Comparison of the WHICAP sample to the 2000 U.S. Census for the zip code catchment area suggests no major biases in the distribution of age, gender, or race-ethnicity between the sample and source population. Eighty-five percent of eligible WHICAP participants who were approached for the study agreed to enroll in SITE. SITE participants were enrolled 2002–2004. 82% of participants completed follow-up over subsequent years.
Measures
In this research, we examined measures collected at the SITE baseline assessment. Bilingual Spanish-English research assistants conducted all assessments.
Depressive symptoms
Depressive symptoms were assessed with the Patient Health Questionnaire-2 (PHQ), a self-report elicitation consistent with DSM-IV criteria (Spitzer et al., 1999). The PHQ-2 asks respondents to report how often they have been bothered by two problems, “little interest or pleasure in doing things” and “feeling down, depressed, or hopeless.” Symptom frequency over the prior 2 weeks is scored “not at all” (0), “several days” (1), “more than half the days,” (2), or “nearly every day” (3). Responses are summed, yielding a score range of 0–6. In assessing the criterion validity of the measure, test developers established the critical value of a score of 0–2 vs. 3 or more. Using mental health professionals’ diagnoses as a gold standard, 90% of patients without a major depressive order had PHQ-2 scores less than 3, while 83% of patients who met criteria for a major depressive disorder had scores of 3 or greater (Kroenke et al., 2003). This sensitivity and specificity is similar to results for the full PHQ-9 (Spitzer et al., 1999). Based on this algorithm, SITE participants with scores of 3 or greater were considered to have possible depression and were excluded. The remainder was considered to have no symptoms (score of 0) or mild symptoms (score 1–2).
Physical Performance
Lower-extremity function was assessed with the Short Physical Performance Battery (SPPB) (Guralnik et al., 1994). The SPPB consists of a 4-meter walk to assess gait speed, progressively more challenging static balance tests (side-by-side, semi-tandem, and full tandem stand), and a chair stand test. Performance is categorized by quartile of performance using population-based norms. Scores across the three domains are summed to yield a score of 0–12, with scores of 10–12 representing best performance. We examined individual components of the SPPB as well as the composite score.
Cognitive Status
To identify dementia, all participants completed a neuropsychological evaluation. This assessment was conducted by the bilingual testers and covered the domains of memory, language, and executive function. Results from cognitive assessments were reviewed in a consensus conference, along with other information, to exclude dementia diagnoses, which were based on NINDS-ADRDA criteria (McKhann et al., 1984).
To assign mild cognitive impairment status, subtests of the memory, language, and executive function assessments were converted to z-scores and summed to create separate composites. Assessment of memory included total and delayed recall from the Selective Reminding Test (Bushke and Fuld, 1974) and recognition from the Benton Visual Recognition Test (Benton, 1955). Assessment of language included performance on the Boston Naming Test (Kaplan, et al., 1983) and repetition and comprehension from the Boston Diagnostic Aphasia Examination (Goodglass and Kaplan, 1983). Assessment of executive function included letter and category fluency (Benton and Hamsher, 1976) and the Trailmaking Test (Reitan, 1958). In the Trails A test, respondents are timed in how long it takes them to draw lines connecting a series of circles in consecutive numeric order. In the Trails B, respondents alternate between a numeric and letter sequence. The verbal fluency tests ask respondents to name as many words as possible in 60 sec beginning with the letters C, F, and L (or Spanish equivalents based on word frequency) and animal names. Composites were converted to T scores using a regression model to adjust for differences in age, education, and race. Respondents scoring below 1.5 SD on any of the composites using these normative corrections were considered to meet criteria for mild cognitive impairment (Manly et al., 2005).
Tests of psychomotor speed included the Grooved Pegboard and Moberg Pick-Up tests. In the pegboard test, respondents are timed placing small steel pegs in 30 notched holes in consecutive order (Lafayette Instruments, Lafayette, IN). The test was stopped at 240 sec and a peg/sec score was calculated to account for people unable to place all pegs in the allotted time. In the Moberg test, people are timed picking up a variety of small items (e.g., coin, screw) and moving them between containers (Moberg, 1958). We did not occlude vision.
Medical and Functional Status
Participants reported physician-diagnosed medical conditions, including myocardial infarction, angina, congestive heart failure, hypertension, diabetes, arthritis, stroke, cancer, and chronic obstructive pulmonary disease (COPD). SITE participants also reported whether they had difficulty in the activities of daily living and upper and lower extremity function using questions from the Women’s Health and Aging Study-I (Guralnik et al., 1995).
Analyses
We examined descriptive statistics for groups defined by mild depressive symptoms. In univariate analyses, we compared the two groups using an independent samples t-test for continuous measures or X2 for proportions. We also developed analysis of variance and regression models to examine the effect of mild depressive symptoms independent of other predictors on measures of disability and cognitive and physical performance.
Results
Among the 335 participants in the SITE cohort with complete neuropsychological characterization, 25 (7.5%) were determined to meet criteria for dementia and were excluded from analyses; an additional 20 (6.0%) were excluded as having depressive symptoms of potential clinical significance (PHQ-2 scores of 3 or greater). Thus a total of 290 participants were available for analysis. Ninety-three (32.1%) reported mild depressive symptoms, with loss of interest in activities or depressed mood “several days” over the past 2 weeks. Of people reporting symptoms, 19 reported only anhedonia, 51 only dysphoria, and 23 both symptoms.
Differences between Participants with and Without Mild Depressive Symptoms
As shown in Table 1, participants reporting mild depressive symptoms did not differ from other participants in sociodemographic features, except for the proportion reporting they lived alone (33.3% of people with mild depressive symptoms, 49.7% otherwise; p < .01). In both groups the average age was about 79 years, with about 70% female. The sample as a whole completed an average of 10 years of school. Hispanics were more likely to report mild depressive symptoms, but differences did not achieve statistical significance (38.4% vs. 22.4% in African-Americans and 28.6% in whites).
Table 1.
Features of SITE Cohort, by Mild Depressive Symptoms Status
| No Symptoms (n=197) |
Mild Depressive Symptoms (n=93) |
|
|---|---|---|
| Sociodemographics | ||
| Age (yr), μ (SD) | 78.9 (5.8) | 78.9 (5.6) |
| Female, % | 65.5 | 76.3 |
| Education (yr), μ (SD) | 10.5 (4.9) | 9.4 (4.7) |
| Live alone, % | 49.7 | 33.3** |
| White, % | 71.4 | 28.6 |
| African-American, % | 77.6 | 22.4 |
| Hispanic, % | 61.6 | 38.4 |
| Medical Status | ||
| Myocardial infarction, % | 8.2 | 11.8 |
| Angina, % | 7.1 | 8.6 |
| Congestive heart failure, % | 3.6 | 5.4 |
| Hypertension, % | 59.9 | 64.6 |
| Diabetes, % | 16.2 | 18.3 |
| Arthritis, % | 58.4 | 73.1* |
| Cancer, % | 15.3 | 24.7 |
| Hip fracture, % | 2.6 | 1.1 |
| Lung disease, % | 8.1 | 9.7 |
| Number of conditions, μ (SD) | 1.79 (1.1) | 2.18 (1.2)** |
| Medications, % | ||
| Antidepressants | 9.8 | 11.2 |
| Anxiolytics | 6.0 | 4.5 |
| Cognitive Status, % | ||
| Mild cognitive impairment | 16.8 | 18.3 |
| Functional Status: Difficulty, % | ||
| Light housework | 4.6 | 7.5 |
| Preparing meals | 3.6 | 3.2 |
| Light shopping | 6.1 | 7.5 |
| Bathing | 3.6 | 7.5 |
| Dressing | 2.5 | 4.3 |
| Using utensils | 1.5 | 2.2 |
| Using toilet | 0.5 | 2.2 |
p < .05,
p < .01 by t-test or X2
Participants reporting mild depressive symptoms were more likely to report most physician-diagnosed medical conditions, but differences were significant only for arthritis (73.1% in the mild depressive symptom group, 58.4% in the group without symptoms; p < .05). In a count across conditions, the mean number (SD) of diagnosed conditions was 2.18 (± 1.1) in the mild symptom group and 1.79 (± 1.2) in people without symptoms (p < .01). The proportion who met criteria for mild cognitive impairment was similar in the two groups (16.8% in people without symptoms, 18.3% in people with symptoms). The two groups did not differ in the proportion taking an antidepressant or anxiolytic medications. The groups were also similar in self-reported IADL and ADL status.
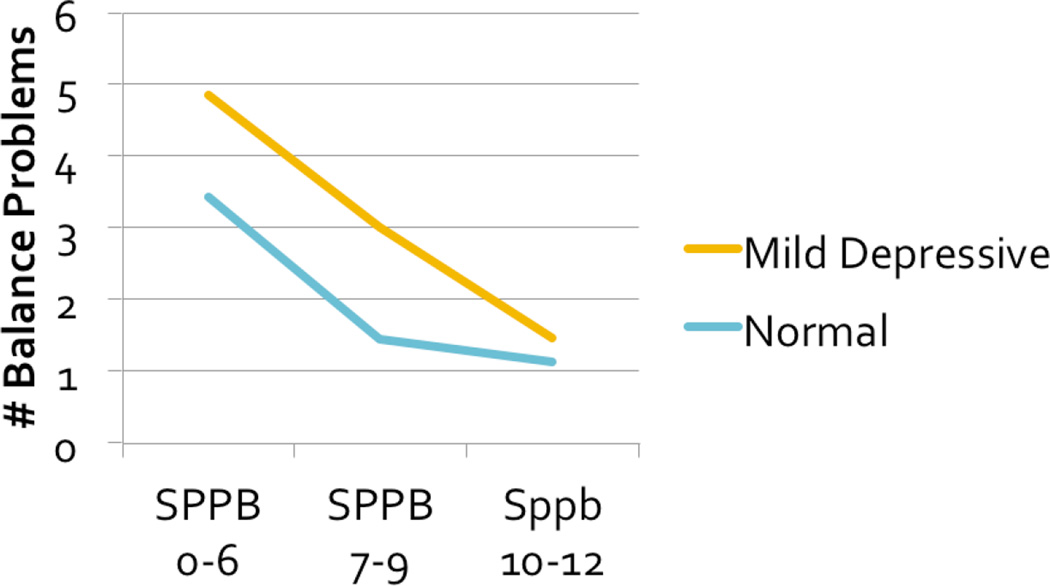
Differences between the groups were more pronounced for self-reported functional limitation, such as mobility limitation or difficulty with upper- and lower-extremity function. As Table 2 shows, people with mild depressive symptoms were significantly more likely to report difficulty with all indicators of mobility and balance except falling. Summing across the balance items (each scored dichotomously), yielded a balance problem scale with scores ranging from 0–7 and a reliability alpha of 0.79. People with mild depressive symptoms reported a significantly greater number of balance problems, even with adjustment for lower extremity performance and number of medical conditions, as shown in Figure 1.
Table 2.
Mild Depressive Symptoms and Self-Reported Functional Limitation
| No Symptoms (n=197) |
Mild Depressive Symptoms (n=93) |
|
|---|---|---|
| Self-Reported Mobility Problems, % | ||
| Walking ¼ mile | 28.9 | 41.9* |
| Walking 10 stair steps | 40.6 | 53.8* |
| Getting out of bed or chair | 16.2 | 29.0* |
| Self-Reported Balance Problems, % | ||
| Problem with balance when walking on level surface? | 34.0 | 52.7** |
| Problem with balance when standing while dressing? | 20.8 | 39.8*** |
| Problem with balance while showering? | 18.3 | 37.6*** |
| Problem with balance when walking down stairs? | 25.9 | 46.2*** |
| Fallen in past 12 months? | 28.4 | 35.5 |
| Afraid you might fall? | 29.1 | 46.7** |
| Limit activities because you might fall? | 18.9 | 32.6** |
| Sum, balance problems (scale, 0–7), μ (SD) | 1.76 (1.97) | 2.91 (2.23)*** |
| Self-Reported Upper Extremity Problems, % | ||
| Raising arms over head | 9.1 | 14.0 |
| Grasping and handling | 9.1 | 19.4* |
| Carrying 10 lbs | 23.9 | 35.5 |
p < .05,
p < .01,
p < .001 by t-test or X2
Figure 1.
Self-Reported Balance Problems and Mild Depressive Symptoms, by Mobility Status
Mild Depressive Symptoms and Slowing Across Multiple Domains
As Table 3 shows, the presence of mild depressive symptoms was associated with slower gait speed (0.73 vs. 0.81 m/sec, p < .01) and time to complete the five chair stands (14.6 vs. 12.1 sec, p < .01), though not in the static balance tests or composite SPPB score. Likewise, people reporting mild depressive symptoms were slower in completing Trails A (113.1 vs. 97.1 sec, p < .05) and B (189.9 vs. 167.3 sec, p < .001). Differences in psychomotor tests were not significant, though people with mild symptoms were slower on both the pegboard and pick-up tests. In analysis of variance models that adjusted for number of disease conditions, the presence of mild depressive symptoms remained a significant predictor of time to complete chair stands (p=.02) but was only marginally associated with gait speed (p=.06).
Table 3.
Subthreshold Depressive Symptoms and Performance Assessment: Lower Extremity Function, Executive Function, Psychomotor Speed
| No Symptoms (n=197) |
Subthreshold Symptoms (n=93) |
|
|---|---|---|
| Lower Extremity Performance Assessment | ||
| 4-meter walk speed, m/sec, μ (SD) | 0.81 (0.28) | 0.73 (0.23)** |
| Able to complete side-by-side stand, % | 100.0 | 100.0 |
| Able to complete semi-tandem stand, % | 82.2 | 82.8 |
| Seconds held, μ (SD) | 8.6 (3.1) | 8.7 (3.1) |
| Able to complete full tandem stand, % | 51.3 | 53.8 |
| Seconds held, μ (SD) | 6.6 (4.0) | 6.7 (4.0) |
| Time to complete chair stand 5×, sec, μ (SD) | 12.1 (6.7) | 14.6 (9.2)** |
| SPPB composite score, μ (SD) | 8.77 (2.8) | 8.22 (2.7) |
| Psychomotor Speed | ||
| Grooved Pegboard, peg/sec, μ (SD) | 7.8 (8.2) | 8.1 (8.4) |
| Moberg Pick-Up Test, sec, μ (SD) | 19.9 (12.5) | 22.0 (22.0) |
| Executive Function | ||
| Trails A, sec, μ (SD) | 97.1 (51.2) | 113.1 (52.9)* |
| Trails B, sec, μ (SD) | 167.3 (51.9) | 189.8 (47.5)*** |
| Letter fluency, CFL, μ (SD) | 29.2 (14.2) | 27.1 (10.8) |
| Category fluency, animals, μ (SD) | 14.4 (4.5) | 13.6 (3.5) |
p < .05,
p < .01,
p < .001 by t-test
Slowing Associated with Mild Depressive Symptoms as a Source of Self-Reported Functional Limitation
In a regression model adjusting for count of medical conditions, the number of self-reported balance problems was significantly associated with mild depressive symptoms (B=1.05, p < .0001). Adding gait speed to the model lowered the regression coefficient for mild depressive symptoms to 0.87, but mild depressive symptoms remained a significant predictor (p < .0001).
In a similar regression model that included number of medical conditions, cognitive slowing (Trails B) was associated with self-reported balance problems (p = .02). Inclusion of depressed mood in the model eliminated this significant association (p = .12).
Discussion
The association between subthreshold depressive symptoms and slowing in physical and cognitive function supports earlier claims that depressive symptoms at any level may indicate ongoing active disease at the biological level (Judd, et al., 2002). Further support for this inference comes from research showing that people with subthreshold depressive symptoms differ in immunologic parameters and other biosignatures (Lotrich et al., 2008; Akiskal et al., 1997).
Slowing in physical and cognitive performance did not fully explain the association between mild depressive symptoms and functional limitation, as represented in self-reported balance problems. Adding gait speed to regression models did not eliminate the effect of depressive symptoms on self-reported balance problems. Thus, reports of functional limitation or disability in people with mild depressive symptoms may represent a reporting bias (i.e., overestimating disability) but also the effects of slowed motor and cognitive abilities. Consistent with this reporting bias, the presence of mild depressive symptoms was associated with poorer self-reported balance but not poorer performance on tests of static balance, such as the semi-tandem and tandem stand. However, these results also suggest that the motor and cognitive slowing associated with mild depressive symptoms may also increase the risk of functional impairment directly.
The cross-sectional association between mild symptoms of depressed mood and lower extremity performance is in accord with longitudinal findings reported for the Established Populations for Epidemiologic Studies of the Aged (EPESE). Relative to older adults with baseline CESD scores of 0–2, declines in gait speed were significantly higher in people with scores 13–19, that is, people with symptoms below the standard depression threshold score of 20 (Penninx et al., 1998). In the EPESE, people with baseline CESD scores 3–6 or 7–12 also showed greater declines in gait speed over 4 years of follow-up relative to people with scores of 0–2, but these differences did not reach statistical significance.
In this research, the significant association between cognitive slowing and self-reported balance problems was not maintained once depressed mood was included in models. This result supports findings reported for the Health, Aging, and Body Composition study, which also showed that the association between executive function and decline in gait speed was no longer significant once depressive symptoms were included in models (Atkinson et al., 2007).
These cross-sectional results point to co-occurring deficits across a range of physiologic systems, in this case cognitive, physical, and affective domains. Thus, subthreshold depression may qualify as a geriatric syndrome in the sense that it is accompanied by deficits in other physiologic systems. A productive test of this formulation would be the design of randomized trials of therapies for subthreshold depressed mood that assess the effect of treatment on gait and cognitive processing speed as well as on depressed mood or progression to frank depression.
Limitations of this research include its cross-sectional design. These data cannot establish the temporal priority of subthreshold depressed mood and slowing in physical and cognitive function. However, the cross-sectional data are helpful for showing that subthreshold symptoms are associated with slowing in these other domains. They demonstrate again as well the need to re-examine established symptom thresholds, since subthreshold disease in this case was associated with physical and cognitive deficits. Subthreshold depressed mood, then, may be part of the broader dysregulation of multiple physiological systems that has been identified for other aspects of aging.
Acknowledgments
This research was supported by the National Institute of Aging (AG18234 and AG024827). Funders had no role in formulating research questions, the design of the research, data collection and analysis, or the decision to publish.
Footnotes
Conflict of Interest
The authors have no financial interest in the conduct or reporting of the study.
Description of Authors’ Roles
S.M. Albert received funding, designed the research plan, conducted study analyses, and drafted the manuscript. J. Bear-Lehman and A. Burkhardt developed study assessments, provided clinical expertise, and reviewed the manuscript for intellectual content.
References
- Akiskal HS, Judd LL, Gallin JC. Subthreshold depressions: clinical and polysomnographic validation of dysthymic, residual, and masked forms. Journal of Affective Disorders. 1997;45:53–63. doi: 10.1016/s0165-0327(97)00059-1. [DOI] [PubMed] [Google Scholar]
- Albert SM, Bear-Lehman J, Burkhardt A, Merete-Roa B, Noboa-Lemonier R. Variation in sources of clinician- and self-rated IADL disability. Journal of Gerontology: Medical Sciences. 2006;61A:826–831. doi: 10.1093/gerona/61.8.826. [DOI] [PubMed] [Google Scholar]
- Atkinson HH, et al. Cognitive function, gait speed decline, and comorbidities: The Health Aging and Body Composition Study. J Gerontol: Med Sci. 2007;62A:844–850. doi: 10.1093/gerona/62.8.844. 2007. [DOI] [PubMed] [Google Scholar]
- Barry LC, Allore HG, Bruce ML, Gill TM. Longitudinal association between depressive symptoms and disability burden among older persons. Journal of Gerontology: Medical Sciences. 2009;64A:1325–1332. doi: 10.1093/gerona/glp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL. The Benton Visual Retention Test. New York: The Psychological Corporation; 1955. [Google Scholar]
- Benton AL, Hamsher KD. Multilingual Aphasia Examination. Iowa City, IA: University of Iowa; 1976. [Google Scholar]
- Bhalla RK, et al. Patterns of mild cognitive impairment after treatment of depression in the elderly. American Journal of Geriatric Psychiatry. 2009;17:308–316. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Castro-Costa E, et al. Prevalence of depressive symptoms and syndromes in later life in 10 European countries. British Journal of Psychiatry. 2007;191:393–401. doi: 10.1192/bjp.bp.107.036772. [DOI] [PubMed] [Google Scholar]
- Cronin-Stubbs D, et al. Six-year effect of depressive symptoms on the course of physical disability in community-living older adults. Archives of Internal Medicine. 2000;160:3074–3080. doi: 10.1001/archinte.160.20.3074. [DOI] [PubMed] [Google Scholar]
- Gallo JJ, Rabins PV, Lyketos CG, Tien AY, Anthony JC. Depression without sadness: Functional outcomes of nondysphoric depression in later life. Journal of the American Geriatrics Society. 1997;45:570–578. doi: 10.1111/j.1532-5415.1997.tb03089.x. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadephia: Lea & Febiger; 1983. [Google Scholar]
- Guralnik JM, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, et al. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. NIH Publ. No. 95-4009. [Google Scholar]
- Hybels CF, Pieper CF, Blazer DG. The complex relationship between depressive symptoms and functional limitations in community-dwelling older adults: The impact of subthreshold depression. Psychological Medicine. 2009;39:1677–1688. doi: 10.1017/S0033291709005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS. The clinical and public health relevance of current research on subthreshold depressive symptoms to elderly patients. American Journal of Geriatric Psychiatry. 2002;10:233–238. [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Phila: Lea & Febiger; 1983. [Google Scholar]
- Katon WJ, et al. Cost-effectiveness of improving primary care treatment of late life depression. Archives of General Psychiatry. 62:1313–1320. doi: 10.1001/archpsyc.62.12.1313. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2. Validity of a two-item depression screener. Medical Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG, Kirshner M, Ferrell RF, Reynolds CF. Serotonin transporter genotype interacts with paroxetine plasma levels to influence depression treatment response in geriatric patients. Journal of Psychiatry and Neuroscience. 2008;33:123–130. [PMC free article] [PubMed] [Google Scholar]
- Lyness JM, et al. Outcomes of minor and subsyndromal depression among elderly patients in primary care settings. Annals of Internal Medicine. 2006;44:496–504. doi: 10.7326/0003-4819-144-7-200604040-00008. [DOI] [PubMed] [Google Scholar]
- Lyness JM, et al. The clinical significance of subsyndromal depression in older primary care patients. American Journal of Geriatric Psychiatry. 2007;15:214–223. doi: 10.1097/01.JGP.0000235763.50230.83. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Bell-McGinty S, Tang M-X, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Archives of Neurology. 2005;62:1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Moberg E. Objective methods for determining the functional value of sensibility in the hand. Journal of Bone and Joint Surgery. 1958;40B:454–476. doi: 10.1302/0301-620X.40B3.454. [DOI] [PubMed] [Google Scholar]
- Penninx BWJH, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJH, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. Journal of the American Medical Association. 1998;279:1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. Journal of the American Medical Association. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- van’t Veer-Tazelaar, et al. Stepped-care prevention of anxiety and depression in late life. Archives of General Psychiatry. 2009;66:297–304. doi: 10.1001/archgenpsychiatry.2008.555. [DOI] [PubMed] [Google Scholar]