Abstract
Emotion dysregulation has been associated with increases in many forms of psychopathology in adolescents and adults. The development of effective emotion regulation skills is important during adolescence, especially as stressful life events increase during this time. The current study examined two emotion regulation strategies, cognitive reappraisal and affective suppression, in interaction with self-report and biological measures of emotional reactivity as predictors of internalizing symptoms. A community sample of adolescents (n = 127), at an age of risk for depression and anxiety, completed self-report measures of emotional reactivity and internalizing symptoms. In addition, they completed a modified social stress task and were assessed on biological measures of reactivity and regulation. Findings suggested that the trait tendency to reappraise was associated with a reduced impact of emotional reactivity on depressive, but not anxiety symptoms. Implications for shared and specific aspects of emotional reactivity and regulation are discussed.
Keywords: Emotion Regulation, Cognitive Reappraisal, Cortisol, Emotional Reactivity, Depressive Symptoms, Anxiety Symptoms
A growing body of research has examined the relationship between emotional processes and psychopathology (Eisenberg, Spinrad & Eggum, 2010). This examination generally has focused on risk factors that divert normal developmental trajectories to those that may lead to maladjustment (Cichetti & Cohen, 2006). The ability to regulate emotions “consists of the extrinsic and intrinsic processes responsible for monitoring, evaluating, and modifying emotional reactions, especially their intensive and temporal features, to accomplish one’s goal” (Thompson, 1994, pp. 27–28). Thus, emotion regulation is an ongoing process of an individual’s pattern of responding to contextual demands (Aldao, 2013). Difficulties with one’s emotional response can impair functioning and contribute to the development of psychopathology (for reviews see Cole, Michel, & Teti, 1994; Kovacs, Joorman, & Gotlib, 2008; Eisenberg et al., 2010; Yap et al., 2007).
Emotion dysregulation has proven to be difficult to define, as it has been associated with concurrent measures of psychopathology and prospective problematic behaviors and emotional responses (Cole, Martin & Dennis, 2004; Keenan, 2000; Mennin et al., 2005). Adding to the intricacy is that emotionality is manifested across multiple systems including subjective experiences, behavioral responses, and physiological changes (Tracy, Klonsky, & Proudfit, 2014). Consistent with recent reviews, the current manuscript considers emotion dysregulation to be an umbrella term that captures a problematic pattern of emotional intensity, duration, and frequency, and a failure to effectively regulate these emotions (Gross & Jazeira 2014). Emotional reactivity has been characterized as the emotional response to an event that may vary between individuals in terms of intensity, the speed at which it reaches peak, and return from this peak back to baseline (Davidson, 1998; Rothbart & Derryberry, 1981). Emotion regulation has been defined as the progression of attending to and activating processes that modulate emotional experiences that can be effortful or implicit (see Sheepes, Suri, & Gross, 2015 for a recent review). Thus, there is an inherent interplay between emotional reactivity and subsequent regulation as conceptually distinct but related constructs.
Emotional reactivity can occur through multiple systems and differ in intensity and duration between individuals. Indeed, these differences have been described in prior research highlighting affective chronometry in measurement of timing of peak response to and recovery from a stressor (Davidson, 1998), and thus, delineating between these two aspects of response are important. Individuals who report more intense and labile emotions report more problem behaviors and more depressive symptoms (Silk, Steinberg, & Morris, 2003). Emotional reactivity also has been linked to clinical levels of anxiety (Carthy et al., 2010) and the development of depressive and anxiety disorders in adulthood (McLaughlin et al., 2010). Additionally, the relationship between one’s perception of stress and internalizing symptoms may be influenced by physiological arousal (Sontag & Graber, 2010). Individuals who biologically reacted more strongly to a social stress task had higher levels of depressive symptoms over time (Morris et al., 2012).
Emotion regulation strategies have been shown to help mitigate the maladaptive response to stress (Moriya & Takahashi, 2013; Vandererhasselt et al., 2014). In his seminal work, Gross (2001) postulated that emotions trigger behavioral and physiological processes that can be modulated at various stages. The two broad stages occur prior to the full emotional response (antecedent-focused) or after the emotional response is underway (response-focused). In line with these stages, cognitive reappraisal is an antecedent-focused strategy that changes one’s cognitions at the outset of an event, whereas expressive suppression attempts to change one’s emotional experiences by inhibiting the response (Gross, 1998). Numerous studies have shown that individuals with a tendency to cognitively reappraise are more effective at regulating their emotions and tend to experience reduced psychopathology, whereas expressive suppression is less effective and associated with higher levels of symptoms (Aldao et al., 2010; D’Avanzato et al., 2013; Moore, Zoellner, & Mollenholt, 2008). Additionally, emotion regulation strategies may modulate physiological responses to stressful tasks (Kim & Hamann, 2012; Lam et al., 2009; Steptoe & Vogele, 1986), yet few studies have examined whether trait cognitive reappraisal modulates the relationship between one’s biological response to stress and symptom presentation. A recent meta-analytic review examined the relationship between emotion regulation strategies and multiple forms of psychopathology (Aldao, Nolen-Hoeksema, & Schweizer, 2010). Results from this review suggest that individuals with poor regulatory processes exhibit higher levels of psychopathology in adulthood (Aldao et al., 2010).
The transdiagnostic association between emotional processes and internalizing symptoms is of particular importance (Hofmann et al., 2012). Depression and anxiety disorders are the most prevalent classes of mental illnesses with high rates of comorbidity (Kessler et al., 2005), which calls into question the relative shared versus distinct characteristics of these two common disorders (Cummings, Caporino & Kendall, 2014). For example, research has highlighted commonalities between generalized anxiety disorder and depression in terms of symptom presentation, higher order emotionality, and possible etiological factors (Mennin et al., 2008). Indeed, there are multiple aspects of emotional processes that relate to both depressive and anxiety disorders, in terms of heightened emotional intensity, poor emotion understanding, negative reactivity, and maladaptive management of emotions (Mennin et al., 2007). Importantly, heightened emotional reactivity and deficits in emotion regulation are not only the product of anxiety and depression, but also a risk factor for these conditions. Heightened levels of emotional reactivity during young adulthood have been shown to predict a lifetime course of both anxiety and depression in a long-term longitudinal study (McLaughlin et al., 2010). Additionally, emotion regulation deficits have been shown to predict both anxiety and depression separately over a five-year period (Berking et al., 2014; Wirtz et al., 2014). Thus, both emotional reactivity and poor emotion regulation may directly impact the development of internalizing symptoms, yet further examination of emotional processes is needed to elucidate their nature as shared or distinct risk factors for anxiety and depression.
The second decade of life is a particularly relevant developmental period within which to study the relationship between emotion processes and psychopathology. Adolescence is a period of increased stress (Ge et al., 1994) and heightened risk for the development of psychopathology, especially anxiety and depression (Cichetti & Rogosch, 2002; Kessler et al., 2005). Adolescence also may mark an important transition in normative development of emotional reactivity (Romeo, 2010). Some researchers report that adolescence is a time of significant increases in emotional reactivity and greater sensitivity to stressors (Casey et al., 2010; Diener, Sandyik, & Larson, 1985; Somerville et al., 2010; Yap et al., 2007). Additionally, adolescence may be a pivotal time during which emotion regulation strategies are particularly needed (Compas, 1987) and emotion regulation may develop in tandem with physical development (Silvers et al., 2012). Emotion regulation strategies may develop in part as a result of brain maturation during adolescence (McRae et al., 2012), suggesting an interplay between biological and environmental factors. Indeed, the tendency to effectively regulate one’s emotions has been shown to mitigate risk factors for the development of internalizing symptoms in early adolescence (Ford et al., 2014). The confluence of developmental processes, increases in environmental stressors, and the increased risk for the development of psychopathology in adolescence makes studying emotion regulation strategies as a means to reduce vulnerability to psychopathological symptoms in response to stress particularly important during this period.
Although prior research has advanced our understanding of the importance of emotional processes in psychopathology, more research is needed to clarify this relationship, especially during the pivotal period of adolescence. Studies have examined the beneficial effects of cognitive reappraisal on self-reported symptoms (e.g., Garfenski & Kraaij, 2006), yet have not examined these tendencies in combination with measures of emotional reactivity. Cognitive reappraisal may be particularly important during times of stress, yet little research examines the effects of cognitive reappraisal in paradigms that include a stressor (Hofmann et al., 2009; Troy et al., 2010) to examine whether the tendency to reappraise modulates the effects of emotionality on symptom presentation. Indeed, in their call to advance the field of affective science, Tracy and colleagues (2014) suggest that research should employ multiple measures of emotional reactivity, focus on the time course of emotional response including rise and return to baseline, assess multiple types of emotion regulation, distinguish between reactivity and regulation, and elucidate disorder-specific patterns of emotional processes.
Taking these suggestions into account, the current study aimed to examine two emotion regulation strategies, cognitive reappraisal and affective suppression (Aldao et al., 2010; Gross, 2001; Gross & John, 2003), in interaction with both self-report and biological (heart rate and cortisol) measures of stress reactivity as predictors of depressive, social anxiety, and physical anxiety symptoms in adolescents. Stress reactivity was assessed in response to a standard social stress paradigm. Consistent with prior research, we hypothesized that heightened emotional reactivity and poor emotion regulation skills would be directly related to higher symptoms. Specifically, we predicted that higher levels of emotional reactivity, measured with self-report and biological indicators, would be directly related to increased levels of both anxiety and depressive symptoms. Additionally, consistent with prior work, we predicted that cognitive reappraisal would be directly associated with decreased symptoms, whereas affective suppression would be associated with increased symptoms. Finally, consistent with theories of emotion (Lazarus & Folkman, 1984), we hypothesized an interaction between these distinct aspects of emotional processes. Namely, we predicted that effective emotion regulation (i.e., tendency to cognitively reappraise) would moderate the relationship between emotional reactivity and both anxiety and depressive symptoms, such that emotional reactivity would be associated with lower symptoms for adolescents with higher trait levels of reappraisal. In contrast, we hypothesized that less effective emotion regulation (i.e., affective suppression) would not moderate the relationship between emotional reactivity and symptoms, as prior experimental research has shown that expressive suppression does not modulate the relationship between arousal and experience of emotion in the short-term (e.g., Gross & John, 2003).
Methods
Participants and Procedures
Participants in the current study included 127 adolescents drawn from a larger longitudinal sample initially recruited from the XXX area when they were 12–13 years old (See XXX et al., 2012 for further recruitment information and inclusion/exclusion criteria). Participants were re-assessed bi-annually in this larger study. The current sample was 49% female, 47% Caucasian, and on average 15.28 years old (SD = 1 year) at the time of this study. A series of independent samples t tests and a chi-square test were conducted to assess potential differences between the overall sample and the current study sample on demographic variables (gender, race, and family income). Participants in the current study did not differ based on gender (t = 1.072, p > .05), race (t = 0.016, p > .05), or income (χ2 = 10.39, p > .05) from the larger sample.
All participants who were scheduled to come back to the laboratory at 3:00 PM or later during the recruitment window for the current study were approached about the inclusion of a new stress task, and those who chose to participate signed additional written assent (and a parent signed additional written consent). Research suggests that the time of day affects cortisol levels (Gunnar and Vazquez, 2001; Kudielka et al., 2004); therefore, adolescents only completed the stress task after 3:00 PM (Mean = 5:07 PM) in order to standardize assessment times. No other inclusion or exclusion criteria were used. Therefore, participants had an equal chance of participating in the current study and were not selected based on additional criteria. After consenting, participants were brought into the interview room. During the baseline period, participants were asked about common factors that influence biological responses to stress and then completed questionnaires until the baseline period was over and the additional stress task was administered.
Social Stress Task
The Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993) is a widely used method to elicit a stress response (Gunnar, Talge, & Herrera, 2009). The original task was modified for adolescents with instructions that participants would be applying for a summer job and to speak about why they should be accepted. Instead of a 2–3 person audience, a video camera was placed in front of the participant, and they were told that their performance would be rated by an expert panel of judges and that those who performed the best would get a prize. After a baseline period lasting between 20–30 minutes, in which participants completed the consent forms and other measures in this study, participants were given instructions for the task. They received 5 min to prepare their speech while alone in the room. Participants then were asked to stand, face the camera, and were prompted to speak for 2 minutes. Standardized prompts were given to the participants if there were long pauses (e.g., 20 seconds), such as “You still have time remaining, please continue.” After the two min speech, an unexpected additional task was introduced as participants were asked to solve a calculation task aloud for 1 minute. They were asked to count backwards from 2,083 to zero in 13-step sequences. They were instructed to calculate as quickly and correctly as possible and if they made a mistake, the interviewer would say “error, 2,083” and they were asked to start over. Following this task, participants were told that the new task was completed and they continued to complete other study measures.
Measures
Biological Reactivity
Biological measurement of the hypothalamic-pituitary-adrenal (HPA) axis stress response used salivary cortisol, and discrete measures of heart rate were used to assess autonomic nervous system (ANS) reactivity. Saliva samples were collected with salivettes (Sarstedt AG & Co., Germany). Participants were instructed to put the salivette into their mouths for two minutes. While the salivette was collecting saliva, the participant’s ANS was assessed to obtain a multi-modal measurement of reactivity to stress. The participant’s heart rate was measured at discrete times using an Omron BP785 cuff. In addition, as a check on the subjective stressfulness at each time point, participants indicated how distressed they felt on a 10-point visual analog scale.
All saliva samples were labeled and stored frozen. Samples were assayed for cortisol using a corticosterone enzyme immunoassay kit (Arbor Assays, Ann Arbor, MI) with intra- and inter-assay coefficients of variation ranging from 6.0%–14.7% and 7.2%–10.9%, respectively. To minimize variability, all samples from each participant were assayed within the same assay batch and all samples were tested in duplicate. Duplicate test values were averaged to create the cortisol score for that time point, with values in picagrams per milliliter (pg/mL). Cortisol values that were returned below the minimum or above the maximum standard curve of comparison were re-run. If these cortisol samples did not have a readable value after they were re-analyzed, they subsequently were not used in analyses (n = 6).
Timing
Biological response to the TSST was assessed at four time points, but measurement of each system only used three of these time points (See Figure 1). As seen in Figure 1, both salivary cortisol and heart rate were measured at the end of the baseline (T1: M = 23.82 min after the participant was in the room, SD = 4.41 min). The ANS is much quicker to respond and recover from a stressor than is the neuroendocrine system. Therefore, heart rate at T2 (immediately after the stress task) was included to measure reactivity (T2: M = 16.43, SD = 3.22 min after the baseline collection). Both heart rate and cortisol were assessed at 30 min (T3: M = 30.59 min, SD = 1.28 min) after the TSST. During this time period, the measurement of HPA axis was aimed at assessing the peak cortisol reactivity, whereas the measure of ANS was aimed at assessing heart rate recovery. Finally, cortisol was measured at 60 min (T4: M = 60.74 min, SD = 2.89 min) after the TSST to measure HPA recovery. These cortisol collection times were based on meta-analytic findings indicating that peak cortisol response occurs 21–40 min following the onset of a stressor and that complete recovery occurs within 41–60 min after stressor offset (Dickerson & Kemeny, 2004). Subjective stress responses were measured at all four time points with the visual analog scale.
Figure 1.
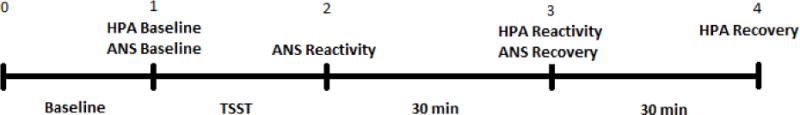
Note: Time 0 = time the participant enters the experiment room (after signing consents); Baseline = during this period participants completed questionnaires in a silent room (Mean time = 23.82 minutes); Time 1 = prior to reading the TSST instructions (measures of HPA, ANS, subjective distress); TSST = Instructions for the task were read, participants had 5 minutes of silent preparation time followed by the stress task (Mean time = 16.43 minutes); Time 2 = immediately following the TSST (measures of ANS and subjective distress); Time 3 = 30 minutes following Time 2 assessment (measures of HPA, ANS, and subjective distress); Time 4 = 30 minutes following Time 3 assessment (HPA and subjective distress).
Emotional Reactivity
The Emotion Reactivity Scale (ERS; Nock, Wedig, Holmberg, & Hooley, 2008) was used to measure trait levels of self-reported emotional reaction to events. The ERS consists of 21 items used to assess one’s emotional sensitivity (e.g., “I tend to get emotional very easily”), intensity (e.g., “I experience emotions very strongly”), and persistence (e.g., “When I am angry/upset, it takes me longer than most people to calm down”). The current study summed scores in each domain and used a total emotional reactivity score, with higher scores indicating higher levels of self-reported emotional reactivity (M = 28.08; SD = 17.73). The ERS has demonstrated good convergent and discriminant construct validity, and criterion-related validity (Nock et al., 2008). Internal consistency was α = .95 in the current study.
Emotion Regulation
The Emotion Regulation Questionnaire (ERQ; Gross & John, 2003) is a 10-item self-report measure of an individual’s use of two emotion regulation strategies: expressive suppression and cognitive reappraisal. Expressive suppression is the tendency to control emotions by not expressing them and was measured using 4-items (e.g., “I keep my emotions to myself”). Cognitive Reappraisal is the tendency to control emotions by changing the way one thinks about the situation and was measured using 6-items (e.g., “When I want to feel less negative emotion, I change the way I’m thinking about the situation”). Participants rated each item using a 7-point Likert scale (1 = strongly disagree; 7 = strongly agree). Each subscale was summed with higher scores indicating greater use of the strategy (Cognitive Reappraisal M = 27.58; SD = 6.08; Expressive Suppression M = 14.65; SD = 4.71). The ERQ has demonstrated high internal consistency and test–retest reliability (Gross & John, 2003). Internal consistency of Cognitive Reappraisal and Expressive Suppression in the current sample was α = .74 and .67, respectively.
Depressive Symptoms
The Children’s Depression Inventory (CDI; Kovacs, 1985) is the most widely used self-rating scale of depressive symptoms in youth. The CDI is designed for use with 7–17 yr. olds and consists of 27 items reflecting affective, behavioral, and cognitive symptoms of depression. Adolescents read a series of three statements (e.g., “I am sad once in a while,” “I am sad many times,” “I am sad all of the time’) and choose which statement best describes them in the past two weeks. In this sample, scores ranged from zero to 40, with higher scores indicating more depressive symptoms (M = 6.79; SD = 6.76). Internal consistency, retest reliability, and convergent and discriminant validity are well established (Klein, Dougherty, & Olino, 2005). Internal consistency was α = .87 in the current study.
Anxiety Symptoms
The Multidimensional Anxiety Scale for Children (MASC; March et al, 1997) is a 39-item self-report inventory of anxiety symptoms including the factors: physical symptoms, social anxiety, harm avoidance, and separation anxiety (March & Albano, 1998). Adolescents are asked to rate how often (e.g., “Never,” “Rarely,” “Sometimes,” or “Often”) they have been thinking and acting recently on the items (e.g., “I feel tense or uptight,” or “I’m afraid other people will think I’m stupid”). In the current study, physical and social anxiety symptoms were used with higher scores indicating more anxiety symptoms (Physical Symptoms M = 7.50; SD = 6.46; Social Anxiety Symptoms M = 8.31; SD 5.88). Retest reliability and good convergent and discriminant validity have been demonstrated (March & Albano, 1998). Internal consistency in this sample was α = .84.
Data Analysis
Consistent with prior studies, log10 transformations were used to establish a more normal distribution of residual cortisol values and to be consistent with prior literature (Gunnar and Talge, 2007; Gunnar et al., 2009). Cook’s distances were inspected to identify influential data points and were all lower than 1, indicating no generally influential values. Consistent with the hypotheses and prior studies (e.g., Harkness et al., 2011), several stress response variables were created from the log transformed cortisol data and raw heart rate data. For cortisol, analysis used (a) ‘cortisol reactivity,’ defined as T3 minus T1 and (b) ‘cortisol recovery,’ defined as T3 minus T4. In addition, for heart rate, (a) ‘heart rate reactivity’ was T2 minus T1 heart rate, and (b) ‘heart rate recovery was T2 minus T3 heart rate.
Results
Preliminary Analyses
Descriptive Statistics
Covariate analyses were conducted first to examine demographic and individual characteristics that may influence the measures of stress response. Consistent with normative changes in daily cortisol levels, correlation analysis revealed that the start time of the social stress task was related to cortisol values, such that earlier start times were related to higher baseline cortisol (Pearson’s r = −.22, p < .05). In addition, gender was correlated with baseline cortisol, such that boys had higher cortisol values than girls (Pearson’s r = −.23, p < .05). Age was significantly related to baseline heart rate (Pearson’s r = −.25, p < .01), with older participants exhibiting lower baseline heart rates than younger participants.
Bivariate correlations between all study variables are shown in Table 1. As expected, depressive symptoms were significantly correlated with both physical and social anxiety symptoms. Additionally, consistent with prior studies, self-reported emotional reactivity was associated with all three types of internalizing symptoms. Interestingly, measures of biological response were not correlated between biological systems (i.e., cortisol variables were not associated with heart rate variables), but each measure of biological reactivity was associated with the same biological measure of recovery. Additionally, only poor cortisol recovery was associated with self-reported emotional reactivity. Of note, trait cognitive reappraisal was not associated with trait expressive suppression, suggesting that participants who have a tendency to use reappraisal as an emotion regulation strategy may not also use suppression.
Table 1.
Correlation of main study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Depressive Symptoms | – | .59*** | .51*** | .60*** | −.20* | .07 | .00 | −.04 | −.08 | −.11 |
| 2 Physical Anxiety Symptoms | – | .61*** | .62*** | −.05 | .05 | −.12 | −.14 | .02 | −.22** | |
| 3 Social Anxiety Symptoms | – | .58*** | −.08 | .07 | .03 | −.05 | .00 | −.17 | ||
| 4 Emotional Reactivity Scale | – | −.06 | .06 | −.16 | −.26** | −.01 | −.12 | |||
| 5 Cognitive Reappraisal | – | .13 | .00 | .02 | .06 | −.06 | ||||
| 6 Expressive Suppression | – | .01 | .03 | .09 | −.04 | |||||
| 7 Cortisol Reactivity | – | .37*** | .01 | −.01 | ||||||
| 8 Cortisol Recovery | – | .09 | −.13 | |||||||
| 9 Heart Rate Reactivity | – | −.48*** | ||||||||
| 10 Heart Rate Recovery | – |
Note:
p < .05
p < .01
p < .001
Manipulation Check
As expected, participants’ distress ratings increased from pre to post stressor on the visual analogue scale (t = 10.21, p < .001, d = .78), which supports the effectiveness of the social stress task in activating distress. Examination of demographic differences in participants’ self-reports of distress revealed that Caucasian participants reported significantly higher levels of distress (increase from pre- to post-stress) compared to African American participants (t = 3.56, p < .01, d = .65). In addition, adolescents who qualified for free/reduced lunch (an indicator of socioeconomic status that takes into account the number of dependents supported on the family’s income) reported less distress (t = 2.29, p < .05, d = .40) than participants who did not qualify for free/reduced lunch. There were no gender or age differences in reported distress reactivity in the sample.
Primary Analyses
Main Effects of Emotional Reactivity
A series of hierarchical regressions were conducted to examine the direct associations of emotional reactivity with symptoms. Self-report and biological measures of reactivity were entered individually to examine whether each was associated with higher levels of depressive, physical anxiety, and social anxiety symptoms, covarying gender, time of assessment, and age. The main effect analyses revealed that self-reported emotional reactivity on the ERS was significantly associated with greater depressive symptoms (b = .23, t = 2.99, p < .001, R2 = .37), physical anxiety symptoms (b = .21, t = 7.99, p < .001, R2 = .43), and social anxiety symptoms (b = .18, t = 7.34, p < .001, R2 = .36). Measures of both cortisol reactivity and recovery were not significantly associated with internalizing symptoms directly. Finally, whereas heart rate reactivity was not significantly associated with internalizing symptoms, poor heart rate recovery was significantly associated with greater physical anxiety symptoms (b = −.21, t = 2.43, p = .02, R2 = .17) and social anxiety symptoms (b = .15, t = 1.97, p = .05, R2 = .09), but not with depressive symptoms (t = 1.10, p > .05).
Main Effects of Emotion Regulation
A series of hierarchical regressions were conducted to examine the direct associations of trait tendencies of emotion regulation with symptoms. Cognitive reappraisal and expressive suppression were entered individually to examine whether each was directly associated with higher levels of symptoms, covarying gender, time of assessment, and age to be consistent with other analyses. Cognitive reappraisal was significantly negatively associated with depressive symptoms (b = −.21, t = 2.16, p = .03, R2 = .07), but not with physical anxiety symptoms (t = .15, p > .05) and social anxiety symptoms (t = .56, p > .05). Contrary to hypotheses, expressive suppression was not significantly associated with any internalizing symptoms.
Interaction of Emotional Reactivity and Emotion Regulation
To examine whether cognitive reappraisal and expressive suppression moderate the relationships between emotional and biological reactivity to stress and internalizing symptoms, hierarchical linear regression was conducted. The main predictor variables of emotion regulation and emotional/biological reactivity were mean centered (Aiken & West, 1991) and then, their interaction was entered in an additional step to examine the moderating effect of emotion regulation strategies on the relationships between emotional/biological reactivity and internalizing symptoms after entering the relevant covariates. To examine the moderation hypotheses, we employed an SPSS macro (MODPROBE) to test whether there was a significant interaction (Hayes & Matthes, 2009). As seen in Table 2, cognitive reappraisal moderated the relationships between emotional/biological reactivity and depressive symptoms. More specifically, as seen in Figures 2 and 3, frequent cognitive reappraisal moderated the relationship between self-reported emotional reactivity (b = −.01, t = 2.83, p < .05, Δ R2 = .04) and poor cortisol recovery (b = .97, t = 2.01, p < .05, Δ R2 = .03) and depressive symptoms. To examine the form of the interactions, follow-up analyses examined the simple slopes at one standard deviation above and below the centered means (Aiken & West, 1991). Analysis revealed that adolescents with a lower tendency to cognitively reappraise, reported higher levels of depressive symptoms when they had higher levels of emotional reactivity than when they had lower levels of reactivity (b = .27, t = 8.65, p < .001). In addition, those with a higher tendency to cognitive reappraise, reported higher depressive symptoms when they had higher levels of emotional reactivity than when they had lower emotional reactivity (b = .13, t = 3.27, p < .01; see Figure 2). The slopes in this figure are significantly different (t = 2.69, p < .01) indicating that those who have a tendency to cognitively reappraise had lower symptoms when they are high in emotional reactivity compared to those with a lower tendency to reappraise. In the second interaction involving cortisol recovery (see Figure 3), analysis revealed that the slope was approaching significance only for lower tendency to reappraise (b = −7.39, t = 1.72, p = .08), such that among adolescents who have a lower tendency to reappraise, those with poorer cortisol recovery had marginally more depressive symptoms compared to those with higher cortisol recovery. Among adolescents with a higher tendency to reappraise, the relationship between cortisol recovery and depressive symptoms was not significant (b = 4.69, t = .99, p >.05).
Table 2.
Interaction of reactivity and cognitive reappraisal predicting symptoms
| Depressive Symptoms | Physical Anxiety Symptoms | Social Anxiety Symptoms | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | S.E. | t | Δ R2 | b | S.E. | t | Δ R2 | b | S.E. | t | Δ R2 | |
|
|
|
|
|
|||||||||
| Regression 1 | .435 | .413 | .352 | |||||||||
| Emotional Reactivity | 0.21 | 0.03 | 7.59*** | 0.21 | 0.03 | 7.52*** | 0.19 | 0.03 | 6.97*** | |||
| Cognitive Reappraisal | −.22 | 0.08 | 2.86** | −.00 | 0.08 | 0.07 | −.05 | 0.07 | 0.75 | |||
| Interaction | −.01 | 0.00 | 2.83** | .038** | −.00 | 0.00 | 0.61 | .002 | −.00 | 0.00 | 1.00 | .006 |
| Regression 2 | .060 | .108 | .078 | |||||||||
| Cortisol Reactivity | 0.46 | 2.41 | 0.19 | −3.36 | 2.25 | 1.49 | −0.05 | 2.08 | 0.02 | |||
| Cognitive Reappraisal | −.23 | 0.11 | 2.17* | −0.03 | 0.09 | 0.28 | −0.03 | 0.09 | 0.38 | |||
| Interaction | 0.33 | 0.41 | 0.80 | .006 | 0.08 | 0.38 | 0.22 | .000 | −0.19 | 0.35 | 0.56 | .003 |
| Regression 3 | .098 | .131 | .086 | |||||||||
| Cortisol Recovery | −1.35 | 3.35 | 0.40 | −1.76 | 3.14 | 0.56 | 0.01 | 2.91 | 0.00 | |||
| Cognitive Reappraisal | −0.21 | 0.10 | 2.09* | −0.03 | 0.09 | 0.31 | −.05 | 0.09 | 0.54 | |||
| Interaction | 0.97 | 0.48 | 2.01* | .034* | 0.64 | 0.45 | 1.41 | .016 | 0.37 | 0.42 | 0.88 | .007 |
| Regression 4 | .081 | .129 | .074 | |||||||||
| Heart Rate Reactivity | −.07 | 0.09 | 0.82 | 0.02 | 0.08 | 0.20 | 0.00 | 0.08 | 0.02 | |||
| Cognitive Reappraisal | −.21 | 0.10 | 2.06* | 0.02 | 0.10 | 0.17 | −.03 | 0.09 | 0.36 | |||
| Interaction | −.01 | 0.02 | 0.71 | .004 | 0.00 | 0.01 | 0.24 | .000 | −.02 | 0.01 | 0.52 | .002 |
| Regression 5 | .094 | .131 | .121 | |||||||||
| Heart Rate Recovery | −.12 | 0.09 | 1.28 | −1.76 | 3.13 | 0.56 | −.13 | 0.08 | 1.65 | |||
| Cognitive Reappraisal | −.22 | 0.10 | 2.19* | 0.03 | 0.10 | 0.31 | −.03 | 0.09 | 0.27 | |||
| Interaction | −.00 | 0.02 | 0.09 | .000 | 0.64 | 0.45 | 1.41 | .016 | 0.03 | 0.01 | 1.83 | .027 |
Note:
p < .05
p < .01
p < .001. Regression analysis included age, sex and baseline time as a covariate. For ease of presentation covariates were excluded from the table. In addition, R2 of the baseline model and interaction model were included. Δ indicates a change in R2 due to the inclusion of the interaction term
Figure 2.
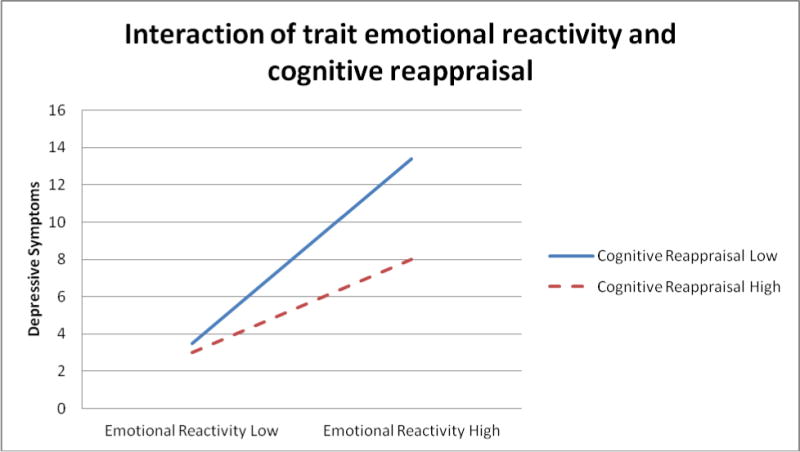
Note: Low and High signify 1 standard deviation above and below the mean.
Figure 3.
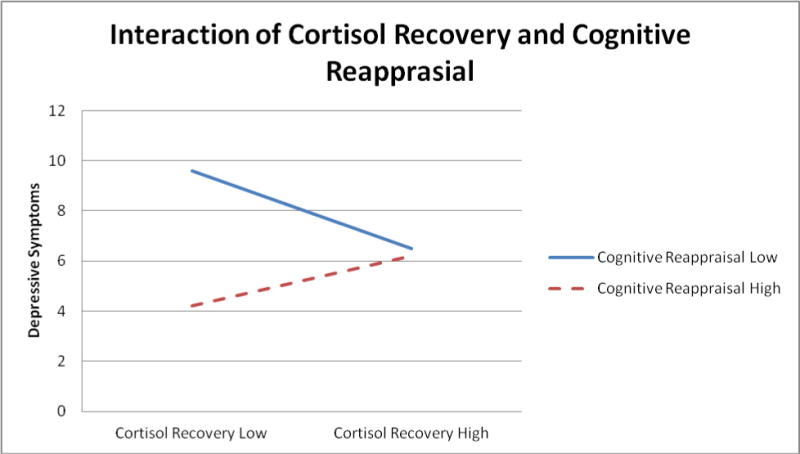
Note: Low and High signify 1 standard deviation above and below the mean.
Additionally, as seen in Table 2, cognitive reappraisal did not influence the relationship between emotional/biological reactivity and either form of anxiety symptoms. The same set of analyses was conducted to examine whether expressive suppression exacerbated the relationships between emotional/biological reactivity and internalizing symptoms. Results indicated that expressive suppression did not moderate the effects of any type of reactivity on any internalizing symptoms.
Finally, post-hoc analyses were conducted to examine the symptom specificity of the results presented (i.e., controlling for the other types of symptoms). The interaction between self-reported emotional reactivity and cognitive reappraisal remained a significant predictor of depressive symptoms after controlling for co-occurring anxiety symptoms (b = −.01, t = 2.89, p < .01, Δ R2 = .04). In contrast, the interaction between poor cortisol recovery and cognitive reappraisal became marginally significant in predicting depressive symptoms after controlling for anxiety symptoms (b = .75, t = 1.78, p = .07, Δ R2 = .02). Finally, the interactions of emotion regulation strategies with emotional/biological reactivity remained non-significant predictors of physical and social anxiety symptoms, controlling for depressive symptoms.
Discussion
The aim of this study was to examine the relationship between the combined effects of emotion regulation strategies and levels of emotional reactivity on internalizing symptoms during adolescence. Findings were largely consistent with study hypotheses and add to the literature highlighting the importance of effective emotion regulation strategies in reducing the impact of emotional reactivity on psychopathology. As expected, trait levels of emotional reactivity and aspects of autonomic response to a social stress task were associated with increased anxiety and depressive symptoms directly. Further, consistent with the main hypothesis of the study, results suggested that high levels of cognitive reappraisal were associated with reduced association between high levels of self-reported trait emotional reactivity and depressive symptoms. Additionally, high levels of cognitive reappraisal were marginally associated with reduced association between poor biological (cortisol) recovery from a stressor and depressive symptoms. These results demonstrate that the tendency to engage in cognitive reappraisal may effectively modulate emotional reactivity at multiple levels of emotional response to stress. Further analysis revealed that the beneficial effect of reappraisal differentially affected adolescents with high levels of emotional reactivity. At lower levels of emotional reactivity, the tendency to cognitively reappraise was not associated with depressive symptoms. For adolescents who had high levels of emotional reactivity and poor biological recovery, high levels of reappraisal were associated with reduced depressive symptoms compared to those with low levels of reappraisal. This is particularly relevant in adolescence, because effective emotion regulation strategies may be greatly needed as both stressors and rates of depression increase during this time. In contrast, cognitive reappraisal did not modulate the association of emotional reactivity or biological recovery with anxiety symptoms, suggesting differences between the impact of cognitive reappraisal on these forms of internalizing symptoms. Whereas prior research suggests that cognitive reappraisal may predict reduced anxiety and depression symptoms over time on its own without consideration of its moderating impact on emotional reactivity (e.g., Berking et al., 2014; Wirtz et al., 2014), the current study found that the trait tendency to reappraise was associated with lower levels of depressive symptoms specifically for adolescents with high emotional reactivity.
It is important to highlight that whereas anxiety and depression are similar in many respects and share features of emotional processes, there are distinct aspects to these disorders as well. Although there is considerable overlap in symptom presentation, there remain distinct cognitive patterns, triggering events (Mennin et al., 2008), and differences in aspects of emotional processes (Aldao et al., 2010; Mennin et al., 2007) between anxiety and depression. Whereas the current study highlights that emotional reactivity may be a shared aspect of both disorders, other components of emotional processes were not similar. Contrary to expectation, an adolescent’s trait frequency to cognitively reappraise events was directly associated with lower depressive symptoms and not anxious symptoms. Although prior research suggests that cognitive reappraisal may be associated with both anxiety and depressive disorders, when internalizing symptoms are subsyndromal, there may be specific beneficial aspects of cognitive reappraisal for depressive symptoms compared to anxiety symptoms. Indeed, in their meta-analysis of emotion regulation across psychopathologies, Aldao and colleagues (2010) found that reappraisal was significantly associated with lower depression symptoms, whereas reappraisal had a non-significant trend association with lower anxiety symptoms. This suggests that the association may be stronger for depression symptoms. However, further examination is needed to make a strong claim for this distinction.
Additionally, and in contrast, poor autonomic recovery was associated with anxiety symptoms and not depressive symptoms. Prior research has found that physiological measures of arousal may differentiate between these groups. For example, Hofmann and colleagues (2010) compared a group of anxious individuals to anxious individuals with depression and found that those with the comorbidity had heightened heart rate variability compared to those with anxiety alone, suggesting the ability of psychophysiological measures to distinguish between these individuals (Hofmann et al., 2010). Indeed, cognitions that are associated with anxiety (i.e., worry) also were more strongly associated with autonomic reactivity compared to cognitions associated with depression (i.e., rumination), highlighting possible functional distinctions between these diagnostic presentations (Aldao, Mennin, & McLaughlin, 2013). Indeed, the stronger association between reappraisal and depression, and the stronger association between ANS reactivity and anxiety may suggest that the emotion regulation measure of cognitive reappraisal in the current sample may not be as effective in dampening the association between biological reactivity to stress and anxiety symptoms. Taken together, findings suggest that it is important to further examine the common and specific aspects of emotional reactivity and regulation in relation to both anxiety and depressive symptoms to help understand the underlying dysfunction and test whether emotion reactivity and regulation are transdiagnostic factors involved in both internalizing disorders.
The results of the present study are consistent with prior theorizing and empirical support for the importance of reappraisal for emotional and psychological well-being. Longstanding theories suggest that the way in which events are appraised influences an individual’s emotional response to those events (Lazarus & Folkman, 1984). Although the current study examined reappraisal, a trait tendency to change one’s initial appraisal, the notion that how individuals views an event will impact their emotional response is consistent. In support of this conceptualization, research suggests that events that are appraised as challenging, threatening, and intense are associated with increased reactivity (Dickerson & Kemeny, 2004). Indeed, numerous studies suggest that those who appraise situations as more negative have heightened physiological responses to events (Denson, Spanovic, & Miller, 2009). The observed effects of antecedent focused cognitive reappraisal in the current study supports the notion that adolescents who have a tendency to reappraise stressful events to be less negative are able to modulate the effect of their emotional response on symptom outcomes. Indeed, research suggests that adaptive reappraisal of stressful events can modulate the physiological response and dampen stress activation (Jamieson et al., 2012). The current study extends these findings and demonstrates that reappraisal may reduce the impact of maladaptive biological recovery on depressive symptom presentation. Additionally, reappraisal dampened associations between both self-reported and biological components of emotional reactivity and symptoms, highlighting the impact of cognitive reappraisal at multiple levels of response.
Interestingly, several findings were unexpected in the current study and did not support the hypotheses. The current study did not obtain an association between expressive suppression and internalizing symptoms directly or in interaction with emotional reactivity (Aldao & Nolen-Hoeksema, 2010; Gullone & Taffe, 2012). Prior research has highlighted the importance of both the context and timing in which emotion regulation strategies are employed (Aldao & Nolen-Hoeksema, 2010). The current study only examined trait dispositional levels of emotion regulation strategies and is unable to draw more specific conclusions regarding the use of these strategies in specific contexts. Indeed, there may be differences between trait tendencies, abilities, spontaneous use of strategies, and instructed means of regulating one’s emotions (Ehring et al., 2010; McRae, 2013). A more fine-grained approach may help to determine the relationship between reactivity, emotion regulation strategies, and symptoms of psychopathology by ascertaining information about perceived stress and either the selected strategies spontaneously employed or the ratio of adaptive versus more maladaptive strategies used when a stressor is appraised negatively. It is promising to note, however, that adaptive strategies (cognitive reappraisal) were associated with reduced symptoms of depression and that maladaptive suppression did not lead to increases in depression or anxiety symptoms in this sample of mid-adolescence.
Although trait emotional reactivity was associated with internalizing symptoms, neither systems of biological reactivity were directly associated, or associated in interaction with poor emotion regulation strategies, with internalizing symptoms. Indeed, only poor heart rate recovery was directly, and poor cortisol recovery in interaction with cognitive reappraisal, was associated with symptoms. It is unclear why the biological reactivity components were not associated with internalizing symptoms, as prior research highlights this as a predictor associated with increased stress and depression/anxiety. The goal of the stress system is to effectively react to a stressor (i.e., allocate appropriate biological resources to combat the stressor) and then effectively return to homeostasis (i.e., quickly recover after the stressor is gone). Dysregulation in the stress system can occur both at the reactivity phase, as well as the recovery phase, of the stress response. Other variables such as chronic stress (Kudielka & Wust, 2010) or a maltreatment history (Harkness, Steward, & Wynne-Edwards, 2011; Heim et al., 2002; Tarullo & Gunnar, 2006) may impact an individual’s biological response to stress and may affect the reactivity component as measured in the current study. However, our findings of the association of poor recovery with depression are consistent with biological models of depression. For example, research highlights that HPA dysregulation may occur at the glucocorticoid receptor site and that those with MDD have a reduced efficiency of these receptors that serve as the conduit for recovery of the stress system (Ising et al., 2005).
Additionally, extensive research has examined the association between the TSST and measures of biological markers of stress that may have influenced the results, particularly measurement of the ANS (Campbell & Ehlert, 2012). The current study assessed ANS reactivity and recovery through a discrete measure of heart rate, which allows for a non-specific indicator of autonomic response. More rigorous continuous measures of heart rate have been used to assess an individual’s peak reactivity and the timing of recovery (Allen et al., 2014), which can use metrics such as heart rate variability, respiratory sinus arrhythmia, and pre-ejection period that would allow sympathetic and parasympathetic nervous system responses to be distinguished. Moreover, evidence suggests that the ANS may habituate quickly to the TSST and the current operationalization of reactivity may be measuring initial recovery (Hellhammer & Schubert, 2012), which should be taken into account when interpreting the results. Finally, participants responded to questionnaires during the baseline period, which may influence physiological responses to the subsequent TSST. Although prior studies using the TSST incorporate varied times and tasks during the baseline, it is important to note that this may influence the relationship between biological reactivity and outcomes in the results (Campell & Ehlert, 2012).
The interpretation of findings from the current study should take into account both its strengths and limitations. The current study included adolescent participants from a diverse community sample, both racially and economically. The non-clinical sampling design enhances the generalizability of these findings to the majority of pre-clinical adolescents who may be at risk for the development of internalizing disorders. Additionally, the current study employed multiple measures of emotionality, both biological and self-report, as well as taking a transdiagnostic approach by measuring symptoms of both anxiety and depression. The present findings also should be interpreted by considering several limitations. Although this study included measurement of emotionality at the biological and self-report levels, it relied upon self-report questionnaires for symptom and emotion regulation strategy assessment, which are subject to reporter bias. The use of clinician rated symptoms of depression and anxiety and continued examination of multi-modal assessment of emotionality in future studies may be helpful to disentangle subjective reporter bias (Monroe and Reid, 2009). The data presented were cross-sectional, and as such, cannot speak to causal implications. Future studies that incorporate prospective designs will help elucidate whether cognitive reappraisal may buffer against the effects of emotional reactivity on the development of depressive and anxiety symptoms. Future research should also incorporate a continuous measure of heart rate to allow for more specific metrics of autonomic response. Finally, although internalizing symptoms during adolescence have been shown to predict internalizing disorders in adulthood (e.g., Pine et al., 1999), it will be beneficial to examine risk for clinical levels and diagnoses of depression and anxiety in future studies.
In summary, emotional reactivity and subsequent regulation strategies are important areas of inquiry to understand risk and protective factors associated with internalizing symptoms in adolescence. The current study built on prior studies by examining emotion reactivity multimodally in relation to emotion regulation strategies and symptoms of both anxiety and depression. The current findings suggest that cognitive reappraisal, but not affective suppression, may be effective in reducing depressive symptoms when adolescents experience high levels of emotional reactivity in response to stressors. The development of a tendency to reappraise is the cornerstone of many forms of therapeutic intervention for internalizing disorders and may be particularly effective at reducing the deleterious effects of maladaptive emotional reactivity on depressive symptoms. Further examination may help to delineate shared and specific aspects of regulation strategies and emotion reactivity on internalizing psychopathology and help understand the influence of context, perception, and the temporal dynamics of this relationship, which is particularly relevant during the pivotal adolescent years.
Acknowledgments
Funding: This study was funded by NIMH grants MH79369 and MH101168 to Lauren B. Alloy and MH099764 to Benjamin G. Shapero.
Footnotes
Conflict of Interest
The authors Benjamin G. Shapero, Lyn Y. Abramson, & Lauren B. Alloy declare that they are no conflict of interest or other financial disclosures. This manuscript presents findings that have not been previously published or submitted elsewhere.
Ethical Approval: The authors have complied with APA ethical standards in the treatment of participants; this study was approved by our Institutional Review Board and is in accordance with the 1964 Helsinki declaration and later amendments. This article does not contain any studies with animals performed by any of the authors
Informed Consent: Informed consent and assent was obtained from all individual participants included in the study prior to study enrollment.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publications, Inc; 1991. [Google Scholar]
- Aldao A. The future of emotion regulation research: Capturing context. Perspectives on Psychological Science. 2013;8:155–172. doi: 10.1177/1745691612459518. [DOI] [PubMed] [Google Scholar]
- Aldao A, Mennin DS, Linardatos E, Fresco DM. Differntial patterns of physical symptoms and subjective processes in generalized anxiety disorder and unipolar depression. Journal of Anxiety Disorders. 2010;24:250–259. doi: 10.1016/j.janxdis.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Aldao A, Mennin DS, McLaughlin KA. Differentiating worry and rumination: Evidence from heart rate variability during spontaneous regulation. Cognitive Therapy and Research. 2013;37:613–619. doi: 10.1007/s10608-012-9485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neuroscience and Biobehavioral Reviews. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Black SK, Young ME, Goldstein KE, Shapero BG, Stange JP, Boccia AS, Matt LM, Boland EM, Moore LC, Abramson LY. Cognitive vulnerabilities and depression versus other psychopathology symptoms and diagnoses in early adolescence. Journal of Clinical Child and Adolescent Psychology. 2012;41:539–560. doi: 10.1080/15374416.2012.703123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berking M, Wirtz CM, Svaldi J, Hofmann SG. Emotion regulation predicts symptoms of depression over five years. Behaviour Research and Therapy. 2014;57:13–20. doi: 10.1016/j.brat.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ehrlert U. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37:1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Carthy T, Horesh N, Apter A, Gross JJ. Patterns of emotional reactivity and regulation in children with anxiety disorders. Journal of Psychopathology and Behavioral Assessment. 2010;32:23–36. [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, Somerville LH. The storm and stress of adolescence: Insights from human imaging and mouse genetics. Developmental Psychobiology. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Cohen DJ. Developmental psychopathology. Hoboken, NJ: 2006. [Google Scholar]
- Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology. 2002;70:6. doi: 10.1037//0022-006x.70.1.6. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Cole PM, Michel MK, Teti LOD. The development of emotion regulation and dysregulation: A clinical perspective. In: Fox NA, editor. The development of emotion regulation: Biological and behavioral considerations. 1994. pp. 73–100. [PubMed] [Google Scholar]
- Compas BE. Coping with stress during childhood and adolescence. Psychological Bulletin. 1987;101(3):393. [PubMed] [Google Scholar]
- Cummings CM, Caporino NE, Kendall PC. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological Bulletin. 2014;140:816–845. doi: 10.1037/a0034733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Avanzato C, Joormann J, Siemer M, Gotlib IH. Emotion regulation in depression and anxiety: Examining diagnostic specificity and stability of strategy use. Cognitive Therapy and Research. 2013;37:968–980. [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–330. [Google Scholar]
- Denson TF, Spanovic M, Miller N. Cognitive appraisals and emotions predict cortisol and immune response: A meta-analysis of acute laboratory social stressors and emotion inductions. Psychological Bulletin. 2009;135:823–853. doi: 10.1037/a0016909. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diener E, Sandvik E, Larsen RJ. Age and sex effects for emotional intensity. Developmental Psychology. 1985;21:542–546. [Google Scholar]
- Ehring T, Tuschen-Caffier B, Schnulle J, Fischer S, Gross JJ. Emotion regulation and vulnerability to depression: Spontaneous versus instructed use of emotion suppression and reappraisal. Emotion. 2010;10:563–572. doi: 10.1037/a0019010. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, Eggum ND. Emotion-related self-regulation and its relation to children’s maladjustment. Annual Review of Clinical Psychology. 2010;6:495–525. doi: 10.1146/annurev.clinpsy.121208.131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford BQ, Mauss IB, Troy AS, Smolen A, Hankin BL. Emotion regulation moderates the risk associated with the 5-HTT gene and stress in children. Emotion. 2014;14:930–939. doi: 10.1037/a0036835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnefski N, Kraaij V. Relationships between cognitive emotion regulation strategies and depressive symptoms: A comparative study of five specific samples. Personality and Individual Differences. 2006;40:1659–1669. [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, Simons RL. Trajectories of stressful life events and depressive symptoms during adolescence. Developmental Psychology. 1994;30:467–483. [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation in adulthood: Timing is everything. Current Directions in Psychological Science. 2001;10:214–219. [Google Scholar]
- Gross JJ, Jazaieri H. Emotion, emotion regulation, and psychopathology: An affective science perspective. Clinical Psychological Science. 2014;2:387–401. [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion Regulation: Conceptual Foundations. In: Gross James J., editor. Handbook of emotion regulation. New York, NY, US: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- Gullone E, Taffe J. Emotion regulation questionnaire for children and adolescents (ERQ-CA): A psychometric evaluation. Psychological Assessment. 2012;24:409–417. doi: 10.1037/a0025777. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM. Neuroendocrine measures in developmental research. In: Schmidt LA, Segalowitz SJ, editors. Developmental Psychophysiology: Theory, Systems, and Methods. Cambridge University Press; Cambridge: 2007. pp. 343–366. [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendicrinology. 2011;36:173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: A multiple regression analysis. Depression and Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Schubert M. The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology. 2012;37:119–124. doi: 10.1016/j.psyneuen.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Heering S, Sawyer AT, Asnaani A. How to handle anxiety: The effects of reappraisal, acceptance, and suppression strategies on anxious arousal. Behaviour Research and Therapy. 2009;47:389–394. doi: 10.1016/j.brat.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Fang A, Asnaani A. Emotion dysregulation model of mood and anxiety disorders. Depression and Anxiety. 2012;29:409–416. doi: 10.1002/da.21888. [DOI] [PubMed] [Google Scholar]
- Ising M, Kunzel HE, Binder EB, Nickel T, Modell S, Holsboer F. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29:1085–1093. doi: 10.1016/j.pnpbp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Jamieson JP, Nock MK, Mendes WB. Mind over matter: Reappraising arousal improves cardiovascular and cognitive responses to stress. Journal of Experimental Psychology. 2012;141:417–422. doi: 10.1037/a0025719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K. Emotion dysregulation as a risk factor for child psychopathology. Clinical Psychological Science & Practice. 2000;7:418–434. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. The effect of cognitive reappraisal on physiological reactivity and emotional memory. International Journal of Psychophysiology. 2012;83:348–356. doi: 10.1016/j.ijpsycho.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer DH. The ‘trier social stress test’ – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klein DN, Dougherty LR, Olino TM. Toward guidelines for evidence-based assessment of depression in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:412–432. doi: 10.1207/s15374424jccp3403_3. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Kovacs M, Joorman J, Gotlib IH. Emotion (dys)regulation and links to depressive disorders. Child Development Perspectives. 2008;3:149–155. doi: 10.1111/j.1750-8606.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Schommer NC, Hellhammer DK, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Wust S. Human models in acute and chronic stress: Assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress. 2010;13:1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- Lam S, Dickerson SS, Zoccola PM, Zaldivar F. Emotion regulation and cortisol reactivity to a social-evaluative speech task. Psychoneuroendocrinology. 2009;34:1355–1362. doi: 10.1016/j.psyneuen.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- March JS, Albano AM. Advances in the assessment of pediatric anxiety disorders. Advances in Clinical Child Psychology. 1998;20:213–241. [Google Scholar]
- March JS, Parker JDA, Sullivan K, Stallings P, Conners C. The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Kubzansky LD, Dunn EC, Waldinger R, Vaillant G, Koenen KC. Childhood social environment, emotional reactivity to stress, and mood and anxiety disorders across the life course. Depression and Anxiety. 2010;27:1087–1094. doi: 10.1002/da.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Hatzenbuehler ML, Mennin DS, Nolen-Hoeksema S. Emotion dysregulation and adolescent psychopathology: A prospective study. Behaviour Research and Therapy. 2011;49:544–554. doi: 10.1016/j.brat.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K. Emotion regulation frequency and success: Separating constructs from methods and time scale. Social and Personality Psychology Compass. 2013;7:289–302. [Google Scholar]
- McRae K, Gross JJ, Weber J, Ochsner KN. The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents, and young adults. Scan. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL, Fresco DM. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behaviour Research and Therapy. 2005;43:1281–1310. doi: 10.1016/j.brat.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Haloway RM, Fresco DM, Moore MT, Heimberg RG. Delineating components of emotion and its dysregulation in anxiety and mood psychopathology. Behavior Therapy. 2007;38:284–302. doi: 10.1016/j.beth.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Fresco DM, Ritter MR. Is generalized anxiety disorder an anxiety or mood disorder? Considering multiple factors as we ponder the fate of GAD. Depression and Anxiety. 2008;25:289–299. doi: 10.1002/da.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Zoellner LA, Mollenholt N. Are expressive suppression and cognitive reappraisal associated with stress-related symptoms? Behaviour Research and Therapy. 2008;9:993–1000. doi: 10.1016/j.brat.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Reid MW. Life stress and major depression. Current Directions in Psychological Science. 2009;18:68–72. [Google Scholar]
- Moriya J, Takahashi Y. Depression and interpersonal stress: The mediating role of emotion regulation. Motivation and Emotion. 2013;37:600–608. [Google Scholar]
- Morris MC, Rao U, Garber J. Cortisol responses to psychosocial stress predict depression trajectories: Social-evaluative threat and prior depressive episodes as moderators. Journal of Affective Disorders. 2012;143:223–230. doi: 10.1016/j.jad.2012.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Wedig MM, Holmberg EB, Hooley JM. The emotion reactivity scale: Development, evaluation, and relation to self injurious thoughts and behaviors. Behavior Therapy. 2008;39:107–116. doi: 10.1016/j.beth.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? American Journal of Psychiatry. 1999;156:133–5. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Adolescence: A central event in shaping stress reactivity. Developmental Psychobiology. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Derryberry D. Development of individual differences in temperament. In: Lamb ME, Brown AL, editors. Advances in developmental psychology. Vol. 1. Hillsdale, NJ: Erlbaum; 1981. [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74:1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JDE, Gross JJ, Remy KA, Ochsner KN. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012;12:1235–1247. doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag LM, Graber JA. Coping with perceived peer stress: Gender-specific and common pathways to symptoms of psychopathology. Developmental Psychology. 2010;46:1605–1620. doi: 10.1037/a0020617. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent Development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Vogele C. Are stress responses influenced by cognitive appraisal? An experimental comparison of coping strategies. British Journal of Psychology. 1986;77:243–255. doi: 10.1111/j.2044-8295.1986.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. In: Fox NA, editor. The development of emotion regulation and dysregulation: Biological and behavioral aspects. Vol. 59. 1994. pp. 25–52. (Monographs of the Society for Research in Child Development). [PubMed] [Google Scholar]
- Tracy JL, Klonsky ED, Proudfit GH. How affective science can inform clinical science: An introduction to the special series on emotions and psychopathology. Clinical Psychological Science. 2014;2:371–386. [Google Scholar]
- Troy AS, Wilhelm FH, Shallcross AJ, Mauss IB. Seeing the silver lining: Cognitive reappraisal ability moderates the relationship between stress and depressive symptoms. Emotion. 2010;10:783–795. doi: 10.1037/a0020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhasselt M, Koster EHW, Onraedt T, Bruyneel L, Goubert L, De Raedt R. Adaptive cognitive emotion regulation moderates the relationship between dysfunctional attitudes and depressive symptoms during a stressful life period: A prospective study. Journal of Behavior Therapy and Experimental Psychiatry. 2014;45:291–296. doi: 10.1016/j.jbtep.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Wirtz CM, Hofmann SG, Riper H, Berking M. Emotion regulation predicts anxiety over a five-year interval: A cross-lagged panel analysis. Depression and Anxiety. 2014;31:87–95. doi: 10.1002/da.22198. [DOI] [PubMed] [Google Scholar]
- Yap MBH, Allen NB, Sheeber L. Using an emotion regulation framework to understand the role of temperament and family processes in risk for adolescent depressive disorders. Clinical Child and Family Psychology. 2007;10:180–196. doi: 10.1007/s10567-006-0014-0. [DOI] [PubMed] [Google Scholar]


